Abstract
Infectious diseases account for between 14 and 17 million deaths worldwide each year. Accurate and rapid diagnosis of bacterial, fungal, viral, and parasitic infections is therefore essential to reduce the morbidity and mortality associated with these diseases. Classical microbiological and serological methods have long served as the gold standard for diagnosis but are increasingly being replaced by molecular diagnostic methods that demonstrate increased sensitivity and specificity and provide an identification of the etiologic agent in a shorter period of time. PCR/LDR coupled with universal array detection provides a highly sensitive and specific platform for the detection and identification of bacterial and viral infections.
Keywords: Ligase detection reaction, Microarray, Multiplexed detection, Bacterial identification, Viral identification
1. Introduction
Molecular diagnostic methods for the identification of infectious agents are increasingly being used in clinical microbiology laboratories, supplementing, and in some cases replacing, classical microbiological techniques (1–4). A majority of these methods are based on detecting specific nucleic acid sequences of the infectious agents. Initial methods relied on the use of nucleic acid probes to identify cultured organisms or for direct detection of the organisms from clinical samples. Nucleic acid amplification based methods offer greater sensitivity and are now being used for diagnosis, identification, and quantitation of infections agents, and in the case of bacteria and fungi, for evaluation of antimicrobial resistance profiles. The FDA has approved several commercial diagnostic kits that utilize a variety of amplification techniques (3). Additionally, some clinical laboratories offer homebrew assays for diagnosis of viral infections.
The ligase detection reaction is a linear amplification technique, where two adjacent oligonucleotides hybridize to a single DNA strand and are ligated by a high fidelity thermostable ligase when there is a perfect match at the junction (5). LDR is an ideal technique for multiplexing as multiple primer pairs can hybridize and ligate along a given template simultaneously without interfering with each other. When coupled with an initial PCR amplification of the target sequence, LDR allows for high sensitivity detection of single base variants with extremely high specificity (6–8). Furthermore, coupling of two orthogonal detection methods reduces the risk of allele dropout in multiplexed PCR amplification and false positives arising from spurious and target independent amplifications.
Attaching a fluorescent label to one of the ligation primers enables the detection of LDR products. The products may be separated by gel or capillary electrophoresis. If the other primer bears a unique zip-code complement, the ligation products can be detected by hybridization to a universal microarray spotted with the unique zip-codes (Fig. 1). Thus, the universal microarray provides a standard platform for the detection of ligation products arising from any LDR reaction in any assay (9). The zip-code oligonucleotides spotted on the array are designed with Tm values that fall within a narrow range to ensure that all ligation products bearing the different zip-code complements hybridize to the array with equal efficiency. The specificity of the array is derived from the LDR, thus false positives and false negatives associated with direct DNA hybridization arrays are obviated. Spotting or printing the zip-code oligonucleotides on a 3-dimensional matrix (such as that available on Codelink activated slides) rather than directly on the glass surface of a slide, increases signal intensities up to a 100-fold when compared to conventional arrays and reduces hybridization times to between 30 min and 2 h (10).
Fig. 1.

Schematic representation of a PCR/LDR/universal array assay. In the example shown, the assay is used to detect the identity of a single base variant (A/C) within a gene of interest. The target gene is amplified using gene specific PCR primers. The PCR amplicon is subjected to an LDR reaction using two upstream primers, one ending in T (complementary to the A allele) with the complement to zip-code 2 attached to its 5′-end and the other ending in G (complementary to the C allele) with the complement to zip-code1 attached to its 5′-end. If the allele present in the sample is C (as shown), then only the “T” primer will ligate to the downstream fluorescently labeled primer. When the ligation products are hybridized to the universal array, a positive signal is seen at zip-code address 1
PCR/LDR/universal array hybridization was first demonstrated for the multiplexed detection of mutations and single base variations in genes implicated in cancer such as BRCA1, BRCA2, K-ras, and p53, both from tumor samples as well as from stool, demonstrating the ability of the technique to detect mutations in the presence of large quantities of normal background DNA (8, 11–15). For example, our laboratory has shown the ability to detect 1 in 100 for a p53 mutation in a wild type sequence, where standard direct hybridization chips are unlikely to be as sensitive (10, 14). Additionally, PCR/LDR/universal array has been used for detection of HLA polymorphisms (16), 21-hydroxylase alleles (7), and determination of CpG island methylation status (17).
The PCR/LDR/universal array platform is ideally suited to develop assays for the detection and identification of infectious disease. For bacterial (or fungal) pathogens, initial PCR amplification of a gene or genes that are conserved across all bacteria (or fungi respectively), can be followed by LDR for the detection of single base variations at multiple loci to identify the pathogen. We have previously demonstrated this technique for the multiplexed identification of a panel of 20 blood borne bacterial pathogens, where the ligation products are detected by capillary electrophoresis (18). The assay is directly transferrable to a universal array format when one set of the ligation primers are redesigned to bear zip-code complements. Others have used PCR/LDR/universal array for identification of bacteria (19) as well as for determining diversity in target bacterial populations (20).
RNA viruses such as hemorrhagic fever viruses present a different challenge. These viruses mutate rapidly and undergo significant sequence drift, making it difficult to design PCR primers that can amplify variant sequences. In these cases, it is prudent to PCR amplify more than one target using multiple PCR primers to account for sequence variations at the primer binding sites. LDR can then be performed to detect the presence of these PCR amplicons using multiple ligation primers at each ligation site to account for sequence variations. We have reported PCR/LDR/universal array assays for the detection of two different RNA viruses, West Nile virus (21), and Dengue virus (Fig. 3) (22).
Fig. 3.

Representative images of universal array detection of Dengue virus serotypes I–IV following hybridization of LDR products from a PCR/LDR assay. LDR primers for each serotype bear zip-code complements to distinct addresses. For correct identification only the addresses specific to a given serotype should provide a positive signal. In this assay, a minimum of two signals are required to identify a given serotype. (see ref. (22) for details)
The PCR/LDR/universal array platform is amenable to incorporation into microfluidic devices. These devices can perform the entire assay, including array hybridization and readout in less than 1 h, making the platform suitable for the development of point of care diagnostic devices (23, 24).
2. Materials
2.1. Microarray Printing and Coupling DNA Probes
Zip-code Oligonucleotides (see Note 1).
aQu Ultrasonic Microarray Pin Cleaning Solution (Genetix, Boston, MA).
3 × 1 in Microscope slides.
384 well plate.
Codelink Activated Slides (SurModics, Inc.).
6× Print buffer: 300 mM sodium phosphate, pH 8.5.
Saturated NaCl humidification chamber.
2.2. Postcoupling and Hybridization
10% Sodium dodecyl sulfate.
20× standard sodium citrate (SSC).
Blocking solution: 50 mM ethanolamine, 0.1 M Tris (pH 9).
Postcoupling wash solution: 4× SSC, 0.1% SDS.
Posthybridization wash solution 1: 4× SSC.
Posthybridization wash solution 2: 2× SSC, 0.1% SDS.
Posthybridization wash solution 3: 0.2× SSC.
Posthybridization wash solution 4: 0.1× SSC.
1× Wash Buffer: 0.3 M Bicine pH 8.0, 0.1% SDS, filter sterilize. Store at room temperature.
Salmon sperm DNA.
ProPlate adhesive seal-strips (Grace Bio-Labs) (see Note 2).
Razor blade.
ProPlate multiarray chamber system (Grace Bio-Labs) (see Note 2).
Shaker.
Heat blocks.
Microcentrifuge.
Slide Spinner (Labnet International, Inc.).
Thermocycler.
Hybridization oven with rotary rocker.
QArrayMini robotic array printer (Genetix, Boston, MA).
ProScanArray microarray scanner (Perkin Elmer, Boston, MA).
2.3. Gene Specific PCR
PCR buffer (10×): 100 mM Tris–HCl buffer, 500 mM KCl, pH 8.0. Store at −20°C.
MgCl2, 25 mM. Store at −20°C.
Deoxynucleoside triphosphates (dNTPs), 0.8 mM. Store at −20°C.
Gene specific PCR primers.
AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). Store at −20°C.
Nuclease free water. Store at room temperature.
DNA templates extracted from blood or serum samples. For detection of viruses, extracted RNA is first converted to cDNA using any commercially available reverse transcription kit.
2.4. Ligase Detection Reaction (LDR) for Detection of Pathogen
10× LDR buffer: 20 mM Tris–HCl buffer, pH 7.6, 100 mM KCl, 10 mM MgCl2. Store at −20°C (see Note 3).
Nicotinamide adenine dinucleotide (NAD+) 10 mM. Store at −20°C.
Dithiothrietol (DTT), 200 mM. Store at −20°C.
Ligation primers. The downstream primers are labeled with fluorescent dye at the 3′ end. The upstream primer bears a specific zip-code complement at the 5′ end. The 5′ end of the upstream primer is also blocked with blocking group. Store stock primer solutions (100 μM) at −20°C.
Product obtained from gene specific PCR detailed in Subheading 2.1.
AK 16D ligase enzyme (expressed from Thermus spp. AK 16D), store at −20°C. The working concentration of the enzyme is 1 μM (see Note 4).
T4 ligase buffer: 50 mM Tris–HCl, 10 mM MgCl2, 10 mM dithiothreitol, 1 mM ATP, 25 μg/ml bovine serum albumin (New England BioLabs, Ipswich, MA). Store at −20°C (see Note 5).
T4 polynucleotide kinase (PNK) enzyme (10 U) (New England BioLabs, Ipswich, MA). Store at −20°C.
3. Methods
The general procedure for carrying out a PCR/LDR/universal array experiment involves the following steps: (1) Array printing and quality control (2) PCR amplification (3) LDR and (4) Array hybridization and scanning. Steps 2 and 3 are outlined in Fig. 1. Arrays are generally printed in batches and a random selection of printed arrays is assayed for print quality. PCR reactions are set up using standard procedures. Sample preparation for PCR should be performed in an area separate from where the reaction mixture for amplification is being assembled. Assembling PCR reactions in a laminar flow cabinet equipped with a UV lamp is recommended to prevent contamination. If possible, a separate set of pipettes designated for PCR setup as well as using pipette tips with aerosol filters for both DNA sample and reaction mixture preparation should be used to further reduce the chance of contamination. PCR primers are designed based on alignments of the target gene sequence, either the same conserved gene from multiple species of organisms, or from multiple isolates of the same species. PCR primers are designed to have Tm values between 65 and 75°C and incorporate the use of degenerate nucleotides to cover for sequence variations (see Note 6). LDR primers are designed to detect single base variations or specific nucleotide positions within the PCR amplicons. As with the PCR primers, the LDR primers use degenerate nucleotides where required to cover for sequence variations (see Note 7).
3.1. Printing and Coupling of Zip-Code Addresses
Prepare the print plate containing a final concentration of 1× print buffer and 25 μM zip-code oligonucleotide in a 384 conical well plate. Add 1 μM fiducial oligonucleotide to the printing mix in each well with each zip-code address. This will be used as a quality control to determine the position and quality of each spot.
Remove Codelink activated slides from sealed package. Unused slides must be stored in a sealed desiccator. Place slides in the QArrayMini robotic array printer (To easily identify the location of the zip-codes spotted in each subarray, place the slides so that the text “Codelink” etched into the slide is at the bottom and each slide is placed flush up and right). Position 1 is for a blotting slide. Fill all empty spaces for each vacuum region. Turn on vacuum. Set the relative humidity <50% and the temperature to 10°C.
Place the print plate in the QArrayMini robotic array printer. Print DNA onto activated slides to produce arrays in desired layout.
Leave the print plate in the QArrayMini robotic array printer overnight to dry out oligonucleotides in wells. Seal plate and store in desiccator between print runs (Resuspend in dH2O at least 1 h prior to print run for each subsequent use).
Place printed slides in slide storage box. Place uncovered slide storage box in saturated NaCl chamber. Seal the NaCl chamber and incubate at room temperature for 4–72 h.
3.2. Postcoupling Processing
Place printed slides in glass coplin jar with prewarmed blocking solution at 50°C for 30 min.
Rinse the slides 2× with ultrapure filtered water.
Wash the slides with 4× SSC, 0.1% SDS (prewarmed to 50°C) for 30 min on the shaker.
Discard wash solution and rinse briefly 2× with ultrapure filtered water.
Spin dry the slides using the Slide Spinner.
Store coupled slides in slide storage boxes at ambient temperature until use. For long-term storage, maintain the slides in a desiccated environment.
3.3. Array Hybridization Quality Control
Randomly select printed slides from each print batch (one from the beginning of the batch, one from the end of the batch, and one or more from the middle of the batch).
Prepare hybridization solution containing 10 fmol fluorescent-labeled zip-code complements, 500 fmol competition primers, 2.5 fmol fiducial complement, 0.1 mg/ml ssDNA, 5× SSC, 0.1% SDS in a total volume of 30 μl. A 6-carboxy-X-rhodamine-labeled fiducial complement included in the hybridization mixture serves as an internal positive control to determine the position and quality of each printed address (Fig. 2).
Prepare the ProPlate multiarray hybridization chamber by affixing the ProPlate multiarray chamber to the Codelink slides, so the printed side is facing the empty chambers.
Add 30 μl hybridization solution to each chamber.
Seal the ProPlate multiarray chamber system with ProPlate adhesive seal-strips.
Incubate the slides in the dark on a rocker platform at 60°C for 2 h.
After incubation, dismantle the ProPlate multiarray chamber system and briefly rinse the slides with 4× SSC.
Wash the slides with prewarmed wash solution I (2× SSC, 0.1% SDS) for 10 min at 60°C.
Wash the slides with wash solution II (0.2% SSC) for 1 min at room temperature.
Wash the slides with wash solution III (0.1% SSC) for 1 min at room temperature.
Spin dry the slides using the Slide Spinner.
Scan the slides and analyze the signal intensities using ProScanArray microarray scanner. The quality control data should provide specific fluorescent signals in the absence of extraneous signals on adjacent addresses (Fig. 2). The requirements for signal intensities from spotted addresses that are statistically significant should be determined (see Note 8).
Fig. 2.
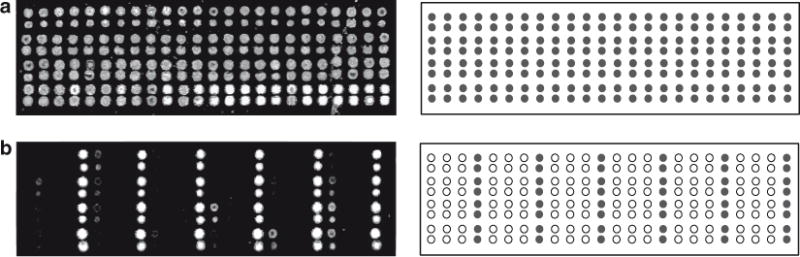
Quality control and validation of arrays post spotting. (Panel a) Hybridization of ROX-labeled fiducial complement to the array. Since the fiducial oligonucleotide is included at every location, all spots should provide a positive fluorescent signal of equal intensity. Note that we double spot each address on the array. (Panel b) Hybridization of FAM-labeled zip-code complements to the addresses in every fourth column (Columns 4, 8, 12, 16, 20, and 24) of the array (addresses 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52, 56, 60, 64, 68, 72, 76, 80, 84, 88, 92, and 96), When no other columns or addresses provide a positive signal, the print run is considered successful. Three other hybridization experiments are carried out using FAM-labeled zip-code complements to the addresses in Columns 1, 5, 9, 13, 17, and 21 combined, Columns 2, 6, 10, 14, 18, and 22 combined, and Columns 3, 7, 11, 15, 19, and 23 combined (data not shown)
3.4. Setup of PCR Amplification
Nucleic acids extracted from human specimens (blood or serum) are used as a template for PCR amplification. For templates stored at −20°C, gently vortex and centrifuge samples and thaw on ice.
Dilute the PCR primers in nuclease-free water to the working concentration (usually 2–5 μM) (see Note 9).
Prepare a master mix containing 2.5 μl of 10× PCR buffer, 2.5 μl of MgCl2, 2.0 μl of dNTPs, 2–5 μl of a 1 μM primer mix containing all primers, 1.25 U of AmpliTaq Gold DNA polymerase, and nuclease-free water. The total volume of the master mix for the PCR reaction is dependent on the volume of DNA template to be added. The recipe provided is for 25 μl reactions. It is best to prepare a sufficient quantity of master mix for multiple reactions and aliquot the mix into separate reaction tubes. Individual DNA templates can then be added to the respective tubes. To set up reactions with larger volumes, such as 50 μl, simply double the volume of reagents used.
Pipette 20–22 μl of the master mix in to thin walled PCR reaction tubes on ice. Add template DNA (amount to be added may be optimized, usually; 10 pg to 1 μg of DNA).
Carry out the PCR reaction in a thermocycler using the following protocol: 10 min at 94°C, followed by 30–40 cycles of 15 s at 94°C, 1 min at 60°C, 1 min at 72°C, followed by a final extension step of 7 min at 72°C, followed by 30 min at 99°C to destroy the Taq polymerase. The PCR products may be stored at 4°C until further processing (see Note 10).
3.5. Setup of LDR
The downstream primers used in an LDR reaction need to be phosphorylated for ligation to occur. Since the upstream ligation primers are blocked at their 5′-termini, a primer mix containing both the upstream and downstream primers is treated with T4 polynucleotide kinase and ATP prior to use (see Note 11).
Prepare a LDR primer mix such that each primer is at a final concentration of 250 or 500 fmol per reaction when used in the LDR.
Mix together, 3 μl of 10× T4 ligase buffer, 10 units of T4 PNK enzyme, and LDR primer mix. Make the volume up to 30 μl with nuclease free water. In a thermocycler, incubate the reaction at 37°C for 1 h followed by 10 min at 65°C, to inactivate the enzyme. Phosphorylated primers may be stored at 4°C for up to a week.
Prepare a LDR master mix containing 2.0 μl of 10× LDR buffer, 0.1 μl of 200 mM DTT, 2 μl of 10 mM NAD+, sufficient phosphorylated primer mix for a final primer concentration of each primer of 250 or 500 fmol, 0.25 μl of 1 μM AK16D ligase, and nuclease-free water (for a total volume of 19 μl). The recipe provided is for a single 20 μl reaction. It is best to prepare a sufficient quantity of master mix for multiple reactions and aliquot the mix into separate reaction tubes. Individual DNA templates can then be added to the respective tubes.
Pipette 19 μl of the master mix into thin walled PCR reaction tubes on ice. Add 1 μl of PCR product from step 5 of Subheading 3.4 above. Carry out the LDR in a thermocycler using the following protocol: 94°C for 2 min followed by 20 cycles of 30 s at 94°C, 4 min at 64°C. The LDR product may be stored at 4°C until hybridization to the array.
3.6. Hybridization of LDR Products
Dilute the entire amount of the ligation products from step 4 of Subheading 3.5 in hybridization buffer: (5× SSC buffer, 0.1% SDS, 0.1 mg/ml salmon sperm DNA (Fisher Scientific), and 5 nM of the fiducial complement in a total volume of 30 μl).
Denature the mixture at 94°C for 3 min and chill on ice.
Repeat steps 3–12 described in Subheading 3.3 above.
Determine the identity of the pathogen based on the zip-code addresses that present fluorescent signals. An example of the identification of four different serotypes of Dengue virus is shown in Fig. 3.
Table 1.
Sequences of zip-code oligonucleotides
| Zip-code sequence | Zip-code complement |
|---|---|
| AGCGAGCGGGAACAGGCCAA | TTGGCCTGTTCCCGCTCGCT |
| GGAACACCACGCAGCGCAGG | CCTGCGCTGCGTGGTGTTCC |
| GCAGTGCTCACCGTCCGCGA | TCGCGGACGGTGAGCACTGC |
| CGGAGTGGCACCAGCGGGAA | TTCCCGCTGGTGCCACTCCG |
| GCAGCAGGCCAAAGCGAGCG | CGCTCGCTTTGGCCTGCTGC |
| GTCCGAGCCCTCACGCAGCG | CGCTGCGTGAGGGCTCGGAC |
| GCAGGACGACGCGGGTGGAA | TTCCACCCGCGTCGTCCTGC |
| TGGCGGTCTGCTGAGCGGTC | GACCGCTCAGCAGACCGCCA |
| GTGGGTCCCGGAAGCGTGCT | AGCACGCTTCCGGGACCCAC |
| GCCTCGAGCCAACACCGCCT | AGGCGGTGTTGGCTCGAGGC |
| TGGCCGGACAGGAGACACGC | GCGTGTCTCCTGTCCGGCCA |
| GCCTGCCTTCACGAGCCCAA | TTGGGCTCGTGAAGGCAGGC |
| GTGGGCGAAGCGGGAACCTC | GAGGTTCCCGCTTCGCCCAC |
| CGGAGCGATCACGTGGCACC | GGTGCCACGTGATCGCTCCG |
| ACGCGACGCACCTGCTCCAA | TTGGAGCAGGTGCGTCGCGT |
| CCTCCCTCACGCGCCTGCAG | CTGCAGGCGCGTGAGGGAGG |
| GTGGACTGAGCGCGGATGGC | GCCATCCGCGCTCAGTCCAC |
| CGAGGCAGACGCGTCCCACC | GGTGGGACGCGTCTGCCTCG |
| TGGCCGAGACTGCAGGAGCG | CGCTCCTGCAGTCTCGGCCA |
| AGCGGACGACTGCGGACGAG | CTCGTCCGCAGTCGTCCGCT |
| GCCTGCGAAGACCCAAGCGA | TCGCTTGGGTCTTCGCAGGC |
| GAGCAGCGACGCCGAGGCAG | CTGCCTCGGCGTCGCTGCTC |
| GCGAGTCCCGAGGGTCCCAA | TTGGGACCCTCGGGACTCGC |
| GGAAAGCGAGCGGCAGCCAA | TTGGCTGCCGCTCGCTTTCC |
| TGGCGGAACAGGACTGCGGA | TCCGCAGTCCTGTTCCGCCA |
| TGGCGGGTTGCTCCTCGTGG | CCACGAGGAGCAACCCGCCA |
| GACGGCCTTGCTAGCGCGGA | TCCGCGCTAGCAAGGCCGTC |
| GCCTGCAGTGCTGGTCCGGA | TCCGGACCAGCACTGCAGGC |
| CCTCCGGAAGACCCTCGCGA | TCGCGAGGGTCTTCCGGAGG |
| GCGAGCAGCAGGGTGGACCA | TGGTCCACCCTGCTGCTCGC |
| GCCTGAGCAGACGGTCGCGA | TCGCGACCGTCTGCTCAGGC |
| GGGTGCCTAGCGGTCCAGCG | CGCTGGACCGCTAGGCACCC |
| CAGGACGCACCAACGCCCAA | TTGGGCGTTGGTGCGTCCTG |
| AGCGCACCCGGAACTGGAGC | GCTCCAGTTCCGGGTGCGCT |
| ACGCGTGGACTGCCTCGAGC | GCTCGAGGCAGTCCACGCGT |
| ACGCCCTCCCAACCTCACGC | GCGTGAGGTTGGGAGGGCGT |
| CACCGCAGCCTCCCAACCAA | TTGGTTGGGAGGCTGCGGTG |
| GGGTTGGCGGAAGGTCGACG | CGTCGACCTTCCGCCAACCC |
| GCGAGCGAACCAGAGCGACG | CGTCGCTCTGGTTCGCTCGC |
| TGGCAGCGTCACGGGTCACC | GGTGACCCGTGACGCTGCCA |
| GGGTGACGAGCGCCAAGCCT | AGGCTTGGCGCTCGTCACCC |
| GCGATGGCAGCGGTGGAGAC | GTCTCCACCGCTGCCATCGC |
| GCAGGCGATGGCTCACGACG | CGTCGTGAGCCATCGCCTGC |
| GTCCTGCTGTGGGCGATGGC | GCCATCGCCCACAGCAGGAC |
| GGTCGTCCGGTCGCCTTGCT | AGCAAGGCGACCGGACGACC |
| ACCAAGCGGCCTCCTCGTCC | GGACGAGGAGGCCGCTTGGT |
| ACGCGGAAGGTCTGGCCAGG | CCTGGCCAGACCTTCCGCGT |
| CCAACGGAGCGACGAGCAGG | CCTGCTCGTCGCTCCGTTGG |
| CGGACAGGGACGGCGATCAC | GTGATCGCCGTCCCTGTCCG |
| GGTCGGGTCAGGCCTCGGAA | TTCCGAGGCCTGACCCGACC |
| CGGAAGCGCGAGACCACACC | GGTGTGGTCTCGCGCTTCCG |
| TCACCCTCTGGCGGAACGGA | TCCGTTCCGCCAGAGGGTGA |
| CCAAAGACAGCGGACGGCGA | TCGCCGTCCGCTGTCTTTGG |
| GGGTGGGTCGAGGCCTGGTC | GACCAGGCCTCGACCCACCC |
| CGGAGTCCTGGCAGCGTGGC | GCCACGCTGCCAGGACTCCG |
| GCGAAGCGACCAAGACCGGA | TCCGGTCTTGGTCGCTTCGC |
| CAGGCACCCACCGCGAAGAC | GTCTTCGCGGTGGGTGCCTG |
| GTCCGCAGCCAACCAAACGC | GCGTTTGGTTGGCTGCGGAC |
| GCAGAGCGTGGCCGAGGTCC | GGACCTCGGCCACGCTCTGC |
| GTGGCGGACGGACGAGTGGC | GCCACTCGTCCGTCCGCCAC |
| GCAGGTGGGACGGGTCGGGT | ACCCGACCCGTCCCACCTGC |
| AGACAGCGGCGAGAGCGGGT | ACCCGCTCTCGCCGCTGTCT |
| CGAGAGCGGTCCCGGAGGTC | GACCTCCGGGACCGCTCTCG |
| CCTCCGAGCACCGACGACGC | GCGTCGTCGGTGCTCGGAGG |
| ACGCCCAAACGCAGACCCAA | TTGGGTCTGCGTTTGGGCGT |
| GGTCCAGGTGGCGGTCGAGC | GCTCGACCGCCACCTGGACC |
| AGCGTCACGAGCCAGGCGGA | TCCGCCTGGCTCGTGACGCT |
| GAGCGTGGCGGAGGTCGGTC | GACCGACCTCCGCCACGCTC |
| GACGGCGAGGGTGCAGGCAG | CTGCCTGCACCCTCGCCGTC |
| CCTCGACGGTCCTGGCTGGC | GCCAGCCAGGACCGTCGAGG |
| GAGCTGCTTGGCGCGACACC | GGTGTCGCGCCAAGCAGCTC |
| GCGACAGGCGGAGAGCGGAA | TTCCGCTCTCCGCCTGTCGC |
| GCAGTGGCGTCCGGGTGAGC | GCTCACCCGGACGCCACTGC |
| CGAGGGAAGTGGGCAGCGGA | TCCGCTGCCCACTTCCCTCG |
| TGGCGAGCGCAGTGGCAGAC | GTCTGCCACTGCGCTCGCCA |
| CGAGGCCTGCAGGGAAAGCG | CGCTTTCCCTGCAGGCCTCG |
| CGAGGTCCGGGTGCGAGAGC | GCTCTCGCACCCGGACCTCG |
| GTGGGAGCGACGCAGGGCAG | CTGCCCTGCGTCGCTCCCAC |
| GGGTGCAGGCCTGTGGGTCC | GGACCCACAGGCCTGCACCC |
| GCCTACGCGAGCGACGGAGC | GCTCCGTCGCTCGCGTAGGC |
| CACCGAGCTGCTGCCTTGGC | GCCAAGGCAGCAGCTCGGTG |
| GGGTGGAAAGCGGAGCGTGG | CCACGCTCCGCTTTCCACCC |
| CAGGCCAAGCAGACGCGACG | CGTCGCGTCTGCTTGGCCTG |
| GCGAGGGTGCGAGGGTTGCT | AGCAACCCTCGCACCCTCGC |
| AGCGGTCCGACGGCCTTCAC | GTGAAGGCCGTCGGACCGCT |
| GTCCCGAGGCAGCGAGAGCG | CGCTCTCGCTGCCTCGGGAC |
| GCAGTCACGGTCAGCGGCCT | AGGCCGCTGACCGTGACTGC |
| GACGCCAACGGACGGAGGGT | ACCCTCCGTCCGTTGGCGTC |
| TCACGCGACACCCGGACACC | GGTGTCCGGGTGTCGCGTGA |
| CGAGCGGAGAGCGAGCCAGG | CCTGGCTCGCTCTCCGCTCG |
| AGCGTGCTGGTCGTGGGCCT | AGGCCCACGACCAGCACGCT |
| AGCGCCAAGGGTCCTCGGGT | ACCCGAGGACCCTTGGCGCT |
| CCAAAGCGAGACCGGAGCGA | TCGCTCCGGTCTCGCTTTGG |
| GACGCACCGAGCACGCACCA | TGGTGCGTGCTCGGTGCGTC |
| TCACCCAAGACGGCAGGCGA | TCGCCTGCCGTCTTGGGTGA |
| GGGTGGTCCGGAGCGAGCAG | CTGCTCGCTCCGGACCACCC |
Acknowledgments
Support for this work was provided by the National Cancer Institute (P01-CA65930) and the National Institute of Allergy and Infectious Diseases (UC1-AI062579).
Footnotes
The zip-code oligonucleotides are synthesized with a 3′-amino modifier and a spacer 18 modifier (phosphoramidite available from Glen Research, alternatively, most commercial oligonucleotide manufactures offer this modification) between the base at the 3′-end and the amino modifier. The zip-code oligonucleotides must be either PAGE or HPLC purified for best results, however, reverse-phase cartridge purification may provide satisfactory results. The sequences of the zip-code oligonucleotides are provided in Table 1.
The ProPlate multiarray chamber system with the ProPlate adhesive strips enables printing of 16 discrete arrays on a single slide. Thus, 16 individual LDR reactions may be hybri dized to the arrays on a slide without cross-contamination of the samples.
We recommend that the 10× LDR buffer be aliquoted in tubes and stored at −20°C. Once an aliquot is thawed for use, it may be stored at 4°C for up to 2 weeks and then discarded.
The AK16D ligase enzyme preparation is normally at a higher concentration than the 1 μM concentration required for the LDR. The enzyme may be diluted to a 1 μM concentration using 1× LDR buffer (tenfold dilution of the 10× LDR buffer) just prior to setting up the LDR reaction. We do not recommend storing the diluted enzyme.
The T4 ligase buffer composition is the same as that of the T4 PNK buffer supplied with the T4 PNK enzyme except that the ligase buffer already contains 1 mM ATP, the phosphate source. If using the PNK buffer, it is necessary to add ATP at a concentration of 1 mM to the buffer. We recommend storing aliquots of either buffer at −20°C for each use of the buffer.
We recommend using the software program Oligo 6 for designing PCR and LDR primers. Oligo 6 calculates Tm values for the primers using the nearest neighbor method. PCR primers should have GC content between 40 and 60% and Tm values of 65–70°C. Where possible, the last 4 bases at the 3′-end of the primers should be SWWC, where S is a strong base such as G or C and W is a weak base such as A or T. The primer sequences should be checked for self-complementary regions or regions complementary to other primers in the reaction mixture, in order to avoid primer-dimer and hairpin formation. No primer should contain more than 3 degenerate positions. For multiplex PCR amplification, universal tail sequences may also be appended to the 5′-ends of forward and reverse PCR primers to prevent the formation of primer dimers. The universal tail sequence commonly used in our laboratory for such applications is CGCTGCCAACTACCGCACATC.
LDR primers are designed to have Tm values of 65–70°C for the upstream primers and 70–75°C for the downstream primers. LDR primers should have no more than 3 degenerate positions in each primer, and there should be no degenerate base within at least 3 bases of the ligation site. In order to adhere to this requirement, it may be necessary to design multiple primers to cover all possible sequence variants. The last base on the upstream primer is the query base and must be perfectly complementary to the template for ligation to occur. As shown in Fig. 1, the upstream LDR primers have zip-code complements attached to their 5′-ends. Additionally, the upstream primers are blocked at the 5′-end with a blocking group such as a C3 spacer or a C6-amino linker. The downstream LDR primers are labeled with a fluorophore (commonly Cy3 or Cy5) at their 3′-end. In a variation, the zip-code complements may be attached to the 3c-ends of the downstream primers followed by a blocking group, and the fluorescent label attached to the 5′-ends of the upstream primers. The sequences of the zip-code oligonucleotides are provided in Table 1.
In general, we consider a signal to be positive when its intensity is at least tenfold higher than the background intensity.
For most PCR protocols, we use between 5 and 10 pmol of each PCR primer. For highly multiplexed PCR reactions, it may be necessary to reduce the amount of each primer to 2 pmol. The optimal amount of each primer is usually determined empirically.
The additional step of incubating the PCR reaction at 99°C is sufficient to inactivate the Taq polymerase. An alternate method involves incubating the PCR reaction with 1 μl of proteinase K (18 mg/ml) at 70°C for 10 min. The proteinase K is then inactivated by incubating at 95°C for 15 min.
While LDR primers may be synthesized with 5′-posphate groups on the downstream primers, we have found that long-term storage of the primers results in degradation of the phosphate groups resulting in progressively weaker ligation signals over time. Therefore, we recommend that primers be freshly phosphorylated by treatment with kinase prior to each LDR reaction. There should be no more than 300 pmol of primers with free 5′-OH ends per 30 μl phosphorylation reaction using 10 units of T4 PNK enzyme.
References
- 1.Boissinot M, Bergeron MG. Toward rapid real-time molecular diagnostic to guide smart use of antimicrobials. Curr Opin Microbiol. 2002;5:478–482. doi: 10.1016/s1369-5274(02)00362-4. [DOI] [PubMed] [Google Scholar]
- 2.Muldrew KL. Molecular diagnos tics of infectious diseases. Curr Opin Pediatr. 2009;21:102–111. doi: 10.1097/MOP.0b013e328320d87e. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA. Molecular approaches to diagnosing and managing infectious diseases: practicality and costs. Emerg Infect Dis. 2001;7:312–318. doi: 10.3201/eid0702.010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procop Gary W. Molecular diagnostics for the detection and characterization of microbial pathogens. Clin Infect Dis. 2007;45:S99–S111. doi: 10.1086/519259. [DOI] [PubMed] [Google Scholar]
- 5.Barany F. The ligase chain reaction in a PCR world. PCR Methods Appl. 1991;1:5–16. doi: 10.1101/gr.1.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc Natl Acad Sci USA. 1991;88:189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day DJ, Speiser PW, White PC, Barany F. Detection of steroid 21-hydroxylase alleles using gene-specific PCR and a multiplexed ligation detection reaction. Genomics. 1995;29:152–162. doi: 10.1006/geno.1995.1226. [DOI] [PubMed] [Google Scholar]
- 8.Khanna M, Cao W, Zirvi M, Paty P, Barany F. Ligase detection reaction for identification of low abundance mutations. Clin Biochem. 1999;32:287–290. doi: 10.1016/s0009-9120(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 9.Favis R, Gerry NP, Cheng YW, Barany F. Applications of the universal DNA microarray in molecular medicine. Methods Mol Med. 2005;114:25–58. doi: 10.1385/1-59259-923-0:25. [DOI] [PubMed] [Google Scholar]
- 10.Gerry NP, Witowski NE, Day J, Hammer RP, Barany G, Barany F. Universal DNA microarray method for multiplex detection of low abundance point mutations. J Mol Biol. 1999;292:251–262. doi: 10.1006/jmbi.1999.3063. [DOI] [PubMed] [Google Scholar]
- 11.Dong SM, Traverso G, Johnson C, Geng L, Favis R, Boynton K, Hibi K, Goodman SN, D’Allessio M, Paty P, Hamilton SR, Sidransky D, Barany F, Levin B, Shuber A, Kinzler KW, Vogelstein B, Jen J. Detecting colorectal cancer in stool with the use of multiple genetic targets. J Natl Cancer Inst. 2001;93:858–865. doi: 10.1093/jnci/93.11.858. [DOI] [PubMed] [Google Scholar]
- 12.Favis R, Barany F. Mutation detection in K-ras, BRCA1, BRCA2, and p53 using PCR/LDR and a universal DNA microarray. Ann N Y Acad Sci. 2000;906:39–43. doi: 10.1111/j.1749-6632.2000.tb06588.x. [DOI] [PubMed] [Google Scholar]
- 13.Favis R, Day JP, Gerry NP, Phelan C, Narod S, Barany F. Universal DNA array detection of small insertions and deletions in BRCA1 and BRCA2. Nat Biotechnol. 2000;18:561–564. doi: 10.1038/75452. [DOI] [PubMed] [Google Scholar]
- 14.Favis R, Huang J, Gerry NP, Culliford A, Paty P, Soussi T, Barany F. Harmonized microarray/mutation scanning analysis of TP53 mutations in undissected colorectal tumors. Hum Mutat. 2004;24:63–75. doi: 10.1002/humu.20069. [DOI] [PubMed] [Google Scholar]
- 15.Fouquet C, Antoine M, Tisserand P, Favis R, Wislez M, Commo F, Rabbe N, Carette MF, Milleron B, Barany F, Cadranel J, Zalcman G, Soussi T. Rapid and sensitive p53 alteration analysis in biopsies from lung cancer patients using a functional assay and a universal oligonucleotide array: a prospective study. Clin Cancer Res. 2004;10:3479–3489. doi: 10.1158/1078-0432.CCR-0994-03. [DOI] [PubMed] [Google Scholar]
- 16.Consolandi C, Busti E, Pera C, Delfino L, Ferrara GB, Bordoni R, Castiglioni B, Bernardi LR, Battaglia C, De Bellis G. Detection of HLA polymorphisms by ligase detection reaction and a universal array format: a pilot study for low resolution genotyping. Hum Immunol. 2003;64:168–178. doi: 10.1016/s0198-8859(02)00685-7. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YW, Shawber C, Notterman D, Paty P, Barany F. Multiplexed profiling of candidate genes for CpG island methylation status using a flexible PCR/LDR/Universal Array assay. Genome Res. 2006;16:282–289. doi: 10.1101/gr.4181406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pingle MR, Granger K, Feinberg P, Shatsky R, Sterling B, Rundell M, Spitzer E, Larone D, Golightly L, Barany F. Multiplexed identification of blood-borne bacterial pathogens by use of a novel 16S rRNA gene PCR-ligase detection reaction-capillary electro phoresis assay. J Clin Microbiol. 2007;45:1927–1935. doi: 10.1128/JCM.00226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busti E, Bordoni R, Castiglioni B, Monciardini P, Sosio M, Donadio S, Consolandi C, Rossi Bernardi L, Battaglia C, De Bellis G. Bacterial discrimination by means of a universal array approach mediated by LDR (ligase detection reaction) BMC Microbiol. 2002;2:27. doi: 10.1186/1471-2180-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castiglioni B, Rizzi E, Frosini A, Sivonen K, Rajaniemi P, Rantala A, Mugnai MA, Ventura S, Wilmotte A, Boutte C, Grubisic S, Balthasart P, Consolandi C, Bordoni R, Mezzelani A, Battaglia C, De Bellis G. Development of a universal microarray based on the ligation detection reaction and 16S rrna gene polymorphism to target diversity of cyanobacteria. Appl Environ Microbiol. 2004;70:7161–7172. doi: 10.1128/AEM.70.12.7161-7172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondini S, Pingle MR, Das S, Tesh R, Rundell MS, Hom J, Stramer S, Turner K, Rossmann SN, Lanciotti R, Spier EG, Munoz-Jordan J, Larone D, Spitzer E, Barany F, Golightly LM. Development of multiplex PCR-ligase detection reaction assay for detection of West Nile virus. J Clin Microbiol. 2008;46:2269–2279. doi: 10.1128/JCM.02335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Pingle MR, Munoz-Jordan J, Rundell MS, Rondini S, Granger K, Chang GJ, Kelly E, Spier EG, Larone D, Spitzer E, Barany F, Golightly LM. Detection and serotyping of dengue virus in serum samples by multiplex reverse transcriptase PCR-ligase detection reaction assay. J Clin Microbiol. 2008;46:3276–3284. doi: 10.1128/JCM.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto M, Barany F, Soper SA. Polymerase chain reaction/ligase detection reaction/hybridization assays using flow-through microfluidic devices for the detection of low-abundant DNA point mutations. Biosens Bioelectron. 2006;21:1915–1923. doi: 10.1016/j.bios.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto M, Hupert ML, Murphy MC, Soper SA, Cheng YW, Barany F. Ligase detection reaction/hybridization assays using three-dimensional microfluidic networks for the detection of low-abundant DNA point mutations. Anal Chem. 2005;77:3243–3255. doi: 10.1021/ac048184d. [DOI] [PubMed] [Google Scholar]


