Abstract
Background and Objectives
Colorectal cancer (CRC) arising in patients under age 30 is a rare disease, and few cases have been reported within Li-Fraumeni kindreds. To determine how often alterations in the p53 pathway genes contribute to disease susceptibility, we have evaluated patients with early onset CRC for the presence of germline variants in the p53 gene and MDM2 SNP309.
Methods
Thirty-five patients with CRC diagnosed before age 30 were included in this study-based on tissue availability. DNA samples from peripheral blood leukocytes were analyzed for constitutional mutations and polymorphisms in p53 as well as polymorphisms in MDM2 SNP309.
Results
No mutations were found in exons 4–10 of the p53 gene. The frequencies of polymorphisms in p53 and in MDM2 SNP309 did not differ from rates previously reported for normal control populations, and no polymorphism in either gene could be associated with early onset CRC.
Conclusions
Neither germline variants in p53 nor MDM2 SNP309 play an underlying role in the development of very early onset CRC. For the large majority of cases, the genetic basis of this disease remains unknown.
Keywords: early onset colorectal cancer, p53, SNP309, genetic syndromes
INTRODUCTION
Genetic studies of colorectal cancer (CRC) have led to the identification of several genes that, when mutated in the germline, lead to a markedly increased susceptibility to the disease [1–4]. Germline mutations of MSH2, MLH1, and MSH6 account for most cases of hereditary non-polyposis colon cancer (HNPCC), while mutations of APC are found in most patients with familial adenomatous polyposis (FAP). In both HNPCC and FAP the onset of CRC occurs at a younger age, and both syndromes can occur sporadically when a germline mutation is acquired de novo.
Colorectal cancer (CRC) is a rare disease in individuals under age 30 years. Based on population-based data from the Surveillance, Epidemiology, and End Results (SEER) database, the age-specific incidence of CRC per 100,000 individuals in patients age 25–29 is 1.6 compared to 327 for patients age 75–79 (SEER Program Web site). The genetic basis for such accelerated onset is poorly understood. In a population-based analysis from Scotland, 35 patients diagnosed under age 40 were identified, and among them only 10 were associated with HNPCC and none with FAP or any other known genetic syndrome [4]. Our group at Memorial Sloan-Kettering Cancer Center (MSKCC) has documented that a large majority of CRCs that occur in adolescents and young adults have no clear hereditary basis [6,7]. Sixty to 70% of cases occur sporadically, with no CRC found in any first degree relatives. Although familial clustering occurs in approximately 30% of patients, only a minority meet the clinical criteria for HNPCC or FAP. Another unexplained characteristic of early onset CRC is aggressive biological behavior. Advanced stage at presentation, high grade histology, and poor prognosis are features of early onset CRC that have been reported in case series from around the world [8–14]. Taken together, these data strongly suggest that among these patients are one or more undiscovered genetic syndromes.
Genetic variants in the p53 gene and its regulator MDM2 have been associated with increased susceptibility and early age of onset sarcoma, breast cancer, and adrenocortical cancer [15–17]. Recently, Wong and colleagues reported evidence from a family registry of Li-Fraumeni syndrome (LFS) kindreds indicating that germline mutations of p53 increase susceptibility to early onset CRC [18]. Among 397 patients within 64 LFS kindreds, 11 (2.8%) developed CRC before age 50. The mean age at diagnosis was 33 years, and four patients were diagnosed under age 21. They concluded that LFS should be considered when a young patient is diagnosed with CRC. Moreover, a fascinating study of LFS patients has recently demonstrated that inheritance of a particular single-nucleotide polymorphism (G-allele of SNP309) in the promoter of the MDM2 has been associated with earlier age of onset of sarcoma [19]. Bond and colleagues have shown that the polymorphic G-allele of SNP309 increases the promoter activity and expression of MDM2, attenuating the p53 pathway and accelerating cancer onset. Analysis of SNP309 in patients with CRC has shown that in select populations (i.e., women or p53 wild type tumors), carriers of the G-allele of SNP309 are diagnosed with malignancy 9 years earlier than non-carriers [20,21].
In the current report, we have evaluated the prevalence of p53 mutations and MDM2 SNP309 in patients with CRC diagnosed at age 30 years or younger. Our goal was to examine whether genetic variants in these important cancer susceptibility genes could account for a subset of early onset CRC.
MATERIALS AND METHODS
Patient Selection
This study was approved by the Institutional Review Board (IRB) and Human Tissue Utilization Committee (HTUC) of MSKCC. It required DNA derived from peripheral blood leukocytes (PBL); all patient identifiers were discarded prior to genetic analysis. Patients from our institution diagnosed with adenocarcinoma of the colon and rectum at age 30 years or younger, for whom PBL was available, were eligible for analysis. We did not exclude patients with known cancer syndromes such as HNPCC, as we felt variants in p53 and/or MDM2 might produce phenocopies of other syndromes or might drive early onset of disease as a “second genetic hit.” Based on PBL availability, the cases included four with HNPCC as per Amsterdam II criteria, four with FAP, one with juvenile polyposis (JP), 1 with ulcerative colitis (UC), and a remaining 25 patients with no known susceptibility to CRC. No patients with LFS were included. All colorectal tumors were documented as invasive adenocarcinoma by review of pathology slides.
Age of diagnosis among the 35 cases ranged from 16 to 30 years. Years of diagnosis were between 1974 and 2005. Disease was staged as per the American Joint Committee on Cancer (AJCC) criteria. Prior to genetic analysis, each case was fully anonymized by assigning a unique research number to protect patient identity.
TP53 Mutation Screening and MDM2 SNP309 Analysis
Genomic DNA was isolated from PBL using DNA isolation kits (Qiagen Inc., Valencia, CA). TP53 mutations and polymorphisms were identified in exons 5–10 using a slight modification of the temporal temperature gradient electropheresis (TTGE) gel technique described by Sorlie et al. [22] Exons 5–10 of p53 were amplified by PCR using GC-clamped primers and conditions previously described [23]. PCR products were separated by TTGE on denaturing gels. The amount of urea-formamide was optimized for each exon as follows: 48% (exon 5A); 53% (exon 5B); 40% (exons 6, 8, 9); 45% (exon 7); 50% (exon 10). All gels were run at 130 V with a temperature range of 54–62°C and rising increments of 1.7 C/hr. Mutant bands were isolated, subjected to PCR using the same primers without a GC-clamp, purified using the QIAQuick PCR Purification Kit (Qiagen), and then analyzed by dideoxy thermal sequencing. Exon 4 was subjected to PCR and direct sequencing without previous screening by TTGE. All cases were compared to cell line or artificially synthesized wild-type (WT) and mutant controls to validate the methodology (exon 5: SW1116, EJ; exon 6: BXPC3; exon 7: MIA; exon 8: SW620; exon 9: SW480; exons 4, 10: oligonucleotide synthesized with point mutations in each exon). Forward and reverse primers for exon 4 were 5′-CGT CAC GAC ACG AAA ACC GTT CTG GTA AGG ACA AGG GTT G-3′ and 5′-CGT CAC GAC ACG AAA CAA AGC CAA AGG GTG AAG AGG AAT C-3′.
SNP309 of MDM2 was analyzed by PCR/LDR [24]. Forward and reverse PCR primers for SNP309 were 5′-CGG GAG TTC AGG GTA AAG GTC AC-3′ and 5′-CAG ACT ACG CGC AGC GTT CAC-3′. Fifty nanograms of DNA were subjected to a PCR reaction (50 μl) containing 20 mM Tris–HCL (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of each flanking primer, 2.5 units of Taq polymerase, 250 μM of dNTP (Invitrogen Corp., Carlsbad, CA). Cell cycle conditions were: 95°C for 5 min; 35 cycles of 95°C for 30 sec, 59°C for 30 sec, and 72°C for 1 min, followed by a final extension at 72°C for 7 min and subsequent cooling of the product to 4°C. Taq polymerase was inactivated by adding 2 μl of proteinase K (20 mg/ml) to each reaction, incubating it at 65°C for 15 min and 95°C for 10 min to denature the proteinase. PCR products were subjected to a ligase detection reaction (LDR) as previously described, using the following primers: G/G Allele 5′ Fam-TAA AAA GGA CCT CCC GCG CCG C 3′; T/T allele: 5′ Fam-AA GGA CCT CCC GCG CCG A-3′; common primer: 5′ Phos-AGC GGC CCC GCA GCC CTA AAA TAA AA-3′. LDR products were electrophoresed on a 3100 Genetic Analyzer, and fluorescent ligation products were analyzed using Gene Scan 3.7 software (Applied Biosystems, Foster City, CA).
RESULTS
Table I shows the clinical features of the study population. Median age of CRC diagnosis was 28 years. Men and women were equally represented. A majority had left-sided primary tumors (77%), and greater than half presented with metastatic disease (51%).
TABLE I.
Clinical Characteristics
| Number of cases | 35 |
| Median age at diagnosis | 28 |
| Age range | 16–30 |
| HNPCC | 4 |
| FAP | 4 |
| JP | 1 |
| UC | 1 |
| Gender | |
| Male | 54% |
| Female | 46% |
| Tumor location | |
| Left-sided | 77% |
| Right-sided | 14% |
| Unknown | 9% |
| Stage | |
| I | 11% |
| II | 11% |
| III | 20% |
| IV | 51% |
| Unknown | 6% |
HNPCC, hereditary non-polyposis colon cancer; FAP-familial adenomatous polyposis; JP, juvenile polyposis; UC, ulcerative colitis.
No patients were found to have germline p53 mutations. One patient had a silent polymorphism at codon 213 (CGA → CGC), and 17 patients harbored codon 72 polymorphisms (15 CGC/CCC, 2 CCC/CCC) that resulted in an amino acid change from the WT arginine (Arg) to polymorphic proline (Pro; Table II, Fig. 1). There was no clustering of age in relation to codon 72 genotypes, and genotype frequencies were not different from most population-based studies (Table III).
TABLE II.
TP53 Genetic Analysis
| Exon | Method | Polymorphism | Mutation |
|---|---|---|---|
| 4A | Sequencing | 17 | 0 |
| 4B | TTGE/sequencing | 0 | 0 |
| 5A | TTGE/sequencing | 0 | 0 |
| 5B | TTGE/sequencing | 0 | 0 |
| 6 | TTGE/sequencing | 1 | 0 |
| 7 | TTGE/sequencing | 0 | 0 |
| 8 | TTGE/sequencing | 0 | 0 |
| 9 | TTGE/sequencing | 0 | 0 |
| 10 | TTGE/sequencing | 0 | 0 |
TTGE, temporal temperature gradient electrophoresis gel.
Fig. 1.
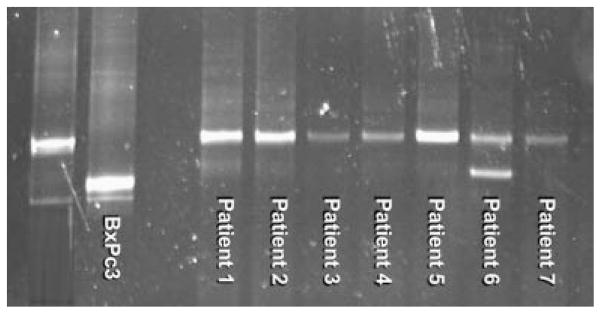
Temporal temperature gradient electrophoresis (TTGE) gel for TP53 exon 6 showing normal and aberrantly migrating bands. All patients possess wild-type bands while patient 6 possesses an additional aberrantly migrating band. Subsequesnt dideoxy sequencing revealed this band to represent a polymorphism in codon 213. Cell lines LoVo and BxPc3 are wild-type and mutant controls, respectively.
TABLE III.
Genotype Frequencies of TP53 Codon 72 Polymorphisms
| Arg/Arg | Arg/Pro | Pro/Pro | |
|---|---|---|---|
| Median age at diagnosis | 28 | 27 | 27 |
| Age range | 18–30 | 16–30 | 26–28 |
| Age 16–27 | 6 | 8 | 1 |
| Age 28–30 | 11 | 6 | 1 |
| Number of cases | 18 | 15 | 2 |
| Percent | 51% | 43% | 6% |
| Overall prevalencea | 14–69% | 28–46% | 3–39% |
Beckman et al. [28].
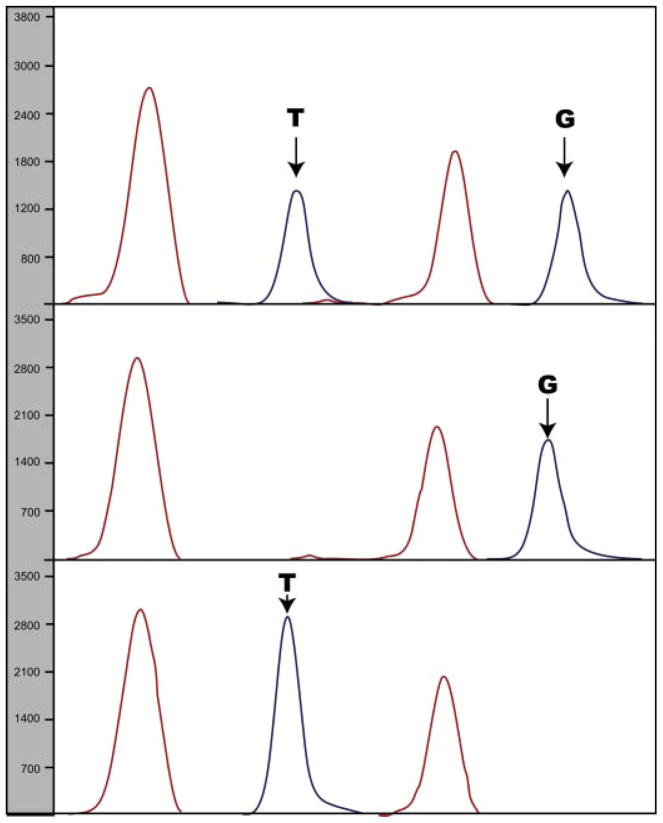
The three different genotypes of the MDM2 SNP309 locus were identified at frequencies similar to published data on normal population, and were as follows: 33%, homozygous WT (T/T); 58%, heterozygous (T/G); 9%, homozygous (G/G; Table IV, Fig. 2). There were no significant differences in age relative to SNP309 genotype.
TABLE IV.
MDM2 SNP309 Genetic Analysis
| T/T | T/G | G/G | P-value | |
|---|---|---|---|---|
| Median age at diagnosis | 28 | 28 | 27 | — |
| Number of cases | 11 | 19 | 3 | — |
| Percent | 33% | 58% | 9% | NS |
| Adult prevalencea | ||||
| CRC | 34% | 48% | 18% | NS |
| Population | 30% | 53% | 17% | NS |
Alhopuro (2005).
Fig. 2.
Electropherogram displaying possible genotypes for MDM2 SNP309. The possible genotypes shown are T/G, G/G, and T/T.
DISCUSSION
The recent report by Wong and colleagues is a reminder that early onset CRC occurs at low frequency in Li-Fraumeni kindreds, and that screening colonoscopy and early workup of colon cancer symptoms should be considered in this population [18]. However, the broader implication of their data is that germline mutations in p53 may be a genetic cause of early onset CRC in any population, since these mutations can occur spontaneously in patients with no family history of cancer. To determine whether genetic variants in p53 pathway genes are associated with this rare disease, we chose to evaluate p53 mutations and MDM2 SNP 309 genotypes in germline DNA from a cohort of patients with early onset CRC. Our results provide no evidence for the association of mutations or polymorphisms in TP53 and MDM2 SNP309 with the development of CRC before age 30. We have analyzed these genes using high quality DNA isolated from PBL with highly accurate, well-controlled methods. Our analysis included genotyping of MDM2 SNP309 and screening of exons 4 through 10 of p53, an area of the gene that covers every case of germline p53 mutation associated with cancer as reported to an international registry (International Agency for Research on Cancer website). By finding no association of these genetic variants in 35 cases, our data suggests that, in cases not associated with LFS, neither p53 germline mutations nor MDM2 SNP309 genotypes are underlying causes in the genetics of very early onset CRC.
In 1993 Bhagirath et al. [5] posed a similar question, and did not find constitutional p53 mutations in any of 35 early onset CRC patients. Their analysis relied on an osmium-based gel that PCR amplified three segments of the gene to screen for mutations encompassing six exons. In the intervening 14 years, more accurate methods have been developed to screen for mutations. We selected TTGE because it isolates the mutant DNA fragment by a highly sensitive electrophoretic method, and is therefore one of the most sensitive techniques available for germline analysis. Furthermore, TTGE has been optimized and successfully applied for detection of p53 mutations by several laboratories [22,25–27]. Our results support the findings of Bhagirath et al. and indicate that, apart from LFS kindreds, constitutional p53 mutations are highly unlikely to account for susceptibility to early onset CRC.
In our study population we encountered frequent variations in the Arg/Pro polymorphism in codon 72 of p53. Among 35 patients, 18 (51%) were homozygous for the wild type allele (Arg/Arg) whereas 43% were heterozygotes (Arg/Pro) and 6% had two polymorphic alleles (Pro/Pro). The prevalence of the polymorphic Pro allele in healthy populations is not well established, but appears to vary from 17% to 63%, depending on ethnicity [28]. Nonetheless, two independent reports have suggested that the Arg/Pro polymorphism in codon 72 of the p53 gene has a strong accelerating effect on onset of CRC in HNPCC patients [29,30]. The first report showed a median age of 40 years for CRC diagnosis of Arg/Pro heterozygotes (N = 28 mutation carriers) compared with 53 years for Arg/Arg wild type cases (N = 57 mutation carriers). The second report showed the opposite trend among 167 HNPCC mutation carriers, with Arg/Arg wild type genotype having the highest median age of onset (41 years) compared to Arg/Pro (36 years) and Pro/Pro (31 years). Although our study is underpowered to address this question, our data does not suggest an association between age of onset and codon 72 genotype. Our data is consistent with the more recent report by Sotamaa et al. [31], which showed no effect of the codon 72 genotype on age of onset in either HNPCC or sporadic CRC.
Since the discovery by Bond et al. that SNP309 of the MDM2 gene can accelerate the onset of sarcoma and breast cancer in LFS patients, there have been several reports assessing the impact of the G allele of SNP309 on the age of CRC onset [16,19–21]. The evidence from studies of sporadic CRCs is controversial, with most showing no overall effect but particular studies implicating the G allele in earlier age of onset among female patients or in patients with CRCs that are WT for p53. In our study, we found the prevalence of the G allele in early onset cases to be no different from that reported in adult CRC patients or in control subjects. Median age of CRC onset in our study was identical for the T/T genotype (N = 11), the T/G genotype (N = 19), and the G/G genotype (N = 3). Thus, we find no evidence to support the idea that MDM2 SNP309 has a significant impact on predisposition to very early onset CRC.
Though we did not find an association between genetic variants in p53 and MDM2 with early onset CRC, one must be cautious in interpreting our data. Our study looked at 35 cases, a small fraction of the 500–600 cases of early onset CRC diagnosed annually in the United States. It is possible that rare cases not represented in our study population may have germline variants in p53 or MDM2 that play a role in cancer susceptibility.
CONCLUSIONS
Early onset CRC is a rare disease about which much remains to be learned. Of the estimated 500–600 cases annually diagnosed before age 30 in the United States, only a minority are accounted for by a known genetic syndrome (SEER Program Web site). The factors influencing susceptibility are unknown, and once diagnosed these cancers follow an aggressive course that is often lethal. Our observations suggest that very early onset CRC is a disease that is genetically and phenotypically distinct from the typical form that occurs in the later decades of life. Additional studies of the genetic basis for early onset CRC are needed, and will likely provide important lessons in the pathogenesis of this disease.
Acknowledgments
Grant sponsor: Warren/Soden/Hopkins Foundation; Grant sponsor: Program Project; Grant number: PO1-CA65930; Grant sponsor: T32 Surgical Oncology Training; Grant number: CA 09501.
The authors gratefully acknowledge the philanthropic support of the Warren/Soden/Hopkins Foundation. This work was also supported by Program Project Grant PO1-CA65930 and T32 surgical oncology training grant CA 09501 of the National Cancer Institute.
References
- 1.Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 2.Alhopuro P, Ylisaukko-Oja SK, Koskinen WJ, et al. The MDM2 promoter polymorphism SMP309T–>G and the risk of uterine leiomyosarcoma, colorectal cancer, and squamous cell carcinoma of the head and neck. J Med Genet. 2005;42:694–698. doi: 10.1136/jmg.2005.031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishel R, Lescoe MK, Rao MR, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1994;77:167. [PubMed] [Google Scholar]
- 4.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 5.Bhagirath T, Condie A, Dunlop MG, et al. Exclusion of constitutional p53 mutations as a cause of genetic susceptibility to colorectal cancer. Br J Cancer. 1993;68:712–714. doi: 10.1038/bjc.1993.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta RV, LaQuaglia MP, Paty PB. Genetic and phenotypic correlates of colorectal cancer in young patients. N Engl J Med. 2000;342:137–138. doi: 10.1056/NEJM200001133420216. [DOI] [PubMed] [Google Scholar]
- 7.LaQuaglia MP, Heller G, Filippa DA, et al. Prognostic factors and outcome in patients 21 years and under with colorectal carcinoma. J Pediatr Surg. 1992;27:1085–1089. doi: 10.1016/0022-3468(92)90565-o. discussion 1089–1090. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Zeid AA, Khafagy W, Marzouk DM, et al. Colorectal cancer in Egypt. Dis Colon Rectum. 2002;45:1255–1260. doi: 10.1007/s10350-004-6401-z. [DOI] [PubMed] [Google Scholar]
- 9.Al-Jaberi TM, Yaghan RJ, El-Heis HA. Colorectal cancer in young patients under 40 years of age. Comparison with old patients in a well defined Jordanian population. Saudi Med J. 2003;24:871–874. [PubMed] [Google Scholar]
- 10.de Silva MV, Fernando MS, Fernando D. Comparison of some clinical and histological features of colorectal carcinoma occurring in patients below and above 40 years. Ceylon Med J. 2000;45:166–168. doi: 10.4038/cmj.v45i4.6722. [DOI] [PubMed] [Google Scholar]
- 11.Kam MH, Eu KW, Barben CP, et al. Colorectal cancer in the young: A 12-year review of patients 30 years or less. Colorectal Dis. 2004;6:191–194. doi: 10.1111/j.1463-1318.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 12.Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90:205–214. doi: 10.1002/bjs.4015. [DOI] [PubMed] [Google Scholar]
- 13.Singh Y, Vaidya P, Hemandas AK, et al. Colorectal carcinoma in Nepalese young adults: Presentation and outcome. Gan To Kagaku Ryoho. 2002;29:223–229. [PubMed] [Google Scholar]
- 14.Vastyan AM, Walker J, Pinter AB, et al. Colorectal carcinoma in children and adolescents—A report of seven cases. Eur J Pediatr Surg. 2001;11:338–341. doi: 10.1055/s-2001-18548. [DOI] [PubMed] [Google Scholar]
- 15.Birch JM, Alston RD, McNally RJ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20:4621–4628. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 16.Bond GL, Hirshfield KM, Kirchhoff T, et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–5110. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 17.Bougeard G, Baert-Desurmont S, Tournier I, et al. Impact of the MDM2 SNP309 and p53 Arg72Pro polymorphism on age of tumour onset in Li-Fraumeni syndrome. J Med Genet. 2006;43:531–533. doi: 10.1136/jmg.2005.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong P, Verselis SJ, Garber JE, et al. Prevalence of early onset colorectal cancer in 397 patients with classic Li-Fraumeni syndrome. Gastroenterology. 2006;130:73–79. doi: 10.1053/j.gastro.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Bond GL, Menin C, Bertorelle R, et al. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006;43:950–952. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menin C, Scaini MC, De Salvo GL, et al. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst. 2006;98:285–288. doi: 10.1093/jnci/djj054. [DOI] [PubMed] [Google Scholar]
- 22.Sorlie T, Johnsen H, Vu P, et al. Mutation screening of the TP53 gene by temporal temperature gradient gel electrophoresis. Methods Mol Biol. 2005;291:207–216. doi: 10.1385/1-59259-840-4:207. [DOI] [PubMed] [Google Scholar]
- 23.Guldberg P, Nedergaard T, Nielsen HJ, et al. Single-step DGGE-based mutation scanning of the p53 gene: Application to genetic diagnosis of colorectal cancer. Hum Mutat. 1997;9:348–355. doi: 10.1002/(SICI)1098-1004(1997)9:4<348::AID-HUMU8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Khanna M, Park P, Zirvi M, et al. Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene. 1999;18:27–38. doi: 10.1038/sj.onc.1202291. [DOI] [PubMed] [Google Scholar]
- 25.Kringen P, Wang Y, Dumeaux V, et al. TP53 mutations in ovarian carcinomas from sporadic cases and carriers of two distinct BRCA1 founder mutations: Relation to age at diagnosis and survival. BMC Cancer. 2005;5:134. doi: 10.1186/1471-2407-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malmer B, Gronberg H, Andersson U, et al. Microsatellite instability, PTEN and p53 germline mutations in glioma families. Acta Oncol. 2001;40:633–637. doi: 10.1080/028418601750444196. [DOI] [PubMed] [Google Scholar]
- 27.Rotterud R, Skomedal H, Berner A, et al. TP53 and p21WAF1/CIP1 behave differently in euploid versus aneuploid bladder tumours treated with radiotherapy. Acta Oncol. 2001;40:644–652. doi: 10.1080/028418601750444213. [DOI] [PubMed] [Google Scholar]
- 28.Beckman G, Birgander R, Sjalander A, et al. Is p53 polymorphism maintained by natural selection? Hum Hered. 1994;44:266–270. doi: 10.1159/000154228. [DOI] [PubMed] [Google Scholar]
- 29.Jones JS, Chi X, Gu X, et al. p53 polymorphism and age of onset of hereditary nonpolyposis colorectal cancer in a Caucasian population. Clin Cancer Res. 2004;10:5845–5849. doi: 10.1158/1078-0432.CCR-03-0590. [DOI] [PubMed] [Google Scholar]
- 30.Kruger S, Bier A, Engel C, et al. The p53 codon 72 variation is associated with the age of onset of hereditary non-polyposis colorectal cancer (HNPCC) J Med Genet. 2005;42:769–773. doi: 10.1136/jmg.2004.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotamaa K, Liyanarachchi S, Mecklin JP, et al. p53 codon 72 and MDM2 SNP309 polymorphisms and age of colorectal cancer onset in Lynch syndrome. Clin Cancer Res. 2005;11:6840–6844. doi: 10.1158/1078-0432.CCR-05-1139. [DOI] [PubMed] [Google Scholar]