Abstract
A challenge to civilization is the growing incidence in the loss of sight and cognition due to increased life expectancy. Therefore, we are confronted with a rise in the occurrence of photoreceptor- and neuronal-survival failure, as reflected mainly by age-related macular degeneration (AMD) and Alzheimer's disease (AD). Nervous system development is driven by neuronal apoptotic cell death and, thereafter, for the entire lifespan of an organism, neurons are post-mitotic cells. In neurodegenerative diseases, apoptosis and other forms of cells death lead to selective neuronal loss. Although age is the main risk factor, not everyone develops these diseases during aging. Despite decades of important findings about neuronal cell death, the specific mechanisms that regulate neuronal survival remain incompletely understood.
Keywords: Neuroprotection, Docosahexaenoic acid, Neuroprotectin D1, Neuroinflammation, Neurodegenerations, Alzheimer's disease
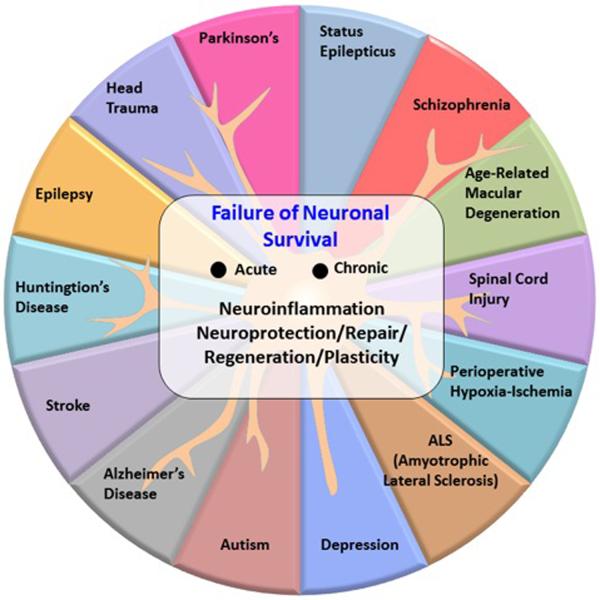
The theme of neuronal survival is a fundamental way to address molecular principle and consequences of dysregulated networks of neuroinflammatory signaling responses to impending disruptions in homeostasis. In addition to AMD and AD, diverse neurological and even some psychiatric diseases involve, as a common event in their initiation and early progression, failure in neuronal survival (Fig 1). Some of these conditions are acute, such as stroke and traumatic brain injury. In other cases, neurons fail to survive in selective areas of the brain in a chronic fashion, such as is the case of Parkinson's disease. Because the bioactivity of docosahexaenoic acid (DHA)-derived mediators known as docosanoids are one of the drivers behind responses at the top of the neuronal survival-signaling pyramid, we and others [1] began exploring the significance of DHA-derived docosanoids as a key to unraveling molecular principles for neuronal survival when cells are confronted with adversities.
Fig. 1.
A failure in neuronal survival takes place in several acute and chronic neurological and psychiatric disorders. A complex series of signaling dysfunctions that comprise neuroinflammation take place early on.
Cellular membranes of the nervous system feature phospholipids richly endowed with DHA acyl chains. Thus the structure, organization and functions of these membranes, particularly the conformation and activity of proteins such as receptors and ion channels are influenced by the environment conformed by pools of highly unsaturated phospholipids. This is the case with presynaptic, postsynaptic, mitochondrial, photoreceptor membranes, or others which display enrichment in unsaturated phospholipids in various proportions. How these pools are segregated and organized in neural cell membranes it is not understood. One membrane selective route of DHA-phospholipids is the ELOV4-mediated elongation pathway, which leads to the formation of very long chain polyunsaturated fatty acids (VLC-PUFAs) such as 38:6 omega 3, which, in turn, results in unique molecular species of phospholipids that establish an intimate relationship with rhodopsin [2]. Moreover, in AMD and in inherited retinal degenerative diseases, a drastic decrease in these VLC-PUFAs has been found [3]. Photoreceptor membranes contain the highest amounts of DHA of the human body and tenaciously retain it during outer segment daily shedding, phagocytosis and recycling [1]. In brain ischemia and at the onset of seizures, phospholipases A2 are activated that, in turn, cleave the docosahexaenoyl chain from phospholipids, creating a free DHA pool that is used to form docosanoids or to be reacylated [4]. Neurotrophins such as BDNF, NGF and PEDF activate synthesis of neuroprotectin D1 (NPD1) (10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid) [5], implying that a phospholipid pool containing DHA was targeted for hydrolysis and that the fatty acid was then channeled to the appropriate enzyme for NPD1 biosynthesis. Even though the significance of phospholipid signaling is becoming clearer, we still have major gaps in our understanding of the molecular interactions that underlie these critical events for neuronal survival. Docosanoids include mediators that promote tissue repair, phagocytic clearance and homeostasis, and that are active participants of an effective, active, well-concerted process of homeostasis restoration after unresolved neuroinflammation resolution; they comprise NPD1, resolvins D1 and D2 (RvD1, RvD2), and maresins [6].
Fundamental questions of molecular principles
The question stated in the title of this review is motivated in part by the following issues: a) in inheritable neurodegenerative diseases, why doesn't the disease manifest during latency?; b) does a cell-specific initial response/s counteract the consequences of mutation/polymorphism expression?; and c) why can the latency period last for decades, for example, in inherited familial forms of Alzheimer's disease and in retinal degenerations including AMD? Because early responses to neurodegenerative diseases engage uncompensated oxidative stress and neuroinflammation, corollaries to these questions are whether a neuroadaptation failure response is involved and also whether there is an impediment to membrane encoding of information for retention and/or release of specific mediators. There are many factors involved, including developmentally-expressed genes, since most of the inherited neurodegenerative diseases remain asymptomatic during development and maturation of the nervous system. Our lab began deciphering aspects of the molecular logic that sustain neuronal survival by uncovering molecular principles (transcriptional signatures) governed by the docosanoid NPD1. Many issues remain to be explored, including the decision-making process involved in storage specificity/retrieval of lipid mediators and the molecular sensors in early stages of neurodegenerations.
How does the nervous system counter disruptions of homeostasis?
The cellular molecular responses of the brain and the entire body to early stages of AD and other neurodegenerations are incompletely understood. Neuroinflammation has a myriad of cellular and molecular components, many of which are necessary for the functional integrity of the nervous system. Nevertheless, when responses go beyond a certain threshold, they became cytotoxic and/or interfere with homeostasis. For example, oxidative stress is needed for cell functions, however uncompensated oxidative stress is a central disruptor of homeostasis. There are several counteracting signals that respond to set in motion neuroprotection, such as repair, plasticity responses, and regenerative induction of stem cells.
In pursuit of the identification of early mechanisms that are set in motion, we have explored how the hippocampus responds in patients with early stages of AD. For example, in the CA1 area, a substantial drop occurs in the abundance of the lipid mediator NPD1 as well as in the expression of the enzymes that catalyze its synthesis: cytosolic phospholipase A2 and 15-lipoxygenase [7]. It is of interest that these changes were selective for the CA1 area and that the donors were patients undergoing early disease stages (limited neuronal loss) and were sampled within 3 hours postmortem. This finding has provided initial validation to the concept that key signaling mechanisms may fail early in AD pathogenesis to sustain homeostasis. Although neuroinflammation has been linked to AD, this is one of the first specific molecular event to be identified for counteracting neuroinflammation that seems to fail.
Systemic chronic inflammation and the brain
Another aspect that deserves attention is the association of chronic peripheral inflammation with the central nervous system (CNS), and of the signaling events that might be harnessed to control consequences of systemic inflammation in AD and other neurodegenerative diseases. The evolving concept is that inflammation resolution engages biosynthesis of endogenous lipid mediators that facilitate the enhancement of cell survival proteins to restore homeostasis. Thus, neuro-lipidomic signaling from DHA-derived lipid mediators would positively modulate unresolved inflammation, microglial activation, uncompensated oxidative stress, failure in the availability of protective proteins, high levels of Aβ peptide, Tau hyperphosphorylation (Tau-HP), neuronal network dysfunctions [8–12], and, in turn, progressive cognitive deficits.
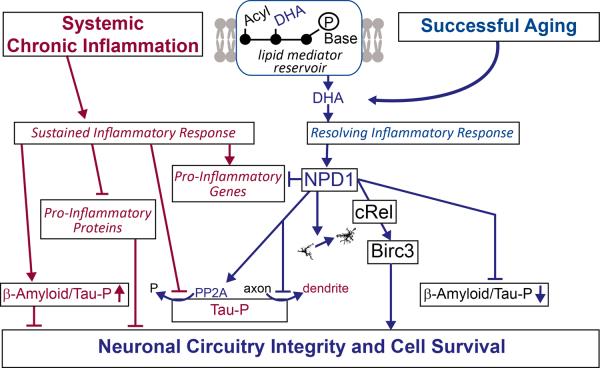
Alzheimer's disease and other neurodegenerative diseases have multifactorial co-morbidities that amplify disease onset and progression. Certain co-morbidities, such as chronic systemic inflammation, are well-defined factors that heighten AD [13–16]. Also, the onset of AD is accelerated by neuroinflammation [17, 18] and is enhanced by systemic immune/inflammatory dysfunctions [19] that trigger early events resembling AD pathology [13, 14]. Since neurodegenerative diseases often involve systemic inflammation [20–22], it is important to understand the significance of DHA-derived lipid mediators that exert inflammation resolution in an AD model undergoing systemic chronic inflammation. We predict that a factor that contributes to heightening AD onset and progression is the sluggish synthesis of docosanoid mediators (Fig.2). Thus systemic chronic inflammation would perturb the ability of cells to synthesize resolving lipid mediators that, in turn, facilitate a non-resolving inflammatory response with downstream negative consequences (Fig.2). A corollary of these predictions is that administered DHA will make the precursor of docosanoids accessible to cells. As a consequence, the synthesis of lipid mediators would be increased and foster a resolving inflammatory response (Fig.2); this, in turn, would counteract systemic chronic inflammation-mediated perturbations in AD. In spite of these observations, we do not yet know the nature of the possible perturbations that may occur during the synthesis of these mediators.
Fig. 2.
Systemic inflammation induces a non-resolving inflammatory milieu (red), whereas successful aging facilitates a counteractive, inflammatory resolving response (blue). The DHA-derived mediator NPD1 induces cRel and BIRC3. NPD1 redirects βAPP processing to the non-amyloidogenic route and inhibits Tau-HP.
The liver plays a key role in supplying DHA to the central nervous system
Omega-3 fatty acids from the diet, linolenic and DHA are taken up by the liver. The liver is endowed with active enzymes to elongate and introduce double bonds in linolenic acid, leading to the formation of DHA. This fatty acid is then shuttled to the nervous system, where it becomes acylated in phospholipids that, in turn, are used in building cell membranes [23]. Since DHA is avidly retained in the nervous system after its arrival, understanding the mechanisms governing such a process is critical for discerning the storage specificity of DHA – the precursor of bioactive docosanoids. The DHA-derived mediator NPD1 induces BIRC3 and other proteins. Decreased abundance of certain proteins results in βAPP processing to yield β-amyloid and Tau-HP. In contrast, induction of certain proteins is neuroprotective, preventing these changes. NPD1 redirects βAPP processing in the non-amyloidogenic route [24].
Lipid-mediated neuronal specific transcriptional modulation
NPD1 induces selective neuronal transcriptional upregulation of c-Rel followed by upregulation of neuronal BIRC3 (baculoviral IAP-inhibitor of apoptosis protein-repeat containing 3), which in turn leads to neuronal cell survival. It is of interest that, after NPD1-medaited c-Rel transcription, nuclear translocation occurs. These events were identified in human retinal pigment epithelial (RPE) cells and in the brain [25]. RPE cells are non-dividing cells and a component of the retina, an integral part of the CNS. Moreover, systemically-administered DHA results in enhanced brain NPD1 biosynthesis, and selective neuronal BIRC3 abundance takes place after experimental ischemic stroke, which is followed by remarkable neurological recovery. cREL is a mediator of NPD1-signaling induction of BIRC3 expression. Our studies reveal an unexpected connection between the lipid mediator and transcriptional regulation by which cREL synthesis and nuclear translocation are activated, followed by selective BIRC3 transcription [25]. This protein displays three BIR domains, as well as a carboxy (C)-terminal RING finger domain depicting ubiquitin ligase (E3) activity [26, 27]. There is little data available on this protein in the CNS. However, based on our results, it is tempting to postulate that the selective upregulation of neuronal BIRC3 by DHA, using cREL as an intracellular messenger in a model of ischemiareperfusion, reveals a transcriptional signature that might underlie a key molecular principle fostering neuronal survival.
Concluding remarks
The concept guiding our current work seeks to identify the molecular logic that supports neuronal functional integrity and survival. Phospholipid signaling stemming from reservoirs of bioactive mediators modulates neuronal transcriptional programs that sustain connectivity and plasticity at the level of neuronal circuits [1]. Moreover, the early engagement of synapses takes place as an early site of dysfunction in neurodegenerative diseases; at the same time, crosstalk of NMDA receptors and resulting signaling plays a role in neuroprotective signaling [28]. The significance of docosanoids in the crosstalk between inflammation and brain circuitry in AD, as counteracted by DHA lipid signaling that sets in motion inflammatory resolution exacerbated by systemic inflammation, is just beginning to be explored [7, 13, 14, 29, 30]. We are learning whether these target genes play a functional role in neuronal survival. DHA counteracts the loss of network connectivity, preventing altered molecular events that lead to synaptic dysfunction and loss of post-synaptic integrity (dendritic spines) to neuronal network aberrant activity that can spread trans-synaptically. Taken together, the evolving data provides evidence that DHA-mediated signaling limits inflammation and slows down the course of early critical disruptions in hippocampal circuits related with AD. Clearly, neuroinflammation induces deterioration of brain functions in AD by increasing dendritic spine vulnerability that leads to neuronal network dysfunction. The understanding of additional specific events and molecular principles that connect unresolved inflammation, microglial activation, uncompensated oxidative stress, failure in the availability of protective proteins, high levels of Aβ peptide, Tau hyper-phosphorylation, neuronal network dysfunctions leading to progressive cognitive deficits is a task for study in various laboratories during the foreseeable future.
In addition, further studies need to be conducted to determine the potential significance of the conditional ablation of neuronal survival proteins in neurodegenerations and to define redundancy mechanisms. This will then allow for future research to determine whether there is redundancy and synergies of inflammation-resolution mediators that target systemic inflammation in AD, and to validate changes observed in lipid mediators through non-invasive assessment of neuronal networks. This could eventually lead to the discovery of possible effective modes of administration for DHA or specific mediators to resolve impending systemic and local inflammatory conditions. Thus, a preventive as well as therapeutic paradigm shift in neurological and retinal diseases may evolve.
Acknowledgements
This works was supported by National Institutes of Health grants GM103340, NS046741 and EY005121 (NGB).
Footnotes
Conflict of Interest: The author declares no conflict of interest.
References
- 1.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aveldaño MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–39. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 3.Logan S, Agbaga MP, Chan MD, Kabir N, Mandal NA, Brush RS, Anderson RE. Deciphering mutant ELOVL4 activity in autosomal-dominant Stargardt macular dystrophy. Proc Natl Acad Sci U S A. 2013;110:5446–51. doi: 10.1073/pnas.1217251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104:13152–7. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepulveda-Falla D, Glatzel M, Lopera F. Phenotypic profile of early-onset familial Alzheimer's disease caused by presenilin-1 E280A mutation. J Alzheimers Dis. 2012;32:1–12. doi: 10.3233/JAD-2012-120907. [DOI] [PubMed] [Google Scholar]
- 9.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–40. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–40. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw J, Saling M, Hopwood M, Anderson V, Brodtmann A. Fluctuating cognition in dementia with Lewy bodies and Alzheimer's disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75:382–7. doi: 10.1136/jnnp.2002.002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra A, Goel RK. Age dependent learning and memory deficit in Pentylenetetrazol kindled mice. Eur J Pharmacol. 2012;674:315–20. doi: 10.1016/j.ejphar.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Sarlus H, Höglund CO, Karshikoff B, Wang X, Lekander M, Schultzberg M, Oprica M. Allergy influences the inflammatory status of the brain and enhances tau-phosphorylation. J Cell Mol Med. 2012;16:2401–12. doi: 10.1111/j.1582-4934.2012.01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarlus H, Wang X, Cedazo-Minguez A, Schultzberg M, Oprica M. Chronic airway-induced allergy in mice modifies gene expression in the brain toward insulin resistance and inflammatory responses. J Neuroinflammation. 2013;10:99. doi: 10.1186/1742-2094-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McManus RM, Higgins SC, Mills KH, Lynch MA. Respiratory infection promotes T cell infiltration and amyloid-β deposition in APP/PS1 mice. Neurobiol Aging. 2014;35:109–21. doi: 10.1016/j.neurobiolaging.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Cheng XS, Zhao KP, Jiang X, Du LL, Li XH, Ma ZW, Yao J, Luo Y, Duan DX, Wang JZ, Zhou XW. Nmnat2 attenuates Tau phosphorylation through activation of PP2A. J Alzheimers Dis. 2013;36:185–95. doi: 10.3233/JAD-122173. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FMJ. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. Neurosci. 2005;25:8843–53. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao X, Cummins DJ, Paul SM. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur J Neurosci. 2001;14:474–82. doi: 10.1046/j.0953-816x.2001.01666.x. [DOI] [PubMed] [Google Scholar]
- 19.Reale M, Iarlori C, Feliciani C, Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. J Alzheimers Dis. 2008;14:147–59. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- 20.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 21.Leung R, Proitsi P, Simmons A, Lunnon K, Güntert A, Kronenberg D, Pritchard M, Tsolaki M, Mecocci P, Kloszewska I, Vellas B, Soininen H, Wahlund LO, Lovestone S. Inflammatory proteins in plasma are associated with severity of Alzheimer's disease. PLoS One. 2013;8:e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 23.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calandria JM, Asatryan A, Balaszczuk V, Knott E, Jun BK, Mukherjee PK, Belayev L, Bazan NG. NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Dis. 2004 doi: 10.1038/cdd.2014.233. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 27.Mace PD, Smits C, Vaux DL, Silke J, Day CL. Asymmetric recruitment of cIAPs by TRAF2. J Mol Biol. 2010;400:8–15. doi: 10.1016/j.jmb.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Stark DT, Bazan NG. Synaptic and extrasynaptic NMDA receptors differentially modulate neuronal cyclooxygenase-2 function, lipid peroxidation, and neuroprotection. J Neurosci. 2011;31:13710–21. doi: 10.1523/JNEUROSCI.3544-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis. 2013;35:697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]