Abstract
Mammalian spermatogenesis, a complex process that involves the movement of developing germ cells across the seminiferous epithelium, entails extensive restructuring of Sertoli–Sertoli and Sertoli–germ cell junctions. Presently, it is not entirely clear how zygotene spermatocytes gain entry into the adluminal compartment of the seminiferous epithelium, which is sealed off from the systemic circulation by the Sertoli cell component of the blood–testis barrier, without compromising barrier integrity. To begin to address this question, it is critical that we first have a good understanding of the biology and the regulation of different types of Sertoli–Sertoli and Sertoli–germ cell junctions in the testis. Supported by recent studies in the field, we discuss how crosstalk between different types of junctions contributes to their restructuring during germ cell movement across the blood–testis barrier. We place special emphasis on the emerging role of desmosome-like junctions as signal transducers during germ cell movement across the seminiferous epithelium.
Keywords: Blood–testis barrier, Tight junction, Ectoplasmic specialization, Desmosome-like junction, Gap junction, Testis
1. Introduction
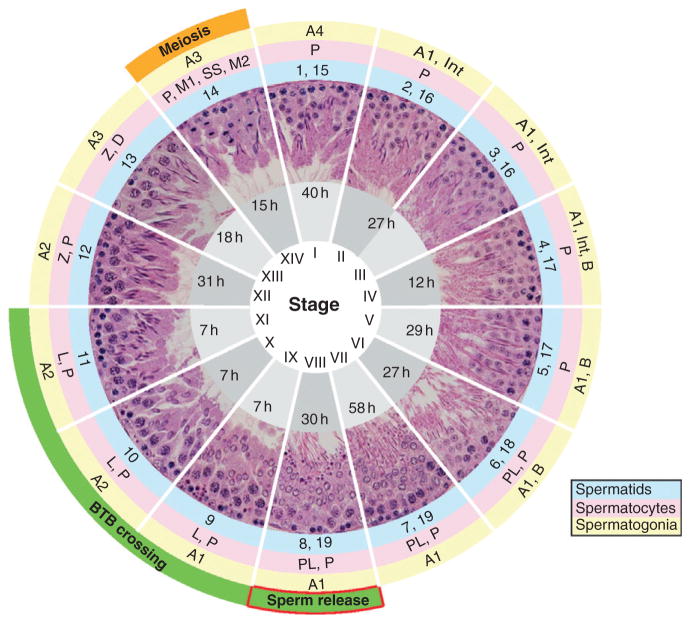
Mammalian spermatogenesis is a continuous process that involves division of type B spermatogonia and spermatocytes (i.e., mitosis and meiosis I/II, respectively), followed by morphogenesis of spermatids (i.e., spermiogenesis) into spermatozoa which are released into the lumen of the seminiferous tubule (i.e., spermiation). Spermatogenesis takes place in the seminiferous epithelium under the strict control of endocrine, paracrine, and autocrine factors (Clermont, 1972; Parvinen, 1982). The stepwise development of spermatozoa from spermatogonia, which takes ~54 days to complete in the rat, occurs in association with Sertoli cells—“nurse-like” cells that are known to extend from the basal lamina to the luminal edge and to provide nutritional and structural support to differentiating germ cells. Moreover, spermatogenesis and germ cell development within the seminiferous epithelium is not at all random. Instead, it is organized into unique cellular associations defined as stages of the seminiferous epithelial cycle (Fig. 5.1). Fourteen stages can be identified in the rat, 12 stages in the mouse, eight stages in the dog, and six stages in the human, and each distinct stage is denoted by a roman numeral. Throughout spermatogenesis, germ cells also traverse the entire height of the seminiferous epithelium. As such, the development of germ cells, the movement of these cells across the seminiferous epithelium, and the remodeling of Sertoli–Sertoli and Sertoli–germ cell junctions throughout spermatogenesis are synchronized (Lie et al., 2009). Thus, it is not surprising that a compromise in any one of these critical cellular events may result in transient or even permanent infertility.
Figure 5.1.
Seminiferous epithelial cycle in the rat testis. These 14 images represent stages of the seminiferous epithelial cycle obtained from paraffin-embedded cross-sections of the adult rat testis stained with hematoxylin and eosin. Stages are noted as roman numerals. Annotations in gray shaded areas indicate the approximate duration of each stage in hours (h). Germ cells are divided into spermatogonia (outer yellow circle), spermatocytes (middle pink circle), or spermatids (inner blue circle). Spermatogonia include types A1–A4, intermediate (Int) and B (yellow circle). Spermatocytes (i.e., preleptotene (PL), leptotene (L), zygotene (Z), pachytene (P), and diplotene (D)), primary spermatocytes in meiosis 1 (M1), secondary spermatocytes (SS), and secondary spermatocytes in meiosis 2 (M2) are also shown (pink circle). Finally, spermatid differentiation spans steps 1–19 (blue circle). Important cellular events are noted in the outermost layer as orange and green shaded areas. Spermiation takes place at stage VIII, concurrent with the transit of preleptotene spermatocytes across the BTB during stages VIII–XI. M1 and M2 take place at stage XIV.
Cell–cell interactions are essential for spermatogenesis, and several different junction types have been described to exist between Sertoli cells, as well as between Sertoli and germ cells (Fig. 5.2). Sertoli cell junctions form an important aspect of the blood–testis barrier (BTB) which physically divides the seminiferous epithelium into a basal and an adluminal compartment. The BTB is located basally within the seminiferous epithelium (i.e., above preleptotene spermatocytes), and it is largely composed of coexisting tight junctions, basal ectoplasmic specializations, desmosome-like junctions, and gap junctions (Fig. 5.2). The BTB is believed to restructure transiently beginning at late stage VIII of the seminiferous epithelial cycle to allow the passage of preleptotene/leptotene spermatocytes across the BTB and the entry of zygotene spermatocytes into the adluminal compartment for continued development, and recent studies have begun to pinpoint several key molecules that are involved in BTB restructuring and germ cell movement. However, the type of adhesive junction present between Sertoli and germ cells depends on the developmental stage of the germ cell: desmosome-like junctions exist between Sertoli cells and pre-step 8 germ cells, whereas apical ectoplasmic specializations exist between Sertoli cells and step 8/post-step 8 spermatids (Fig. 5.2).
Figure 5.2.
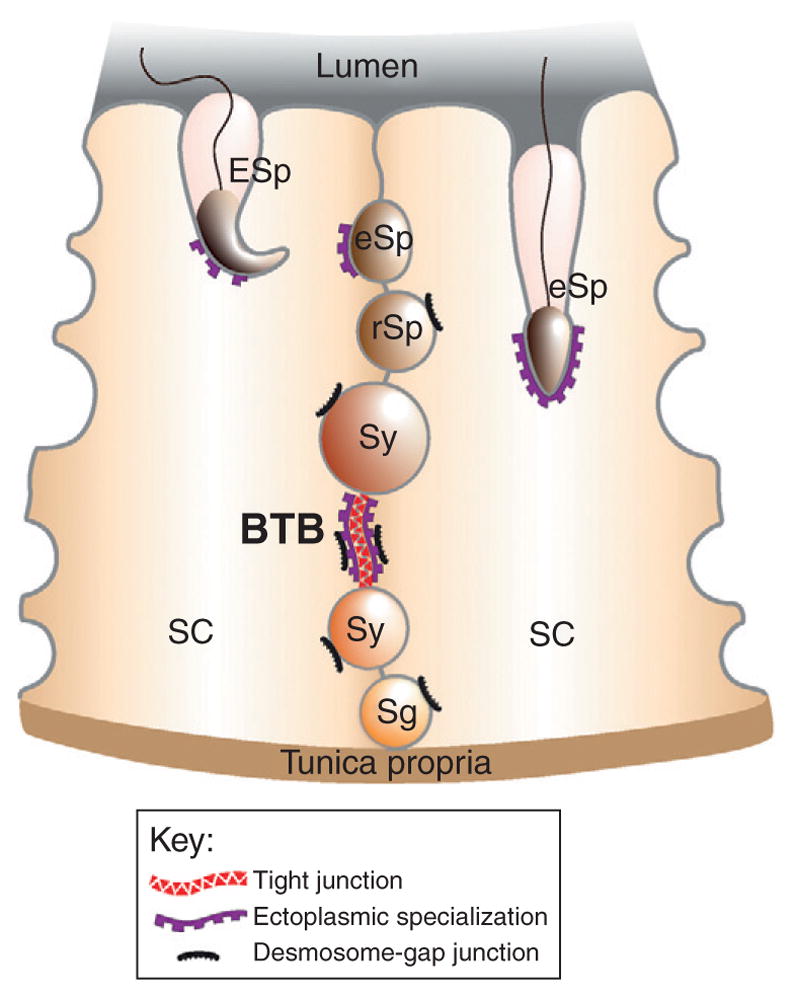
Sertoli–Sertoli and Sertoli–germ cell junctions. Two columnar Sertoli cells are shown sitting atop the tunica propria in the seminiferous epithelium. The BTB is constituted by adjacent Sertoli cells and composed of coexisting tight junctions, basal ectoplasmic specialization, and desmosome–gap junctions. Desmosome–gap junctions are found between Sertoli cells and all germ cells up to, but not including, step 8 spermatids, whereas the apical ectoplasmic specialization is found between Sertoli cells and all step 8–19 spermatids. Gap junctions and hemidesmosomes (a type of cell–matrix junction) are not illustrated since these junction types were not discussed in great detail. Also, it is also worth noting that two different stages of the seminiferous epithelial cycle are shown within a single panel (i.e., left, stage VII; right, stage VI) for the sake of simplicity, but this does not accurately represent the in vivo situation. Abbreviations: BTB, blood–testis barrier; SC, Sertoli cell; Sg, spermatogonium; Sy, spermatocyte; rSp, round spermatid; eSp, elongating spermatid; ESp, elongated spermatid.
In this chapter, we will focus on the biology of desmosome-like junctions in germ cell movement and spermatogenesis. As mentioned above, desmosome-like junctions are present at the BTB and at the Sertoli cell–pre-step 8 germ cell (i.e., spermatogonia, spermatocytes, and round spermatids) interface. At both sites, their morphology is not characteristic of mature desmosomes such as those found in stress-bearing tissues (hence the name “desmosome-like”; alternatively, they can be defined as “desmosome-gap”), but characteristic of wound-edge desmosomes which exhibit considerably less adhesive strength, suggesting that desmosome-like junctions may have an unconventional function in the testis (Russell, 1977a). For instance, two recent reports have described crosstalk between proteins at the desmosome and proteins at the tight junction or gap junction (Li et al., 2009; Lie et al., 2010), and these reports essentially showed that desmosome-like junctions—in addition to conferring adhesion—can also function as a platform for signal transduction. As the basis of our review, we will first summarize what is known about the structure, function, and regulation of conventional desmosomes. Next, we will examine point-by-point how desmosome-like junctions found in the seminiferous epithelium are different from conventional desmosomes found in other epithelia, followed by a general update of cell junction dynamics in the testis. Finally, we will discuss how crosstalk among desmosome-like junctions, tight junctions, ectoplasmic specializations, and gap junctions facilitates the movement of preleptotene/leptotene spermatocytes across the BTB from stages VIII to XI of the seminiferous epithelial cycle.
2. Structure, Function, and Regulation of Conventional Desmosomes
Desmosomes are intermediate filament-based junctions known to confer robust cell–cell adhesion. They are particularly prominent in tissues subjected to enormous mechanical stress such as the heart and skin, but their presence in other organs has also been reported (Delva et al., 2009; Garrod and Kimura, 2008; Green and Gaudry, 2000; Green and Simpson, 2007; Stokes, 2007). By electron microscopy, desmosomes are easily identifiable as a pair of electron dense plaques with each plaque lying adjacent to the plasma membrane and an intermediate dense line marking the extracellular space. Desmosomal adhesion between adjacent epithelial cells is mediated by trans-interactions of single-pass transmembrane proteins known as the desmosomal cadherins (i.e., desmogleins and desmocollins). The cytoplasmic tails of desmosomal cadherins are then tethered to intermediate filaments via proteins from the armadillo (i.e., plakoglobin and plakophilin) and plakin (i.e., desmoplakin, periplakin, and envoplakin) families. Together, proteins from these three families assemble a functional desmosome (Table 5.1; Fig. 5.3). It is also important to note that the basic architecture of the desmosome closely resembles that of the adherens junction, an actin-based cell–cell junction. In the following sections, we will discuss the molecular components (Section 2.1), unique structural features (Section 2.2), regulation (Section 2.3), and signaling role (Section 2.4) of desmosomes.
Table 5.1.
Tissue expression and functions of desmosomal proteins
| Expression in seminiferous epithelium (Lie et al., 2010) | Expression in other tissuesa | Phenotypes in knockout animals | Related hair/skin diseases | Related heart disease | |
|---|---|---|---|---|---|
| Desmosomal cadherins | |||||
| Desmoglein-1 | GC | E, I (γ isoform) (Brennan et al., 2004) | Pemphigus foliaceus (Emery et al., 1995), pemphigus vulgaris (Emery et al., 1995), SPPK (Rickman et al., 1999) | ||
| Desmoglein-2 | SC, GC | E, H, I, L (Schafer et al., 1994) | Apparently normal blastocysts with altered desmoplakin distribution which die shortly after implantation (Eshkind et al., 2002) | ARVC (Pilichou et al., 2006) | |
| Desmoglein-3 | nil | E (Tsunoda et al., 2003) | Hair loss, lesions in the oral cavity and traumatized skin (Koch et al., 1997) | Pemphigus vulgaris (Amagai et al., 1996) | |
| Desmoglein-4 | GC | E (Whittock and Bower, 2003) | Localized autosomal recessive hypotrichosis (Kljuic et al., 2003) | ||
| Desmocollin-1 | GC | E (Legan et al., 1994) | Fragile epidermis, abnormal epidermal proliferation and differentiation, hair loss (Chidgey et al., 2001) | ||
| Desmocollin-2 | SC, GC | E, H, I, L (Lorimer et al., 1994; Nuber et al., 1995; Wang et al., 2010) | ARVC (Heuser et al., 2006) | ||
| Desmocollin-3 | SC | E (Legan et al., 1994) | Conditional KO in epidermis—intraepidermal blistering, hair loss (Chen et al., 2008) | ||
| Armadillo proteins | |||||
| Plakoglobin | SC, GC | E, H, I, L (Cowin et al., 1986) | Embryonic lethal due to heart defect; skin blistering in late survivors (Bierkamp et al., 1996; Ruiz et al., 1996) | Naxo’s disease—SPPK (McKoy et al., 2000) | Naxo’s disease—ARVC (McKoy et al., 2000) |
| Plakophilin-1 | SC | E (Kapprell et al., 1988) | Skin fragility syndrome (Ersoy-Evans et al., 2006) | ||
| Plakophilin-2 | SC, GC | E, H, I, L (Mertens et al., 1996) | ARVC (Gerull et al., 2004) | ||
| Plakophilin-3 | nil | E, H (fetal), I (Bonne et al., 1999; Schmidt et al., 1999) | Defective hair formation, abnormal proliferation, apoptosis, and differentiation in epidermis (Sklyarova et al., 2008) | ||
| Plakins | |||||
| Desmoplakin | SC, GC | E, H, I, L (Angst et al., 1990; Wang et al., 2010) | KO—embryonic lethal due to defective desmosomal assembly (Gallicano et al., 1998); Conditional KO in epidermis—epithelium peeling after mild mechanical stress, desmosomes lack intermediate filament attachment (Vasioukhin et al., 2001) | SPPK (Norgett et al., 2000) | ARVC (Norgett et al., 2000) |
| Envoplakin | n.d. | E (Kim et al., 1997; Ruhrberg et al., 1996) | Slight delay in barrier formation in the epidermis during embryonic development (Maatta et al., 2001) | Paraneoplastic pemphigus (Sonnenerg and Liem, 2007) | |
| Periplakin | n.d. | E (Ruhrberg et al., 1997) | No discernible abnormalities (Aho et al., 2004) | Paraneoplastic pemphigus (Sonnenerg and Liem, 2007) | |
Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; E, epidermis; GC, germ cell; H, heart; I, intestine; KO, knockout; L, liver; n.d., not determined; SC, Sertoli cell; SPPK, striate palmoplantar keratoderma.
These are only selected examples of tissues expressing desmosomal genes/proteins, and this table is not meant to be exhaustive.
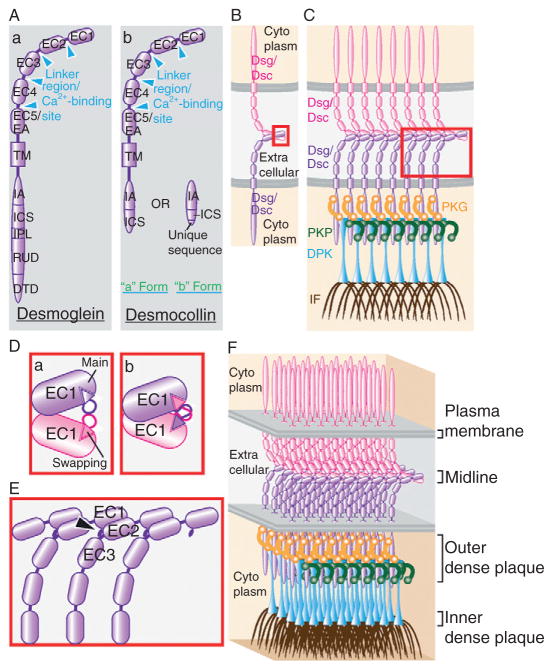
Figure 5.3.
Domain structure of desmosomal cadherins and the structure of desmosomes. (A) Different domains in desmosomal cadherins. From the N-terminus, desmogleins (a) and desmocollins (b) are composed of the following domains: extracellular (extracellular cadherin repeat, EC 1–5; connected by linker regions which contain Ca2+-binding sites), transmembrane (TM), and cytoplasmic (intracellular anchor (IA), intracellular cadherin-like sequence (ICS), intracellular proline-rich linker (IPL), repeat unit domain (RUD), desmoglein terminal domain (DTD)). Desmocollins do not contain IPL, RUD, and DTD, and the shorter “b form” has a truncated ICS followed by a unique sequence. It is worth noting that EC5 is sometimes known as the extracellular anchor (EA). (B) Trans-interaction of desmogleins (Dsg) or desmocollins (Dsc) between two opposing membranes. The boxed area represents the site of adhesion and is magnified in (D). (C) Organization of desmosomal cadherins (Dsg/Dsc), cytoplasmic plaque proteins and intermediate filaments (IF) at a desmosome. Plakoglobin (PKG) links desmosomal cadherins to desmoplakin (DPK), while plakophilins (PKP) link individual DPK molecules together laterally, which in turn tethers the desmosomal plaque to intermediate filaments. Boxed area is magnified in (E). (D) Strand-swap mechanism of cadherin adhesion. This diagram shows the EC1 domains of two trans-interacting cadherins (see B), before (a) and after (b) they undergo symmetrical strand swapping. Each molecule inserts its “swap domain,” also known as A strand, into the “main domain” of its partner. (E) Mechanism of Ca2+ independence. Within the compact arrangement in desmosomes (see C), the Ca2+-binding site between EC2 and 3 on each molecule is protected by a small β-strand in EC1 of the neighboring molecule (arrowhead). This results in the entrapment of bound Ca2+, even in low Ca2+ medium. (F) Compact arrangement within a desmosome. This dense arrangement of proteins leads to a few desmosomal-specific features discernable under the electron microscope. The midline in the extracellular space consists of the ends of desmosomal cadherin where the EC1 domains engage with each other. The outer dense plaque includes desmosomal cadherin cytoplasmic tails, plakoglobin, plakophilins, and the plakin domain of desmoplakin. The inner dense plaque is made up of the plakin repeat domains (PRD) of desmoplakin. The area in between outer and inner dense plaques consists of the rod domain of desmoplakin.
2.1. Molecular components of desmosomes
Members of the three desmosomal protein families (i.e., desmosomal cadherins, armadillo proteins, and plakins) exhibit variable and unique tissue expression patterns. For instance, desmoglein-1 and desmocollin-1 are expressed abundantly in the superficial differentiated layers of the epidermis, whereas desmoglein-3 and desmocollin-3 are expressed mainly in the basal layer (Green and Simpson, 2007). As for the seminiferous epithelium in the testis, constitutive desmosomal proteins such as desmoglein-2, plakoglobin, and plakophilin-2 are expressed by both Sertoli and germ cells (Li et al., 2009; Lie et al., 2010; Section 3). In addition to the three desmosomal protein families, we will also briefly discuss intermediate filaments.
2.1.1. Desmosomal cadherins
The cadherin superfamily is composed of type I and II cadherins, desmosomal cadherins, protocadherins, atypical cadherins, and large cadherins (Halbleib and Nelson, 2006; Shapiro and Weis, 2009). Type I cadherins (i.e., E-, N-, P-, and R-cadherins)—the classic cadherins—are well-studied transmembrane proteins that promote Ca2+-dependent cell–cell adhesion via cis- (i.e., homodimerization of cadherins on the same cell) and trans- (i.e., homodimerization of cadherins on opposing cells) interactions. Relatively less is known about type II cadherins (i.e., cadherins 5–12), except that they appear to be structurally related to type I cadherins. The desmosomal cadherin family includes desmogleins-1 to -4 and desmocollins-1 to -3, and both desmogleins and desmocollins are critical for desmosome function. Each desmocollin gene can also give rise to variants (i.e., a and b forms) which are the result of differential splicing. Moreover, desmosomal cadherins are closely related in both structure and function to classic cadherins. For example, desmosomal cadherins possess five N-terminally located extracellular cadherin repeats (i.e., EC1 to 5; EC1 is membrane distal, and EC5 is membrane proximal) that are linked together by Ca2+-binding sites similar to classic cadherins. Ca2+ binding (the concentration of Ca2+ in the extracellular milieu is in the millimolar range) is critical for cell adhesion, as it rigidifies the connection between cadherin repeats, resulting in a curved and rod-like ectodomain (Holthofer et al., 2007; Shapiro and Weis, 2009; Fig. 5.3). The accepted idea is that cell–cell adhesion is mediated by two trans-interacting EC1 domains, one from each cadherin molecule (Boggon et al., 2002). While Ca2+ binding is critical for cadherin function per se, mature desmosomes are in fact Ca2+ independent (i.e., they are resistant to disruption by divalent cation chelation; Garrod et al., 2005). Therefore, conventional desmosomes are defined as hyperadhesive, which essentially means that these structures are capable of facilitating strong adhesion (Garrod and Kimura, 2008; Section 2.2). Because of structural and functional homologies between classic and desmosomal cadherins, the following sections will also include discussions on classic cadherins when applicable.
Presently, it is not entirely clear whether stable desmosomal adhesion is mediated by homophilic or heterophilic interactions, or whether they involve a combination of both types of interactions. To better understand interactions between different desmosomal cadherin ectodomains, Waschke and colleagues performed a series of in vitro experiments at the molecular biology level. These studies utilized atomic force microscopy to examine the adhesive force between two interacting desmosomal cadherin molecules. It was shown that desmogleins-1 (Waschke et al., 2005) and -3 (Heupel et al., 2008) were each capable of mediating homophilic trans-interactions. In addition, a heterophilic interaction between desmoglein-1 and desmocollin-3 was noted (Spindler et al., 2009). These results were expanded at the cellular level by an in vitro adhesive force assay (i.e., laser tweezer trapping) in which microbeads coated with a desmosomal cadherin were seeded atop keratinocytes, followed by application of a laser beam to displace microbeads. In this experiment, specific interactions between desmoglein-1–desmoglein-1, desmoglein-3–desmoglein-3, and desmoglein-1–desmocollin-3 were demonstrated when deadhesion was induced by pemphigus (an autoimmune disease that causes skin and mucosa to blister) autoantibodies (Heupel et al., 2008; Spindler et al., 2009; Waschke et al., 2005). In other studies, aggregation assays were used to better understand trans-interactions in cells expressing desmosomal cadherins. For instance, cells expressing desmoglein-1 and desmocollin-2a failed to aggregate (Kowalczyk et al., 1996), whereas those expressing desmoglein-3 only aggregated weakly (Amagai et al., 1994). Furthermore, overexpression of desmocollin-1a in desmoglein-2-expressing cells caused the latter protein to transit from the cytoplasm to the cell surface, demonstrating that desmoglein-2 can be recruited to the plasma membrane by desmocollin-1a. However, the nature of their interaction (i.e., cis or trans) was not defined (Chitaev and Troyanovsky, 1997).
By far, the best approach used to investigate how cadherin molecules interact is to visualize their three-dimensional structures. To date, the ectodomains (either partial or full) of several classic cadherins have been studied by X-ray crystallography, but some of these results remain mootable (Al-Amoudi and Frangakis, 2008). At present, the structure of the C-cadherin (a maternally encoded cadherin found in Xenopus laevis cleavage-stage embryos and oocytes; Choi et al., 1990; Ginsberg et al., 1991; Levine et al., 1994) ectodomain obtained by X-ray crystallography at 0.31-nm resolution reveals that it assumes a curved conformation and that trans-interacting cadherin molecules engage in symmetrical strand swap (Boggon et al., 2002). This means that in the presence of Ca2+, two cadherin monomers present on opposing plasma membranes swap their EC1 N-terminal β-strands, thereby resulting in trans-dimer formation. Adhesion is stabilized by a conserved tryptophan side chain (Trp2) that is inserted into the hydrophobic pocket in the other molecule (Boggon et al., 2002; Fig. 5.3). While strand swapping appears to be characteristic of classic cadherins, it is probably also used by desmosomal cadherins (Posy et al., 2008; Shapiro and Weis, 2009). However, in the absence of Ca2+, cadherin molecules interact with each other in cis, instead of in trans. Regardless, cis-dimers utilize the same strand swapping mechanism as trans-dimers to mediate adhesion through their N-terminal ends (Troyanovsky et al., 2003). Moreover, the flexibility of the linker regions when Ca2+ is depleted is likely to be an important factor as well (Pokutta et al., 1994). While cis dimer formation induced by Ca2+-depletion has little significance in vivo because Ca2+ can only be depleted under experimental in vitro conditions, desmosomal cadherins are hypothesized to participate in lateral cis-interactions in vivo under a back-to-back arrangement, and this may be important for the tight arrangement of cadherins which is needed for robust cell adhesion (Garrod and Chidgey, 2008; Section 2.2).
The next questions we ask are: how are cadherin molecules arranged laterally within the plasma membrane of one cell, and how does this arrangement contribute to desmosomal adhesion? Based on the three--dimensional visualization of the C-cadherin ectodomain, cadherin molecules within the plasma membrane of one cell are arranged back-to-back at regular intervals, and they interact with each other in cis (Boggon et al., 2002). The combination of cis-interactions within one cell with trans-interactions between two opposing cells results in a lattice which is somewhat similar to a stack of zippers arranged in parallel at the cell–cell interface (Boggon et al., 2002). Interestingly, when homology models for desmoglein-2 and desmocollin-2 were generated using the C-cadherin crystal structure as a template, it was shown that the three-dimensional arrangement of desmosomal cadherins also generated a similar lattice (Garrod et al., 2005). As such, this arrangement of desmosomal cadherins is believed to confer hyperadhesion in desmosomes (Section 2.2). It is hoped that studies in the future make available the crystal structures of desmosomal cadherins so that these hypothetical models can be validated.
On a final note relating to desmosomal cadherin distribution, useful information has also been obtained from cryoelectron tomography experiments which helped to define the arrangement of desmosomal cadherin molecules within desmosomes in situ. Desmosomes visualized in close-to-native conditions using vitreous sections of human epidermis displayed a regular but periodic arrangement of cadherin molecules along the midline (Al-Amoudi et al., 2004, 2007). In contrast, when freeze-substituted resin-embedded sections of newborn mouse epidermis were used, an irregular arrangement of desmosomal cadherin molecules was observed with clustering of their N-terminal ends (He et al., 2003). Owen et al. (2008) sought to settle this discrepancy in data by using the same tissue but processing it in two different ways. Tomographic slices were obtained from both frozen and freeze-substituted sections of cow snout epidermis. In both cases, desmosomes exhibited irregular midlines in the extracellular space, illustrating that discrete groups of molecules are present rather than a uniform arrangement (Owen et al., 2008). At this point, additional studies using a higher resolution are needed to further reconcile these conflicting observations.
2.1.2. Armadillo proteins
Proteins from the armadillo family that constitute the desmosome include plakoglobin and members of the p120 catenin subfamily, namely plakophilins-1 to -3 (Delva et al., 2009; Green and Gaudry, 2000; Green and Simpson, 2007). Another member of the p120 catenin subfamily, p0071 (also known as plakophilin-4), has also been hypothesized to be a component of both desmosomes and adherens junctions (Hatzfeld and Nachtsheim, 1996; Hatzfeld et al., 2003). However, its presence at the desmosome is somewhat arguable at this point (Hatzfeld, 2007; Hofmann et al., 2008, 2009). Thus, this protein will not be discussed any further. Armadillo proteins are unique because they are characterized by a series of armadillo repeats which are flanked by N-terminal head and C-terminal tail domains. These proteins display variable tissue expression patterns, and they localize to desmosomes, as well as to nuclei. Therefore, they are defined as multifunctional scaffolding proteins with dual roles in cell adhesion and signal transduction (Bass-Zubek et al., 2009; Hatzfeld, 2007; Schmidt and Jager, 2005).
2.1.2.1. Plakoglobin
Plakoglobin (also known as γ-catenin) is an adaptor protein found in both desmosomes and adherens junctions, and it also localizes to nuclei in various cells (Delva et al., 2009; Green and Gaudry, 2000). Proteins that interact with plakoglobin include classic and desmosomal cadherins, α-catenin, plakophilins, and desmoplakin (Choi et al., 2009; Hatzfeld, 2007). Plakoglobin is critical for the early stages of desmosomal assembly. For example, in keratinocytes isolated from plakoglobin knockout mice, the incorporation of desmosomal proteins such as desmogleins-1 and -2 into the plasma membrane was impaired (Yin et al., 2005a). This phenotype, which could not be rescued by β-catenin, was reversed by the reexpression of plakoglobin. In another related study published by a separate group of investigators, keratinocytes from plakoglobin knockout mice were still capable of clustering desmosomal cadherins on the cell surface and forming desmosome-like junctions, except that in this case β-catenin appeared to compensate for the loss in plakoglobin. However, β-catenin still failed to recruit sufficient amounts of plakophilin-1 and desmoplakin to desmosomes, thereby resulting in thin and sparse cytoplasmic plaques that were weakly connected to intermediate filaments (Acehan et al., 2008). Moreover, during Ca2+-induced junction assembly, plakoglobin was found to first associate with desmocollin-3, which preceded binding of desmocollin-3 to desmoglein-3 (Aoyama et al., 2009). These studies exemplify the important role of plakoglobin in recruiting desmosomal components to the desmosome which are needed for junction assembly.
Desmosomal cadherins are known to bind to plakoglobin’s armadillo domain, and they generally do not bind to β-catenin (Choi et al., 2009). Thus, plakoglobin is thought to be largely responsible for the vertical linkage of desmosomal cadherins to desmoplakin, in contrast to plakophilin which facilitates lateral linkage between desmoplakins (Hatzfeld, 2007; Kowlczyk et al., 1999; Section 2.1.2.2). Plakoglobin is also an important regulator of cell adhesion and motility. For instance, the adhesive strength of desmosomes was shown to be downregulated by phosphorylation of plakoglobin following activation of the epidermal growth factor receptor (EGFR). This resulted in the dissociation of desmoplakin, which is an important prerequisite for cell movement during wound healing (Yin et al., 2005a). Finally, plakoglobin also plays an important role in several signaling cascades during the processes of cell motility, cell proliferation, and apoptosis, as well as in the pathogenesis of pemphigus vulgaris (de Bruin et al., 2007; Yin et al., 2005b; Section 2.3). All these processes involve the interaction of cytoplasmic plakoglobin with various kinases and the inhibition of Wnt–β-catenin signaling by nuclear plakoglobin (Garcia-Gras et al., 2006).
2.1.2.2. Plakophilins
Plakophilins-1 to -3 have been shown to localize to the nuclei of various cells irrespective of the presence of desmosomes, but their incorporation into these structures was found to be tissue specific (Bass-Zubek et al., 2009; Hatzfeld, 2007; Schmidt and Jager, 2005). For instance, plakophilin-1 is expressed predominantly in the differentiated layers of the epidermis (Bass-Zubek et al., 2009), whereas plakophilin-2 is found in desmosomes in almost all desmosome-bearing cells, including those in simple epithelia (e.g., colon) and the proliferating layer of complex epithelia, as well as in nonepithelial cells such as cardiomyocytes (Bass-Zubek et al., 2009). Finally, plakophilin-3 is found in desmosomes of both simple and complex epithelia (Bonne et al., 1999). The functional significance of plakophilin in the structural integrity of desmosomes is demonstrated by several human diseases. For instance, mutations in plakophilin-1 result in ectodermal dysplasia/skin fragility syndrome (McGrath et al., 1997), whereas mutations in plakophilin-2 associate with arrhythmogenic right ventricular cardiomyopathy (Gerull et al., 2004).
Coimmunoprecipitation, in vitro binding assays, and yeast two-hybrid systems have been used to identify plakophilin-interacting proteins. Plakophilins are unique in that they bind to almost all other proteins within the desmosome, including desmosomal cadherins, plakoglobin, desmoplakin, and intermediate filament proteins (Bonne et al., 2003; Chen et al., 2002; Hatzfeld et al., 2000; Hofmann et al., 2000; Kowlczyk et al., 1999). However, intermediate filaments do not appear to be decorated by plakophilins in vivo (Hofmann et al., 2000). Plakophilin-2 also binds to the adherens junction adaptors β-catenin (Chen et al., 2002) and αT-catenin (Goossens et al., 2007). In essence, this broad myriad of interacting proteins enables plakophilins to function as important hubs within desmosomes by stabilizing other desmosomal components at the cell–cell interface (Hatzfeld et al., 2000; Kowlczyk et al., 1999).
The interaction between plakophilins and desmoplakin is of particular importance because the plakophilin head domain contains at least two desmoplakin-binding sites (Bonne et al., 2003). This is thought to confer lateral linkage between individual desmoplakin molecules, which are multidomain proteins that tether the whole desmosome to the intermediate filament network. Indeed, plakophilin-1-deficient keratinocytes formed fewer Ca2+-independent desmosomes (South et al., 2003), a cellular phenomenon that requires a tight array of desmosomal proteins (Garrod and Kimura, 2008). A downregulation of plakophilin also resulted in the dissociation of desmoplakin and in the disassembly of the junctional complex (Grossmann et al., 2004; Pieperhoff et al., 2008). Furthermore, plakophilin-1 is known to compete with plakoglobin for desmoplakin binding. Interestingly, both armadillo proteins are required for the clustering of desmosomal components into punctate structures, which otherwise would display a continuous distribution along the cell surface and possibly abrogate robust desmosomal adhesion (Bornslaeger et al., 2000).
As versatile adaptors with several binding partners, it is not surprising that plakophilins also interact with the actin cytoskeleton, as well as with the actin-based adherens junction. Plakophilin-1 binds to actin filaments via its armadillo domain, which is different from the domain required for desmoplakin interaction (i.e., the N-terminal head domain; Hatzfeld et al., 2000). Moreover, decoration of actin filaments by plakophilin-1 has been observed in both normal and overexpressed cells, and the upregulation of plakophilin-1 induced the formation of actin-containing structures such as filopodia and cell protrusions (Hatzfeld et al., 2000). As mentioned above, plakophilin-2 also binds to αT-catenin in the area composita, a specialized junction found at intercalated disks in the heart that contains both adherens junction and desmosome components (Goossens et al., 2007). This interaction may strengthen cell–cell adhesion by providing the actin-based cadherin–catenin complex with an additional opportunity to link to the intermediate filament network. It is also worth noting that plakophilin-2 interacts with noncadherin-bound β-catenin and promotes β-catenin–T cell factor (TCF) signaling (Chen et al., 2002).
Plakophilins also localize to cell nuclei, but their exact roles within this structure have yet to be defined. One study showed that plakophilin-2 was present in nuclear particles containing the largest subunit of RNA polymerase III and that it was also found in the holoenzyme (Mertens et al., 2001). RNA polymerase III is responsible for the transcription of rRNA and tRNA, which may explain in part the constitutive nuclear localization of plakophilin-2. This is in contrast to the transient nuclear localization of another armadillo protein, β-catenin, which is imported to the nucleus only upon the induction of Wnt signaling that activates the transcription of Wnt target genes (Clevers, 2006). Also, recent studies have suggested a role for plakophilins in translation. Plakophilin-3 was detected in cytoplasmic particles containing poly (A)-binding, fragile-X-related, and ras-GAP-SH3-binding proteins, all of which are RNA-binding proteins. When cells were exposed to stress, these proteins together with plakophilin-1 or -3 were found within stress granules that are known to accumulate stalled translational initiation complexes (Hofmann et al., 2006). In addition, plakophilin-1 was shown to associate with eukaryotic translation initiation factor 4A1 (eIF4A1), and plakophilin-1 overexpression enhanced its translational activity by promoting adenosine triphosphatase activity (Wolf et al., 2010). Taken collectively, these findings show the involvement of plakophilins in translation.
2.1.3. Plakins
Plakins are large multidomain proteins that link junction proteins to the cytoskeleton. They comprise an N-terminal plakin domain for binding adaptor proteins, a central coiled-coil domain for dimerization and several C-terminal plakin repeat domains for intermediate filament binding. The plakin repeat domain comprises a series of plakin repeat motifs that are characteristic of this family (Jefferson et al., 2004; Leung et al., 2002; Sonnenerg and Liem, 2007). In addition to this basic structure, nondesmosomal plakins can also contain an actin-binding domain, spectrin repeats, and a Gas-2-related domain for microtubule binding, which enable plakins to mediate crosstalk between cytoskeletal networks. Similar to members of the armadillo protein family, plakins are involved in both cell adhesion and signaling. For instance, as a constitutive component of the desmosome, desmoplakin is needed for the late stages of desmosomal assembly. Moreover, desmoplakin is downregulated during wound healing and morphogenesis, which destabilizes desmosomes. Interestingly, this is mediated by the proteolytic action of caspases (Aho, 2004). Plakins are also known to participate in important signaling pathways by acting as platforms for kinase activity (van den Heuvel et al., 2002; Sections 2.3 and 2.4).
To date, desmoplakin, periplakin, and envoplakin have been reported to localize to desmosomes, plectin, and bullous pemphigoid antigen 1 (BPAG-1) to associate with hemidesmosomes and microtubule actin cross-linking factor (MACF) to associate with focal adhesions. However, the localization of epiplakin is presently unclear (Jefferson et al., 2004; Leung et al., 2002; Sonnenerg and Liem, 2007). Desmoplakin is a putative constituent of the desmosomal plaque and is found in all epithelia and in cardiomyocytes, while periplakin and envoplakin are found in complex epithelia but not in simple epithelia and nonepithelial cells such as cardiomyocytes.
The function of desmoplakin is to tether cytoplasmic plaque proteins (e.g., plakoglobin) and the cytoplasmic tails of desmosomal cadherins to intermediate filaments. As mentioned above, individual desmoplakin molecules are linked laterally by plakophilins (Leung et al., 2002). To investigate the role of desmoplakin in desmosomal assembly, Godsel et al. (2005) studied the dynamics of desmoplakin incorporation by visualizing desmoplakin tagged to green fluorescent protein using time lapse imaging. Their findings demonstrated that desmoplakin binding to intermediate filaments was not required for the early stages of desmosomal assembly. Specifically, it was reported that desmoplakin was incorporated into discrete puncta in a de novo manner, followed by their aggregation. Thereafter, cytoplasmic particles containing desmoplakin, as well as plakophilin-2, were visible, and these were shuttled toward the cell surface via the actin network. In cells expressing desmoplakin with a deleted plakin repeat domain, which affected binding to intermediate filaments, the translocation of cytoplasmic particles to the plasma membrane was interrupted at first but was then restored to normal. Conversely, phosphorylation-deficient desmoplakins, which showed an increase in intermediate filament binding, were somewhat delayed in their recruitment to the cell surface (Godsel et al., 2005), illustrating that premature binding to intermediate filaments can affect the assembly of desmosomes.
Another study reported similar findings when desmosome-like junctions were formed in keratinocytes from epidermis-specific desmoplakin knockout mice. In this case, desmosomes were improperly connected to intermediate filaments, lacked the inner dense plaque, and associated with few desmosomal cadherins and plaque proteins (Vasioukhin et al., 2001). This scenario was equivalent to an early stage of normal desmosome assembly during which time cadherin engagement and recruitment of adaptors occurs but not the attachment of plaque proteins to intermediate filaments. Progression through this stage would require desmoplakin-mediated binding to intermediate filaments, as reflected by the formation of partial desmosomes in desmoplakin-null keratinocytes. Furthermore, these cells were incapable of forming an epithelium because adherens junction assembly was halted after the engagement of E-cadherin at cell–cell contacts. The formation of adhesion zippers and actin organization was also adversely affected (Vasioukhin et al., 2001). In essence, these studies emphasize the requirement of desmoplakin for intermediate filament linkage which completes desmosome assembly, as well as the critical role of desmosomes in stabilizing adherens junctions by acting as spot welds to hold apposing membranes together. This facilitates remodeling of the actin cytoskeleton and the sealing of membranes via the formation of mature adherens junctions (i.e., the adhesion belt).
2.1.4. Intermediate filaments
To conclude this section on desmosomal proteins, we include a brief discussion on intermediate filaments. In the cytoplasm, intermediate filaments extend from the perinuclear region to the cell periphery where they link to cell junctions, namely desmosomes and hemidesmosomes. Intermediate filaments are filamentous hetero- or homopolymers, and they do not exhibit polarity, a characteristic of actin microfilaments and microtubules. This is because intermediate filament protein dimers are arranged in antiparallel (Herrmann et al., 2007, 2009; Kim and Coulombe, 2007). Intermediate filaments belong to a large family of proteins comprising ~70 genes in the human which exhibit tissue-specific expression, and they are categorized into five subtypes (Herrmann et al., 2007). For instance, keratins belong to subtypes 1 and 2, but vimentin belongs to subtype 3. Keratins are generally expressed by epithelial cells, whereas vimentin is generally expressed by mesenchymal cells (Eriksson et al., 2009; Kim and Coulombe, 2007). In this regard, it is worth noting that Sertoli cells in the seminiferous epithelium are an exception to this rule: Sertoli cell intermediate filaments comprise vimentin, while keratins are only expressed during their development (Vogl et al., 2008).
2.2. Unique features of desmosomes
2.2.1. Ca2+ independence
Ca2+ independence is a unique characteristic of mature desmosomes, and this feature is not shared by other types of junctions such as adherens and tight junctions (Garrod and Kimura, 2008). Ca2+ dependence is an inherent characteristic of cadherin molecules because their rod-like conformation can only be maintained when Ca2+ ions are bound to their Ca2+ binding sites at linker regions (Leckband and Prakasam, 2006). This conformation favors trans-dimer formation between opposing cell surfaces to confer cell–cell adhesion. Otherwise, Ca2+-free cadherin molecules would become flexible. This relaxed conformation favors the formation of cis- instead of trans-dimers (Troyanovsky et al., 2003). The mechanism of Ca2+ independence was revealed by structural information relating to the cadherin ectodomain. As discussed previously, a homology model of the desmocollin-2 ectodomain structure was obtained (Garrod et al., 2005) by using the C-cadherin crystal structure as a template (Boggon et al., 2002). From this model, the three-dimensional packing of the desmocollin-2 ectodomain resulted in rows of back-to-back cis-interacting desmosomal cadherin molecules distributed at regular intervals on each cell surface. Within this arrangement, the Ca2+-binding site present between EC2 and EC3 on one cadherin molecule was protected by a small β-helix found in EC1 from the neighboring cadherin molecule, thereby trapping the bound Ca2+ ions even in a low Ca2+ environment (Garrod et al., 2005). Nevertheless, Ca2+ independence per se is only the end product of this tight cadherin arrangement, and this property has no relevance in vivo because a Ca2+-depleted environment can only be created in vitro. Hyperadhesion, however, is the endpoint of the compact arrangement of cadherins (Section 2.2.2), and it can only be attained by mature desmosomes which are also Ca2+ independent. Thus, Ca2+ independence can be a useful tool to experimentally study the adhesive status of desmosomes (Garrod and Kimura, 2008).
2.2.2. Hyperadhesion
It is hypothesized that hyperadhesion (and thus Ca2+ independence) is a characteristic of mature desmosomes in vivo such as those found in the epidermis and heart, thus enabling desmosomes in these tissues to endure great physical stress (Garrod et al., 2005). Mature desmosomes are typified by a dense midline in the extracellular space, indicative of the regular arrangement of tightly packed cadherin molecules. During morphogenesis and wound healing, desmosomal adhesion is downregulated by intracellular signals to allow cell motility, giving rise to desmosomal plaques with no discernable midlines due to the irregular arrangement of cadherin molecules. However, both halves of the desmosome were still attached. The connection between hyperadhesion and Ca2+ independence was demonstrated by Garrod et al. (2005). Mature desmosomes in the epidermis in vivo were resistant to disruption after prolonged exposure to low-Ca2+ medium, while wound-edge desmosomes split into two halves. Interestingly, wound-edge desmosomes associated with protein kinase C (PKC)-α, suggesting that PKC-α-mediated inside–out signals downregulate desmosomal adhesion (Garrod et al., 2005). Epithelial cells cultured at high density in vitro could also attain Ca2+ independence over time after reaching confluency. The adhesive strength of desmosomes before and after the acquisition of Ca2+ independence was experimentally determined by an adhesion assay using intact cell sheets (Kimura et al., 2007). Indeed, when HaCaT cell sheets cultured for 2 days were subjected to rotational shear force, sheets were fragmented into several more pieces, as compared to cell sheets cultured for 6 days which had acquired Ca2+ independence (Kimura et al., 2007). This validated the association between adhesive strength and Ca2+ dependence. Furthermore, in agreement with in vivo findings, the use of a PKC-α/β inhibitor or PKC-α antisense oligonucleotides could rapidly reverse desmosomes to the Ca2+-independent state without affecting the levels of desmosomal proteins, which is an extremely useful method for manipulating desmosomal adhesion (Kimura et al., 2007; Wallis et al., 2000).
Hyperadhesion not only strengthens cell–cell adhesion in stress-bearing tissues but has also recently been shown to attenuate the disruptive effects of pemphigus vulgaris serum (Cirillo et al., 2010). When keratinocytes were treated with the PKC-α/β inhibitor Go6976, a hyperadhesive state was rapidly induced. Interestingly, subsequent exposure of treated cells to pemphigus vulgaris serum resulted in significantly less cell–cell detachment then in non-Go6976-treated cells. It was proposed that the compact cadherin arrangement in hyperadhesive desmosomes helped to protect the epitopes from being targeted by autoantibodies, thereby attenuating their disruptive effects. To investigate whether hyperadhesion is solely responsible for this protective effect, the authors prevented hyperadhesion by pretreating cells with blocking peptides of cadherin adhesion recognition sites. In this case, the PKC inhibitor could still partially attenuate the effects of the autoantibodies, illustrating that other aspects were also responsible for the pathogenesis of pemphigus vulgaris (Cirillo et al., 2010). These results illustrate that the maintenance of hyperadhesion can provide new insights into the treatment of pemphigus vulgaris.
2.3. Regulation of desmosomal adhesion
Desmosome function is under strict regulation by multiple factors. For instance, desmosomal adhesion can be downregulated at the wound edge during healing to prepare cells for migration. Desmosomal adhesion is also regulated by numerous signaling cascades that involve kinases and proteases, and many of these molecules are known to directly interact with desmosomal plaque proteins. Cirillo and colleagues compiled interactions between desmosomal and cellular proteins in keratinocytes by a systems biology approach, and classified these interactions into six functional subnets (Cirillo and Prime, 2009). Apart from membrane proteins, adaptors, and cytoskeletal proteins, the three main types of proteins that desmosomal proteins are known to interact with are kinases, phosphatases, and proteases, illustrating that desmosome function is regulated by these protein families. For example, phosphorylation of cytoplasmic plaque proteins by kinases was shown to introduce negative charges on the former. These proteins then repelled each other, thereby perturbing adhesive strength (Garrod and Chidgey, 2008). Indeed, there are other important examples in which kinases were able to downregulate desmosomal adhesion, and these are discussed in Section 2.3.1.
2.3.1. Regulation by kinases
2.3.1.1. PKC
PKC-mediated downregulation of desmosomal adhesion and its reversion to Ca2+ dependence were discussed previously, and readers are asked to refer to Section 2.2.2. After treating urinary bladder carcinoma cells with the PKC-α/β inhibitor Go6976, cell adhesion was shown to be strengthened. Interestingly, this was accompanied by an increase in the number of desmoplakin-positive spot welds at the cell–cell interface (Koivunen et al., 2004), findings which may suggest that more desmoplakin molecules were recruited to desmosomes or that the actual number of desmosomes increased. Since these effects—which appeared to be specific to desmosomes—were not affected by the actin disruptor cytochalasin-D, it was concluded that PKC regulates desmosomes (Koivunen et al., 2004). On the contrary, desmoplakin failed to be recruited to desmosomes when it was not phosphorylated by PKC-α (Bass-Zubek et al., 2008). Moreover, in SCC-9 and A431 epithelial cell lines, desmoplakin recruitment required the formation of a multiprotein complex comprising plakophilin-2, desmoplakin, and PKC-α. The disruption of this multiprotein complex by plakophilin-2 silencing rendered desmoplakin less phosphorylated which then associated with the intermediate filament network (Bass-Zubek et al., 2008).
2.3.1.2. Src
Like many adherens and tight junction proteins, desmosomal proteins can also be regulated by Src. Remarkably, phosphorylation of plakoglobin by Src appears to elicit opposite effects in adherens junctions and in desmosomes. When plakoglobin was phosphorylated on Tyr-643 (equivalent to Tyr-654 on β-catenin), its association with E-cadherin and α-catenin decreased, whereas its association with desmoplakin increased (Miravet et al., 2003). The role of Src in the regulation of desmosomes was also illustrated by the pathogenesis of the autoimmune disease pemphigus vulgaris (Miravet et al., 2003). Exposure of keratinocytes to desmosomal protein autoantibodies led to the activation of Src which peaked at ~30 min, followed by the activation of EGFR and p38 mitogen-activating protein kinase (MAPK). Contrary to the hypothesis that acantholysis (the loss of adhesion between keratinocytes) is induced by antidesmoglein-1 or -3 antibodies, these investigators demonstrated that these proteins were not involved in the initial events of deadhesion. Instead, they showed that cell–cell detachment was triggered by nondesmoglein antibodies, eliciting a signaling cascade involving Src, which downregulated desmosomal adhesion via inside–out signaling (Miravet et al., 2003).
2.3.1.3. EGFR
In squamous cell carcinoma cells cultured in low Ca2+ medium which only permitted the formation of E-cadherin-based adhesion, the inhibition of EGFR resulted in a shift from a fibroblastic to a more epithelial cell phenotype resulting from an induction of desmosomal assembly (Lorch et al., 2004). This was demonstrated by the expression and recruitment of desmoglein-2 and desmocollin-2 to the cell surface, a decrease in the phosphorylation of desmoglein-2 and plakoglobin, and an increase in desmoglein-2 and desmoplakin in the triton-insoluble fraction. These effects were specific to desmosomes because EGFR activation affected neither the phosphorylation states nor the localization of E-cadherin and β-catenin (Lorch et al., 2004).
2.3.2. Regulation by proteases
2.3.2.1. Caspases
Caspases are a family of cysteine proteases that serve as primary effectors during apoptosis to proteolytically dismantle most cellular structures, including the cytoskeleton, cell junctions, mitochondria, endoplasmic reticulum, Golgi, and the nucleus (Taylor et al., 2008). They exist as inactive zymogens in normal cells, but they are rapidly processed into active proteases in response to apoptotic signals. The initial activation of major caspases such as caspases 3 and 7 leads to the activation of another set of caspases, which in turn activate more caspases in this cascade. Together, they mediate proteolysis of cellular proteins (Taylor et al., 2008). Many desmosomal proteins are targets of caspases during apoptosis, including desmogleins-1 to -3 (Cirillo et al., 2008; Nava et al., 2007; Weiske et al., 2001), plakoglobin (Weiske et al., 2001), plakophilin-1 (Weiske et al., 2001), and desmoplakin (Aho, 2004; Nava et al., 2007; Weiske et al., 2001). Cleavage of these proteins produces two or more intracellular and extracellular fragments, which are then further processed into smaller fragments and internalized from the cell surface, resulting in the disintegration of desmosomal structure. These cleaved proteins can have biological activity, and they appear to be required for the propagation of apoptotic signals, illustrating that desmosomal proteolysis plays a role in the regulation of pathogenesis and normal development (Section 2.4.4).
2.3.2.2. Matrix metalloproteases and ADAMs
The matrix metalloprotease (MMP) family of proteases—with collagenase being the founding member—plays an important role in disease and in development. Some MMPs such as MMP 14 (also known as membrane type 1-MMP) are membrane bound (Page-McCaw et al., 2007), whereas A Disintegrin And Metalloproteases (ADAMs) comprise a related family of proteases which specialize in the shedding of membrane-bound proteins (Huovila et al., 2005). Desmosomal proteins that are cleaved by MMPs or ADAMs include desmogleins-1 to -3 and desmocollin-3 (Dusek et al., 2006; Klessner et al., 2009; Santiago-Josefat et al., 2007; Weiske et al., 2001). Moreover, desmoglein-2 was found to be targeted by a broad spectrum of proteases, including MMPs and ADAMs 9, 10, 15, and 17, as well as caspase 3. While MMP and ADAM 17 generated a 100-kDa fragment encompassing the cytoplasmic tail, transmembrane, and extracellular juxtamembrane domains, further processing of this fragment required ADAM 10, whose knockdown led to the accumulation of this 100 kDa fragment (Klessner et al., 2009). In normal cells, ADAM 17 is located at the dorsal cell surface or within intracellular vesicles. Thus, it is sequestered away from desmoglein-2 which is present at the cell surface. Interestingly, EGFR can stabilize internalized desmoglein-2 during apoptosis, which would otherwise become available for ADAM-mediated proteolytic cleavage (Klessner et al., 2009; Santiago-Josefat et al., 2007).
2.3.2.3. Serine proteases
In the epidermis, terminal differentiation is a process that produces the outermost cornified layer of apoptotic cells. These cells possess modified desmosomes called corneodesmosomes, which contain desmoglein-1 and desmocollin-1, as well as another extracellular glycoprotein known as corneodesmosin (Candi et al., 2005). The desquamation of these apoptotic cells is mediated by the cleavage of corneodesmosomal proteins by serine proteases, including stratum corneum chymotryptic enzyme (SCCE) and stratum corneum tryptic enzyme (SCTE), until cells no longer retain their adhesive properties and detach (Ovaere et al., 2009). Desmoglein-1, desmocollin-1, and corneodesmosin are all targeted by these two serine proteases (Caubet et al., 2004; Simon et al., 2001). Interestingly, SCTE was unable to cleave recombinant corneodesmosin in vitro, suggesting that this enzyme may act through the activation of another protease. Indeed, SCTE is capable of proteolytically processing pro-SCCE into its mature active form, which in turn cleaves corneodesmosin (Caubet et al., 2004). The activity of these serine proteases is under the regulation of inhibitors such as lymphoepithelial kazal-type inhibitor (LEKTI), whose deficiency led to increased SCCE and SCTE activity and the Netherton syndrome, a skin disease (Descargues et al., 2005).
2.4. Desmosome-mediated signaling
Emerging evidence suggests that desmosomes also function as signaling centers (Delva et al., 2009; Green and Gaudry, 2000; Green and Simpson, 2007), especially since not all tissues possess mature desmosomes. However, many investigators prefer not to use the terminology “desmosome signaling” because it may be confused with the type of signaling triggered by integrins. Nevertheless, desmosomal proteins are capable of transducing signals via both genomic and nongenomic pathways, as well as indirectly affecting the localization of signaling molecules.
2.4.1. Desmosomal proteins as scaffolds for kinases
Many desmosomal proteins, especially adaptors such as plakoglobin and plakophilin-2, are known to physically interact with kinases (Cirillo and Prime, 2009). While some of these proteins are substrates for kinases, they can also tether kinases to desmosomes or intermediate filaments. The interaction between PKC-α and desmosomal plaque proteins was discussed previously (Garrod et al., 2005; Wallis et al., 2000), and readers are asked to refer to Section 2.2.2. Among the several plaque proteins that bind to PKC-α, plakophilin-2 was reported to form a complex with PKC-α and desmoplakin in SCC-9 and A431 epithelial cell lines (Bass-Zubek et al., 2008). Disruption of this complex by plakophilin-2 knockdown not only blocked desmoplakin recruitment but also resulted in the release of PKC-α and in the increase in phosphorylation of PKC-α substrates, indicating a role for plakophilin-2 in the sequestration of PKC-α (Bass-Zubek et al., 2008). In addition, the C-terminal domain of the desmosomal plakin, periplakin, was shown to interact with protein kinase B (PKB, also known as Akt; van den Heuvel et al., 2002). Overexpression of this domain resulted in a decrease in nuclear PKB, thereby reducing PKB-dependent Forkshead transcription factor activity.
Downstream of these kinases, desmosomes can also regulate other important signaling molecules such as Src and MAPK through which numerous pathways converge and diverge. For instance, an increase in cell motility was noted in keratinocytes isolated from the skin of plakoglobin knockout mice. This effect was suppressed by using PP2, a Src family inhibitor or U0126, a MAPK kinase 1/2 inhibitor (Yin et al., 2005b). Furthermore, desmoglein-2 was found to associate with Src in a recent study from our lab when Sertoli cell and seminiferous tubule lysates were used for coimmunoprecipitation. Interestingly, simultaneous knockdown of desmoglein-2 and desmocollin-2 by RNA interference (RNAi) led to the relocation of Src from the Sertoli cell surface to the cytoplasm, suggesting that desmoglein-2 is a docking site for Src (Lie et al., 2010; Fig. 5.4). Concurrent with the mislocalization of Src, a decrease in the integrity of the tight junction barrier was noted, possibly resulting from the disruptive effects of Src on occludin–zonula occludens-1 (ZO-1; Kale et al., 2003) and connexin-43–ZO-1 (Gilleron et al., 2008) interactions, which are critical for barrier dynamics (Li et al., 2009). Another example of cadherin–kinase interaction is that between desmoglein-1 and EGFR. Desmoglein-1 was required for the suppression of the EGFR-Erk1/2 (a MAPK) signaling pathway during the terminal differentiation of keratinocytes, which exemplifies the importance of desmosomal signaling in tissue development (Getsios et al., 2009).
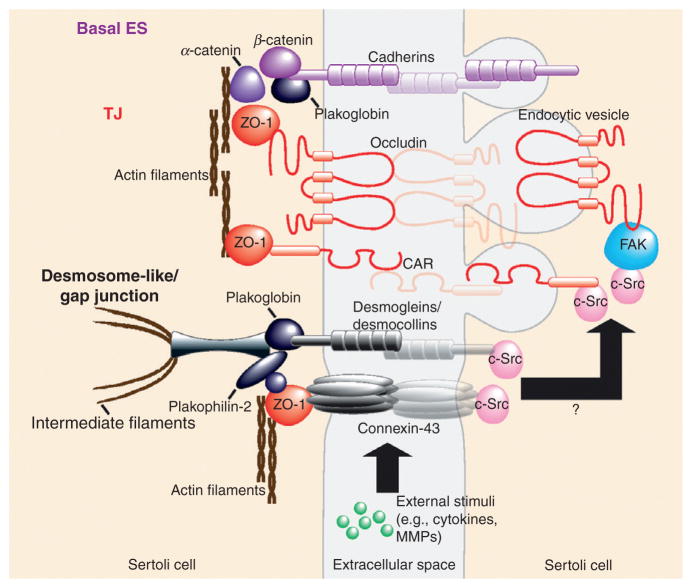
Figure 5.4.
Crosstalk between different junction types at the BTB as a mechanism to regulate and to maintain the integrity of the immunological barrier. The BTB is constituted by adjacent Sertoli cells near the basement membrane and is composed of coexisting tight junctions (i.e., occludin), basal ectoplasmic specializations (i.e., N-cadherin), desmosome-like junctions (i.e., desmogleins, desmocollins, and plakophilins), and gap junctions (i.e., connexin-43). The physiological significance for the coexistence of these junctions at the BTB has remained unknown for decades. Recent studies, however, have demonstrated that these junctions provide an efficient and effective means to induce restructuring of the BTB to facilitate the transit of preleptotene spermatocytes at stage VIII of the seminiferous epithelial cycle of spermatogenesis. It is likely that external stimuli (e.g., cytokines and MMPs) initiate disruption of two multiprotein complexes: (i) desmoglein-2/desmocollin-2 at the desmosome-like junction and (ii) connexin-43/plakophilin-2 at the gap junction. Since c-Src is an integrated component of both junctions (black arrow), this activates the c-Src signaling cascade, thereby eliciting changes in protein distribution (e.g., occludin, CAR, and N-cadherin) at the Sertoli–Sertoli cell interface as demonstrated in recent studies using RNA.
2.4.2. Desmosomal proteins as signal propagators for kinases
While many desmosomal proteins are regulated by kinases (Section 2.3.1), they can still propagate signals via downstream pathways and feedback loops. Plakoglobin is a good example, acting both as a substrate and as an effector of kinases (Berkowitz et al., 2005; Chernyavsky et al., 2007). During the pathogenesis of pemphigus vulgaris, nondesmoglein antibodies were shown to trigger acantholysis, followed by the activation of Src and EGFR (Berkowitz et al., 2005; Chernyavsky et al., 2007). This in turn downregulated desmosomal adhesion by phosphorylating desmosomal proteins (Garrod and Chidgey, 2008; Yin et al., 2005b; Section 2.3.1). One of the targets of these kinases is plakoglobin because plakoglobin-deficient keratinocytes were unaffected by pemphigus antibodies (Dusek et al., 2007). In addition, interesting changes relating to the phosphorylation state and intracellular localization of plakoglobin were also noted. For instance, depletion of nuclear plakoglobin was shown to mediate the transcription of c-myc (Williamson et al., 2006) and Wnt target genes (Garcia-Gras et al., 2006). Thus, desmosomes are not only targets of kinases (Section 2.3.1); they can also act as their effectors by eliciting downstream signaling.
2.4.3. Desmosomal proteins as regulators or components of transcriptional machinery
Many desmosomal proteins such as plakoglobin and plakophilin are also found in the nucleus, even in nondesmosome-bearing cells where they appear to participate in the regulation of gene expression (Delva et al., 2009; Green and Gaudry, 2000; Green and Simpson, 2007). As discussed previously, nuclear plakophilin-2 is a component of RNA polymerase III holoenzyme which is responsible for the synthesis of rRNA and tRNA, thereby explaining its constitutive nuclear localization (Mertens et al., 2001). However, a balance exists among the distribution of armadillo proteins in junctions, the cytoplasm, and the nucleus in desmosome-bearing cells which essentially prevents gene expression from being disrupted (Bass-Zubek et al., 2009; Hatzfeld, 2007; Schmidt and Jager, 2005). For instance, plakoglobin is a target of multiple kinases (Cirillo and Prime, 2009). During pemphigus vulgaris, the increased rate of plakoglobin turnover at the desmosome, possibly mediated by Src and EGFR, led to the depletion of plakoglobin from the nucleus (Williamson et al., 2006). However, phosphorylation of Tyr-549 by Fer kinase increased plakoglobin’s association with adherens junctions (Miravet et al., 2003).
The transit of plakoglobin to the nucleus is known to inhibit two genomic pathways. First, plakoglobin is required for the suppression of mitogenic c-myc transcription. Depletion of nuclear plakoglobin—and thus c-myc transcription—appeared to cause proliferation, as well as a weakening of cell–cell adhesion (Williamson et al., 2006). This illustrates that desmosomal proteins are capable of eliciting downstream signals and generating feedback loops to regulate cell–cell adhesion. Similar effects were also seen in plakoglobin knockout keratinocytes, which failed to accumulate plakoglobin in their nuclei and did not undergo acantholysis in response to pemphigus vulgaris antibodies (de Bruin et al., 2007; Dusek et al., 2007). Second, plakoglobin antagonizes with β-catenin in the canonical β-catenin–Wnt signaling pathway by inhibiting the transcriptional activity of β-catenin–Tcf/Lef1. As discussed previously, the armadillo proteins plakoglobin and β-catenin are homologous, both in their sequences and in their function as adaptors in desmosomes and/or adherens junctions. However, nuclear plakoglobin and β-catenin are antagonistic in that they compete for the transcription factors Tcf/Lef1 (Garcia-Gras et al., 2006). By binding to different domains of Tcf-4, they can elicit opposite effects on its transcriptional activity (Solanas et al., 2004). This ligand specificity exhibited by plakoglobin and β-catenin is determined by their N- and C-terminal tails, whereas the binding site is found in the armadillo domain (Solanas et al., 2004). While β-catenin supports transcription of Wnt target genes via Tcf/Lef1 binding, plakoglobin renders these transcription factors less efficient in DNA binding, thereby reducing their transcriptional activity (Garcia-Gras et al., 2006). Taken collectively, these results illustrate that desmosomes also act as signaling centers to transduce signals outside–in and inside–out and that these involve genomic or nongenomic pathways and feedback loops.
2.4.4. Biologically active fragments from the proteolysis of desmosomal proteins
Desmosomal proteins are proteolytically cleaved during apoptosis and tissue remodeling by caspases, MMPs, ADAMs, and serine proteases (Section 2.3.2), and some of these cleavage products have biological activity. For instance, the cleavage of desmoglein-2 during apoptosis by caspase 3 yields 100 and 60 kDa products. However, during this time, full-length desmoglein-2 was shown to increase (Nava et al., 2007), and this appeared to be mediated by the 60 kDa product because its overexpression in normal cells was found to upregulate desmoglein-2 and caspase activity, thereby enhancing apoptotic signals (Nava et al., 2007). The necessity of these proteolytic products for the propagation of apoptotic signals was also reflected by the knockdown of desmoglein-1 (Dusek et al., 2006) and desmoglein-2 (Nava et al., 2007), two caspase 3 targets which protected epithelial cells from apoptosis. Furthermore, desmoplakin was also shown to be cleaved by caspases 2 and 4 during terminal differentiation of the epidermis, and one of these cleavage products was found to remain in the cornified envelope, suggesting that it may possess a yet unknown function (Aho, 2004). Thus, desmosomal proteins participate in the regulation of apoptosis during disease and development.
3. Desmosome-Like Junctions in the Seminiferous Epithelium
Desmosomes were first described in the testis in 1977, but ultrastructurally they do not resemble bona fide desmosomes such as the ones found in the skin and heart (Russell, 1977a). As such, they were classified as “desmosome-like” junctions, in part because they lacked a clearly defined dense midline which is characteristic of conventional desmosomes (Russell, 1977a). At the time, this observation seemingly implied that desmosomes in the seminiferous epithelium are of the Ca2+-dependent type and not likely to mediate robust cell adhesion—features that may be needed to facilitate germ cell movement across the BTB, as well as partially across the seminiferous epithelium. Desmosome-like junctions exist between Sertoli cells at the BTB, and between Sertoli and all germ cell types up to, but not including, step 8 spermatids (Fig. 5.2). Sertoli–germ cell desmosome-like junctions first appear within the seminiferous epithelium at the start of spermatogenesis on postnatal day ~5 in rodents. Once spermatids begin to elongate (i.e., step 8) on postnatal day ~30, the desmosome-like junction is completely replaced by the apical ectoplasmic specialization, revealing that there is no functional overlap between these two junction types at the Sertoli–germ cell interface (Russell, 1977a). At the BTB, however, desmosome-like junctions have been shown to coexist and to cofunction with tight junctions, basal ectoplasmic specializations, and gap junctions (Fig. 5.2).
A recent study from our laboratory aimed to better understand the biology of desmosome-like junctions at the BTB. A survey of desmosomal gene expression indicated that Sertoli and germ cells are equipped with all the necessary components to form functional desmosomes. Constitutive desmosomal proteins such as desmoglein-2, plakophilin-2, plakoglobin, and desmoplakin were shown to be abundantly expressed, while differentiation-specific proteins such as desmoglein-1 were absent in Sertoli cells (Lie et al., 2010). The immunofluorescent staining of desmoglein-2 in the testis showed a punctate pattern of localization at the BTB with some colocalization with the putative BTB protein N-cadherin, illustrating that the desmosome-like junction is an integral component of the BTB. Furthermore, desmoglein-2, desmocollin-2, and plakoglobin were indeed capable of forming a multi-protein complex, which also contained Src as shown by coimmunoprecipitation experiments (Lie et al., 2010).
Interestingly, it has been suggested that gap junctions are incorporated into the desmosomal plaque based on the observation that the width of the extracellular space within desmosomes occasionally converged from 14–18 to 3–5 nm (McGinley et al., 1979; Russell, 1977a). This observation led to the use of the terminology “desmosome-gap junction” to describe desmosomes in the testis (Russell et al., 1983). The notion that desmosomes and gap junctions are physically intermixed at the BTB was addressed by one of our recent studies. The desmosomal plaque protein plakophilin-2 was found to associate with the gap junction protein connexin-43 at the BTB where it mediated crosstalk with tight junctions and basal ectoplasmic specializations during junction reassembly (Li et al., 2009). Moreover, functional RNAi studies of desmosomal proteins at the BTB have been conducted, and they illustrated that desmosome-like junctions can regulate cell adhesion and junction reassembly by interacting with tight junctions, basal ectoplasmic specializations, and gap junctions (Section 5).
4. Junction Complexes in the Seminiferous Epithelium
4.1. Tight junctions
The ultrastructural presence of tight junctions between Sertoli cells at the BTB was reported in the 1970s, but it took until 1998 for occludin to be first identified as a putative BTB protein in the mouse testis (Moroi et al., 1998). Since then, however, other transmembrane proteins, namely claudin, junctional adhesion molecule (JAM), and coxsackie and adenovirus receptor (CAR, a tight junction and ectoplasmic specialization protein), as well as their cytoplasmic adaptor protein ZO-1, have also been identified as constituent proteins of the BTB (Mruk and Cheng, 2004b). Occludin is by far the best studied tight junction protein in different epithelia and endothelia, but its exact function within this junction is not yet known because of contradictory results from in vitro and in vivo experiments (Shin et al., 2006). While occludin has been linked to several signal transduction cascades such as those involving Raf-1 (Li and Mrsny, 2000; Wang et al., 2007) and RhoA (Matter et al., 2005), it does not appear to be indispensable for tight junction function because occludin-deficient mice possessed morphologically normal tight junctions (Saitou et al., 2000). Moreover, the significance of occludin in BTB function and mammalian spermatogenesis is not entirely known owing to the fact that occludin-deficient mice were found to be sterile, but also because occludin is not expressed by the human testis (Moroi et al., 1998). Recently, an occludin–ZO-1–focal adhesion kinase (FAK) protein complex was found to regulate BTB integrity (Siu et al., 2009; Fig. 5.4). Interestingly, knockdown of FAK, a nonreceptor tyrosine kinase, by RNAi was shown to desensitize Sertoli cells from the adverse effects of cadmium on the permeability barrier (Siu et al., 2009), demonstrating that FAK is an important regulator of BTB restructuring in vivo. It is worth noting, however, that FAK does not phosphorylate proteins per se. Instead, activated FAK is autophosphorylated, thereby binding to Src, which can then phosphorylate additional sites on FAK (Ilic et al., 1997) or possibly its binding proteins such as occludin (Siu et al., 2009). Indeed, FAK silencing would render occludin non- or less-phosphorylated (Siu et al., 2009), possibly sequestering it to the basolateral membrane. Additional studies are needed to investigate the role of Src within the occludin–ZO-1–FAK multiprotein complex.
Claudins are structural proteins responsible for creating charge-selective pores within tight junctions, and the overall paracellular ion permeability characteristics of an epithelium are defined by the pattern of claudin expression. Claudin 2, for example, is typically found in leaky epithelia, most notably in proximal renal tubules (Kiuchi-Saishin et al., 2002) but not in seminiferous tubules of the testis (Mruk and Cheng, 2004b) which is well known for its tight blood–tissue barrier. In the seminiferous epithelium, claudin expression (i.e., claudins 1, 3, 4, 5, 8, and 11) has been reported by several investigators, and up until recently, claudin expression in the testis was restricted to Sertoli and endothelial cells (Morita et al., 1999; Morrow et al., 2009). Morrow et al. (2009) now report that spermatogonia and preleptotene spermatocytes also express claudin 5. Claudin 5 immunoreactivity was highest during stage VIII of the seminiferous epithelial cycle, coinciding with an early stage of preleptotene spermatocyte transit across the BTB. This is in agreement with another related study which also reported an elevated level of claudin 3 at stage VIII (Meng et al., 2005). Morrow et al. (2009) also demonstrate that claudin 5 expression in the seminiferous epithelium depended on the presence of germ cells. These findings are intriguing for several reasons. First, germ cells lack tight junctions. Thus, it is not immediately known why germ cells would express a tight junction protein, but it is possible that claudin is participating in some aspect of germ cell movement across the BTB and/or contributing to the maintenance of the immunological barrier. As hypothesized by Morrow et al., germ cell claudin 5 may be working in concert with membrane-type matrix metalloproteases (MT-MMPs) to activate soluble-type MMPs which may be needed to cleave proteins at the BTB during preleptotene spermatocyte transit (Fritz et al., 1993; Longin et al., 2001; Oku et al., 2006; Siu et al., 2003; Fig. 5.4). A stimulation of MT-MMP-mediated pro-MMP-2 activation has also been noted with claudins 1, 2, and 3 in 293 T cells (Miyamori et al., 2001). Second, the observation that Sertoli cell expression of claudin 5 is dependent on the presence of germ cells within the epithelium (Morrow et al., 2009) may suggest that germ cells (i.e., preleptotene spermatocytes) facilitate their transit across the BTB by regulating claudin. Indeed, germ cells are known to regulate several Sertoli cell proteins (Boitani et al., 1981; Morrow et al., 2009; Nicholls et al., 2009). The role of germ cells in Sertoli cell junction restructuring is an exciting new area that needs further investigation.
4.2. Ectoplasmic specializations
The ectoplasmic specialization is a testis specific, actin-based junction that is found at two sites within the seminiferous epithelium: (i) between opposing Sertoli cells at the BTB (defined as the basal ectoplasmic specialization) and (ii) between Sertoli cells and elongating/elongated spermatids in the adluminal compartment (defined as the apical ectoplasmic specialization; Mruk and Cheng, 2004a; Vogl et al., 2008; Fig. 5.2). In the rat, the apical ectoplasmic specialization appears when spermatids reach step 8 of spermiogenesis (i.e., stage VIII), which marks the elongation of round spermatids, and it disappears when spermatids reach step 19 prior to spermiation (Russell, 1977b). The apical ectoplasmic specialization is known to structurally and functionally replace the desmosome-like junction as the only adhesive structure found between Sertoli and germ cells at these developmental stages. Ultrastructurally, the ectoplasmic specialization consists of a layer of hexagonally packed actin microfilaments found between the Sertoli cell plasma membrane and a network of endoplasmic reticulum, a hallmark feature observed in electron micrographs of the apical and basal ectoplasmic specialization. However, the ectoplasmic specialization has not been observed to exist in elongating/elongated spermatids. In recent years, the ectoplasmic specialization has been the focus of several studies because of its unique hybrid-like character. Although generally defined as an adhesive structure constituted by anchoring junction proteins (i.e., cadherin and nectin), the apical ectoplasmic specialization has also been shown to be composed of proteins normally found within tight junctions and focal contacts (a type of cell–matrix junction; Mruk and Cheng, 2004a; Mruk et al., 2008). In the remainder of this section on the ectoplasmic specialization, we will discuss recent findings relating to classic cadherins.
There are numerous studies reporting cadherin function to be regulated by cytokines, androgens, estrogens, protein kinases, and phosphatases, and small GTPases, as well as by phosphorylation, changes in protein–protein interactions and endocytosis (Braga et al., 1997; Delva and Kowalczyk, 2009; Le et al., 1999; Lee et al., 2003; MacCalman et al., 1997; Mosesson et al., 2008; Mruk and Cheng, 2004b; Nagafuchi et al., 1993). Recent studies demonstrate a novel mechanism for cadherin regulation via the proteolytic cleavage of type I and type II cadherins by metalloproteases (i.e., ADAMs) and γ-secretase, resulting in the generation of biologically active peptides having diverse functions including roles in cell adhesion (Marambaud et al., 2002, 2003; McCusker and Alfandari, 2009; Reiss and Saftig, 2009; Section 2.3.2). So far, more than 50 cell surface proteins have been shown to undergo ectodomain shedding by proteases. For instance, the cleavage of E- or N-cadherin by ADAM10 was shown to produce an extracellular N-terminal fragment (defined as NTF) and a membrane-bound C-terminal fragment (defined as CTF1). Interestingly, CTF1 was processed rapidly by the γ-secretase complex to produce a cytoplasmic fragment (defined as CTF2), which caused the relocation of β-catenin from the cell surface to the cytoplasm (Marambaud et al., 2002, 2003). Furthermore, this cytoplasmic fragment was found to maintain its association with β-catenin and to activate cyclin D1 (a protein known to promote progression through the G1–S phase of the cell cycle) transcription in the nucleus via the Wnt signaling pathway (Maretzky et al., 2005). Cyclins D1 and D2 upregulation was also demonstrated in another study when a soluble E-cadherin fragment generated by ADAM15 was shown to interact with ErbB (a receptor tyrosine kinase) and to activate downstream signaling (Najy et al., 2008), suggesting a connection between cell adhesion and cell differentiation/division. In terms of biological activity, the NTF of N-cadherin has been shown to associate with the extracellular matrix, suggesting a role in cell movement (Cifuentes-Diaz et al., 1994; Paradies and Grunwald, 1993; Utton et al., 2001). Indeed, the NTF of cadherin 11 (generated via ADAMs 9 and 13) was demonstrated to promote cell migration in the X. laevis neural crest (McCusker et al., 2008). Equally important, ADAMs were found to colocalize and coimmunoprecipitate with cadherins in various cell types (Ham et al., 2002). At this point, studies are warranted to determine if ADAM-mediated cadherin cleavage has any role in BTB restructuring and germ cell movement.
4.3. Gap junctions
The study of gap junctions at the BTB and at the Sertoli–germ cell interface was investigated recently, and this study showed that connexin-43 is a primary component of gap junctions at the BTB (Li et al., 2009). In line with earlier observations which showed gap junctions to be intermixed with desmosomes in the testis (McGinley et al., 1979; Russell, 1977a), connexin-43 was shown to interact with plakophilin-2. Together they regulate junction reassembly at the BTB by interacting with tight junctions and basal ectoplasmic specializations (Li et al., 2009; Section 5.4).
5. Junctional Interplay at the BTB: Tight Junctions, Ectoplasmic Specializations, Desmosome-Like and Gap Junctions
A unique feature of the seminiferous epithelium is the coexistence of different junctions at the BTB, namely tight junctions, basal ectoplasmic specializations, desmosome-like junctions and gap junctions, and these are all located basally in the seminiferous epithelium. This is in contrast to other epithelia where the apical junctional complex contains a belt of tight junctions, followed by a discrete belt of adherens junctions. The coexistence of different junction types at the BTB represents an important mechanism for protecting tight junction barrier integrity while permitting the transit of spermatocytes across the BTB. Evidence to support crosstalk among these junction types at the BTB is becoming increasingly available.
5.1. Crosstalk between adherens and tight junctions
Based on elegant studies in other in vitro and in vivo systems, the general consensus is that tight junction organization requires prior assembly of adherens junctions and that, once assembled, adherens junctions can influence tight junction function (Rajasekaran et al., 1996). A good example of this is the upregulation of claudin 5 (Taddei et al., 2008) and the control of tight junction permeability (Corada et al., 1999) by vascular endothelial (VE)-cadherin in endothelial cells. While it is not yet known if cadherin can upregulate claudin or other tight junction proteins at the BTB, these findings would provide needed insight on the mechanism behind germ cell transit across the BTB. Interestingly, tight junction and basal ectoplasmic specialization junctional complexes at the BTB were shown to interact via their adaptors. It comes as little surprise that ZO-1 is involved in this crosstalk because it is known to transit between both junction types (Ikenouchi et al., 2007; Itoh et al., 1999). Moreover, tight junctions and basal ectoplasmic specializations are engaged via the association between ZO-1 and α-catenin, and this protein–protein interaction is believed to contribute to tight junction barrier impermeability in basal conditions (Yan and Cheng, 2005). However, when the seminiferous epithelium was being restructured following the administration of adjudin (a chemical entity that specifically affects Sertoli–germ cell adhesion in the testis, leading to germ cell loss and transient infertility), tight junction, and basal ectoplasmic specialization adaptors dissociated from each other, which protected the integrity of tight junction barrier while permitting restructuring of ectoplasmic specializations (Yan and Cheng, 2005).
5.2. Crosstalk between adherens and desmosome-like junctions
Evidence is also emerging to support crosstalk between adherens junctions and desmosomes. For decades, the regulation of actin-based cell junctions (i.e., tight junctions and adherens junctions/ectoplasmic specializations) was presumed to be separate from the regulation of intermediate filament-based junctions (i.e., desmosomes), but it is now well accepted that stable cell adhesion requires cues from both actin and intermediate filaments (Getsios et al., 2004; Huen et al., 2002). Similar to tight junctions in epithelial cells, the assembly of desmosomes requires preexisting adherens junctions. Indeed, in an epithelial cell line null for classic cadherins, overexpression of both E-cadherin and plakoglobin was required for desmosome formation (Lewis et al., 1997), illustrating the significance of these proteins in desmosome organization. Interestingly, plakoglobin was shown to localize to both junction sites in epithelial cells and reported to mediate crosstalk between adherens junctions and desmosomes (Cowin et al., 1986). In the testis, restructuring of basal ectoplasmic specializations and desmosome-like junctions during the movement of preleptotene spermatocytes may be mediated by plakoglobin since this protein was found to localize to the BTB where it was incorporated into desmosome-like junctions together with desmoglein-2 and desmocollin-2 (Lie et al., 2010; Mruk et al., 2008), but additional functional studies would be needed to support this notion.
5.3. Crosstalk between tight and desmosome-like junctions
While considerably less is known regarding crosstalk between tight junctions and desmosomes in other epithelia, a recent study in the intestinal epithelium has demonstrated JAM-C (a tight junction protein) localization at the desmosome and proposed JAM-C involvement in neutrophil transepithelial migration at this site (Zen et al., 2004). This is in agreement with our recent findings which demonstrated a compromise in tight junction permeability barrier function following the knockdown of desmosomal proteins. Simultaneous knockdown of desmoglein-2 and desmocollin-2 by RNAi in cultured Sertoli cells having an assembled barrier—one which mimicked the BTB in vivo—resulted in a marked decrease in tight junction barrier function when assessed by transepithelial electrical resistance (TER) measurements (Lie et al., 2010). This was mediated in part by an acceleration in the endocytosis of CAR, as well as the reduction and mislocalization of ZO-1 and Src, demonstrating junctional interplay between the tight junction, ectoplasmic specialization, and desmosome-like junction at the BTB. This might provide an efficient mechanism for transient junction disassembly to permit the movement of spermatocytes across the BTB during spermatogenesis. Germ cells in transit may release factors to stimulate the proteolytic cleavage of desmosomal cadherins in Sertoli and/or germ cells without affecting other junction complexes. Desmosomal cadherins are specifically targeted by the action of multiple proteases such as MMPs and ADAMs during junction remodeling (Bech-Serra et al., 2006; Klessner et al., 2009). Downregulation of desmosomal cadherins would then increase the rate of CAR turnover at the BTB, facilitating the formation of Sertoli–germ cell CAR–CAR interactions to substitute for the Sertoli cell tight junction barrier, which is transiently compromised likely due to Src-mediated dissociation of occludin–ZO-1 interactions (Lie et al., 2010; Fig. 5.4).
5.4. Interplay of tight, adherens, desmosome-like, and gap junctions
Physical and functional coupling of desmosome-like and gap junctions have been detected at the BTB, which are crucial for mediating crosstalk with tight junctions and basal ectoplasmic specializations during junction assembly and maintenance. Simultaneous knockdown of plakophilin-2 and connexin-43 (a gap junction protein known to bind plakophilin-2) resulted in compelling changes, including the reduction in CAR and N-cadherin cell surface protein levels and the mislocalization of occludin and ZO-1 from the cell surface, which perturbed barrier function in Sertoli cells in vitro (Li et al., 2009; Fig. 5.4). These effects were not produced by the knockdown of either plakophilin-2 or connexin-43 alone. This is in line with another report in which plakophilin-2 was silenced in ventricular myocytes and epicardial cells. In this case, connexin-43 was shown to be affected, resulting in a loss of gap junction function (Oxford et al., 2007). While the central paradigm appears to support functional overlap between tight junctions, ectoplasmic specializations, and desmosome-like and gap junctions, additional studies are needed to elucidate the functional significance of their coexistence at the BTB and the underlying mechanisms of their interactions.
6. Concluding Remarks
In this chapter, we have discussed recent findings relating to tight junctions, ectoplasmic specializations, and desmosome-like and gap junctions in the seminiferous epithelium of the mammalian testis. While we have a good appreciation of the molecular components of these junctions, additional functional studies are needed to better understand their regulation during spermatogenesis, including BTB restructuring and germ cell movement because many questions remain unanswered. What role does the basal ectoplasmic specialization have in the maintenance of BTB integrity during spermatogenesis? How do preleptotene spermatocytes contribute to BTB restructuring? What other tight junction proteins are expressed by preleptotene spermatocytes? How is crosstalk mediated between desmosome-like and gap junctions in the seminiferous epithelium? What is the role of Src within the occludin–ZO-1–FAK multiprotein complex at the BTB? Finally, what specific signaling events are involved in the disassembly of Sertoli–germ cell desmosome-like junctions at stage VII of the seminiferous epithelial cycle, followed immediately by the assembly of apical ectoplasmic specialization at stage VIII? Herein, we have also provided a model of crosstalk between different junctions at the BTB with an emphasis on the role desmosome-like junctions, which should serve as a framework for future studies.
Acknowledgments
The authors’ research is supported by NICHD, NIH (R03HD061401-01 to DDM; R01HD056034, R03HD051512, and U54HD029990 Project 5 to CYC).
References
- Acehan D, Petzold C, Gumper I, Sabatini D, Muller E, Cowin P, et al. Plakoglobin is required for effective intermediate filament anchorage to desmosomes. J Invest Dermatol. 2008;128:2665–2675. doi: 10.1038/jid.2008.141. [DOI] [PubMed] [Google Scholar]
- Aho S. Plakin proteins are coordinately cleaved during apoptosis but preferentially through the action of different caspases. Exp Dermatol. 2004;13:700–707. doi: 10.1111/j.0906-6705.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- Aho S, Li K, Ryoo Y, McGee C, Ishida-Yamamoto A, Uitto J, et al. Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Mol Cell Biol. 2004;24:6410–6418. doi: 10.1128/MCB.24.14.6410-6418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amoudi A, Frangakis AS. Structural studies on desmosomes. Biochem Soc Trans. 2008;36:181–187. doi: 10.1042/BST0360181. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Norlen LP, Dubochet J. Cryo-electron microscopy of vitreous sections of native biological cells and tissues. J Struct Biol. 2004;148:131–135. doi: 10.1016/j.jsb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Amagai M, Karpati S, Klaus-Kovtun V, Udey MC, Stanley JR. Extracellular domain of pemphigus vulgaris antigen (desmoglein 3) mediates weak homophilic adhesion. J Invest Dermatol. 1994;102:402–408. doi: 10.1111/1523-1747.ep12372164. [DOI] [PubMed] [Google Scholar]
- Amagai M, Koch PJ, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J Invest Dermatol. 1996;106:351–355. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- Angst BD, Nilles LA, Green KJ. Desmoplakin II expression is not restricted to stratified epithelia. J Cell Sci. 1990;97:247–257. doi: 10.1242/jcs.97.2.247. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Yamamoto Y, Yamamguchi F, Kitajima Y. Low to high Ca2+-switch causes phosphorylation and association of desmocollin 3 with plakoglobin and desmoglein 3 in cultured keratinocytes. Exp Dermatol. 2009;18:404–408. doi: 10.1111/j.1600-0625.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, et al. Plakophilin 2: a critical scaffold for PKCα that regulates intercellular junction assembly. J Cell Biol. 2008;181:605–613. doi: 10.1083/jcb.200712133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass-Zubek AE, Godsel LM, Delmar M, Green KJ. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol. 2009;21:708–716. doi: 10.1016/j.ceb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Serra JJ, Santiago-Josefat B, Esselens C, Saftig P, Baselga J, Arribas J, et al. Proteomic identification of desmoglein 2 and activated leukocyte cell adhesion molecule as substrates of ADAM17 and ADAM10 by difference gel electrophoresis. Mol Cell Biol. 2006;26:5086–5095. doi: 10.1128/MCB.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, et al. Desmosome signaling. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Murrym J, Chappuis-Flament S, Wong EW, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Boitani C, Ritzen EM, Parvinen M. Inhibition of rat Sertoli cell aromatase by factor(s) secreted specifically at spermatogenic stages VII and VIII. Mol Cell Endocrinol. 1981;23:11–22. doi: 10.1016/0303-7207(81)90113-1. [DOI] [PubMed] [Google Scholar]
- Bonne S, van Hengel J, Nollet F, Kools P, van Roy F. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J Cell Biol. 1999;112:2265–2276. doi: 10.1242/jcs.112.14.2265. [DOI] [PubMed] [Google Scholar]
- Bonne S, Gilbert B, Hatzfeld M, Chen X, Green KJ, Van Roy F. Defining desmosomal plakophilin-2 interactions. J Cell Biol. 2003;161:403–416. doi: 10.1083/jcb.200303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, et al. Plakophilin 1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell–cell borders. J Cell Sci. 2000;114:727–738. doi: 10.1242/jcs.114.4.727. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan D, Hu Y, Kljuic A, Choi YS, Joubeh S, Bashkin M, et al. Differential structural properties and expression patterns suggest functional significance for multiple mouse desmoglein 1 isoforms. Differentiation. 2004;72:434–449. doi: 10.1111/j.1432-0436.2004.07208009.x. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, et al. Degradation of cornodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ. Protein binding and functional characterization of plakophilin 2. J Biol Chem. 2002;277:10512–10522. doi: 10.1074/jbc.M108765200. [DOI] [PubMed] [Google Scholar]
- Chen J, Den Z, Koch PJ. Loss of desmocollin 3 in mice leads to epidermal blistering. J Cell Sci. 2008;121:2844–2849. doi: 10.1242/jcs.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis. J Biol Chem. 2007;282:13804–13812. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- Chidgey M, Brakebusch C, Gustafsson E, Cruchley A, Hail C, Kirk S, et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J Cell Biol. 2001;155:821–832. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell–cell adhesion. J Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Sehgal R, McCrea P, Gumbiner B. A cadherin-like protein in eggs and cleaving embryos of Xenopus laevis is expressed in oocytes in response to progesterone. J Cell Biol. 1990;110:1575–1582. doi: 10.1083/jcb.110.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Gross JC, Weis WI. Interactions of plakoglobin and β-catenin with desmosomal cadherins: basis of selective exclusion of α- and β-catenin from desmosomes. J Biol Chem. 2009;284:31776–31788. doi: 10.1074/jbc.M109.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development. 1994;120:1–11. doi: 10.1242/dev.120.1.1. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Desmosomal interactome in keratinocytes: a systems biology approach leading to an understanding of the pathogenesis of skin disease. Cell Mol Life Sci. 2009;66:3517–3533. doi: 10.1007/s00018-009-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo N, Lanza M, De Rosa A, Cammarota M, La Gatta A, Gombos F, et al. The most widespread desmosomal cadherin, desmoglein 2, is a novel target of caspase 3-mediated apoptotic machinery. J Cell Biochem. 2008;103:598–606. doi: 10.1002/jcb.21431. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Lanza A, Prime SS. Induction of hyper-adhesion attenuates autoimmune-induced keratinocyte cell–cell detachment and processing of adhesion molecules via mechanisms that involve PKC. Exp Cell Res. 2010;316:580–592. doi: 10.1016/j.yexcr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- de Bruin A, Caldelari R, Williamson L, Suter MM, Hunziker T, Wyder M, et al. Plakoglobin-dependent disruption of the desmosomal plaque in pemphigus vulgaris. Exp Dermatol. 2007;16:468–475. doi: 10.1111/j.1600-0625.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Delva E, Kowalczyk A. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- Dusek RL, Getsios S, Chen F, Park JK, Amargo EV, Cryns VL, et al. The differentiation-dependent desmosomal cadherin desmoglein 1 is a novel caspase-3 target that regulates apoptosis in keratinocytes. J Biol Chem. 2006;281:3614–3624. doi: 10.1074/jbc.M508258200. [DOI] [PubMed] [Google Scholar]
- Dusek R, Godsel LM, Chen F, Strohecker AM, Getsios S, Harmon R, et al. Plakoglobin deficiency protects keratinocytes from apoptosis. J Invest Dermatol. 2007;127:792–801. doi: 10.1038/sj.jid.5700615. [DOI] [PubMed] [Google Scholar]
- Emery DJ, Diaz LA, Fairley JA, Lopez A, Taylor AF, Giudice GJ. Pemphigus foliaceus and pemphigus vulgaris auto-antibodies react with the extracellular domain of desmoglein-1. J Invest Dermatol. 1995;104:323–328. doi: 10.1111/1523-1747.ep12665364. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Parllari H, et al. Introducing intermediate filaments: from discovery to disease. J Clin Invest. 2009;119:1763–1771. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersoy-Evans S, Erkin G, Fassihi H, Chan I, Paller AS, Surucu S, et al. Ectodermal dysplasia—skin fragility syndrome resulting from a new homozygous mutation, 888delC, in the desmosomal protein plakophilin 1. J Am Acad Dermatol. 2006;55:157–161. doi: 10.1016/j.jaad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Eshkind L, Tian Q, Schmidt A, Franke WW, Windoffer R, Leube RE. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur J Cell Biol. 2002;81:592–598. doi: 10.1078/0171-9335-00278. [DOI] [PubMed] [Google Scholar]
- Fritz IB, Tung PS, Ailenberg M. Proteases and antiproteases in the seminiferous tubule. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Cache River Press; Clearwater: 1993. pp. 217–235. [Google Scholar]
- Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, et al. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 1998;143:2009–2022. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, et al. Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Garrod D, Kimura TE. Hyper-adhesion: a new concept in cell–cell adhesion. Biochem Soc Trans. 2008;36:195–201. doi: 10.1042/BST0360195. [DOI] [PubMed] [Google Scholar]
- Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, et al. Molecular reorganization of Cx43, ZO-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci. 2008;121:4069–4078. doi: 10.1242/jcs.033373. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, DeSimone D, Geiger B. Expression of a novel cadherin (EP-cadherin) in unfertilized eggs and early Xenopus embryos. Development. 1991;111:315–325. doi: 10.1242/dev.111.2.315. [DOI] [PubMed] [Google Scholar]
- Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LP, Huen AC, et al. Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J Cell Biol. 2005;171:1045–1059. doi: 10.1083/jcb.200510038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens S, Janssens B, Bonne S, De Rycke R, Braet F, van Hengel J, et al. A unique and specific interaction between αT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci. 2007;120:2126–2136. doi: 10.1242/jcs.004713. [DOI] [PubMed] [Google Scholar]
- Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, et al. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol. 2004;167:149–160. doi: 10.1083/jcb.200402096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Ham C, Levkau B, Raines EW, Herren B. ADAM15 is an adherens junction molecule whose surface expression can be driven by VE-cadherin. Exp Cell Res. 2002;279:239–247. doi: 10.1006/excr.2002.5606. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M. Plakophilins: multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Nachtsheim C. Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci. 1996;109:2767–2778. doi: 10.1242/jcs.109.11.2767. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Haffner C, Schulze K, Vinzens U. The function of plakophilin 1 in desmosome assembly and actin filament organization. J Cell Biol. 2000;149:209–222. doi: 10.1083/jcb.149.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Green KJ, Sauter H. Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J Cell Sci. 2003;116:1219–1233. doi: 10.1242/jcs.00275. [DOI] [PubMed] [Google Scholar]
- He W, Cowin P, Stokes DL. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Bar H, Kreplak L, Strelkov S, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772–1783. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heupel W, Zillikens D, Drenckhahn D, Waschke J. Pemphigus vulgaris IgG directly inhibit desmoglein 3-mediated transinteraction. J Immunol. 2008;181:1825. doi: 10.4049/jimmunol.181.3.1825. [DOI] [PubMed] [Google Scholar]
- Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2006;79:1081–1088. doi: 10.1086/509044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann I, Mertens C, Brettel M, Nimmrich V, Schnolzer M. Interaction of plakophilins with desmoplakin and intermediate filmament proteins: an in vitro analysis. J Cell Sci. 2000;113:2471–2483. doi: 10.1242/jcs.113.13.2471. [DOI] [PubMed] [Google Scholar]
- Hofmann I, Casella M, Schnolzer M, Schlechter T, Spring H, Franke WW. Identification of the junctional plaque protein plakophilin 3 in cytoplasmic particles containing RNA-binding proteins and the recruitment of plakophilins 1 and 3 to stress granules. Mol Biol Cell. 2006;17:1388–1398. doi: 10.1091/mbc.E05-08-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann I, Kuhn C, Franke WW. Protein p0071, a major plaque protein of non-desmosomal adhering junctions, is a selective cell-type marker. Cell Tissue Res. 2008;334:381–399. doi: 10.1007/s00441-008-0725-2. [DOI] [PubMed] [Google Scholar]
- Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW. Protein p0071—an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J Cell Sci. 2009;122:21–24. doi: 10.1242/jcs.043927. [DOI] [PubMed] [Google Scholar]
- Holthofer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. Int Rev Cytol. 2007;264:65–163. doi: 10.1016/S0074-7696(07)64003-0. [DOI] [PubMed] [Google Scholar]
- Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, et al. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila AJ, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz R. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Damsky C, Yamamoto T. Focal adhesion kinase: at the cross-roads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and α-catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, Liem RKH. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol. 2004;5:542–553. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- Kapprell HP, Owaribe K, Franke WW. Identification of a basic protein of Mr 75,000 as an accessory desmosomal plaque protein in stratified and complex epithelia. J Cell Biol. 1988;106:1679–1691. doi: 10.1083/jcb.106.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- Kim S, Kwon YD, Lee IJ, Lee IJ, Chang SN, Lee TG. cDNA cloning of the 210-kDa paraneoplastic pemphigus antigen reveals that envoplakin is a component of the antigen complex. J Invest Dermatol. 1997;109:365–369. doi: 10.1111/1523-1747.ep12336235. [DOI] [PubMed] [Google Scholar]
- Kimura TE, Merritt AJ, Garrod DR. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J Invest Dermatol. 2007;127:775–781. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Klessner JL, Desai BV, Amargo EV, Getsios S, Green KJ. EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol Biol Cell. 2009;20:328–337. doi: 10.1091/mbc.E08-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O’Shaughnessy R, Mahoney MG, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, et al. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C α/β inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64:5693–5701. doi: 10.1158/0008-5472.CAN-03-3511. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Borgwardt JE, Green KJ. Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin-plakoglobin complexes. J Invest Dermatol. 1996;107:293–300. doi: 10.1111/1523-1747.ep12363000. [DOI] [PubMed] [Google Scholar]
- Kowlczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, et al. The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. J Biol Chem. 1999;274:18145–18148. doi: 10.1074/jbc.274.26.18145. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Leckband D, Prakasam A. Mechanism and dynamics of cadherin adhesion. Annu Rev Biomed Eng. 2006;8:259–287. doi: 10.1146/annurev.bioeng.8.061505.095753. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Legan PK, Yue KKM, Chidgey MAJ, Holton JL, Wilkinson RW, Garrod DR. The bovine desmocollin family: a new gene and expression patterns reflecting epithelial cell proliferation and differentiation. J Cell Biol. 1994;126:507–518. doi: 10.1083/jcb.126.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CL, Green KJ, Liem RKH. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 2002;12:37–45. doi: 10.1016/s0962-8924(01)02180-8. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kinter C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Wahl JK, III, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem Sci. 2009;34:366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longin J, Guillaumot P, Chauvin MA, Morera AM, Le Magueresse-Battistoni B. MT1-MMP in rat testicular development and the control of Sertoli cell proMMP-2 activation. J Cell Sci. 2001;114:2125–2134. doi: 10.1242/jcs.114.11.2125. [DOI] [PubMed] [Google Scholar]
- Lorch JH, Klessner J, Park JK, Getsios S, Wu YL, Stack MS, et al. Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. J Biol Chem. 2004;279:37191–37200. doi: 10.1074/jbc.M405123200. [DOI] [PubMed] [Google Scholar]
- Lorimer JE, Hall LS, Clarke JP, Collins JE, Fleming TP, Garrod DR. Cloning, sequence analysis and expression pattern of mouse desmocollin 2 (DSC2), a cadherin-like adhesion molecule. Mol Membr Biol. 1994;11:229–236. doi: 10.3109/09687689409160432. [DOI] [PubMed] [Google Scholar]
- Maatta A, DiColandrea T, Groot K, Watt FM. Gene targeting of envoplakin, a cytoskeletal linker protein and precursor of the epidermal cornified envelope. Mol Cell Biol. 2001;21:7047–7053. doi: 10.1128/MCB.21.20.7047-7053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCalman CD, Getsios S, Farookhi R, Blaschuk OW. Estrogens potentiate the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology. 1997;138:41–48. doi: 10.1210/endo.138.1.4831. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, et al. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, et al. A CBP binding transcriptional repressor produced by the PS1/ε-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- McCusker CD, Alfandari D. Life after proteolysis: exploring the signaling capabilities of classical cadherin cleavage fragments. Commun Integr Biol. 2009;2:155–157. doi: 10.4161/cib.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CD, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2008;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley DM, Posalaky Z, Provaznik M, Russell LD. Gap junctions between Sertoli and germ cells of rat seminiferous tubules. Tissue Cell. 1979;11:741–754. doi: 10.1016/0040-8166(79)90028-4. [DOI] [PubMed] [Google Scholar]
- McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, et al. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet. 1997;17:240–244. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2421. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Hofmann I, Wang Z, Teichmann M, Chong SS, Schnolzer M, et al. Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci USA. 2001;98:7795–7800. doi: 10.1073/pnas.141219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Castano J, Raurell I, Franci C, Dunach M, et al. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates β-catenin-mediated transcription. Mol Cell Biol. 2003;23:7391–7402. doi: 10.1128/MCB.23.20.7391-7402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamori H, Takino T, Kobayashi Y, Tokai H, Itoh Y, Seiki M, et al. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem. 2001;276:28204–28211. doi: 10.1074/jbc.M103083200. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, et al. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cell testes. Am J Physiol Cell Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Morrow CMK, Tyagi G, Simon L, Carnes K, Murphy KM, Cooke PS, et al. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor Ets-variant 5 and contributes to blood-testis barrier function. Biol Reprod. 2009;81:871–879. doi: 10.1095/biolreprod.109.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Cell–cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Tsukita S, Takeichi M. Transmembrane control of cadherin-mediated cell–cell adhesion. Semin Cell Biol. 1993;4:175–181. doi: 10.1006/scel.1993.1021. [DOI] [PubMed] [Google Scholar]
- Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava P, Laukoetter MG, Hopkins AM, Laur O, Gerner-Smidt K, Green KJ, et al. Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium. Mol Biol Cell. 2007;18:4565–4578. doi: 10.1091/mbc.E07-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls PK, Harrison CA, Gilchrist RB, Farnworth PG, Stanton PG. Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology. 2009;150:2481–2490. doi: 10.1210/en.2008-1048. [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JR, Common J, Purkis PE, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- Nuber UA, Schafer S, Schmidt A, Koch PJ, Franke WW. The widespread human desmocollin Dsc2 and tissue-specific patterns of synthesis of various desmocollin subtypes. Eur J Cell Biol. 1995;66:69–74. [PubMed] [Google Scholar]
- Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 γ2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66:5251–5257. doi: 10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009;34:453–463. doi: 10.1016/j.tibs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Owen GR, Acehan D, Derr KD, Rice WJ, Stokes DL. Cryoelectron tomography of isolated desmosomes. Biochem Soc Trans. 2008;36:173–179. doi: 10.1042/BST0360173. [DOI] [PubMed] [Google Scholar]
- Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin-43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies NE, Grunwald GB. Purification and characterization of NCAD90, a soluble endogenous form of N-cadherin, which is generated by proteolysis during retinal development and retains adhesive and neurite-promoting function. J Neurosci Res. 1993;36:33–45. doi: 10.1002/jnr.490360105. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pieperhoff S, Schumacher H, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. V The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol. 2008;87:399–411. doi: 10.1016/j.ejcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- Posy S, Shapiro L, Honig B. Sequence and structural determinants of strand swapping in cadherin domains: do all cadherins binding through the same adhesive interface? J Mol Biol. 2008;378:954–968. doi: 10.1016/j.jmb.2008.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Saftig P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Rickman L, Simrak D, Stevens HP, Hunt DM, King IA, Bryant SP, et al. N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma. Hum Mol Genet. 1999;8:971–976. doi: 10.1093/hmg/8.6.971. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Hajibagheri MAN, Simon M, Dooley TP, Watt FM. Envoplakin, a novel precursor of the cornified envelope that has homology to desmoplakin. J Cell Biol. 1996;134:715–729. doi: 10.1083/jcb.134.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Hajibagheri MAN, Parry DAD, Watt FM. Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J Cell Biol. 1997;139:1835–1849. doi: 10.1083/jcb.139.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, et al. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977a;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on rat Sertoli ectoplasmic (“junctional”) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977b;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Russell LD, Tallon-Doran M, Weber JE, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: III. A study of specific cellular relationships. Am J Anat. 1983;167:181–192. doi: 10.1002/aja.1001670204. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Josefat B, Esselens C, Bech-Serra JJ, Arribas J. Post-transcriptional up-regulation of ADAM17 upon epidermal growth factor receptor activation and in breast tumors. J Biol Chem. 2007;282:8325–8331. doi: 10.1074/jbc.M608826200. [DOI] [PubMed] [Google Scholar]
- Schafer S, Koch PJ, Franke WW. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994;211:391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Jager S. Plakophilins—hard work in the desmosome, recreation in the nucleus? Eur J Cell Biol. 2005;84:189–204. doi: 10.1016/j.ejcb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Pratzel S, Rode M, Rackwitz H, Franke WW. Plakophilin 3—a novel cell-type-specific desmosomal plaque protein. Differentiation. 1999;64:291–306. doi: 10.1046/j.1432-0436.1999.6450291.x. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Simon M, Jonca N, Guerrin M, Haftek M, Bernard D, Caubet C, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001;276:20292–20299. doi: 10.1074/jbc.M100201200. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklyarova T, Bonne S, D’hooge P, Denecker G, Goossens S, De Rycke R, et al. Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol. 2008;128:1375–1385. doi: 10.1038/sj.jid.5701189. [DOI] [PubMed] [Google Scholar]
- Solanas G, Miravet S, Casagolda D, Castano J, Raurell I, Corrionero A, et al. β-Catenin and plakoglobin N- and C-tails determine ligand specificity. J Biol Chem. 2004;279:49849–49886. doi: 10.1074/jbc.M408685200. [DOI] [PubMed] [Google Scholar]
- Sonnenerg A, Liem RKH. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- South AP, Wan H, Stone MG, Dopping-Hepenstal PJC, Purkis PE, Marshall JF, et al. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J Cell Sci. 2003;116:3303–3314. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- Spindler V, Heupel W, Efthymidadis A, Schmidt E, Eming ER, Rank C, et al. Desmocollin 3-mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. J Biol Chem. 2009;284:30556. doi: 10.1074/jbc.M109.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. Desmosomes from a structural perspective. Curr Opin Cell Biol. 2007;19:565–571. doi: 10.1016/j.ceb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Troyanovsky RB, Sokolov E, Troyanovsky SM. Adhesive and lateral E-cadherin dimers are mediated by the same interface. Mol Cell Biol. 2003;23:7965–7972. doi: 10.1128/MCB.23.22.7965-7972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda K, Ota T, Aoki M, Yamada T, Nagai T, Nakagawa T, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J Immunol. 2003;170:2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- Utton MA, Eickholt B, Howell FV, Wallis J, Doherty P. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J Neurochem. 2001;76:1421–1430. doi: 10.1046/j.1471-4159.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel APJ, de Vries-Smits AMM, van Weeren PC, Dijkers PF, de Bruyn KMT, Riedl JA, et al. Binding of protein kinase B to the plakin family member periplakin. J Cell Sci. 2002;115:3957–3966. doi: 10.1242/jcs.00069. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman J. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Advances in Experimental Medicine and Biology Landes Bioscience; Austin, TX: 2008. pp. 186–211. [DOI] [PubMed] [Google Scholar]
- Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The α isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ, et al. Raf1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor Slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- Wang Q, He J, Meng L, Liu Y, Pu H, Ji J. A proteomics analysis of rat liver membrane skeletons: the investigation of actin-and cytokeratin-based protein components. J Proteome Res. 2010;9:22–29. doi: 10.1021/pr900102n. [DOI] [PubMed] [Google Scholar]
- Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiske J, Schoneberg T, Schroder W, Hatzfeld M, Tauber R, Huber O. The fate of desmosomal proteins in apoptotic cells. J Biol Chem. 2001;276:41175–41181. doi: 10.1074/jbc.M105769200. [DOI] [PubMed] [Google Scholar]
- Whittock NV, Bower C. Genetic evidence for a novel human desmosomal cadherin, desmoglein 4. J Invest Dermatol. 2003;120:523–530. doi: 10.1046/j.1523-1747.2003.12113.x. [DOI] [PubMed] [Google Scholar]
- Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, et al. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Krause-Gruszczynska M, Birkenmeier O, Ostareck-Lederer A, Huttelmaier S, Hatzfeld M. Plakophilin 1 stimulates translation by promoting eIF4A1 activity. J Cell Biol. 2010;188:463–471. doi: 10.1083/jcb.200908135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Getsios S, Caldelari R, Godsel LM, Kowalczyk AP, Muller EJ, et al. Mechanisms of plakoglobin-dependent adhesion. J Biol Chem. 2005a;280:40355–40363. doi: 10.1074/jbc.M506692200. [DOI] [PubMed] [Google Scholar]
- Yin T, Getsios S, Caldelari R, Kowalczyk AP, Muller EJ, Jones JCR, et al. Plakoglobin suppresses keratinocyte motility through both cell–cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2005b;102:5420–5425. doi: 10.1073/pnas.0501676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]