Abstract
T cell development in the thymus is a highly regulated process that critically depends upon productive signaling via the pre T cell Receptor (preTCR) at the β-selection stage, and via the TCR for selection from the CD4+ CD8+ double positive stage to the CD4 or CD8 single positive stage. ShcA is an adapter protein expressed in thymocytes, and is required for productive signaling through the preTCR, with impaired signaling via ShcA leading to a developmental block at the β-selection checkpoint. However, the role of ShcA in subsequent stages of T cell development has not been addressed. Here, we generated transgenic mice (CD4-Cre/ShcFFF mice) that specifically express a phosphorylation-defective dominant negative ShcA mutant (ShcFFF) in late T cell development. Thymocytes in CD4-Cre/ShcFFF mice progressed normally through the β-selection checkpoint, but displayed a significant reduction in the numbers of single positive CD4+ and CD8+ thymocytes. Furthermore, CD4-Cre/ShcFFF mice, when bred with transgenic TCR mouse strains, had impaired signaling through the transgenic TCRs. Consistent with defective progression to the single positive stage, the CD4-Cre/ShcFFF mice also had significant peripheral lymphopenia. Moreover, these CD4-Cre/ShcFFF mice develop attenuated disease in CD4+ T cell dependent experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS). Collectively, these data identify an important role for the adapter protein ShcA in later stages of thymic T cell development and in peripheral T cell-dependent events.
Introduction
T cell development in the thymus is a highly regulated process that involves the coordinated expression of cell surface receptors and distinguishable selection steps. Development proceeds from the most immature CD4−CD8− double-negative (DN) stage (which can further be subdivided into DN1 through DN4), to the CD4+CD8+ double positive (DP) stage, and then to the CD4 or CD8 single positive (SP) stage (1, 2). Expression and productive signaling through the preTCR and TCR are essential for proper T cell development and progression through the two sequential developmental checkpoints (1, 3–6). Signaling via the preTCR allows thymocytes to progress through the first developmental checkpoint, the β-selection checkpoint, and undergo further differentiation to the DP stages of development (3). The quality of TCR signaling at the DP stage controls progression through the next checkpoints that involve positive and negative selection of DP thymocytes leading to single positive CD4 or CD8 thymocytes (1, 7, 8). The duration and strength of TCR signaling determines the fate of each thymocyte; thymocytes that receive intermediate TCR activation are generally positively selected, while thymocytes that receive strong TCR activation or very weak/no TCR activation undergo apoptosis (1). Furthermore, stronger and more persistent signaling via MHC class II-restricted TCRs promotes the CD4-lineage commitment, while intermittent signaling via MHC class I-restricted TCRs is linked to the development of the CD8-lineage cells (7, 8).
T cell development and function are impaired by disruption of genes encoding components of the preTCR/TCR as well as downstream signaling molecules, including adapter proteins (1, 9). ShcA is a ubiquitously expressed adapter protein that has an N-terminal phosphotyrosine-binding (PTB) domain, a central proline-rich (CH1) domain and a C-terminal Src-homology 2 (SH2) domain (10, 11). ShcA is essential during embryonic development as deletion of the Shc1 gene (encoding ShcA) leads to lethality at day e11.5 due to cardiac defects (12, 13). During T cell development, ShcA is essential for progression through the β-selection checkpoint (14–17). In developing thymocytes, ShcA is phosphorylated on three conserved tyrosine residues within the CH1 domain downstream of the preTCR, and links receptor activation to the Ras-MAPK pathway (14–16). In fact, ShcA is required for 70% of ERK1/2 phosphorylation in DN3 thymocytes, with ERK signaling being essential for further thymocyte development (14, 15). Additionally, ShcA is required for productive signaling through the preTCR; thymocytes either lacking the expression of ShcA (Lck-Cre/Shc1fl/fl) or expressing the phosphorylation-defective ShcA transgene (Lck-Cre/ShcFFF) from the DN2/DN3 stage, have a developmental block at the DN3 stage of development (14, 15). Furthermore, in mature T cells, ShcA is phosphorylated after CD3 stimulation, and in vitro studies have shown that ShcA affects functions such as IL2 production (18–20).
While previous studies have highlighted the requirement for ShcA in the DN to DP transition, (14–17), the nearly complete block in development at the β-selection checkpoint in the Lck-Cre/ShcFFF transgenic mouse line has precluded us from using this mouse line to study the role of ShcA in subsequent developmental stages. In this report, we used CD4-Cre-mediated expression of the phosphorylation-defective ShcFFF transgene, in which the crucial ShcA tyrosine residues within the CH1 domain were mutated to phenylalanine (15). The CD4-Cre transgenic mouse line expresses the Cre recombinase from the late DN4/early DP stage, allowing normal progression at the β-selection checkpoint (21). We now find that ShcA function is important during DP to SP transition. CD4-Cre/ShcFFF mice have reduced thymic cellularity with fewer SP thymocytes, and display impaired positive selection in mice expressing either endogenous or transgenic TCRs. CD4-Cre/ShcFFF mice also have alterations in the peripheral T cell compartment, with an overall lymphopenia and skewing of the CD4:CD8 T cell ratio. Peripheral lymphopenia leads to a functional defect in immunity, as CD4-Cre/ShcFFF mice develop attenuated disease in the CD4 T cell driven experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) and display impaired expansion of TH1 and TH17 T cells under ex vivo skewing conditions.
Materials and Methods
Mice
All mice used were on the C57BL/6J background unless otherwise noted. C57BL/6J wild-type mice, TCRα deficient mice, the Rosa26STOP-EYFP reporter mice, and the CD4-Cre, OT-I, OT-II, DO11.10, and H-Y transgenic mice were purchased from the Jackson Laboratories or Taconic (6, 21–25). The inducible ShcFFF transgenic mouse line has been previously described (14, 15). The LckF505 transgenic mouse line and the Rag2p-eGFP mouse line were kindly provided by Dr. Roger Perlmutter’s laboratory and Dr. Pamela Fink’s laboratory, respectively, and have been previously described (26–28). PCR was performed on tail DNA or sorted cell populations. Mice were bred and housed in a specific pathogen-free environment, and all animal experiments were approved by the University of Virginia Animal Care and Use Committee.
Flow Cytometry
Thymocytes, splenocytes, and lymphocytes were isolated from 4- to 6-week old mice (littermates) for analysis of T cell development. DP and DN compartments were analyzed by staining thymocytes with antibodies specific for CD4, CD8, CD3, Thy1.2, CD25, CD44, CD28, TCRβ, CD5, CD24, and CD69 as well as lineage markers (CD11b, CD11c, B220, Gr1, Ly6G, and Ter119) as described previously (17). Expression of the transgenic T cell receptor in the different transgenic lines was analyzed via staining for KJ1.26, Vβ5.1/2, T3.70, and Vα3 for the DO11.10, OT-II, H-Y, and OT-I transgenic mice, respectively. Absolute numbers were determined via enumeration with the hemocytometer, followed by flow cytometry analysis or by inclusion of reference counting beads (Sephrotec). Viability and apoptosis were evaluated by staining with Annexin V and 7AAD (Invitrogen), according to manufacturer’s instructions. Splenocytes and lymphocytes were stained with antibodies specific for CD3, CD4, CD8, FoxP3, CD62L, CD44, and CCR7. All antibodies used were obtained from eBioscience unless otherwise noted. FACSCanto (BD Biosciences) was used for flow cytometry and results were analyzed by the FlowJo software (TreeStar Inc.).
Migration
Migration of thymocytes was performed using the Transwell system. Briefly, thymocytes were isolated from 4–6 week old mice and resuspended in RPMI with 0.5 % BSA (Sigma) at 107 cells/ml. One million thymocytes (100 µl) were placed in the upper chamber of a Transwell with the 5µM pore size (Costar). Migration medium (RPMI with 0.5 % fatty-acid free BSA) or varying concentrations of the indicated cytokine in 600 µl of migration media were placed in the bottom well. An additional well contained 106 thymocytes (in 500 µl of media) and represented the ‘input’ for determining of the fraction of migrating cells. Cells were allowed to migrate for 3 hours in the 37°C incubator. After migration, the cells in the bottom chamber as well as the input well were collected, the bottom chambers were rinsed with ice-cold PBS and the cells were stained with cell surface markers and analyzed via flow cytometry with inclusion of reference counting beads (Sephrotec) and the percent migration was determined.
Experimental Autoimmune Encephalomyelitis
EAE immunization was performed as previously described (29). For optimal EAE induction, 10 week-old female mice were immunized subcutaneously into the lower back with 100 µg MOG35–55 peptide (CS Bio Co), emulsified in equal volume of complete Freund’s adjuvant (Sigma) supplemented with heat killed M. tuberculosis (clone H37RA) (Difco) for a total of 400 µg H37RA per mouse. Mice received 200 ng of pertussis toxin (List Biologicals) intraperitoneally on day 0 and 1 after immunization. For sub-optimal EAE induction, mice were immunized subcutaneously with 75 µg MOG(35–55) in CFA supplemented with H37RA but received only a single intraperitoneal injection of 200 ng of pertussis toxin on day 0. The mice were weighed and scored daily on a 5-point scale: 0-no clinical signs; 1-paralyzed tail; 2-mild hindlimb paresis; 3-severe hindlimb paresis; 4-hindlimb paralysis; 5 quadriplegia/moribund. Brain and spinal cord leukocytes were isolated on day 28 post-injection using Percoll (GE Healthcare) gradient centrifugation. Isolated cells were identified via staining with antibodies specific for CD4, CD45, CD11b, and B220 followed by flow cytometry.
TH17 and TH1 in vitro differentiation
TH17 and TH1 skewing in vitro was performed by using total lymphocytes or selecting CD4+ T cells from spleens and lymph nodes of 4-week old mice (Miltenyi Biotec). The cells were skewed towards the TH17 lineage for 4 days on 1µg/ml anti-CD3 and 2µg/ml anti-CD28 coated plates along with 0.3ng/ml TGF-β1 (R&D Systems), 20ng/ml IL-6 (R&D Systems), 10ng/ml IL-23 (eBioscience), 10µg/ml anti-IL4 (eBioscience), and 10µg/ml anti-IFNγ (eBioscience) in IMDM supplemented with 10% FBS, 50µM β-Mercaptoethanol, 2-mM L-glutamine, non-essential amino acids, 1 mM sodium pyruvate, and 10 mM Hepes. After 4 days, cells were collected for analysis. Cells analyzed by intracellular cytokine staining were stimulated with 50 ng/ml PMA and 1 µM Ionomycin along with GolgiStop (BD Pharmingen) for 5 hours prior to staining. Intracellular staining for IL-17A (BD Pharmingen) and IFNγ (eBioscience) was performed by fixing the cells in 4% paraformaldehyde followed by permeabilization with 0.1% Saponin. TH1 skewing was performed by culturing total lymphocytes or CD4+ cells on 1µg/ml anti-CD3 and 2 µg/ml anti-CD28 coated plates along with 100U/ml IL-2 (Peprotech), 10ng/ml IL-12 (Ebiosciences), and 10 µg/ml anti-IL4 (eBioscience) and analysis was performed on day 7 as described above for TH17 cells.
T cell stimulation and proliferation
For CD3/CD28 stimulation, 80,000 purified CD4+ T cells (purified using a MACS kit, Miltenyi Biotec) were stimulated with anti-CD3/anti-CD28 beads (Dynabeads, Life Technologies) according to the manufacturer’s protocol for indicated times. T cells were stained with 5 µM CFSE (Molecular Probes) prior to stimulation and proliferation was assessed via CFSE dilution. Stimulations were also performed by culturing cells with 50 ng/ml PMA (phorbol 12-myristate 13-acetate; Calbiochem) with 500ng/ml ionomycin (Calbiochem), 5µg anti-CD3 (BD Pharmingen), or with 5µg anti-CD3 (BD Pharmingen) and 2µg anti-CD28 (BD Pharmingen). All stimulations were performed in 200 µl RPMI 1640 medium (supplemented with 10 % FBS, 50 µM β-Mercaptoethanol, 2 mM L-glutamine, and 1 % pennicillin/streptomycin) in round bottom 96-well plates and cultured at 5% CO2 at 37°C.
Immunohistochemistry and Immunofluorescence
For immunohistochemistry, thymi were fixed by immersion in 10 % neutral buffered formalin (Fisher) and embedded in paraffin blocks. For histological analysis of the spinal cord, mice were perfused with 4 % paraformaldehyde in phosphate buffered saline (PBS) and the sacral, lumbar, thoracic, and cervical parts of the spinal cord were fixed in 4% paraformaldehyde in PBS and embedded in paraffin blocks. Sections were processed for immunohistochemistry using standard techniques. Images were acquired on an Olympus SZX12 low magnification microscope equipped with an Olympus DP70 digital camera. Quantification of cell number was enumerated using the NIH Image-J software.
Immunoblotting and Immunoprecipitation
Immunoprecipitation was performed from thymocytes or splenocytes lysed using RIPA buffer containing protease inhibitors (Calbiochem). Lysates were incubated with anti-Flag agarose beads (Sigma) or anti-ShcA antibody (BD) followed by protein A/G agarose beads (Santa Cruz). Beads were washed and eluted by boiling in SDS sample buffer containing β-ME and analyzed via SDS-PAGE and immunoblotting for ShcA (BD) or anti-tyrosine.
Ex vivo survival assays
Thymocytes isolated from DO11.10 mice were incubated with the A20 B cell line along with OVA peptide (comprised of amino acids 323–339). After 8 and 20 hours, thymocytes were stained with CD4, CD8, Annexin V, and 7AAD, according to manufacturer’s instructions.
Quantitative PCR
Total RNA was extracted from thymocytes and selected CD4+ T cells using a QIAshredder and RNeasy kit (Qiagen) followed by reverse transcription using the SuperScript III (Invitrogen) kit. Quantitative PCR was performed using the TaqMan Gene Expression assays (Applied Biosystems) on a StepOnePlus system (Applied Biosystems). TaqMan gene expression probes were used for gene analysis of mouse IL-2 and HPRT. Each sample was performed in duplicate, target transcripts were normalized to HPRT mRNA as an internal control gene, and the relative expression of each target gene was calculated using the comparative cycling method with StepOne v2.1 software (Applied Biosystems).
Statistical analysis
Statistical comparisons were performed using student’s two-tailed t-test or a two-way ANOVA (clinical scores for EAE) using GraphPad Prism version 4.0. Results with a p-value lower than 0.05 were considered significant.
Results
CD4-Cre/ShcFFF mice have defects in T cell development
We have previously used Lck-Cre/ShcFFF and Lck-Cre/Shcfl/fl transgenic mice to demonstrate an absolute requirement for ShcA in β-selection(14–17). To determine whether ShcA phosphorylation is required in later stages of T cell development, we crossed our dominant negative ShcFFF transgenic mouse line to the CD4-Cre transgenic mouse line (21). The ShcFFF transgenic construct allows specific expression of a phosphorylation defective ShcFFF transgene downstream of the ubiquitous EF-1α promoter; however, to keep basal expression of the ShcFFF transgene silent and to allow Cre-mediated transgene expression, a floxed STOP cassette has been inserted between the promoter and the ShcFFF coding sequence (14, 15). To confirm the fidelity of the CD4-Cre transgenic mouse line in our hands, we first crossed the CD4-Cre mouse line to the Rosa26STOP-EYFP and analyzed eYFP expression in the different thymic subsets. We found that eYFP is expressed in late DN4, DP, CD4 SP, and CD8 SP but not in DN3 thymocytes and importantly, the CD4-Cre affects both the CD4 and CD8-lineages (Supplemental Fig. 1B) (30). Therefore, we expected that the CD4-Cre/ShcFFF mice would allow us to bypass the requirement for ShcA phosphorylation during β-selection, yet facilitate investigation of ShcA function in late T cell development.
We first observed that CD4-Cre/ShcFFF mice have altered T cell development with a decrease in overall thymic cellularity and a striking reduction in the percentage of the CD4 SP compartment with no change in the percentage of the CD8 SP compartment (Fig. 1A, Supplemental Fig. 1E). CD4-Cre/ShcFFF mice showed a reduction in the absolute number of the DP, CD4 SP, and CD8 SP thymocytes (Fig. 1A, Table I). Although there was a slight decrease in the absolute number of DP thymocytes, the defect in the SP compartment was more profound than the defect in the DP compartment, suggesting that the reduction in SP thymocytes is likely not entirely due to the reduced numbers of DP thymocytes.
Figure 1. Defect in late thymic development in thymocytes expressing the ShcFFF transgene from the DN/DP stage of development.
(A) Left, profile of thymi isolated from 4-to-6 week old CD4-Cre/ShcFFF or control mice analyzed by flow cytometry for CD4 and CD8 (top panel), and CD44 and CD25 expression (bottom panel) within DN thymocytes (CD4 CD8 B220 Gr1 Ter119 CD11b CD11c). Right, total thymic cellularity and absolute numbers of each subset (n>12 mice per genotype, age-matched littermate controls, **p<0.01, ***p<0.001).
(B) Cell surface expression of CD24 and CD5 in CD4 SP and CD8 SP thymocytes isolated from 4-to-6 week old CD4-Cre/ShcFFF or control mice (representative of n>5 mice per genotype).
(C) Expression of TCRβ and CD69 on total thymocytes from CD4-Cre/ShcFFF or control mice to identify thymic subsets undergoing positive selection. Right, quantification of population 4 and 5 (n>5 mice per genotype).
Table 1.
Absolute numbers (± standard deviations) of T cell subsets. Thymi and spleen from mice of the indicated genotypes were analyzed by flow cytometry and numbers were determined based on their fractions within the total population. For the transgenic TCR setting, the cells were first gated on cells expressing the transgenic TCR before estimating the specific subsets. C/S refers to the CD4-Cre/ShcFFF transgenic mouse line.
| Control | CS | DO11.10 | OT-II | H-Y | OT-I | dLckF505 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | CS | Control | CS | Control | CS | Control | CS | Control | CS | |||
| Total Thymocytes | 191 ± 67 | 119 ± 35 | 143 ± 27 | 101± 33 | 106 ± 16 | 49 ± 24 | 95 ± 4 | 67 ± 15 | 117 ± 25 | 60 ± 12 | 220 ± 27 | 157 ± 36 |
| DN | 6.3 ± 2.6 | 5.8 ± 2.2 | 24 ± 11 | 23 ± 7 | 17 ± 6 | 12 ± 5 | 19 ± 2 | 17 ± 6 | 15 ± 3.5 | 10 ± 2 | 7 ± 1 | 6.6 ± 2 |
| DP | 146 ± 47.9 | 90 ± 24.8 | 74 ± 23 | 46 ± 20 | 61 ± 6 | 27 ± 14 | 45 ± 3 | 34 ± 5 | 57 ± 12 | 20 ± 4 | 108 ± 21 | 97 ± 30 |
| CD4 SP | 16.9± 6 | 7.8 ± 2.7 | 42 ± 12 | 23 ± 8 | 30 ± 4 | 11 ± 5 | - | - | - | - | 110 ± 10 | 52 ± 12 |
| CD8 SP | 5.8 ± 2.1 | 3.3 ± 1.4 | - | - | - | - | 23 ± 6 | 12 ± 3 | 19 ± 3 | 10 ± 1.5 | 6.2 ± 1.8 | 5.5 ± 1.2 |
| Total Splenocytes | 84 ± 11.7 | 72 ± 17.5 | 87.5 ± 16 | 69 ± 17 | 59 ± 2* | 54 ± 6* | 71 ± 18 | 59 ± 12 | 87 ± 21 | 100 ± 29 | 88 ± 13 | 70 ± 10 |
| CD4 T cells | 8.9 ± 2.4 | 3.2 ± 1.8 | 8.5 ± 1.6 | 6 ± 2 | 5 ± 0.2 | 4 ± 0.4 | - | - | - | - | 12 ± 1.1 | 7.7 ± 1 |
| CD8 T cells | 5 ± 1.8 | 1.7 ± 1.7 | - | - | - | - | 5 ± 1.2 | 4.4 ± 2 | 3.6 ± 1.8 | 1 ± 0.8 | 2.1 ± 0.1 | 1.9 ± 0.5 |
refers to n=4 mice analyzed.
The development of the CD4-lineage is often more sensitive to alterations in TCR signaling as CD4-lineage commitment requires stronger and more sustained signaling than CD8-lineage commitment (7, 8, 31–33). We noted that in the CD4-Cre/ShcFFF mice, the development of CD4 SP thymocytes was more affected than CD8 SP thymocytes, leading to an alteration in the ratio of CD4 SP to CD8 SP thymocytes (Supplemental Fig. 1E). Moreover, the phenotypic maturation of CD4 SP and CD8 SP thymocytes also appeared to be impaired in the CD4-Cre/ShcFFF mice based on cell surface staining for CD24 and CD5 (Figure 1B) (34–36). Both CD4 SP and CD8 SP thymocytes had reduction in the percentage of mature CD24lo SP thymocytes and an alteration in the ratio of mature (CD24lo) to immature (CD24hi) SP thymocytes (Fig. 1B, Supplemental Fig. 1F). Additionally, expression of the maturation and adhesion molecule CD5 was decreased in the CD8 SP thymocytes (Fig. 1B). CD5 levels correlate with TCR signal strength, however it is also possible that these CD5lo CD8 SP thymocytes represent immature CD8 SP thymocytes (ISP), and there might be additional defects in the transition from ISP to DP thymocytes in the CD4-Cre/ShcFFF transgenic mice (Fig. 1B) (36). Collectively, the impairment in CD4 SP and CD8 SP compartments, with a more profound defect in the CD4 SP compartment, suggests that the CD4-Cre/ShcFFF mice have a defect in TCR signaling and transition from DP to SP stages of thymocyte development.
To further understand the specific developmental stages affected in the CD4-Cre/ShcFFF mice, we subdivided thymocytes into five developmental stages based on the expression of TCRβ and CD69 (31–33). Populations 1 (TCRβloCD69lo) and 2 (TCRβintCD69lo) represent the most immature pre-positive selection DN and DP thymocytes (31). CD4-Cre/ShcFFF mice have a slight increase in the percentages of both of these subsets (data not shown). Population 3 (TCRintCD69int) has partially up-regulated CD69 and is thought to be undergoing positive selection (37). We found a slight reduction in this population in the CD4-Cre/ShcFFF mice (data not shown). Additionally, CD4-Cre/ShcFFF mice had a significant reduction in the composition of post-positive selection thymocytes (population 4, TCRhiCD69hi) and mature thymocytes (population 5, TCRhiCD69lo) (Fig. 1C). Collectively, these data suggested that CD4-Cre/ShcFFF mice have deficiencies in late T cell development, likely due to altered positive selection.
Signaling though transgenic TCRs is impaired in CD4-Cre/ShcFFF mice
Developmental defects in positive selection are often better revealed in mice carrying a transgenic TCR, since the normal TCR repertoire may undergo compensatory changes that can mask defects in thymocyte selection (32, 38, 39). Therefore, we crossed the CD4-Cre/ShcFFF mice to two different MHC class II-restricted TCR transgenic lines: OT-II and DO11.10. The developmental defects due to ShcFFF expression were more pronounced in these TCR transgenic backgrounds (Table I). CD4-Cre/ShcFFF mice expressing either the OT-II or the DO11.10 transgenic TCR had an overall reduced thymic cellularity and decreased numbers in the DP and CD4 SP thymic compartments (Table I). Additionally, staining with antibodies specific for the transgenic TCRs demonstrated a specific reduction in thymocytes expressing the OT-II transgenic TCR (Fig. 2A, 2B), with fewer CD4 SP transgenic Vβ5.1/2hi cells (Supplemental Fig. 2), although we did not find a defect in percentage of cells expressing the D011.10 transgenic TCR.
Figure 2. ShcFFF expression impairs the integrity of transgenic TCR signaling.
(A–D) Surface expression of CD4 and CD8 in control and CD4-Cre/ShcFFF mice on the indicated transgenic TCR backgrounds (top panels). Cell surface expression of indicated transgenic TCRs (bottom panel) (n>2 mice per genotype).
(E) Percent annexin V+ DP thymocytes after incubation with the A20 B cell line and the indicated concentration of OVA peptide for 8 hours (left panel). Recovery of DP thymocytes after incubation for 20 hours (right panel) (representative of n=2 experiments).
(F) Cell surface expression of CD4 and CD8 in thymocytes from CD4-Cre/ShcFFF or control mice on the TCRα deficient background.
(G) Surface marker expression of CD4 and CD8 on thymocytes from control and CD4-Cre/ShcFFF mice on the LckF505 transgenic background (n=4 mice per genotype).
Previously, we have demonstrated that the block in early T cell development in mice expressing the ShcFFF transgene is essentially complete such that any DP, SP, or peripheral T cells observed in the Lck-Cre/ShcFFF mice arise due to incomplete Cre-mediated recombination at the loxP sites and, in turn, fail to express the ShcFFF transgene (i.e. essentially wild type cells) (14–16). To determine whether peripheral T cells in the DO11.10/CD4-Cre/ShcFFF mice were similar escapees without Cre-mediated deletion of the STOP cassette, we assessed the expression of the transgene-encoded, FLAG-tagged ShcFFF protein by immunoblotting. We were able to detect expression of FLAG-ShcFFF in the lysates from the thymus, but not lysates of peripheral CD4+ T cells (Supplemental Fig. 2D). This suggested that in DO11.10/CD4-Cre/ShcFFF mice, ShcA phosphorylation is required for the development of CD4+ T cells and that the peripheral T cells detected in these animals either do not express the ShcFFF transgene or express low levels of the protein that are undetectable via immunoblotting.
DO11.10 mice have a MHC class II-restricted TCR that recognizes the ovalbumin (OVA) peptide, and it has been shown that in vivo intraperitoneal injection of OVA peptide or ex vivo treatment of thymocytes with the OVA peptide leads to apoptosis of DP thymocytes (23, 40). To test a potential defect in signaling through the transgenic TCR in the DO11.10/CD4-Cre/ShcFFF triple transgenic mice, we assessed peptide-induced apoptosis of DP thymocytes from these mice. In an ex vivo assay, thymocytes from DO11.10/CD4-Cre/ShcFFF mice had reduced apoptosis of DP thymocytes after 8 hours and, in turn, increased recovery of DP thymocytes after 20 hours (Fig. 2E), suggestive of reduced signaling via the transgenic TCR in these thymocytes. Collectively, these data suggest a defect in signaling through MHC class II-restricted TCRs in the CD4-Cre/ShcFFF mice. These transgenic TCR studies further implied a crucial role for ShcA phosphorylation during the DP to SP stage of development, since even the enhanced signaling through the transgenic TCR failed to rescue the developmental defects in CD4-Cre/ShcFFF mice.
Next, we investigated whether ShcA phosphorylation is required for CD8-lineage commitment and positive selection. In CD4-Cre/ShcFFF mice, we found a less profound defect in the development of CD8 SP compared to CD4 SP thymocytes with no apparent change in the percentage and a reduction in the absolute numbers of CD8 SP thymocytes (Supplemental Fig. 1E and Table I). To further address this in the context of transgenic TCRs, we crossed our CD4-Cre/ShcFFF mice to two different MHC class I-restricted mouse lines: OT-I and H-Y transgenic TCR lines. We found a defect in CD8 SP T cell development due to impaired ShcA mediated signaling with a slight decrease in overall thymic cellularity in the CD4-Cre/ShcFFF mice expressing either the H-Y or OT-I transgenic TCR and a decrease in DP and CD8 SP thymocytes (Table I) with fewer transgenic TCRhi CD8 SP thymocytes (Fig. 2C, 2D, Supplemental Fig. 2A).
To assess negative selection in thymocytes expressing the ShcFFF transgene, we analyzed thymocyte development in male H-Y/CD4-Cre/ShcFFF mice. The H-Y transgenic mouse line encodes an MHC class I-restricted transgenic TCR that recognizes a male antigen (24). While in female H-Y transgenic mice, positive selection leads to the generation of CD8 SP thymocytes, in the male H-Y transgenic mice, negative selection leads to a decrease in overall thymic cellularity and very few DP and SP thymocytes (24). Negative selection appeared normal in the H-Y/CD4-Cre/ShcFFF mice with greatly reduced thymic cellularity due to negative selection and a decrease in the expression of CD4 and CD8 comparable to control male H-Y mice without ShcFFF (Supplemental Fig. 2C). Collectively, these data suggest that there is also a defect in the CD8-T cell lineage, albeit less profound than the one observed in the CD4-lineage, both in the context of endogenous and transgenic TCRs.
CD4-Cre/ShcFFF mice do not display obvious defects within DP thymocyte subset
Defects in TCR signaling and positive selection often lead to a relative accumulation and increased percentage of DP thymocytes. However, we found that there was a reduction in the absolute number of DP thymocytes in the CD4-Cre/ShcFFF mice (Table I). Therefore, we next assessed whether there was an additional defect within the DP compartment. First, we crossed the transgenic CD4-Cre/ShcFFF mouse line to the TCRα deficient mouse line. Previous studies have shown that thymocytes from TCRα deficient mice can undergo normal DN —> DP transition, but are blocked at the DP stage due to their inability to generate the αβTCR and undergo positive selection (6). We found that in the TCRα deficient CD4-Cre/ShcFFF mice there was only a slight difference in the total cellularity compared to control TCRα deficient mice (Fig. 2F). This suggested that the reduced thymic cellularity in the CD4-Cre/ShcFFF mice is not likely to be reflective of a major defect in DP thymocytes, and is mainly due to defects in the DP to SP transition. Furthermore, no defects in the survival of DP thymocytes isolated from CD4-Cre/ShcFFF transgenic mice were revealed by Annexin V and 7AAD staining of freshly isolated thymocytes, or thymocytes undergoing spontaneous and anti-CD3 mediated apoptosis under ex vivo conditions (Supplemental Fig. 2E). Based on these data, we conclude that the primary defect in the CD4-Cre/ShcFFF mice is at the stage of DP to SP transition, although a modest reduction in the number of DP thymocytes is observed.
ShcA is downstream of p56lck in DP thymocytes
The protein tyrosine kinase p56lck (Lck) in an important signaling molecule in T cells and signals downstream of both the preTCR and the TCR(16). Lck activity is required for preTCR and TCR signaling, as the constitutively active LckF505 transgene can rescue thymic development defects resulting from the absence of either the preTCR or TCR (42–44). Previous studies have shown that during TCR stimulation, Lck phosphorylates ShcA on the same three crucial tyrosine residues that are mutated in the ShcFFF transgene (16). To determine whether ShcA acts downstream of Lck and whether a constitutively active version of Lck (LckF505) can bypass the need for ShcA mediated signaling, we crossed CD4-Cre/ShcFFF mice to the LckF505 transgenic mouse line. Despite the known augmented signaling in the LckF505 context (42–44), LckF505/CD4-Cre/ShcFFF mice also displayed significantly impaired T cell development compared to control mice expressing LckF505 alone, suggesting that LckF505 transgene is unable to fully rescue T cell development in the context of impaired ShcA signaling (Fig 2G, Table I). These data suggest that ShcA acts downstream of Lck in DP thymocytes and that ShcA phosphorylation is necessary for Lck mediated downstream signaling.
CD4-Cre/ShcFFF display defects in thymic organization and cell trafficking
Next, we performed H&E staining on thymic sections and found the thymic architecture considerably altered in CD4-Cre/ShcFFF mice, with reduction in the area representing both the cortex and the medulla (Fig. 3A, B). Furthermore, the medulla was fragmented and disorganized, as is often seen in mice with defects in positive selection (31, 45), and the cortex to medulla area ratio was higher in the CD4-Cre/ShcFFF mice (7.9) compared to control (3.6) (Fig. 3A).
Figure 3. Defect in thymic organization and cell trafficking in CD4 Cre/ShcFFF mice.
(A) Representative H&E staining of thymi isolated from CD4-Cre/ShcFFF or control mice. R-value is calculated as the area of the cortex divided by the area of the medulla.
(B) Area of the cortex and medulla of thymi isolated from CD4-Cre/ShcFFF or control mice (n>3 mice per genotype).
(C) Cell surface staining for CCR7 on DP CD3Hi thymocytes isolated from CD4-Cre/ShcFFF or control mice (n=2 mice of each genotype).
(D) Migration of DP CD3Hi thymocytes to CCL19 and CCL21 from CD4-Cre/ShcFFF or control mice. Left, representative plot of CD3 expression of gated DP thymocytes after migration to CCL19 and CCL21. Right, migration of DP CD3Hi thymocytes from CD4-Cre/ShcFFF mice to CCL19 and CCL21 (normalized to control, n>4 mice per genotype).
Previous studies have shown that ShcA acts downstream of CXCR4 and is required for CXCR4-mediated migration in T cells and thymocytes (17, 46). After positive selection, thymocytes upregulate the CCR7 receptor and migrate from the cortex towards the medulla via a gradient of CCL19 and CCL21 expressed by the medullary epithelial cells (47, 48). First, we found that ShcA was phosphorylated in thymocytes after stimulation with a CCR7 ligand (Supplemental Fig. 3A). We then assessed the migration of DP thymocytes from CD4-Cre/ShcFFF mice. Interestingly, the thymocytes from CD4-Cre/ShcFFF mice had reduced migration toward the CCR7 ligands CCL19 and CCL21 (Fig. 3D). Notably, this was not due to altered CCR7 expression on these thymocytes, as the post-positively selected TCRhi DP thymocytes from CD4-Cre/ShcFFF mice had expression levels of CCR7 comparable to control mice (Fig. 3C). These findings suggest that the thymic architecture is likely altered due to both defects in positive selection and the subsequent migration of DP thymocytes. It is noteworthy that the defect in CCR7 migration is likely underestimated in the above assay as many of the post-positively selected DP thymocytes may represent ‘escapees’ that still retain the STOP cassette and do not express the ShcFFF transgene.
CD4-Cre/ShcFFF mice have a reduced peripheral T cell compartment
Next, we investigated the peripheral T cell compartment in CD4-Cre/ShcFFF mice. The CD4-Cre/ShcFFF mice had a significant lymphopenia and reduction of both the number and percentage of CD4+ and CD8+ T cells in the lymph nodes and spleen (Fig. 4A, 4B, Table 1). Additionally, while the CD4 to CD8 ratio was 2:1 in wild type mice, this ratio was roughly 1:1 in CD4-Cre/ShcFFF mice (Fig. 4B). Lymphopenia did not appear to be due to improper accumulation of T cells in non-lymphoid peripheral tissues, as we also saw reduced numbers of T cells in other tissues such as the lung and blood (data not shown). As has been seen previously in lymphopenic conditions, we also found a slight increase in the percentage of the ‘memory like’ CD62LloCD44hi CD4 T cells, which is often the result of homeostatic proliferation of T cells (Supplemental Fig. 3B). We next investigated whether the peripheral T cells in the CD4-Cre/ShcFFF mice were Cre ‘escapees’ by assessing the deletion of the STOP sequence. Although this PCR based approach was non-quantitative, we were able to detect some excision of the STOP cassette in peripheral T cells from CD4-Cre/ShcFFF (Supplemental Fig. 1G). However, we were unable to detect the Flag-tagged ShcFFF transgene-encoded protein by western blotting (Supplemental Fig. 1G). Thus, while some peripheral T cells have undergone Cre-mediated excision of the STOP cassette, many of them likely do not express the ShcFFF transgene at protein levels detectable via immunoblotting (Supplemental Fig. 1H). Additionally, we did not observe a proliferative defect in these peripheral T cells from CD4-Cre/ShcFFF mice after optimal anti-CD3/anti-CD28 stimulation ex vivo (Supplemental Fig. 3C). Furthermore, we did not observe any defects in IL2 production after ex vivo stimulation of CD4+ T cells with anti-CD3, anti-CD3/anti-CD28, or PMA/Ionomycin stimulation (Supplemental Fig. 3D) or in a time-course of anti-CD3/anti-CD28 stimulation (Supplemental Fig. 3E).
Figure 4. Phenotype of peripheral T cells in CD4 Cre/ShcFFF mice.
(A) Left, CD4 and CD8 cell surface staining of cells isolated from the lymph node or spleen of CD4-Cre/ShcFFF or control mice. Right, total cellularity and absolute numbers of CD4 and CD8 T cells from the spleen (n>12 mice of each genotype).
(B) Percentage of CD4 and CD8 T cells in the spleen from control or CD4-Cre/ShcFFF mice (n>18 mice per genotype).
(C) Left, cell surface staining of CD4 and CD8 on cells isolated from the spleen of CD4-Cre/ShcFFF mice or control mice expressing the OT-I transgenic TCR. Middle panel, cell surface staining of CD4 and CD8 after gaiting on Vα3hi splenocytes. Left panel, staining of splenocytes with the Vα3 to assess for the expression of the OT-I transgenic TCR (n>2 mice per genotype).
(D) Generation of Rag 2p-eGFP CD4-Cre/ShcFFF mice and equation used to calculate the emigration ratio.
(E) Quantification of the emigration ratio (GFP+ CD4 or CD8 Splenocytes/GFP+ CD4 or CD8 SP thymocytes) from control or CD4-Cre/ShcFFF mice (n>15 mice per genotype).
A similar impairment was seen in peripheral T cells in the CD4-Cre/ShcFFF mice on the background of MHC class I and MHC class-II restricted transgenic TCRs, with reduced splenic cellularity and reduction in CD8+ or CD4+ peripheral T cells (Table I). In the OT-I CD4-Cre/ShcFFF mice, we found a substantial reduction in the numbers of CD8+ T cells as well as reduction in the percentage of cells staining for the OT-I transgenic TCR (Fig. 4B).
Since the CD4-Cre/ShcFFF mice have an overall reduction in thymic cellularity with a further reduction in CD4 SP thymocytes, initially we thought it is not too surprising that the CD4-Cre/ShcFFF mice have a defect in the peripheral T cell compartment. Many genetic mouse models with disruption in genes required for positive selection, such as Themis and Tespa deficient mice, also have defects in the peripheral T cell compartment (31–33, 45). However, we found that the defect in peripheral T cell compartment appeared to be disproportionate and could not be exclusively due to the reduced thymic cellularity (Table I). We hypothesized that the CD4-Cre/ShcFFF mice have an additional defect in the emigration of the CD4 and CD8 SP thymocytes from the thymus into the periphery. Previously, it has been shown that the Rag2 eGFP transgenic mice (which express enhanced GFP under the expression of the Rag2 promoter) can be used to identify recent thymic emigrants (RTE) in the periphery (26, 49, 50). Furthermore, thymocytes from Rag2-eGFP mice express GFP in the late DN and DP stages of development, but the GFP expression briefly persists in peripheral T cells and, therefore, GFP+ T cells in the periphery represent the RTEs (Supplemental Fig. 3F) (26, 49, 50). To identify recent thymic emigrants in the context of impaired ShcA signaling, we crossed the CD4-Cre/ShcFFF mice to the Rag2-eGFP transgenic mice (Fig. 4D). We calculated the emigration ratio for the Rag2-eGFP CD4-Cre/ShcFFF mice and Rag2-eGFP control mice by dividing the absolute number of GFP+ CD4+ or CD8+ splenocytes by the number of GFP+ CD4 SP or GFP+ CD8 SP thymocytes (Fig. 4C). The emigration ratio for both CD4+ and CD8+ T cells was reduced in the CD4-Cre/ShcFFF mice, and therefore the CD4-Cre/ShcFFF mice appeared to have an additional defect in the emigration of mature SP thymocytes into the periphery (Fig. 4D, E). However, we did not observe an accumulation of these SP subsets in the thymus, which is often found in mice with defects in thymic emigration (51) most likely a consequence of the concurrent defects in positive selection and a reduction in the numbers of CD4 SP and CD8 SP thymocytes.
Lymphopenia persists in adult CD4-Cre/ShcFFF mice and these mice display attenuated disease in the experimental autoimmune encephalomyelitis (EAE) model
T cell developmental defects are often more striking in younger animals and certain phenotypes in ShcA mutant mice (in the context of brain) are only present in young mice (52). Therefore, we asked whether the peripheral lymphopenia is transient or if it persists in adult CD4-Cre/ShcFFF mice. When we analyzed 10 to 12 week old CD4-Cre/ShcFFF mice, the reduced numbers of CD4+ and CD8+ T cells in the spleen and lymph nodes still remained (Fig. 5A). Next, we subcutaneously immunized the CD4-Cre/ShcFFF mice with MOG(35–55) and collected the draining lymph nodes on day 8 after immunization. In the CD4-Cre/ShcFFF mice, we found reduced number and percentage of the CD4+ and CD8+ T cells after immunization with MOG(35–55) (Fig. 5B). Therefore, the peripheral lymphopenia in the CD4-Cre/ShcFFF mice persists with age, as well as after immunization.
Figure 5. Peripheral lymphopenia in 10 week old CD4 Cre/ShcFFF mice, and after EAE immunization.
(A) Left, cell surface expression of CD4 and CD8 isolated from the spleen or lymph node from 10-week old CD4-Cre/ShcFFF mice or control mice. Right, absolute numbers of CD4 and CD8 T cells (n=10 mice for each genotype).
(B) Left, cell surface expression of CD4 and CD8 on cells isolated from the draining lymph node of CD4-Cre/ShcFFF or control mice on day 8 after MOG(35–55) immunization (n=3, 5 of CD4-Cre/ShcFFF or control mice respectively).
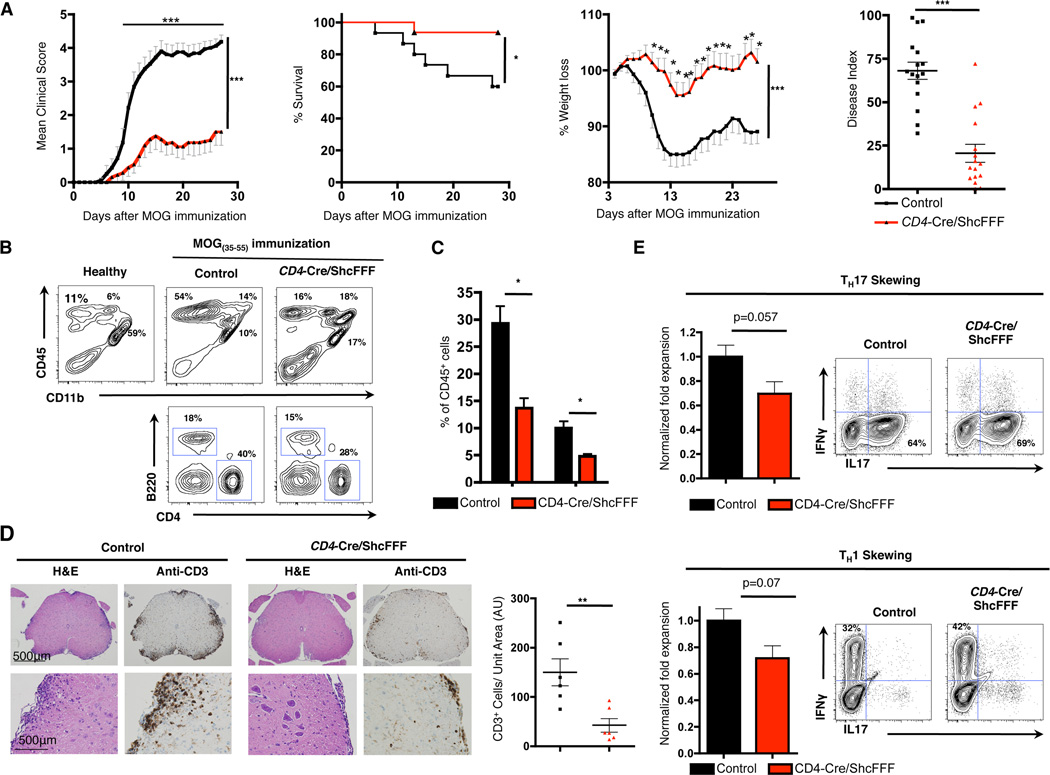
To test whether the lymphopenia of the CD4-Cre/ShcFFF mice leads to attenuated disease in the context of autoimmunity, we chose to use the CD4+ T cell driven experimental autoimmune encephalomyelitis (EAE) model (53). The EAE model recapitulates many of the clinical, pathological, and immunological aspects of the human disease multiple sclerosis (MS). In MS and EAE infiltration of autoreactive T cells into the central nervous system (CNS leads to inflammation, demyelination and progressive disability (53, 54). Furthermore, as shown in Fig. 5B, 8 days after immunization with the MOG(35–55) peptide, CD4-Cre/ShcFFF mice have reduced numbers of CD4+ and CD8+ T cells in the draining lymph nodes (Fig. 5B). We immunized CD4-Cre/ShcFFF and control mice with MOG(35–55) to induce EAE, and monitored disease severity and weight loss over a period of 28 days. We found that CD4-Cre/ShcFFF mice developed strikingly attenuated disease with a significant reduction in disease severity and weight loss as well as improved survival (Fig. 6A) (in 3 independent experiments with a combined total of n=16 mice per genotype, p<0.0001 for clinical scores by 2-way ANOVA). Additionally, the CD4-Cre/ShcFFF had a lower incidence of disease with 25% of CD4-Cre/ShcFFF mice never exhibiting any signs of disease (defined as score of 1 or greater) and a lower overall maximum score and disease index (area under the curve) (Fig. 6A, Table II). Of the CD4-Cre/ShcFFF mice that did exhibit signs of disease, the day of onset was significantly delayed from an average of 10.1 days for control mice to an average of 14.1 days for CD4-Cre/ShcFFF mice (Table II). We also found that the CD4-Cre/ShcFFF transgenic mice developed attenuated disease in a model of sub-optimal EAE induction (Supplemental Fig. 4D).
Figure 6. CD4 Cre/ShcFFF are mostly resistant to EAE.
(A) Mean clinical scores, survival, weight loss, and disease index of CD4-Cre/ShcFFF and control mice immunized for EAE. Compiled data are from 3 experiments with a total of n=16 mice of each genotype.
(B) Cell surface staining of CD45, CD4, CD11b, and B220 on cells isolated from the spinal cord and brain of CD4-Cre/ShcFFF or control mice on day 28 after MOG immunization (n=3 mice of each genotype).
(C) Percentage of CD4+ and B220+ cells within the CD45+ cells isolated from the spinal cord and brain of CD4-Cre/ShcFFF or control mice on day 28 after MOG immunization (n=3 mice of each genotype).
(D) Left, immunohistochemistry for CD3 and H&E staining on spinal cord sections from CD4-Cre/ShcFFF or control mice on day 28 after immunization with EAE. Right, quantification of number of CD3+ T cells from spinal cord sections (n=6 mice of each genotype).
(E) Left, normalized fold expansion of CD4 T cells under TH17 or TH1 skewing condions from CD4-Cre/ShcFFF or control mice. Right, representative IL17 and IFNγ intracellular staining after TH17 or TH1 skewing (n= 5 mice per genotype).
Table II.
Parameters of disease in CD4-Cre/ShcFFF mice
| Genotype | Incidence | Day of Onset | Mean Max | Disease Index |
Mortality |
|---|---|---|---|---|---|
| Control | 100% (16/16) | 10.1 ± 3.0 | 4.5 ± .45 | 68.1 ± 19.8 | 37.5% (6/16) |
| CD4 Cre/ShcFFF | 75% (12/16) | 14.1 ± 5.4 | 2.4 ± 1.6 | 20.5 ± 20.6 | 6.3% (1/16) |
At chronic stage of the disease (day 28), we assessed the composition of the immune infiltrate in the CNS by analyzing the cells isolated from the brains and spinal cords of CD4-Cre/ShcFFF and control mice. CD4-Cre/ShcFFF mice had overall fewer mononuclear cells isolated from the brain and spinal cords (Supplemental Fig. 4B). There was a decrease in the percentage of CD4+ and B220+ lymphocytes in the CD4-Cre/ShcFFF mice compared to control mice (Fig 6B, 6C). Overall, the CD4-Cre/ShcFFF mice had a trend of reduced total numbers of CD4+ T cells, B cells, macrophages, and microglia in the brain and spinal cord at the chronic stage of the disease (Supplemental Fig. 4B). Histological analysis by H&E staining and immunohistochemistry confirmed that the CD4-Cre/ShcFFF mice had fewer loci of immune infiltration and fewer CD3+ T cells in the spinal cords (Fig. 6E).
Previous studies have shown that CD4+ T cells, and in particular TH17 cells, play a crucial role in the initiation and development of multiple sclerosis and EAE. The peripheral lymphopenia in the CD4-Cre/ShcFFF transgenic mice clearly contributed to the attenuated disease in the EAE model. We next investigated whether the peripheral T cells from the CD4-Cre/ShcFFF transgenic mice had a defect in ex vivo TH17 and TH1 skewing. CD4+ T cells isolated from CD4-Cre/ShcFFF transgenic mice had an overall defect in expansion when cultured under TH17 and TH1 skewing conditions (Fig. 6E, Supplemental Fig. 4C). We found fewer absolute numbers of the TH17 and TH1 cells after skewing CD4+ T cells isolated from CD4-Cre/ShcFFF transgenic mice as compared to CD4+ T cells isolated from littermate controls (Fig. 6E). Although there was an overall defect in their expansion, we found that peripheral CD4+ T cells from CD4-Cre/ShcFFF transgenic mice cells that differentiated into TH17 and TH1 lineage had no defects in the production of IL-17 or IFNγ (Fig. 6E). We next investigated whether the CD4+ T cells skewed under TH17 or TH1 skewing conditions were Cre ‘escapees’ by assessing the deletion of the STOP sequence. We were able to detect excision of the STOP cassette, which suggests that at least some of these TH1 and TH17 cells expressed the ShcFFF transgene (data not shown). Collectively, these data demonstrated that lymphopenia and the impaired expansion of TH17 and TH1 cells in the CD4-Cre/ShcFFF mice lead to a net functional deficit in CD4+ T cells manifested as attenuated disease in the EAE model.
Discussion
In this study, we demonstrate a requirement for ShcA function in late T cell development, beyond the previously reported requirement for ShcA in preTCR signaling and progression through the β-selection checkpoint (14, 15). To bypass the requirement for ShcA in early T cell development, we generated the CD4-Cre/ShcFFF transgenic mice, which express the ShcFFF transgene from the DN4/DP stage of development (21). We find that the CD4-Cre/ShcFFF transgenic mice show two notable defects in late T cell development: a defect in the absolute number and percentage of CD4 SP thymocytes, and a persistent peripheral lymphopenia.
Productive signaling through the TCR in DP thymocytes is required for positive selection and the development of mature SP thymocytes. Additionally, sustained signaling through the TCR is essential for the development of CD4 SP thymocytes, and the CD4 SP compartment is more sensitive to defects in TCR signaling than the CD8 SP compartment (1). Through several approaches, we find that impaired ShcA mediated signaling affects the development of mature CD4 SP thymocytes. We also found that the impairment in positive selection and development of CD4 SP thymocytes due to impaired ShcA signaling is more profound than the development of CD8 SP thymocytes, although the CD8 SP compartment is also impaired. To overcome the issue that developmental defects in positive selection can be masked by compensatory changes in the TCR repertoire, we also analyzed the CD4-Cre/ShcFFF mice in the context of transgenic TCR expression and found similar defects during positive selection. Furthermore, antigenic-peptide induced signaling via the transgenic TCR in the DP thymocytes was also decreased in DO11.10/CD4-Cre/ShcFFF transgenic mice. Lastly, although the main defects in thymic development in the CD4-Cre/ShcFFF transgenic mice were found in the SP compartment, we also found a slight reduction in the number of DP thymocytes, which may be due to impairment in the transition from the ISP to the DP stage of thymocyte development. Collectively, these data demonstrated that ShcA phosphorylation is required for optimal signaling through the TCR in DP thymocytes for their progression into CD4 and CD8 SP T cells. In addition to the TCR and preTCR, many chemokine and cytokine receptors are critical for normal thymocyte development and survival. Previously, we have demonstrated that ShcA is required for optimal signaling through CXCR4 during the DN stage of development (17) and here we find decreased CCR7-mediated chemotaxis in the DP compartment. Therefore, it is possible that ShcA-mediated signaling through other chemokine and cytokine receptors may also contribute to the thymic development defects found in the CD4-Cre/ShcFFF transgenic mice.
The CD4-Cre/ShcFFF mice also had impairment in the peripheral T cell compartment, with reduced numbers and an alteration in the ratio of CD4+ to CD8+ T cells. Peripheral lymphopenia can be partially explained by the defects in the thymic CD4 SP and CD8 SP compartments. However, we found that the defect in the peripheral compartment is disproportionate to the thymic defect. Our data suggests that the peripheral compartment in CD4-Cre/ShcFFF mice may also be partially altered due to reduced emigration of thymocytes into the periphery. Despite extensive investigation, we were unable to find a definitive explanation for the disproportionate peripheral defect; there was no significant defect in ex vivo S1P mediated migration of CD4+CD62Lhi SP thymocytes (data not shown), and we did not observe an apparent survival or proliferative defect in peripheral T cells. However, we found that T cells isolated from CD4-Cre/ShcFFF mice had impaired expansion under TH17 and TH1 skewing conditions, suggesting that cytokine signaling required for the lineage specific differentiation of CD4 T cells may also be at least partially dependent on ShcA phosphorylation. Importantly, peripheral lymphopenia and impaired expansion in CD4-Cre/ShcFFF mice result in functional impairment, as CD4-Cre/ShcFFF mice develop attenuated disease in the CD4 T cell driven EAE mouse model. We currently cannot definitively discern whether the majority of peripheral T cells are Cre ‘escapees’ or whether the peripheral CD4-Cre/ShcFFF T cells express very low levels of the transgene (detectable by the excision of the STOP cassette but not by immunoblot of the transgene-encoded protein). Since CD4 expression has been reported in some cells of the central nervous system (55), we cannot exclude whether ShcFFF transgene expression in other cell types may have also contributed to the attenuated disease in the EAE model. However, the persistent peripheral lymphopenia and impaired expansion of T cells under TH17 and TH1 skewing conditions lead us to conclude that ShcA phosphorylation directly contributes to peripheral T cell function and to the attenuated disease in the EAE model in the CD4-Cre/ShcFFF transgenic mice.
Development of αβ T cells critically depends on productive signaling through the preTCR in DN thymocytes and the TCR in DP thymocytes as well as other chemokine and cytokine receptors. Disruption of the components of the preTCR and TCR, or of downstream signaling molecules, leads to impairment in T cell development and results in the block of developmental progression. Along with our previous studies on the essential role of the adapter protein ShcA in signaling via the preTCR, the studies presented here on the role of ShcA in signaling via αβTCR suggest that ShcA critically contributes to the progression through β-selection and positive-selection checkpoints.
Supplementary Material
Acknowledgments
We thank members of the Ravichandran laboratory for their helpful suggestions and for critically reading this manuscript. We thank Dr. Roger Perlmutter and Dr. Pamela Fink for generously providing the LckF505 and Rag2-eGFP transgenic mice lines, respectively. Additionally, we thank the UVA Flow Cytometry core, UVA Gene Targeting and Transgenic Facility, UVA Research Histology Core, and the UVA Biorepository and Tissue Research Facility.
Funding: This work was supported by GM55761 (to K.S.R.) and by a F30 Fellowship (to M.W.B.) and support to M.W.B. via the MSTP program and the T32 Immunology Training Grant.
Abbreviations used in this article
- DN
double negative
- DP
double positive
- EAE
experimental autoimmune encephalomyelitis
- ISP
immature single positive
- MS
multiple sclerosis
- MOG35–55
myelin oligodendrocyte glycoprotein 35–55
- pMHC
peptide-MHC
- RTE
recent thymic emigrants
- SP
single positive
- S1P
sphingosine 1-phosphate
- WT
wild type
References
- 1.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nature immunology. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruisbeek AM, Haks MC, Carleton M, Wiest DL, Michie AM, Zuniga-Pflucker JC. Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunology today. 2000;21:637–644. doi: 10.1016/s0167-5699(00)01744-8. [DOI] [PubMed] [Google Scholar]
- 4.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pfllucker JC. Obligatory Role for Cooperative Signaling by Pre-TCR and Notch during Thymocyte Differentiation. The Journal of Immunology. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 5.Gascoigne NR, Palmer E. Signaling in thymic selection. Current opinion in immunology. 2011;23:207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 7.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nature reviews. Immunology. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasutomo K, Doyle C, Miele L, Fuchs C, Germain RN. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404:506–510. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
- 9.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annual Review of Immunology. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 10.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 11.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci PG. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 12.Lai KM, Pawson T. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes & development. 2000;14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderlaan RD, Hardy WR, Kabir MG, Pasculescu A, Jones N, deTombe PP, Backx PH, Pawson T. The ShcA phosphotyrosine docking protein uses distinct mechanisms to regulate myocyte and global heart function. Circulation research. 2011;108:184–193. doi: 10.1161/CIRCRESAHA.110.233924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trampont P, Zhang L, Ravichandran KS. ShcA Mediates the Dominant Pathway to Extracellular Signal-Regulated Kinase Activation during Early Thymic Development. Molecular and cellular biology. 2006;26:9035–9044. doi: 10.1128/MCB.00988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Camerini V, Bender TP, Ravichandran KS. A nonredundant role for the adapter protein Shc in thymic T cell development. Nature immunology. 2002;3:749–755. doi: 10.1038/ni820. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Lorenz U, Ravichandran KS. Role of Shc in T-cell development and function. Immunological reviews. 2003;191:183–195. doi: 10.1034/j.1600-065x.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. CXCR4 acts as a costimulator during thymic [beta]-selection. Nat Immunol. 2010;11:162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravichandran KS, Lee KK, Songyang Z, Cantley LC, Burn P, Burakoff SJ. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 19.Pratt JC, van den Brink MR, Igras VE, Walk SF, Ravichandran KS, Burakoff SJ. Requirement for Shc in TCR-mediated activation of a T cell hybridoma. Journal of immunology. 1999;163:2586–2591. [PubMed] [Google Scholar]
- 20.Pacini S, Ulivieri C, Di Somma MM, Isacchi A, Lanfrancone L, Pelicci PG, Telford JL, Baldari CT. Tyrosine 474 of ZAP-70 is required for association with the Shc adaptor and for T-cell antigen receptor-dependent gene activation. The Journal of biological chemistry. 1998;273:20487–20493. doi: 10.1074/jbc.273.32.20487. [DOI] [PubMed] [Google Scholar]
- 21.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A Critical Role for Dnmt1 and DNA Methylation in T Cell Development, Function, and Survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 22.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based agr]- and bgr]-chain genes under the control of heterologous regulatory elements. Immunology and cell biology. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 25.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 26.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nature immunology. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 27.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 28.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Thymic tumorigenesis induced by overexpression of p56lck. Proceedings of the National Academy of Sciences. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nature protocols. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 30.Shi J, Petrie HT. Activation Kinetics and Off-Target Effects of Thymus-Initiated Cre Transgenes. PloS one. 2012;7:e46590. doi: 10.1371/journal.pone.0046590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesourne R, Uehara S, Lee J, Song KD, Li L, Pinkhasov J, Zhang Y, Weng NP, Wildt KF, Wang L, Bosselut R, Love PE. Themis, a T cell-specific protein important for late thymocyte development. Nature immunology. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JAH, Munshi A, Huang YH, Hu J, Fox HS, Sauer K, Acuto O, Gascoigne NRJ. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nature immunology. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson AL, Aravind L, Shulzhenko N, Morgun A, Choi S-Y, Crockford TL, Lambe T, Domaschenz H, Kucharska EM, Zheng L, Vinuesa CG, Lenardo MJ, Goodnow CC, Cornall RJ, Schwartz RH. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nature immunology. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crispe IN, Bevan MJ. Expression and functional significance of the J11d marker on mouse thymocytes. Journal of immunology. 1987;138:2013–2018. [PubMed] [Google Scholar]
- 35.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 Expression Is Developmentally Regulated By T Cell Receptor (TCR) Signals and TCR Avidity. The Journal of experimental medicine. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park C-S, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine Tuning of TCR Signaling by CD5. The Journal of Immunology. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita I, Nagata T, Tada T, Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection. International Immunology. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- 38.Fischer A, Malissen B. Natural and engineered disorders of lymphocyte development. Science. 1998;280:237–243. doi: 10.1126/science.280.5361.237. [DOI] [PubMed] [Google Scholar]
- 39.Phee H, Dzhagalov I, Mollenauer M, Wang Y, Irvine DJ, Robey E, Weiss A. Regulation of thymocyte positive selection and motility by GIT2. Nature immunology. 2010;11:503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T Cells Recognize a C-Terminal Ovalbumin 323–339 Epitope. The Journal of Immunology. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 41.Mingueneau M, Kreslavsky T, Gray D, Heng T, Cruse R, Ericson J, Bendall S, Spitzer MH, Nolan GP, Kobayashi K, von Boehmer H, Mathis D, Benoist C, Best AJ, Knell J, Goldrath A, Jojic V, Koller D, Shay T, Regev A, Cohen N, Brennan P, Brenner M, Kim F, Rao TN, Wagers A, Rothamel K, Ortiz-Lopez A, Bezman NA, Sun JC, Min-Oo G, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Blair DA, Dustin ML, Shinton SA, Hardy RR, Laidlaw D, Collins J, Gazit R, Rossi DJ, Malhotra N, Sylvia K, Kang J, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S. The transcriptional landscape of alphabeta T cell differentiation. Nature immunology. 2013;14:619–632. doi: 10.1038/ni.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Hoyos G, Sohn SJ, Rothenberg EV, Alberola-Ila J. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity. 2000;12:313–322. doi: 10.1016/s1074-7613(00)80184-3. [DOI] [PubMed] [Google Scholar]
- 43.Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 44.Sohn SJ, Forbush KA, Pan XC, Perlmutter RM. Activated p56lck directs maturation of both CD4 and CD8 single-positive thymocytes. Journal of immunology. 2001;166:2209–2217. doi: 10.4049/jimmunol.166.4.2209. [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Zheng M, Lei L, Ji J, Yao Y, Qiu Y, Ma L, Lou J, Ouyang C, Zhang X, He Y, Chi J, Wang L, Kuang Y, Wang J, Cao X, Lu L. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nature immunology. 2012;13:560–568. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 46.Patrussi L, Ulivieri C, Lucherini OM, Paccani SR, Gamberucci A, Lanfrancone L, Pelicci PG, Baldari CT. p52Shc is required for CXCR4-dependent signaling and chemotaxis in T cells. Blood. 2007;110:1730–1738. doi: 10.1182/blood-2007-01-068411. [DOI] [PubMed] [Google Scholar]
- 47.Kwan J, Killeen N. CCR7 Directs the Migration of Thymocytes into the Thymic Medulla. The Journal of Immunology. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 48.Ueno T, Hara K, Willis MS, Malin MA, Hopken UE, Gray DH, Matsushima K, Lipp M, Springer TA, Boyd RL, Yoshie O, Takahama Y. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 49.Fink PJ. The Biology of Recent Thymic Emigrants. Annual Review of Immunology. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 50.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11:544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 52.McFarland KN, Wilkes SR, Koss SE, Ravichandran KS, Mandell JW. Neural-specific inactivation of ShcA results in increased embryonic neural progenitor apoptosis and microencephaly. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7885–7897. doi: 10.1523/JNEUROSCI.3524-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 54.Compston A, Coles A. Multiple sclerosis. The Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 55.Omri B, Crisanti P, Alliot F, Marty MC, Rutin J, Levallois C, Privat A, Pessac B. CD4 expression in neurons of the central nervous system. Int Immunol. 1994;6(3):377–385. doi: 10.1093/intimm/6.3.377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.