Abstract
Adjudin (1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide; formerly called AF-2364) has been shown to inhibit spermatogenesis by disrupting anchoring junctions at the Sertoligerm cell interface. This, in turn, leads to germ cell loss from the seminiferous epithelium, and transient infertility. Adjudin’s efficacy in inhibiting spermatogenesis, the recovery of spermatogenesis after cessation of the drug, and side effects were examined in adult male Japanese rabbits. The pharmacokinetics profiles of adjudin in rabbits after oral administration and after intravenous injection were compared. Rabbits received 25 mg/kg adjudin once weekly for 4 consecutive weeks either by intravenous injection or by gavage. Vehicle-treated rabbits were used as controls. At 1, 2, 3, 4, and 8 weeks after treatment, testes were removed for microscopic examination to assess the status of spermatogenesis. Four weeks after intravenous cessation of adjudin, the recovery of spermatogenesis also was monitored. Blood was withdrawn after first administration to measure plasma concentrations of adjudin by high-performance liquid chromatography. Four weeks after intravenous treatment, examination of testis sections showed rapid exfoliation of elongated/elongating spermatids and the presence of large multinucleated cells; more than 95% of germ cells were absent from the seminiferous epithelium. Intravenous treatment showed a more severe disturbance of spermatogenesis compared with gavage treatment, which was correlated with bioavailability of the drug. The areas under the curve for intravenous injection and gavage were 20.11 ± 1.90 and 2.23 ± 0.45 mg?h?L−1, respectively. These results illustrate the potential of adjudin as a male contraceptive, and the efficacy is associated with the bioavailability of the drug.
Keywords: Male contraception, testis, Sertoli-germ cell adhesion
During spermatogenesis, preleptotene and leptotene spermatocytes residing below the blood-testis barrier, which is formed by the inter-Sertoli cell tight junctions near the basal lamina, will migrate from the basal to the adluminal compartment of the seminiferous epithelium for further development (Russell, 1977; Mruk and Cheng, 2004b). This process is associated with extensive restructuring of cell-cell actin-based anchoring junctions. One such anchoring junction is the ectoplasmic specialization, which is found between Sertoli and germ cells at step 8 and beyond (Mruk and Cheng, 2004b). Thus, the ectoplasmic specialization could be a specific target for male contraception, because its disruption can lead to germ cell loss from the epithelium. Adjudin (1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide; formerly known as AF-2364) is a derivative of lonidamine, which specifically targets the apical ectoplasmic specialization (Cheng et al, 2005). Adjudin has been shown to remarkably disrupt anchoring junctions between Sertoli cells and elongated/ elongating, and round spermatids in adult rats (Cheng et al, 2005). Although it also affects anchoring junctions between Sertoli cells and spermatocytes but to a lesser extent, adjudin does not seem to affect the blood-testis barrier and adherens junctions between Sertoli cells and spermatogonia (Mruk and Cheng, 2004b; Lee and Cheng, 2005; Lee et al, 2005). Extensive studies from rat models showed that adjudin-induced infertility is not associated with substantial changes in serum levels of follicle-stimulating hormone, luteinizing hormone, and testosterone (Cheng et al, 2001). Furthermore, adjudininduced inhibition of spermatogenesis is also reversible, and normal spermatogenesis and fertility resume progressively after cessation of the compound (Cheng et al, 2001). Although adjudin-induced disruption of spermatogenesis has been extensively evaluated in rats, the antifertility and pharmacokinetics profiles have not been examined in other species. The present study evaluated the effects on fertility and recovery of spermatogenesis after cessation of treatment. The pharmacokinetics profiles after intravenous and oral administration of the adjudin were compared in adult male Japanese rabbits.
Materials and Methods
Chemicals and Animals
Adjudin was synthesized as described previously (Cheng et al, 2001). The compound was suspended in corn oil and used at a concentration of 25 mg/mL for gavage treatment. For intravenous injection, the compound was suspended in ethanol/DMSO (2:1, vol/vol). Carbamazepine, the internal standard, was purchased from Sigma Co (St Louis, Missouri). All other chemicals and reagents were high-performance liquid chromatography (HPLC) grade. Male adult Japanese rabbits (2.3–2.6 kg) were raised from Wenzhou Medical College Laboratory Animal Center (Wenzhou, China).
Animal Treatment
Adult male rabbits were divided into 3 groups: vehicle control, 25 mg/kg per week intravenous administration, and 25 mg/kg per week oral administration. The 25 mg/kg per week dose was selected based on the minimal effective dose of gavage in rats (Cheng et al, 2001). All animals were housed at Wenzhou Medical College Laboratory Animal Research Center. All experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wenzhou Medical College and were in accordance with the Guide for the Care and Use of Laboratory Animals. Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum. Control animals were treated with intravenous injection ethanol/DMSO (2:1, vol/vol) solution. Animals were treated intravenously or orally with 25 mg/kg per week adjudin (1 injection each week) for 4 consecutive weeks. Some animals were housed for an additional 4 weeks after cessation of intravenous or oral treatment. Blood samples (0.5,1 mL) were directly collected into the heparinized tube from the marginal ear vein at predetermined times before or after the first administration of adjudin and centrifuged at 800 × g for 10 minutes. Plasma samples were stored at −20°C for further analysis.
Sample Preparation and HPLC Detection of Adjudin
The plasma concentrations of adjudin were measured using reversed-phase HPLC and a diode array detector (DAD). Adjudin and the internal standard (carbamazepine, 160 ng) were extracted twice from 300 μL plasma by mixing with an organic solvent (n-hexane to acetoacetate, 4:1, vol/vol) and centrifugation at 2500 × g for 5 minutes. After evaporation of the solvent under nitrogen gas at 50°C, the residue was reconstituted in mobile phase A (100 μL), and a 40-μL aliquot was injected. Chromatographic separations were performed by an Agilent HPLC system (Agilent, Santa Clara, California) equipped with a quaternary pump and autosampler coupled to a DAD detector (G1315). Chromatographic separations were achieved on an XDB-C-18 column (inner diameter, 4.56150 mm; particle size 5 μm; Agilent). The mobile phases used were: phase A solution with acetonitrile, water, and trifluoroacetic acid at 5:95:0.1 (vol/vol/vol), and phase B solution with acetonitrile, water, and trifluoroacetic acid at 95:5:0.1 (vol/vol/vol) operated under isocratic conditions at a flow rate of 1.0 mL/min at 30°C with following A and B ratios: 0–3.5 minutes, A/B = 66:34; 3.5–5.5 minutes, A/B = 60:40; and 5.5–7.5 minutes, A/B = 40:60. Adjudin and internal standard were detected at 302 nm. Carbamazepine and adjudin were separated with different retention times (Figure 1). Calibration standards of adjudin were prepared by serial dilution to obtain concentrations 0.125, 0.25, 0.5, 1.0, 2.5, 5, 10, and 20 μg/mL (Figure 2). Stock solutions were prepared weekly throughout the validation period and on each day of sample analysis. Calibration curves were prepared by spiking blank plasma. Calibration curves were linear over the range 0.15 to 20 μL/mL (r = 0.99) (Figure 2). Adjudin quality control samples corresponding to low, medium, and high concentrations on the calibration curve were 0.25, 1, and 5 μL/ mL, respectively. The intra-assay coefficients of variation at 0.25, 1, and 5 mg/L of adjudin were 3.15%, 2.88%, and 1.04% (n 5 5), respectively. The interassay coefficients of variation at the above concentrations were 7.41%, 11.21%, and 3.55% (n 5 5 in all cases), respectively. The method is selective for adjudin, with no interference from other plasma components. The recoveries of 0.25, 1, and 5 mg/L are listed in Table 1.
Figure 1.
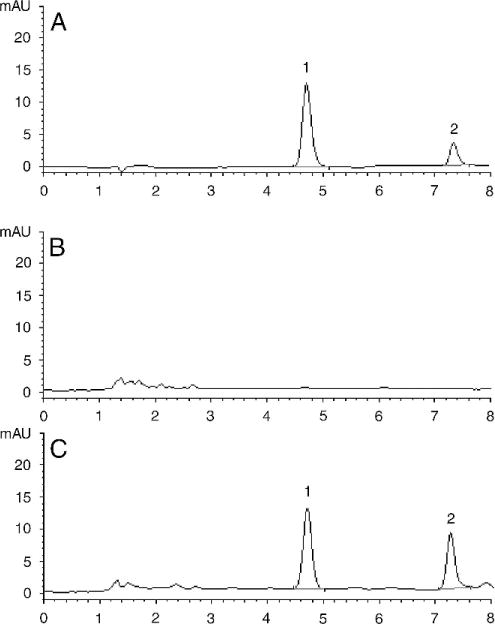
Chromatographic separation of adjudin and carbamazepine (internal control). Adjudin and carbamazepine peaks were well separated. (A) Chromatograph of carbamazepine (peak 1, retention time 4.7 minutes) and adjudin (peak 2, retention time 7.2 minutes) dissolved in mobile phase (x-axis is the retention time in minutes and y-axis is the value of absorbance at 302 nm). (B) Chromatograph of blank plasma without adjudin and/or carbamazepine. (C) Chromatograph of adjudin and carbamazepine in a rabbit plasma sample. mAU indicates milli-absorbance unit.
Figure 2.
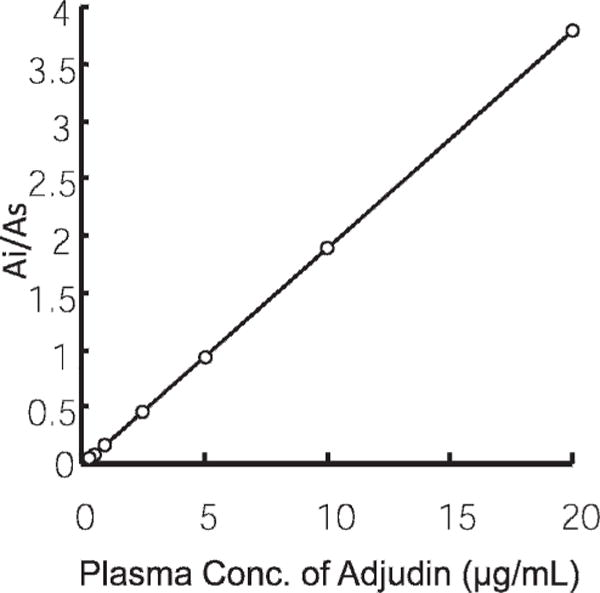
Standard curve of adjudin. This is estimated by the highperformance liquid chromatography method as described in “Materials and Methods.” Ai indicates absorbance of adjudin; As, absorbance of the internal standard, carbamazepine.
Table 1.
Recoveries of adjudin in rabbit plasmaa
| Added, mg/L | Relative Recovery, %
|
Absolute Recovery, %
|
||
|---|---|---|---|---|
| Recovery | RSD | Recovery | RSD | |
| 0.25 | 104.88 ± 8.86 | 8.451 | 51.913 ± 1.244 | 2.397 |
| 1.00 | 108.76 ± 8.40 | 7.724 | 53.889 ± 1.791 | 3.323 |
| 5.00 | 101.20 ± 1.53 | 1.510 | 54.843 ± 1.862 | 3.40 |
Abbreviation: RSD, rising single dose scheme.
Mean ± SEM, n = 5.
Histologic Procedures
The animals were anesthetized by 25% ethyl carbamate (4 mL/ kg), and testes were removed immediately at specified time points after adjudin treatment. Testes and epididymedes were examined by light microscopy to assess the status of spermatogenesis. In brief, 6-μm–thick cross sections were prepared and mounted on glass slides and stained with hematoxyslin and eosin.
Data Processing
Following intravenous or oral administration, the adjudin plasma concentration-time data were fitted to any compartmental models (Drug and Statistics, version 2.0, Clinical and Drug Research Center, Anhui, China), which were then used to calculate different pharmacokinetics parameters with the noncompartment model. All pharmacokinetics parameters were summarized using descriptive statistics. The 95% confidence intervals for the differences between the means of the pharmacokinetics parameters were calculated using the commercially available computer program Confidence Interval Analysis (Altman et al, 2000).
Results
Pharmacokinetics of Adjudin in Male Rabbits
In the pharmacokinetics study shown in Figure 1, we developed an HPLC method to measure the plasma concentrations of adjudin using carbamazepine as internal control. The method detected plasma adjudin with good precision, and the detection limit for adjudin was 125 ng/mL in plasma. The standard curve used to calibrate adjudin and to interpolate unknown samples is shown in Figure 2. Table 1 also illustrates the recovery of adjudin in rabbit plasma samples, confirming the validity of this HPLC-based method to quantify adjudin in rabbit plasma samples.
Concentrations of adjudin in rabbit plasma peaked at 7.669 mg/L 15 minutes after a single intravenous injection of 25 mg/kg adjudin. The plasma adjudin level declined rapidly thereafter, and it became undetectable in rabbits within 24 hours. The mean plasma concentration-time profile for intravenous administration was best described by a 2-compartment curve (Figure 3). Pharmacokinetics parameters of adjudin based on the curves are shown in Table 2. The overall elimination constant (k21) was 2.363 ± 2.062 h−1, and the terminal half-life (t½β) of adjudin was 2.284 ± 0.599 hours. This proved that adjudin was rapidly distributed to peripheral tissues. We calculated the area under the curve (AUC), maximum concentration (Cmax), and time to maximum concentration (tmax) for rats treated orally with the same doses of adjudin as for intravenous injection. The AUC results showed that oral administration is about 8.055% ± 1.453% as effective as intravenous injection (Table 2; Figure 3A vs 3B). Moreover, the t½β (5.645 ± 1.512 hours) of adjudin in the systemic circulation after gavage administration was longer than that of intravenous administration (2.284 ± 0.599 hours; Table 2), showing that orallyadministered adjudin had a slower clearance rate.
Figure 3.
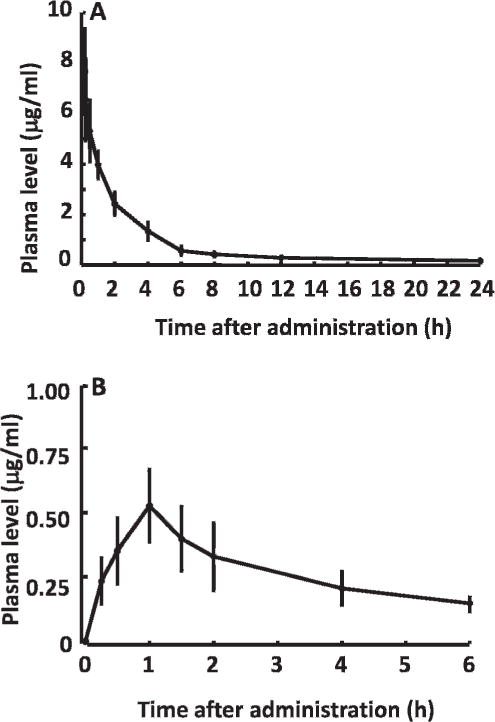
Plasma time curve of adjudin after intravenous or oral administration of single dose of 25 mg/kg adjudin on 6 male Japanese rabbits. (A) Adjudin plasma profile after intravenous administration. (B) Adjudin plasma profile after gavage. Adjudin level in plasma was estimated by HPLC as shown in Figures 1 and 2.
Table 2.
Pharmacokinetics parameters after single-dose 25 mg/kg intravenous or oral administration of adjudin in male rabbit plasmaa
| Parameter | Oral | Intravenous |
|---|---|---|
| k10/h−1 | 0.162 ± 0.036 | 0.480 ± 0.068 |
| k12/h−1 | 0.019 ± 0.028 | 2.428 ± 2.951 |
| k21/h−1 | 0.152 ± 0.044 | 2.363 ± 2.062 |
| t½α/h | 4.876 ± 1.435 | 0.305 ± 0.207 |
| t½β/h | 5.645 ± 1.512 | 2.284 ± 0.599 |
| tmax/h | 1 | 0.1 |
| Cmax/mg·L−1 | 0.526 ± 0.140 | 7.669 ± 1.690 |
| AUC(0–24)/mg·h·L−1 | 1.647 ± 0.452 | 9.611 ± 2.185 |
| AUC(0–∞)/mg·h·L−1 | 2.230 ± 0.450 | 20.411 ± 1.803 |
| MRT(0–t)/h | 2.437 ± 0.120 | 4.598 ± 0.605 |
| MRT(0–∞)/h | 4.689 ± 1.534 | 5.845 ± 1.236 |
Abbreviations: AUC, area under the curve; MRT, mean residence time.
Mean ± SEM, n = 6.
Efficacy of Adjudin
The efficacy of adjudin in depleting germ cells from the seminiferous epithelium of rabbit testes versus normal rabbits (Figure 4A) was evaluated by histologic analysis of testis and epididymis sections. About 70% of tubules were devoid of sperm 2 weeks after intravenous administration of adjudin (Figure 4B). Four weeks after intravenous treatment, more than 95% of round and elongate spermatids and more than 50% of spermatocytes had disappeared (Figure 4C). Sperm numbers in the epididymis were significantly reduced after 2 weeks of intravenous treatment versus control (normal) rabbits (Figure 5A, B). No sperm except some cellular debris were found in the epididymis after 4 weeks of intravenous administration (Figure 5C).
Figure 4.
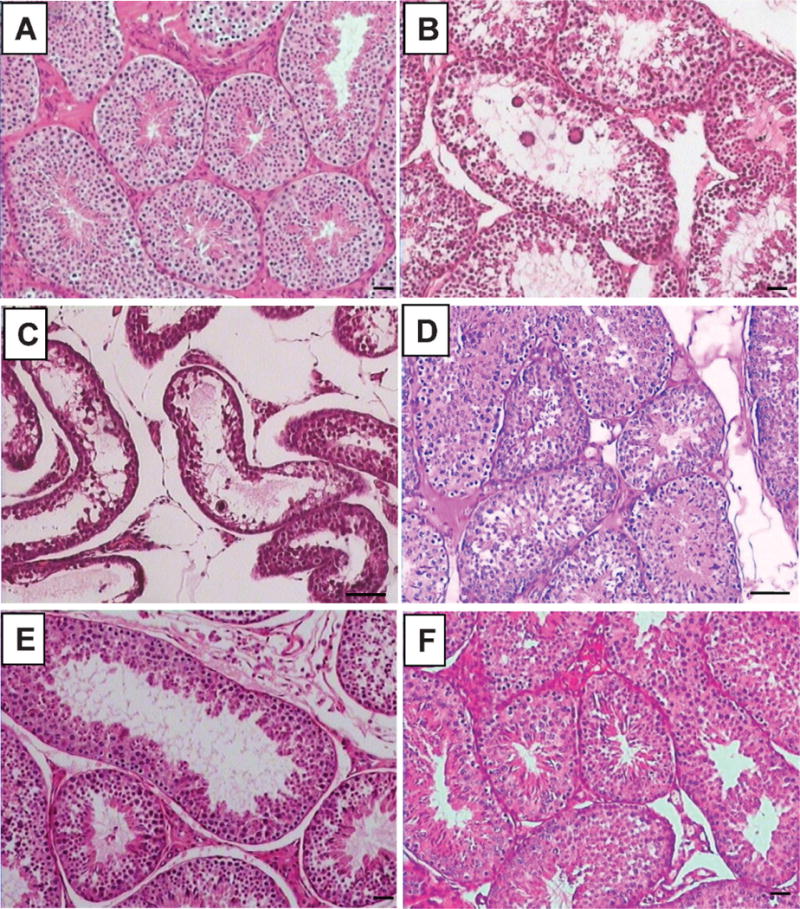
Testis sections after intravenous or oral administration of adjudin. Adult rabbits were treated with adjudin (25 mg/kg b.w.) intravenously or orally and killed 2, 3, 4, or 8 weeks after treatment for histologic analysis by hematoxylin and eosin staining. Cross section of control (A), 2 (B) or 4 (C) weeks after IV administration, 4 weeks (D) after oral administration, or 4 weeks after cessation of adjudin by intravenous (E) or oral (F) administration. Scale bar 5 50 mm.
Figure 5.

Epididymis sections after intravenous or oral administration of adjudin. Adult rabbits were treated with adjudin (25 mg/kg b.w.) intravenously or orally and killed 2, 3, 4, or 8 weeks after treatment for histologic analysis by hematoxylin and eosin staining. Cross section of control (A), 2 (B) or 4 (C) weeks after intravenous administration, 4 weeks (D) after oral administration, or 4 weeks after cessation of adjudin by intravenous (E) or oral (F) administration. Scale bar = 50 μm.
The effects of the 4-week oral administration were comparable to those of the 2-week intravenous administration, as shown in Figure 4D, and sperm numbers in the epididymis section (Figure 5D). This suggests that oral administration is far less effective than intravenous administration.
Systemic Toxicity
Treatment had no effects on body weight (Table 3) or behavior, and it did not disturb gastrointestinal function.
Table 3.
Body weights of rabbits before and after administration of adjudina
| Body weights, kg
|
||||
|---|---|---|---|---|
| Groups | 0 wk | 2 wk | 4 wk | 8 wk |
| Control | 2.45 ± 0.12 | 2.50 ± 0.11 | 2.60 ± 0.09 | 2.71 ± 0.03 |
| Intravenous | 2.41 ± 0.06 | 2.46 ± 0.06 | 2.60 ± 0.06 | 2.65 ± 0.05 |
| Oral | 2.47 ± 0.08 | 2.53 ± 0.09 | 2.61 ± 0.08 | 2.67 ± 0.06 |
Mean ± SE; n = 9 for each group between 0 and 4 weeks, and n = 3 at 8 weeks.
Recovery of Spermatogenesis
Four weeks after cessation of intravenous treatment, 50% of tubules showed signs of spermatogenesis recovery (Figure 4E). Some spermatozoa were also found in the epididymal lumen (Figure 5E). In comparison, the recovery was faster in the gavage group, as spermatogenesis was not completely inhibited after 4 weeks of oral administration.
Discussion
The present study demonstrated for the first time that adjudin effectively depleted elongating/elongated spermatids from the seminiferous epithelium in rabbit testes. This was followed by the loss of most spermatocytes from the seminiferous epithelium, as shown in an earlier report (Chen et al, 2003). The efficacy of the drug as a male contraceptive, however, is dependent on its bioavailability. Pharmacokinetic data showed that only about 8% of the drug was bioavailable after oral administration of the drug, which was consistent with an earlier report in rats that assessed the tissue disruption of [3H]-adjudin following its administration by gavage (Cheng et al, 2005).
The adjudin-induced, stage-dependent germ cell loss possibly was caused by activation of the mechanism that causes the cleavage of anchoring junctions between late spermatids and Sertoli cells (Mruk and Cheng, 2004a; Cheng et al, 2005). Indeed, the adjudin-induced disturbance of junction restructuring during spermatogenesis is associated with a complex process regulated by a multitude of molecules and MAPK-dependent signal transduction pathways (Xia and Cheng, 2005; Xia et al, 2007). Adjudin-induced changes in the steady-state levels of junction proteins that are the crucial proteins of the disrupted signaling pathways (eg, the ERK signaling pathway) appear to be associated with testin, because a disruption of Sertoli-germ cell anchoring junctions is associated with a significant surge in testin expression both in vivo and in vitro (Cheng et al, 2001). Significant changes in expression of multiple genes were detected by approximately 8 hours after administration of adjudin in a subsequent study using gene profiling techniques in adult rats (Xia et al, 2007). As in rats, orally administrated adjudin had low bioavailability in rabbits. As reported herein, in rabbits the orally administrated bioavailability of adjudin was about 8% of that dose given intravenously. We suggest that adjudin is poorly absorbed or extensively metabolized in the gastrointestinal tract. The pharmacokinetics profiles in rabbits were similar to those in rats (Cheng et al, 2005). When [3H]-adjudin was administered orally to adult rats, the drug was found to distribute uniformly among the testis, kidney, liver, brain, heart, small intestine, prostate, epididymis, seminal vesicles, pancreas, and thyroid after its absorption via the gastrointestinal tract (Cheng et al, 2005). These findings show that adjudin is not specifically taken up by any organ. By 72 to 96 hours after administration, adjudin had been virtually removed via the urine from all organs examined. Our data in rabbits have confirmed these earlier findings in rats, because 24 hours after a single intravenous injection of adjudin at 25 mg/kg body weight (b.w.), the serum adjudin level was already undetectable. Earlier studies in which adjudin was given by gavage showed that less than 5% of [3H]-adjudin was taken up by all organs combined and only 0.035% of administered [3H]-adjudin reached the testis (Cheng et al, 2005); these studies corroborate our finding that this compound has a low oral bioavailability. The mean plasma concentration-time profile for intravenous administration as reported herein was best described by a 2-compartment curve and was consistent with an extensive distribution in peripheral organs.
In summary, adjudin induced germ cell loss from the seminiferous epithelium in rabbits, as previously shown for rats, and it is a potential male contraceptive when delivered intravenously or orally. The pharmacokinetics analysis, however, indicates that this drug has low bioavailability when delivered orally.
Acknowledgments
Supported in part by grants from the National Institutes of Health (National Institute of Child Health and Human Development U54 HD029990 Project 5 and U01 HD045908) to C.Y.C. and the Wenzhou Medical College Fund to R.-S.G.
References
- Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. 2. London: BMB Books; 2000. Confidence interval analysis [software included with book purchase] [Google Scholar]
- Chen YM, Lee NP, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- Lee NP, Mruk DD, Wong CH, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/beta-catenin (CATNB) signaling pathway: an in vitro and in vivo study. Biol Reprod. 2005;73:458–471. doi: 10.1095/biolreprod.105.040766. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Unraveling the molecular targets pertinent to junction restructuring events during spermatogenesis using the Adjudin-induced germ cell depletion model. J Endocrinol. 2007;192:563–583. doi: 10.1677/JOE-06-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]


