Abstract
The world’s population is nearing 6.8 billion, and we are in need of a male contraceptive that is safe, effective, reversible and affordable. Hormonal approaches, which employ different formulations of testosterone administered in combination with other hormones, have shown considerable promise in clinical trials, and they are currently at the forefront of research and development. However, the long-term effects of using hormones throughout a male’s reproductive life for contraception are unknown, and it may take decades before this information becomes available. Because of this, many investigators are aiming to bring a nonhormonal male contraceptive to the consumer market. Indeed, there are several distinct but feasible avenues in which fertility can be regulated without affecting the hypothalamus-pituitary-testis axis. In this review, we discuss several approaches for fertility control involving the testis that one day may lead to the development of a nonhormonal male contraceptive.
Keywords: Male contraceptive, Testis, Adjudin, Cell adhesion, Spermatogenesis
1. Introduction
At the writing of this review, the world’s population is estimated to be 6.8 billion and rising rapidly. Still, safe, effective, reversible and affordable contraception continues to be an unmet need for many couples. While most women commonly assume the responsibility of family planning, surveys have shown that men in general are interested in playing a greater role [1,2]. This is probably because none of the currently available contraceptive options for men (i.e., condom, vasectomy, withdrawal or periodic abstinence) are ideal. For instance, there are concerns that vasectomy associates with an increased incidence of epididymitis or orchitis resulting from the development of anti-sperm antibodies [3–5]. As for the condom, which has been used for at least 400 years, the failure rate is unacceptably high at 10–18% with typical use [6]. Thus, studies that aim to identify new and better solutions for fertility control in males are a priority.
Presently, there are several male contraceptive approaches being investigated, including delivery of hormones which affect sperm production, partially blocking the vas deferens to prevent the passage of sperm, immunocontraceptives and administration of different compounds that can arrest spermatogenesis, thereby leading to infertility. Of these, hormonal contraceptive methods have gained the most ground since many regimens (e.g., testosterone enanthate, testosterone decanoate, testosterone undecanoate) have been tested in clinical trials and shown to be effective [7–9]. The premise for hormonal male contraception is that exogenous administration of different testosterone formulations in combination with other hormones (e.g., etonogestrel implants or depot medroxyprogesterone acetate injections) can suppress the secretion of pituitary luteinizing hormone (LH) and follicle stimulating hormone (FSH), resulting in azoospermia in many, but not all, men. While hormonal contraceptives appear to be safe, additional studies are needed to investigate the long-term effects of testosterone administration not only on androgen-dependent organs such as the prostate but also on overall male health since contraceptives would be used by healthy individuals for extended periods of time.
Besides hormonal male contraceptives, there is also an interest to develop nonhormonal contraceptives and several different approaches are under investigation. For example, studies from our own laboratory on adjudin have shown this compound to be effective in inducing germ cell loss from the seminiferous epithelium in rats and rabbits by specifically affecting germ cell adhesion in the testis but not in other organs, resulting in transient infertility [10–14]. Other studies employing transcriptional profiling of genes in the testis aim to identify novel targets which may halt sperm production, development or function. Thus, the need for non-hormonal male contraceptives is also being addressed by several different lines of research. Herein, we focus on the current status of nonhormonal male contraceptive research and development.
2. Prospects for nonhormonal male contraception
2.1. Reversible inhibition of sperm under guidance
Reversible inhibition of sperm under guidance (RISUG) involves injection of steric maleic anhydride (SMA) combined with dimethyl sulfoxide (DMSO) into the vas deferens to create a partial obstruction, and as sperm passes, its plasma and acrosomal membranes, midpieces and tails are severely destroyed, resulting in infertility [15,16]. RISUG may be a promising alternative to vasectomy since infertility was shown to be reversed after short-term vas occlusion in langur monkeys and rats [16–18]. A recent report also describes a novel formulation defined as smart RISUG that was synthesized by dispersing iron oxide and copper particles into SMA-DMSO, thus allowing detection of the implant by X-ray and magnetic imaging [19]. Interestingly, magnetic particles have been shown to agglomerate and to bind proteins [20], whereas copper particles have been demonstrated to displace molecules on the surface of sperm [21]. This formulation also appears to provide improved spermicidal action over the original formulation of RISUG [19]. The safety and efficacy of RISUG in humans has been previously shown [22,23], and RISUG is presently undergoing Phase III clinical trials in India [24]. However, additional studies are needed on RISUG.
2.2. Contraceptive vaccines
Immunocontraception involves the use of antigens/antibodies to target different aspects of gamete production and function as a means of inducing infertility [25–29]. For example, vaccines based on luteinizing hormone-releasing hormone/gonadotropin releasing hormone (GnRH) have been tested, and they have been shown to affect testosterone production, resulting in a decrease in testis and prostate weights [30,31]. Immunocontraception that targets different sperm antigens to affect fertilization and fertility has also been investigated. For instance, immunization of male monkeys with human recombinant epididymal protease inhibitor (EPPIN), a serine protease inhibitor expressed by the testis and epididymis [32–34], altered sperm progressive motility (as the ability of anti-EPPIN-treated spermatozoa to utilize cAMP was compromised [35]) and resulted in reversible infertility [36,37]. Additional studies reported that EPPIN binds to semenogelin I [38], a protein in the coagulum that renders sperm immobile [39], and anti-EPPIN antibodies appear to affect the interaction between EPPIN and semenogelin [40]. However, after nearly 3 decades of research, there are no immunocontraceptives on the market for human use. This is because contraceptive vaccines have been shown to be ineffective in inducing long-term infertility in all subjects which is probably due to differences in host immune responses. There are other obstacles as well. The entire spermatozoon cannot be used for the development of a vaccine because it contains several antigens that are also likely to be expressed by other cells in the body, that is, the antigen must be sperm-specific. Moreover, any antigen that is to be used for immunocontraception has to be present on the cell surface [41]. Equally important, the antigen has to be able to raise high-titer antibodies following administration and to interrupt fertility. Faced with these challenges, many investigators are currently re-examining the feasibility of using immunocontraception for fertility control. For instance, genetically engineered antibodies (e.g., multi-epitope vaccines and single chain variable fragment antibodies) that are likely to enhance efficacy may hold promise in the future [42].
2.3. Ca++ channel blockers
Ca++ is critical for sperm motility, capacitation and the acrosome reaction [43] and many Ca++-permeable channels have been identified in sperm including cyclic nucleotide-gated channels, voltage-gated channels and transient receptor potential channels [44–46]. In addition to these Ca++ channels, sperm expresses unique Ca++ channels, CAT-SPERS, which localize to the principal piece [47–49]. All four CatSper genes are indispensable for male fertility as a disruption in any one of these genes failed to initiate hyperactivation, resulting in sterility [48–52]. Indeed, HC-056456, a CATSPER channel blocker, was recently shown to prevent hyperactivated motility in an in vitro study [53]. As such, targeting CATSPER function may be a promising approach for male contraceptive development since effects would very likely be restricted to the testis. Along similar lines, nifedipine is a widely prescribed anti-hypertension drug that blocks Ca++ influx into sperm and affects sperm membrane cholesterol, thereby compromising fertility [54]. The Ca++ blockers verapamil and diltiazem are also known to affect sperm motility in rats [55]. While calcium blockers such as nifedipine which affect male fertility have passed extensive safety tests in humans, the use of anti-hypertension drugs for the control of male fertility does not appear to be an appealing contraceptive approach. At present, additional research is needed to identify drugs that would block CATSPER but not other ion channel proteins.
2.4. Indenopyridines
CDB-4022 [4aRS,5SR,9bRS]-2-ethyl-2,3,4,4a,5,9b-hex-ahydro-8-iodo-7-methyl-5-[4-carbomethoxyphenyl]-1H-indeno[1,2-c]pyridine-hydrochloride], an indenopyridine, is a compound that was initially developed as an antihistamine but subsequently shown to elicit antispermatogenic effects (i.e., germ cell loss from the seminiferous epithelium and testis atrophy) in mice, rats, dogs and monkeys [56–62]. In the rat, CDB-4022 (a racemic mixture of l and d isomers) induced irreversible infertility [61], but in the monkey l-CDB-4022 caused reversible oligospermia [63], illustrating variability in the way different species respond to CDB-4022. Interestingly, infertility was reversed in the rat when the administration of CDB-4022 was preceded by the administration of a GnRH agonist or antagonist to suppress endogenous testosterone [60,62]. CDB-4022 appears to target the Sertoli cell [59], as evidenced by the many parameters of Sertoli cell function that were shown to be affected within hours of CDB-4022 administration. For example, there was a significant decrease in serum inhibin B and FSH in rats treated with l-CDB-4022, suggestive of a disruption in spermatogenesis, but activin A, testosterone (however, in another study, serum testosterone was shown to decline in CDB-4022-treated adult rats [64]) and LH remained unchanged [60,61]. Equally important, the micro-tubule network, which gives the Sertoli cell its columnar shape, was disrupted and the steady-state levels of several adhesion proteins (e.g., cadherin, catenin, nectin, afadin and integrin β1) were shown to change in the rat testis following l-CDB-4022 treatment [65]. In addition to these biochemical changes, l-CDB-4022 also activated the mitogen activated protein kinase (MAPK) pathway in that an increase in the levels of phosphorylated extracellular signal-regulated kinases 1 and 2 [ERK1/2] was observed [65]. The MAPK pathway is comprised of several serine/threonine kinases that respond to extracellular stimuli and control diverse cellular events such as mitosis, differentiation, proliferation and cell survival [66–69] so that l-CDB-4022 likely targets some important aspect of Sertoli cell function that inadvertently leads to germ cell loss from the seminiferous epithelium.
2.5. Analogues of indazole-3-carboxylic acid
2.5.1. Adjudin
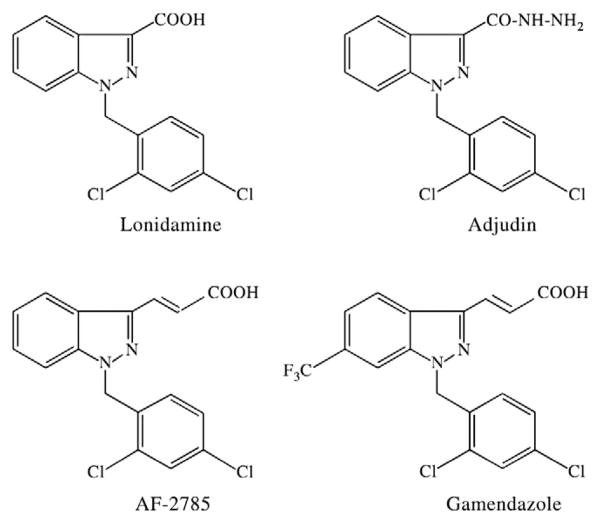
Adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is an analog of lonidamine [1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid], a chemotherapeutic agent known to inhibit the aerobic glycolysis of tumor cells [70,71], that causes reversible infertility in rats, rabbits and dogs without affecting the hypothalamic-pituitary-testicular axis [10,11,13,14,72] (Fig. 1). In essence, adjudin affects fertility by disrupting Sertoli-germ cell junctions, and once this occurs, germ cells slough the seminiferous epithelium prematurely. Once adjudin is cleared from the systemic circulation (i.e., by 24–48 h) and its adverse effects in the seminiferous epithelium of the testis have culminated, fertility begins to bounce back gradually [i.e., by 82 days following two oral doses at 50 mg/kg body weight (b.w.) administered 1 week apart] [12]. This suggests that adjudin, at least at this dose, does not destroy spermatogonial stem cells. It is worth noting that cell adhesion was not affected in other organs when examined histologically [11,13]. Moreover, many of the significant gene and protein changes (e.g., RhoB) that were demonstrated in the testis were not observed in other organs such as the brain and kidney [73]. When [3H]-adjudin was administered to adult rats, less than 0.05% was uptaken by the testis [12]. Instead, the greatest percentage of [3H]-adjudin that was recovered was from the serum (~3.5%), followed by the muscle (~0.8%), liver (~0.6%) and small intestine (~0.2%) 3 h after administration; and interestingly, less than 6% [3H]-adjudin was recovered in total [12]. These results are interesting for another reason: oral administration of adjudin (50 mg/kg b.w. for 29 consecutive days) in a subchronic toxicity test performed by licensed toxicologists resulted in adverse effects in two of these organs, namely, muscle atrophy and liver inflammation, in male (but not female) rats [13]. Nevertheless, such adverse effects would preclude adjudin from becoming a marketable male contraceptive. In addition, adjudin was shown to have low bioavailability in the rat, but the cause(s) (i.e., first-pass metabolism, insufficient time for absorption in the gastrointestinal tract, hydrolysis by digestive enzymes, etc.) for this is not known. Micronization by pulverization, which decreased adjudin’s particle size to ~50 μm, did improve dissolution rate in the gastrointestinal tract since only 16 mg/kg b.w. was needed to cause reversible infertility versus the original formulation which required 50 mg/kg b.w.
Fig. 1.
Chemical structures of Ionidamine, adjudin, AF-2785 and gamendazole.
In order to circumvent adverse effects (i.e., muscle atrophy and liver inflammation) that were noted in the subchronic toxicity test, adjudin was delivered directly to the testis via conjugation to a recombinant FSH mutant (note: the mutant’s hormonal activity, but not its ability to bind the FSH receptor present on the Sertoli cell surface, was deleted) which served as a “carrier.” When this FSH mutant was used to deliver adjudin to the testis, reversible infertility was induced when 0.5 mcg adjudin–FSH mutant was administered intraperitoneally to adult rats, representing a substantial increase in selectivity and efficacy over the oral administration of adjudin at 50 mg/kg b.w. Moreover, this approach by-passed issues with toxicity and bioavailability since adjudin was delivered directly to the testis. However, this approach is prohibitively expensive for a male contraceptive because of the cost associated with the production of recombinant protein and its conjugation to adjudin. In line with this previous study, we are currently making good progress in appropriately modifying the original formulation of adjudin so that the effective dose needed to induce reversible infertility in rats can be lowered several-fold, thereby minimizing or even completely eliminating side effects noted in the muscle and liver in the subchronic toxicity test [13].
On a final note, a recently published study on drug transporters and adjudin has helped us to better understand how this compound enters and exits the testis [74]. While adjudin is a small, lipophilic compound (335.18 g/mol) that should easily cross the blood-testis barrier via diffusion, findings from a study using [3H]-adjudin to assess the tissue distribution and bioavailability following its administration by gavage have shown that adjudin is not freely permeable to the blood-testis barrier [12]. Instead, it appears that adjudin is utilizing drug influx and efflux pumps present on the basolateral surface of Sertoli cells and at the site of the blood-testis barrier to enter and exit the seminiferous epithelium. In this recent study from our laboratory, the steady-state levels of several efflux pumps, namely permeability glycoprotein (P-glycoprotein, encoded by the Abcb1 gene), multiple drug resistance protein 1 (encoded by Mrp1 gene) and ATP-binding cassette sub-family G member 1 (encoded by Abcg1 gene) increased [74] at the time [3H]-adjudin peaked in the testis (i.e., 3–24 h) [12]. Interestingly, we found that P-glycoprotein, which localized at the blood-testis barrier in untreated control testes, increased its association with other integral membrane proteins present at the blood-testis barrier, namely occludin, claudin-11 and junctional adhesion molecule-A (JAM-A) at the time [3H]-adjudin was highest in the testis [74]. These results may suggest that an increase in P-glycoprotein association with occludin, claudin-11 and JAM-A occurs so that the integrity of the immunological barrier can be reinforced, thus prohibiting adjudin from entering the seminiferous epithelium. However, adjudin still enters the seminiferous epithelium to cause germ cell loss, possibly using a drug transporter that was not investigated in our study. If one can specifically “shut down” the drug pump (s) being used by adjudin to enter the muscle and liver, side effects may also be alleviated.
2.5.2. Gamendazole
Gamendazole [trans-3-(1-benzyl-6-(trifluoromethyl)-1H-indazol-3-yl)acrylic acid] (Fig. 1) is an analogue of indazole-3-carboxylic acid (selected from a screen of 150 lonidamine analogues) that was previously shown to affect spermatogenesis in different species [10,11,75,76] and to cause infertility in rats at single doses ranging from 1.5 to 25 mg/kg b.w. when administered orally or intraperitoneally [77]. For instance, infertility was achieved by 3 weeks in seven out of seven rats receiving a single oral dose of gamendazole at 6 mg/kg b.w., followed by the recovery of fertility which was observed by 9 weeks in four out of these seven animals. At 25 mg/kg b.w. of gamendazole, no gross histopathological changes (e.g., inflammation, necrosis, hemorrhage or tumors) were observed in any organ in all treated rats when compared to untreated rats. Similar to CDB-4022 and adjudin, it was concluded that the Sertoli cell was the primary target of gamendazole because this compound was shown to decrease the level of serum inhibin B in vivo, as well as to inhibit its production in Sertoli cells in vitro [77,78]. Importantly, gamendazole did not affect serum testosterone levels, but a transient increase in serum FSH was noted within 1 week of treatment [78]. Using proteomic and molecular techniques, Tash et al. [77] also showed that biotinylated gamendazole was able to bind to heat shock protein HSP 90-β (encoded by Hsp90ab1 gene) and eukaryotic translation elongation factor 1 α 1 (encoded by Eef1a1 gene) in the testis, as well as in Sertoli cells and ID8 ovarian cancer cells. Moreover, gene microarray experiments showed an up-regulation in interleukin 1 and nuclear factor κB inhibitor α in the testis within hours of gamendazole treatment [77], suggestive of inflammation and apoptosis since both genes are known to play active roles in these cellular processes [79,80]. H2-gamendazole (3-[1-(2,4-dichlorobenzyl)-6-trifluoromethyl-1H-indazol-3-yl]-propionic acid), another analogue of lonidamine, is also known to possess anti-spermatogenic effects but this compound remains to be tested for safety and efficacy.
In this respect, it is important to note that mortalities were noted in three out of five adult rats, representing 60% of animals dosed with gamendazole at 200 mg/kg b.w [78]. However, no deaths were reported when rats were treated with adjudin, even when a comparable single dose or consecutive doses were administered by gavage including a subchronic toxicity with 10 male and 10 female rats in which each adult rat received 50 mg/kg b.w. via gavage daily for 29 days (Ref. [12,13], Mruk and Cheng, unpublished findings). While the authors of the gamendazole study did not discuss in great detail why deaths were observed at this higher dose, the trifluoromethyl group attached to the indazole ring of gamendazole may explain this compound’s toxicity as this is one key difference between gamendazole and adjudin. Indeed, there are several examples in the literature in which the trifluoromethyl group is associated with severe toxicity (i.e., death). One example being hydramethylnon, a trifluoromethyl aminohydrazone and pesticide used in baits (i.e., Combat®) to control insects in both indoor and outdoor settings that disrupts cellular respiration by inhibiting the electron transport chain in mitochondria, thereby resulting in death usually within 3–4 days after treatment [81,82]. This compound was shown to be equally harmful to aquatic life; in rainbow trout, the LC50 was estimated to 0.16 mg/L water 96 h after exposure. Interestingly, the testis was also shown to be a target for hydramethylnon. For example, mice fed daily for 18 months with this compound at 3.8 mg/kg b.w. developed severe testicular lesions, while in rats and dogs, testicular atrophy was also observed [83].
Gamendazole’s toxicity may also be the result of the acrylic acid group as this is a second key difference between gamendazole and adjudin. Mortalities in two out of six adult rats, representing ~30% of animals, were also noted following administration of 200 mg/kg b.w. AF-2785, 1-(2,4-dichlorobenzyl)-1H-indazole-3-acrylic acid (Fig. 1), another lonidamine analogue that also contains an acrylic acid group at the indazole ring as well [78]. In a separate and unrelated subchronic study to assess the toxicity of acrylic acid, deaths were also reported in Wistar male and female rats: 50% of rats died when they were dosed orally with 150 mg/kg b.w. acrylic acid five times per week for 3 months, whereas this mortality rate increased to 75% when 375 mg/kg b.w. was administered via this same regimen [84]. At this point, it remains to be determined if rodents, but not other mammals such as rabbits and dogs, are especially sensitive to compounds containing one or both of these chemical modifications, thereby resulting in death in some animals, as species differences can exist. Interestingly, lonidamine which contains a carboxylic acid group did not produce a single mortality as previously observed by us and recently reported by Tash et al. [78]. Regardless, lonidamine is considered to be more toxic than adjudin; and while it is associated with several side effects, it is presently being used to kill tumor cells that have been sensitized by X-irradiation [85–87]. Thus, we conclude compounds having an acrylic acid group to be most toxic and even fatal if a high dose is used (i.e., gamendazole, AF-2785), immediately followed by compounds having a carboxylic acid group (i.e., lonidamine) and finally followed by those having a carbohydrazide group (i.e., adjudin). Nevertheless, much additional research is needed — research that not only focuses on gamendazole’s mechanism of action but also on the screening of new indazole-3-carboxylic acid entities because a male contraceptive pill that can cause mortalities in approximately 60% of subjects, albeit when administered at a high dose, is not likely to sit well with the consumers, the general public and pharmaceutical companies.
3. Future perspectives and concluding remarks
In this review, we have discussed the current status of research relating to nonhormonal male contraception, including RISUG, contraceptive vaccines, Ca++ channel blockers, indenopyridines and indazole-3-carboxylic acid analogues. While several of these approaches appear to show promise, additional research is needed so that a safe, effective, reversible and affordable male contraceptive can be brought to the consumer market. Of these criteria, safety is the most important since male contraceptives would be used voluntarily by otherwise healthy individuals for extended periods of time. In recent years, there has also been increasing involvement to control wildlife (i.e., deer) and companion animal (i.e., cats and dogs) populations using approaches other than surgical sterilization which is costly, risky and difficult to perform in a field setting (as is the case for wildlife) so that male contraceptives that are safe but irreversible should not be disregarded as having no clinical use. At present, we remain hopeful that continued research will identify a nonhormonal contraceptive that is safe, effective, reversible and affordable in the near future.
Acknowledgments
Research conducted in the authors’ laboratory was supported by NICHD, NIH (R01HD056034 and U54HD029990, Project 5 to CYC).
References
- 1.Martin CW, Anderson RA, Cheng L, et al. Potential impact of hormonal male contraception: cross-cultural implications for development of novel preparations. Hum Reprod. 2000;15:637–45. doi: 10.1093/humrep/15.3.637. [DOI] [PubMed] [Google Scholar]
- 2.Eberhardt J, van Wersch A, Meikle N. Attitudes towards the male contraceptive pill in men and women in casual and stable sexual relationships. J Fam Plann Health Reprod Care. 2009;35:161–5. doi: 10.1783/147118909788707986. [DOI] [PubMed] [Google Scholar]
- 3.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Immune-related disease before and after vasectomy: an epidemiological database study. Hum Reprod. 2007;22:1273–8. doi: 10.1093/humrep/dem010. [DOI] [PubMed] [Google Scholar]
- 4.Massey FJ, Berstein GS, O’Fallon WM, et al. Vasectomy and health. Results from a large cohort study. JAMA. 1984;252:1023–9. doi: 10.1001/jama.252.8.1023. [DOI] [PubMed] [Google Scholar]
- 5.Kohler TS, Fazili AA, Brannigan RE. Putative health risks associated with vasectomy. Urol Clin North Am. 2009;36:337–45. doi: 10.1016/j.ucl.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Trussell J. Contraceptive failure in the United States. Contraception. 2004;70:89–96. doi: 10.1016/j.contraception.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Gu YQ, Liang X, Wu W, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 8.Handelsman DJ, Farley TM, Peregoudov A, Waites GM. Factors in nonuniform induction of azoospermia by testosterone enanthate in normal men. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril. 1995;63:125–33. [PubMed] [Google Scholar]
- 9.Hay CJ, Brady BM, Zitmann M, et al. A multicenter Phase IIb study of a novel combination on intramuscular androgen (testosterone decanoate) and oral progestogen (etonogestrel) for male hormonal contraception. J Clin Endocrinol Metab. 2005;90:2042–9. doi: 10.1210/jc.2004-0895. [DOI] [PubMed] [Google Scholar]
- 10.Cheng CY, Silvestrini B, Grima J, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–61. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- 11.Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–8. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CY, Mruk D, Silvestrini B, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–61. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–8. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 14.Hu GX, Hu LF, Yang DZ, et al. Adjudin targeting rabbit germ cell adhesion as a male contraceptive: a pharmacokinetic study. J Androl. 2008;30:87–93. doi: 10.2164/jandrol.108.004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohiya NK, Manivannan B, Mishra PK. Ultrastructural changes in the spermatozoa of langur monkeys Presbytis entellus after vas occlusion with styrene maleic anhydride. Contraception. 1998;57:125–32. doi: 10.1016/s0010-7824(98)00011-0. [DOI] [PubMed] [Google Scholar]
- 16.Lohiya NK, Suthar R, Khandelwal A, et al. Sperm characteristics and teratology in rats following vas deferens occlusion with RISUG and its reversal. Int J Androl. 2009 doi: 10.1111/j.1365-2605.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- 17.Koul V, Srivastava A, Guha SK. Reversibility with sodium bicarbonate of styrene maleic anhydride, an intravasal injectable contraceptive, in male rats. Contraception. 1998;58:227–31. doi: 10.1016/s0010-7824(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 18.Manivannan B, Mishra PK, Lohiya NK. Ultrastructural changes in the vas deferens of langur monkeys Presbytis entellus entellus after vas occlusion with styrene maleic anhydride and after its reversal. Contraception. 1999;59:137–44. doi: 10.1016/s0010-7824(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 19.Jha RK, Jha PK, Guha SK. Smart RISUG: a potential new contraceptive and its magnetic field-mediated sperm interaction. Int J Nanomed. 2009;4:55–64. doi: 10.2147/ijn.s4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Kohler N, Zhang M. Surface modification of super-paramagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–61. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 21.Roblero L, Guadarrama A, Lopez T, Zegers-Hochschild F. Effect of copper ion on the motility, viability, acrosome reaction and fertilizing capacity of human spermatozoa in vitro. Reprod Fertil Dev. 1996;8:871–4. doi: 10.1071/rd9960871. [DOI] [PubMed] [Google Scholar]
- 22.Guha SK, Singh G, Ansari S, et al. Phase II clinical trial of a vas deferens injectable contraceptive for the male. Contraception. 1997;56:245–50. doi: 10.1016/s0010-7824(97)00142-x. [DOI] [PubMed] [Google Scholar]
- 23.Guha SK, Singh G, Anand S, et al. Phase I clinical trial of an injectable contraceptive for the male. Contraception. 1993;48:367–75. doi: 10.1016/0010-7824(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RS, Rajanna A, Singh BK, Mathur AK, Mukherjee AK. Current status of development of RISUG: an intravasal injectable male contraceptive. In: Sharma RS, Rajanna A, Rajalakshmi M, editors. Proceedings of the Conference on Recent Advances and Challenges in Reproductive Health Research. New Delhi, India: Indian Society for the Study of Reproduction and Fertility; 2007. pp. 127–40. [Google Scholar]
- 25.Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar GP. Recent advances in contraceptive vaccine development: a mini-review. Hum Reprod. 2005;20:3271–83. doi: 10.1093/humrep/dei256. [DOI] [PubMed] [Google Scholar]
- 26.Aitken RJ. Immunocontraceptive vaccines for human use. J Reprod Immunol. 2002;57:273–87. doi: 10.1016/s0165-0378(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 27.Frayne J, Hall L. The potential use of sperm antigens as targets for immunocontraception; past, present and future. J Reprod Immunol. 1999;43:1–33. doi: 10.1016/s0165-0378(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 28.Anderson RA, Baird DT. Male contraception. Endocr Rev. 2002;23:735–62. doi: 10.1210/er.2002-0002. [DOI] [PubMed] [Google Scholar]
- 29.Suri A. Family of sperm associated antigens: relevance in sperm-egg interaction and immunocontraception. Soc Reprod Fertil. 2007;63 (Suppl):433–43. [PubMed] [Google Scholar]
- 30.Simms MS, Scholfield DP, Jacobs E, et al. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br J Cancer. 2000;83:443–6. doi: 10.1054/bjoc.2000.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Zhang ZF, Jing CX, Wu FL. Intraperitoneal administration of gonadotropin-releasing hormone-PE40 induces castration in male rats. World J Gastroenterol. 2008;14:2106–9. doi: 10.3748/wjg.14.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian ZH, Zhang J, Ding XL, et al. Localization of epididymal protease inhibitor in adult rat and its transcription profile in testis during postnatal development. Asian J Androl. 2009;11:731–9. doi: 10.1038/aja.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson RT, Sivashanmugam P, Hall SH, et al. Cloning and sequencing of human eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene. 2001;270:93–102. doi: 10.1016/s0378-1119(01)00462-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Widgren EE, Richardson RT, O’Rand MG. Characterization of an eppin protein complex from human semen and spermatozoa. Biol Reprod. 2007;77:476–84. doi: 10.1095/biolreprod.107.060194. [DOI] [PubMed] [Google Scholar]
- 35.O’Rand MG, Widgren EE, Beyler S, Richardson RT. Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: effect on cyclic adenosine monophosphate. Biol Reprod. 2009;80:279–85. doi: 10.1095/biolreprod.108.072942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Rand MG, Widgren EE, Sivashanmugam P, et al. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306:1189–90. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- 37.O’Rand MG, Widgren EE, Wang Z, Richardson RT. Eppin: an epididymal protease inhibitor and a target for male contraception. Soc Reprod Fertil. 2007;63(Suppl):445–53. [PubMed] [Google Scholar]
- 38.Wang Z, Widgren EE, Sivashanmugam P, O’Rand MG, Richardson RT. Association of eppin with semenogelin on human spermatozoa. Biol Reprod. 2005;72:1064–70. doi: 10.1095/biolreprod.104.036483. [DOI] [PubMed] [Google Scholar]
- 39.Robert M, Gagnon C, Semenogelin I. A coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–60. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Rand MG, Widgren EE, Wang Z, Richardson RT. Eppin: an effective target for male contraception. Mol Cell Endocrinol. 2006;250:157–62. doi: 10.1016/j.mce.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Naz RK. Vaccine for contraception targeting sperm. Immunol Rev. 1999;171:193–202. doi: 10.1111/j.1600-065x.1999.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 42.Naz RK. Development of genetically engineered human sperm immunocontraceptives. J Reprod Immunol. 2009;83:145–50. doi: 10.1016/j.jri.2009.06.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darszon A, Lopez-Martinez P, Acevedo JJ, Hernandez-Cruz A, Trevino CL. T-type Ca2+ channels in sperm function. Cell Calcium. 2006;40:241–52. doi: 10.1016/j.ceca.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Darszon A, Acevedo JJ, Galindo BE, et al. Sperm channel diversity and functional multiplicity. Reproduction. 2006;131:977–88. doi: 10.1530/rep.1.00612. [DOI] [PubMed] [Google Scholar]
- 45.Florman HM, Arnoult C, Kazam IG, Li C, O’Toole CM. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod. 1998;59:12–6. doi: 10.1095/biolreprod59.1.12. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Hum Reprod Update. 2005;12:253–67. doi: 10.1093/humupd/dmi050. [DOI] [PubMed] [Google Scholar]
- 47.Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: characterization of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol. 2003;1:53–67. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin JL, O’Doherty AM, Wang S, et al. Catsper3 and Catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol Reprod. 2005;73:1235–42. doi: 10.1095/biolreprod.105.045468. [DOI] [PubMed] [Google Scholar]
- 49.Qi H, Moran MM, Navarro B, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren D, Navarro B, Perez G, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–9. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci U S A. 2001;98:12527–31. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol. 2008;52:607–13. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlson AE, Burnett LA, del Camino D, et al. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLoS ONE. 2009;4:e6844. doi: 10.1371/journal.pone.0006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benoff S, Cooper GW, Hurley I, et al. The effect of calcium ion channel blockers on sperm fertilization potential. Fertil Steril. 1994;62:606–17. [PubMed] [Google Scholar]
- 55.Morakinyo AO, Iranloye BO, Adegoke OA. Anti-reproductive effect of calcium channel blockers on male rats. Reprod Med Biol. 2009;8:97–102. doi: 10.1007/s12522-009-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodel C, Suter K. Reversible inhibition of spermatogenesis with an indenopyridine (20-438) Arch Toxicol. 1978;1(Suppl):323–6. [PubMed] [Google Scholar]
- 57.Cook CE, Wani MC, Jump JM, et al. Structure-activity studies of 2,3,4,4a,5,9b-hexahydroindenol[1,2-c]pyridines as antispermatogenic agents for male contraception. J Med Chem. 1995;38:753–63. doi: 10.1021/jm00005a003. [DOI] [PubMed] [Google Scholar]
- 58.Cook CE, Jump JM, Zhang P, et al. Exceptionally potent antispermatogenic compounds from 8-halogenation of (4aRS,5SR,9bRS)-hexahydroindeno-[1,2-c]pyridines. J Med Chem. 1997;40:2111–2. doi: 10.1021/jm970268+. [DOI] [PubMed] [Google Scholar]
- 59.Hild SA, Reel JR, Dykstra MJ, Mann PC, Marshall GR. Acute adverse effects of indenopyridine, CDB-4022, on the ultrastructure of Sertoli cells, spermatocytes and spermatids in rat testes: comparison to the known Sertoli cell toxicant, di-n-pentylphthalate (DPP) J Androl. 2007;28:621–9. doi: 10.2164/jandrol.106.002295. [DOI] [PubMed] [Google Scholar]
- 60.Hild SA, Attardi BJ, Reel JR. The ability of a gonadotropin-releasing hormone antagonist, acyline, to prevent irreversible infertility induced by the indenopyridine, CDB-4022, in adult male rats: the role of testosterone. Biol Reprod. 2004;71:348–58. doi: 10.1095/biolreprod.103.026989. [DOI] [PubMed] [Google Scholar]
- 61.Hild SA, Reel JR, Larner JM, Blye RP. Disruption of spermatogenesis and Sertoli cell structure and function by the indenopyridine CDB-4022 in rats. Biol Reprod. 2001;65:1771–9. doi: 10.1095/biolreprod65.6.1771. [DOI] [PubMed] [Google Scholar]
- 62.Hild SA, Meistrich ML, Blye RP, Reel JR. Lupron depot prevention of antispermatogenic/antifertility activity of the indenopyridine, CDB-4022, in the rat. Biol Reprod. 2001;65:165–72. doi: 10.1095/biolreprod65.1.165. [DOI] [PubMed] [Google Scholar]
- 63.Hild SA, Marshall GR, Attardi BJ, et al. Development of l-CDB-4022 as a nonsteroidal male oral contraceptive: induction and recovery from severe oligospermia in the adult male cynomolgus monkey (Macaca fascicularis) Endocrinology. 2007;148:1784–96. doi: 10.1210/en.2006-1487. [DOI] [PubMed] [Google Scholar]
- 64.Chen YC, Cochrum RK, Tseng MT, et al. Effects of CDB-4022 on Leydig cell function in adult male rats. Biol Reprod. 2007;149:1850–60. doi: 10.1095/biolreprod.106.059204. [DOI] [PubMed] [Google Scholar]
- 65.Koduri S, Hild SA, Pessaint L, Reel JR, Attardi BJ. Mechanism of action of l-CDB-4022, a potential nonhormonal male contraceptive, in the seminiferous epithelium of the rat testis. Endocrinology. 2008 doi: 10.1210/en.2007-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–6. [PubMed] [Google Scholar]
- 67.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–6. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 68.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 69.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 70.Floridi A, Paggi MG, Marcante ML, et al. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J Natl Cancer Res. 1981;66:497–9. [PubMed] [Google Scholar]
- 71.Floridi A, Paggi MG, D’Atri S, et al. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res. 1981;41:4661–6. [PubMed] [Google Scholar]
- 72.Zhou HY, Hu GX, Hu LF, et al. Adjudin targeting dog germ cell adhesion as a male contraceptive. Albuquerque (NM): American Society for Andrology Annual Meeting; 2008. (Abstract) [Google Scholar]
- 73.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 74.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–87. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coulston F, Dougherty WJ, LeFevre R, Abraham R, Silvestrini B. Reversible inhibition of spermatogenesis in rats and monkeys with a new class of indazole-carboxylic acids. Exp Mol Pathol. 1975;23:357–66. doi: 10.1016/0014-4800(75)90029-5. [DOI] [PubMed] [Google Scholar]
- 76.De Martino C, Malcorni W, Bellocci M, Floridi A, Marcante ML. Effects of AF1312 TS and lonidamine on mammalian testis. A morphological study. Chemotherapy. 1981;27(Suppl 2):27–42. doi: 10.1159/000238043. [DOI] [PubMed] [Google Scholar]
- 77.Tash JS, Chakrasali R, Jakkaraj SR, et al. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BETA) and EEF1A1 (eFF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol Reprod. 2008;78:1139–52. doi: 10.1095/biolreprod.107.062679. [DOI] [PubMed] [Google Scholar]
- 78.Tash JS, Attardi BJ, Hild SA, et al. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127–38. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 79.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 80.Pasparakis M. Regulation of tissue homeostasis by NF-kB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–88. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 81.California Agency Environmental Protection. Summary of toxicological data: hydramethylnon. Sacramento (CA): Department of Pesticide Regulation, Medical Toxicology Branch; 2000. Available at: http://www.calepa.ca.gov. [Google Scholar]
- 82.Kamrin MA, editor. Pesticide profiles: toxicity, environmental impact, and fate. Boca Raton (FL): CRC/Lewis Publishing; 1997. pp. 589–629. [Google Scholar]
- 83.United States Agency Environmental Protection. Integrated Risk Information System Database. Washington, DC: 1995. pp. 10–14. ( http://www.epa.gov/NCEA/iris) [Google Scholar]
- 84.Hellwig J, Deckardt K, Freisberg KO. Subchronic and chronic studies of the effects of oral administration of acrylic acid to rats. Food Chem Toxicol. 1993;1993:1–18. doi: 10.1016/0278-6915(93)90172-u. [DOI] [PubMed] [Google Scholar]
- 85.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 86.De Lena M, Lorusso V, Latorre A, et al. Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37:364–8. doi: 10.1016/s0959-8049(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 87.Di Cosimo S, Ferretti G, Papaldo P, et al. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today (Barc) 2003;39:157–74. doi: 10.1358/dot.2003.39.3.799451. [DOI] [PubMed] [Google Scholar]