Ectodermal appendages such as feathers, hair, mammary glands, salivary glands, and sweat glands form branches allowing a much-increased surface for functional differentiation and secretion. There are similarities among these branching organs, not only in their embryonic origin and their architecture, but also in repetitive deployment of some central signaling molecules such as BMPs, TGF-β, FGF, and MMPs, which are used in all branching organs. Details of the molecular and architectural pathways of branching, however, are context dependent. The difference in branching morphogenesis in vivo is based on chemical, mechanical, and geometric cues in the mesenchymal stroma that “sculpt” epithelial progenitors (see, for example, Nelson et al., 2006). Here, we choose the mammary gland and feathers to demonstrate these principles (Widelitz et al., 2007).
Once a branched appendage develops, its structure needs to be retained through the life of the individual. In ectodermal branching organs, much of the lush morphogenesis happens after birth. By undergoing cyclic involution and growth, mammary glands and feathers renew their branching phenotypes coupled to body hormone status and seasonal changes for the best possible functional performance (Chuong et al., 2012). Some of the molecules mediating organ specificity remain similar to those involved in organ development, yet they differ in that the former now must prevent the organs from losing their structural and functional identities, ensuring that the mammary gland and the feather, while both branching, remain distinct from each other (Bhat and Bissell, 2014).
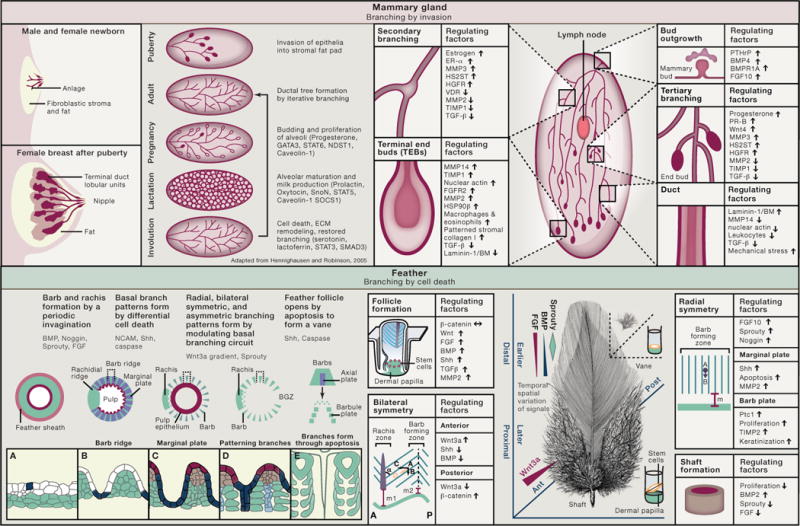
Mammary Gland: Branching by Invasion
Male and female mammals are born with a rudimentary mammary gland referred to as an “anlage.” During puberty, under the action of ovarian hormones, the embryonic anlage undergoes extensive branching by invading into the mammary fat pad in the female and ceases after expanding to the outer limits of the mesenchymal fat pad. The tree-like epithelial network is made of a bilayer of luminal duct or luminal milk-secreting cells, surrounded by myoepithelial cells and basement membrane. Myoepithelial cells provide structural and functional support for their luminal counterparts and, along with the stroma, are responsible for synthesis and organization of the basement membrane. During pregnancy, the alveolar compartment proliferates and expands to prepare for lactation, during which alveolar luminal cells synthesize milk proteins. Milk is ejected by systematic contraction of myoepithelial cells in response to suckling-mediated release of oxytocin. After weaning, the mammary gland involutes.
During branching, epithelial cells have to mobilize the necessary machinery for invasion of the growing ducts into the fat pad and the formation of secondary and tertiary branches to complete the eventual adult mammary architecture. This relies on the activities of a number of matrix metalloproteinases (MMPs) (Fata et al., 2004). Although MMPs’ proteolytic activity is central to all branching structures, recent findings show that the signaling function of a number of them is through domains other than their catalytic domains (e.g., Correia et al., 2013; Mori et al., 2013).
Whereas the epithelial compartment of human breast is separated from fat tissue by interstitial stroma, mammary epithelial structures in mouse are embedded directly in fat tissue, which distinguishes formation of mammary cancers in mice and humans.
Feathers: Branching by Differential Cell Death
The basal layer of the cylindrical epithelia of feather filament forms periodic invaginations and segregates into alternating zones of cells destined for proliferation and death. Each valley becomes a marginal plate that will undergo programmed cell death, creating space between barbs. Each ridge becomes a barb ridge with bilaterally positioned barbule plates and centrally positioned axial plates. Axial plate cells will eventually disappear to give space to opening barbules. The initial periodic patterning is triggered by activators and inhibitors that involve BMP signaling, whereas the apoptosis of marginal plates involves Shh signaling and caspase-3. At the follicular level, there is another round of apoptosis including pulp epithelia lying internal to the filament cylinder, feather sheath enclosing the filament cylinder, and barb generative zone located in the posterior follicle. Thus, feather branches open up after the branching pattern is sculpted by programmed cell deaths (Chang et al., 2004).
Feathers in different body regions serve different functions and have different branching patterns. The radially symmetric downy feathers mainly in the ventral trunk maintain the endothermy of the animal, whereas the bilaterally symmetric vaned feathers seen in the dorsal trunk and tail are used for communication. The bilateral-asymmetric feathers in the wing allow aerodynamic flight. The developing feathers begin as a cylinder, and their complex shape forms from the distal to proximal end. Feather stem cells form a ring located above the dermal papilla, a mesenchymal signaling center at the follicle base. Epithelial progenitors are displaced upward and, after a distance (marked as m, m1, m2), will undergo branching morphogenesis. The morphology (shaft or different branching patterns) reflects the cues received from the follicular microenvironment when progenitor cells are generated. A Wnt3a gradient is responsible for the tilting of the stem cell ring and parallel barb ridges toward the anterior side, thus converting radially symmetric feathers to bilaterally symmetric ones (Yue et al., 2006).
ABBREVIATIONS
- Ant
anterior
- BGZ
barb generative zone
- BM
basement membrane
- BMP
bone morphogenetic protein
- BMPR1A
BMP receptor 1A
- ECM
extracellular matrix
- ER-α
estrogen receptor α
- FGF
fibroblast growth factor
- FGFR2
FGF receptor 2
- GATA-3
GATA-binding protein 3
- HGFR
hepatocyte growth factor receptor
- HS2ST
heparan sulfate 2-O-sulfotransferase
- HSP90β
heat-shock protein 90 β
- MMP
matrix metalloproteinase
- NCAM
neural cell adhesion molecule
- NDST1
N-deacetylase/N-sulfotransferase 1
- Post
posterior
- PR-B
progesterone receptor-B
- Ptc1
patched homolog 1
- PTHrP
parathyroid hormone-related protein
- Shh
Sonic hedgehog
- SnoN
Ski-related novel protein N
- SOCS1
suppressor of cytokine signaling 1
- STAT
signal transducer and activator of transcription
- TGF-β
transforming growth factor-β
- TIMP1
tissue inhibitor of metalloproteinases 1
- VDR
vitamin D receptor
- WNT
int/Wingless
References
- Bhat R, Bissell MJ. WIREs Dev Biol. 2014;3:147–163. doi: 10.1002/wdev.130. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. J Invest Dermatol. 2004;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Randall VA, Widelitz RB, Wu P, Jiang TX. Physiology (Bethesda) 2012;27:61–72. doi: 10.1152/physiol.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. Genes Dev. 2013;27:805–817. doi: 10.1101/gad.211383.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson G. Nature Reviews Molecular Cell Biology. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD, Nelson CM, Chen CS, Zhang H, Bascom JL, et al. Development. 2013;140:343–352. doi: 10.1242/dev.084236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB, Veltmaat JM, Mayer JA, Foley J, Chuong CM. Semin Cell Dev Biol. 2007;18:255–266. doi: 10.1016/j.semcdb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Proc Natl Acad Sci USA. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]


