Abstract
Health neuroscience is a new field that is at the interface of health psychology and neuroscience. It is concerned with the interplay between the brain and physical health over the lifespan. This review provides a conceptual introduction to health neuroscience, focusing on its major themes, representative studies, methodologies, and future directions.
Health Neuroscience: Definition and Scope
How does the brain influence physical health? How does physical health influence the brain? We propose that these are inseparable and open questions, and a new field at the interface of health psychology and neuroscience is poised to answer them—a field called health neuroscience. But what is this new field and what are its conceptual themes, goals, and methods? What are its challenges and opportunities moving forward? This review addresses these questions and highlights recent studies illustrating health neuroscience approaches to understanding the dynamic interplay between the brain and physical health over the lifespan.
Here we adopt the definition of health as the absence of physical or mental illness, disease, pain, or discomfort. However, we would expand on this by arguing that health could be viewed as the absence, or paucity, of physiological (e.g., insulin resistance), social (e.g., loneliness), cognitive (e.g., slow processing speed), or emotional (e.g., anxiety) risk factors. Our reasoning is that these may explain the etiology, prevention, and progression of disease and specific endpoints. As discussed below, we distinguish physical health from mental health with the awareness that there are ambiguous boundaries, and significant comorbidities, between physical and mental health conditions (i.e., between cardiovascular disease (CVD) and depression) along with clear physical (i.e., biological) substrates for mental health conditions (see below). These definitions provide some clarity to what we view as the defining attributes of health neuroscience (see below).
With several ongoing international efforts to better understand brain function (e.g., Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative), we can expect that neuroscience and its methodologies will continue to play central roles in psychology. Indeed, the widespread integration of neuroscience into the fabric of psychology has been fostered over the last few decades by the rapid ascendance of neuroimaging technologies and their applications to the study of cognition, emotion, social behavior, personality, psychopathology, development, aging and other areas of inquiry. Over this same time period, we have gained a deeper and multilevel understanding of the biological, psychological, and social (biopsychosocial) determinants of physical health. This understanding has been enabled by the growth of health psychology and its allied areas of research and clinical practice (e.g., social epidemiology and behavioral medicine). Notwithstanding the ascendance of neuroscience and the simultaneous—but largely independent—traction of health psychology, we still lack answers to many fundamental questions about the brain and physical health. Accordingly, we believe that now is the time to formally define a new and integrative field of health neuroscience.
More precisely, health neuroscience can be defined as an emerging field focused on understanding how the brain affects and is affected by physical health. This definition artificially splits the brain from the other organs of the body; however, we make this conceptual separation because both research and education in neuroscience and psychology has traditionally been conducted independently from other fields of physiology and medicine. It is precisely this false distinction between the brain and body that research in health neuroscience is positioned to refute. Thus, to test hypotheses about the inter-dependent nature of the brain and body we necessarily distinguish them in our definition of health neuroscience. From this perspective, health neuroscience is thematically rooted in health psychology, while adopting neuroscience methodologies for studying brain function and structure in human and non-human animal models. It can be distinguished from the broader area of health psychology in that it has primary theoretical and empirical foci on the brain, and it differs from other areas of neuroscience in its primary focus on physical health. A chief goal of health neuroscience is to characterize bidirectional and dynamic brain-behavior and brain-physiology relationships that are determinants, markers, and consequences of physical health states across the lifespan. The motivation behind this goal is that a better understanding of these relationships will provide mechanistic insights into how the brain links multilevel genetic, biological, psychological, behavioral, social, and environmental factors with physical health—especially vulnerability to and resilience against clinical illnesses. Moreover, such mechanistic insights will also provide new cross-disciplinary platforms to develop brain-based prevention and intervention efforts to improve physical health, inform health policies, and promote successful development and aging.
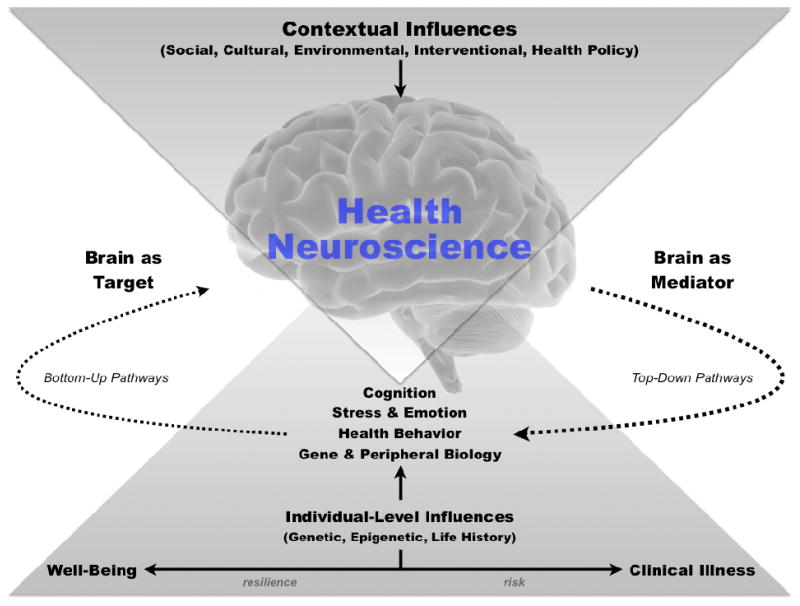
Figure 1 illustrates key concepts of health neuroscience. Here, the brain is considered as the central organ that affects and is affected by states of health, which span a continuum from optimal well-being to clinical illness. In this regard, heath neuroscience research may conceptualize the brain as the primary or ‘top-down’ determinant of downstream mediating processes that proximally influence physical health states (right side of Figure 1). Downstream mediating processes would thus be considered as emergent phenomena under the control of the brain, and could include processes related to cognition and decision-making, stress and emotion, health behaviors, and facets of peripheral physiology. Likewise, health neuroscience approaches may conceptualize the brain as a target organ that is affected by health states via ‘bottom-up’ pathways. For example, health neuroscience studies may examine changes in brain structure and function that result from smoking, systemic inflammation, or other factors related to physical health (left side of Figure 1; and see below for more examples). Finally, health neuroscience approaches conceptualize these brain↔health relationships and pathways as subject to the contextually modifying influences of social, cultural, environmental, and other higher-level factors, as well as the modifying influences of life histories, genetics, and other individual-level factors (see top and bottom of Figure 1). In sum, health neuroscience studies may operationalize direct measurements of brain function and structure (e.g., from neuroimaging or electrophysiological recording methods) as predictor (independent) or outcome (dependent) variables, depending on the particular brain↔health relationship(s) under investigation or targeted by intervention.
Figure 1.
Health neuroscience is an interdisciplinary field at the interface of health psychology and neuroscience. Thematically, health neuroscience is concerned with understanding how the brain influences and is influenced by physical health across the lifespan—extending along a continuum shown at the bottom of the figure of optimal states of health and well being to states of disease risk, symptom expression, and clinical illness. Distal contextual influences at the top of the figure are viewed to impact physical health via downstream effects that are mediated by the brain, including social (e.g., familial and peer networks), cultural (e.g., valued group identities and shared practices), environmental (e.g., counties, neighborhoods, workplaces, etc.), interventional (e.g., efforts to change physical activity, diets, lifestyles, psychological states, etc.), and health policy (e.g., laws affecting the distribution of health resources, public health messaging and campaigns, etc.) influences. Proximal influences at the bottom of the figure are viewed to impact physical health via direct and interactive effects on the brain, as well as via mediating processes that affect and are affected by the brain, including genetic, epigenetic, developmental, and aging influences. Processes that bi-directionally and dynamically link the brain to states of health throughout life include factors that are widely studied in health psychology, but also studied historically in separate fields of study; namely, cognitive, stress, emotion, health behavioral, peripheral physiological, and gene expression processes. Health neuroscience studies are diverse and integrative, insofar as these processes are viewed as being regulated by the brain via top-down (efferent) pathways and as influencing the brain via bottom-up (afferent) pathways. In this way, health neuroscience studies conceptualize measurements of brain function and structure as outcome variables that are dependent on bottom-up pathways and as independent variables that determine health processes via top-down pathways.
There may be questions about the position of mental health (i.e., psychopathology) within the definition of health neuroscience. Indeed, research on psychopathology and mental health may be included as a part of health neuroscience to the extent that the research questions involve the examination of reciprocal associations or comorbidities between mental and physical health. For example, psychopathology (e.g., depression) is often coexistent, and may predict health outcomes (e.g., obesity) or be modified by health behaviors (e.g., physical activity and reduced risk for depression). Insofar as the neural substrates mediating these relationships are of interest, such topics would fall under the auspices of health neuroscience.
Empirical Illustrations
To illustrate applications of the above concepts we will highlight health neuroscience studies and end by considering some major methodological, computational, and conceptual challenges and opportunities in health neuroscience.
Stress, Emotion, and Social Factors
For decades, health psychologists have been interested in the connection between physical health and stress, emotion, and a vast spectrum of social factors (e.g., Adler & Matthews, 1994). Such interest has led to conceptualizations of psychological and social determinants of health that emphasize health-related behaviors and peripheral biological mediators as common downstream pathways to health endpoints (e.g., Cohen, Janicki-Deverts, & Miller, 2007). For example, anger, anxiety, and depression are thought to confer risk for cardiovascular disease by leading to disadvantageous health behaviors (e.g., smoking) and to alterations in systemic inflammation, neuroendocrine outflow, and autonomic physiology that adversely impact the heart and vasculature (Suls & Bunde, 2005). A health neuroscience approach builds on this conceptual framing in several ways. First, as indicated on the right side of Figure 1, health neuroscience studies are integrating theoretical models and empirical findings from social, cognitive, and affective neuroscience to consider the brain as the central, top-down regulator (i.e., determinant) of behaviors and parameters of peripheral physiology that impact physical health (e.g., B.S. McEwen & Gianaros, 2010). Second, these models and findings consider stress, emotion, and social processes as being functionally instantiated in neural circuits that also influence health behaviors and peripheral physiology (e.g., Eisenberger & Cole, 2012). Finally, as illustrated on the left side of Figure 1, health neuroscience studies are beginning to consider the bottom-up influences of health behaviors and peripheral physiology on brain systems and circuits that mediate stress, emotion, social, and other behavioral processes (e.g., Critchley & Harrison, 2013).
To elaborate, recent studies incorporating peripheral physiological recordings have shown that exaggerated cardiovascular reactions (e.g., large rises in heart rate and blood pressure) to acute psychological stressors (e.g., time-pressured cognitive tasks with negative feedback and social evaluative threat paradigms) are associated with concurrent alterations in stress-induced neural activity within the amygdala (Gianaros et al., 2008) and medial prefrontal cortex (Wager et al., 2009). Importantly, exaggerated cardiovascular reactions have been established by health psychologists to confer risk for cardiovascular disease (Chida & Steptoe, 2010). And both the amygdala and anatomically-networked regions of the medial prefrontal cortex participate in stress- and emotion-related processes, as well as in the regulation of peripheral physiology (Phelps & LeDoux, 2005; Roy, Shohamy, & Wager, 2012). Accordingly, health neuroscience findings are furthering our understanding of the brain systems involved in stress-related factors (cardiovascular stress reactions) important for physical health (e.g., cardiovascular disease). Moreover, such health neuroscience research has been extended to show that neural activity changes within the anterior cingulate cortex, as evoked by the cognitive regulation of negative emotions, associate with the severity of preclinical atherosclerosis in major blood vessels and that this association is mediated by systemic inflammation (Gianaros, Marsland, Kuan, et al., 2013). This work identifying a brain-body pathway to atherosclerosis complements a growing movement in health psychology toward emotion regulation interventions to improve physical health and is consistent with some models of allostatic load that describe the impact of stress on the brain, mind and body (B. S. McEwen & Gianaros, 2011) (McEwen & Gianaros, 2011). In addition to health neuroscience studies of stress and emotion, there is emerging work on social factors and interpersonal processes known to predict wide ranging health outcomes, including work on socioeconomic status (Gianaros, Marsland, Sheu, Erickson, & Verstynen, 2013), social ties and support (Eisenberger, 2013), and social discrimination (Akdeniz et al., 2014). Collectively, the work highlighted above promises to increase our brain-based understanding of how stress, emotion, and social factors “get under our skin” to influence our physical health (Miller, Chen, & Cole, 2009).
Health Behaviors
Health psychology holds a longstanding interest in health behaviors that increase (e.g., smoking) or decrease (e.g., mammography screening) health risks. Emerging health neuroscience studies extend this interest to conceptualize the brain as both a determinant and target of behaviors linked to physical health. For example, greater brain activity evoked by rewarding food cues predicts subsequent weight gain over six months (Demos, Heatherton, & Kelley, 2012). Moreover, weight gain may subsequently alter the function of brain systems that are responsive to food-related reward cues: women who gained weight over a six-month period had reduced striatal reward responses when consuming a milkshake (Stice, Yokum, Blum, & Bohon, 2010). Moreover, recent work suggests that weight gain negatively affects the structure of gray (Raji et al., 2010) and white matter tissue (Verstynen et al., 2013; Verstynen et al., 2012), illustrating bottom-up influences of physical health states on the brain.
Emerging neuroscience research is also informing psychological models of health behavior change. For example, increased neural activity in brain regions important for inhibitory-control processes predict smoking cessation among smokers attempting to quit (Berkman, Falk, & Lieberman, 2011). The brain may also mediate the efficacy of targeted health messages for health behavior change. For example, greater activity in the medial prefrontal cortex predicts subsequent sunscreen use (Falk, Berkman, Mann, Harrison, & Lieberman, 2010), healthy food choices (Hare, Malmaud, & Rangel, 2011), and smoking cessation (Chua et al., 2011). Moreover, some of this work suggests that brain activity may predict broader success of national public health ad campaigns (Falk, Berkman, & Lieberman, 2012).
Interventions
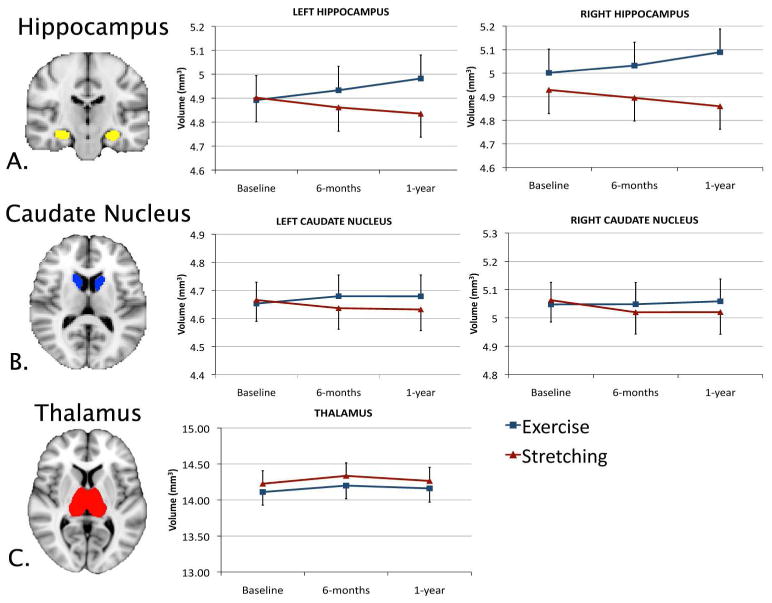
Recent studies are also characterizing the role of the brain in determining how behavioral interventions (e.g., aerobic exercise, mindfulness meditation training) improve health. For example, randomized controlled trials (RCTs) of aerobic exercise training may increase hippocampal volumes ((Erickson et al., 2011) Figure 2), functional correlations in the activity within brain networks (Voss et al., 2010) and task-related functional activity in regions involved in executive functions, memory, and attention (Colcombe et al., 2004). Importantly, these changes to the brain account for exercise-related improvements in cognitive performance (Erickson et al., 2009; Erickson et al., 2011), which have implications for understanding how health behaviors associate with dementia and memory problems in adulthood via brain-body pathways (Erickson et al., 2010). Recent RCTs also suggest that mindfulness meditation interventions can reduce pain and stress reactivity, possibly via functional alterations in cortical and limbic brain circuits important for stress and emotion regulation (Creswell, 2014). These are only some of many other examples (i.e., pain management; (Hong et al., 2013)) that illustrate how health neuroscience approaches are extending the study of major topics in health psychology, particularly by conceptualizing and considering the bidirectional relationships between the brain and health behaviors.
Figure 2.
In this figure, Erickson and colleagues (2011) demonstrated that a 1 year randomized exercise intervention resulted in an increase in the size of the hippocampus relative to the control group, but no significant changes in the size of the caudate nucleus or thalamus. This example illustrates how a health behavior intervention (i.e., exercise) affects brain structure in ways that predict cognitive functioning.
Methodological and Computational Considerations
The conceptual and empirical goals of health neuroscience engender multilevel and often expansive methodological approaches—which, in a single study, may span genetic, molecular, organ systems, psychological, behavioral, social, and environmental levels of analysis. Accordingly, the computational challenges posed by health neuroscience may be expanded in size and complexity relative to other fields of psychology and neuroscience. This is particularly true when employing popular neuroimaging tools and analysis pipelines for studying brain↔health relationships. Most “off-the-shelf” analytical tools for neuroimaging data are tasked with estimating functional responses or morphological (structural) features of volumetric parcels of space resulting in up to several hundreds of thousands of correlated observations per subject. This not only results in challenges to control for the possibility of reporting chance findings and other quantitative issues affecting study inferences, but also forces health neuroscientists to confront so-called “Big Data” problems. Thus, in size alone, typical neuroimaging data sets can easily run into several gigabytes of data per person. When scaled to larger sample sizes typical of health psychology studies (e.g., hundreds of subjects), this can easily mushroom into terabytes or more of data per study. Indeed, the challenge of scaling to population-level neuroscience studies is an immensely complex topic that has been detailed elsewhere (Falk et al., 2013). Computational challenges may be further compounded by the use of analytic approaches that examine both individual brain areas and neural network dynamics across hundreds or thousands of connected brain areas. Furthermore, many questions in health neuroscience are often mediational in nature (e.g., Does the brain mediate the relationship between a health behavior intervention and improvements in physical health?). This means applying computationally demanding techniques often used in health psychology, like mediation analysis and structural equation modeling, to neuroimaging data, which could result in estimating hundreds of thousands of hierarchical or mediational models across the brain. In fact, such techniques have already been used to elucidate complex relationships like the influence of psychosocial factors on the neural representation of pain (Atlas, Bolger, Lindquist, & Wager, 2010) or the influence of social and physiological systems on global myelin integrity (Verstynen et al., 2013). These analytical approaches, along with machine learning techniques designed to refine hypothesis generation on neuroimaging data sets (Voytek & Voytek, 2012; Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) can dramatically expand the utility of assessing complex brain-health relationships.
Opportunities and Future Directions
As health neuroscience continues to grow, we anticipate an expansion of our knowledge about the role(s) of the brain in physical health and the reciprocal relationships between physical and mental health via brain pathways. Such knowledge will be driven in large part by questions that are cross-fertilized by interdisciplinary perspectives. For example, common questions asked in cognitive neuroscience (e.g., What are the neural correlates of response inhibition?) can be extended as health neuroscience questions (e.g., Do the neural correlates of response inhibition relate to health behaviors, such as smoking cessation? (Berkman et al., 2011)). Likewise, traditional social-affective neuroscience questions on the neural correlates of emotion regulation can be extended to questions about how the neural systems supporting emotion regulation also relate to health relevant aspects of hypothalamic-pituitary-adrenal axis functioning (Urry et al., 2006) or behavioral treatment efficacy (Lieberman et al., 2004). Hence, what will define creative future health neuroscience approaches and questions is whether there is a brain-based focus on physical health mediators and outcomes.
As this field grows, we also anticipate the continued refinement of conceptual and analytical perspectives with respect to the parent disciplines of health psychology and neuroscience and allied fields of study. We maintain that in health neuroscience, the brain can be thought of as an outcome, or dependent, variable—but in a unique interpretive framework. Thus, some studies noted above have examined the extent to which health behaviors (e.g., engagement in exercise) influence brain morphology, function, or integrity (Figure 2). However, in addition to conceptualizing the brain as an outcome, health neuroscience also views the brain as a potential mediator of health outcomes or as a predictor of health behaviors (Figure 1). Thus, health neuroscience envisions the brain as an important node that could be positioned either as a predictor, mediator, or outcome depending on the particular framework of the research question being studied. And, we expect that work on the horizon will fill in the details of this general conceptual framework.
We acknowledge that we selectively reviewed only a manageable subsample of possible studies illustrative of health neuroscience approaches and that there are other avenues and directions by which to incorporate neuroscience methods, theories, and models into topics of health psychology. We fully recognize the importance of integrative questions, methods, and findings from other disciplines relevant to health neuroscience and also recognize the importance of circumspect interpretations of studies that are correlational in nature and the need for methodological rigor when designing and interpreting the results from health neuroscience studies.
Over a decade ago, Sung and colleagues (Sung et al., 2003) argued that the most exciting science in the 21st century is likely to evolve among, not within, traditional disciplines. As a field situated among the traditional disciplines of health psychology and neuroscience, our hope is that our initial description of health neuroscience results in the growth of this exciting interdisciplinary area. As this new field is defined and shaped, we face important questions about how to fund this research, how to effectively train individuals in both neuroscience and health, and to build programs of research that can offer mechanistic and translational impacts. Nonetheless, health neuroscience investigations are poised to address longstanding questions in both health psychology and neuroscience—by helping the field develop new mechanistic models, and in understanding factors that confer risk and protection for physical health outcomes. In doing so, health neuroscience can have a significant impact on improving and transforming public health.
Acknowledgments
This research was supported grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK095172), the National Heart Lung and Blood Institute (HL089850)
Contributor Information
Kirk I. Erickson, Department of Psychology and Center for the Neural Basis of Cognition, University of Pittsburgh
J. David Creswell, Department of Psychology and Center for the Neural Basis of Cognition, Carnegie Mellon University.
Timothy D. Verstynen, Department of Psychology and Center for the Neural Basis of Cognition, Carnegie Mellon University
Peter J. Gianaros, Department of Psychology and Center for the Neural Basis of Cognition, University of Pittsburgh
Recommended Reading
- Adler N, Matthews K. Health psychology: why do some people get sick and some stay well? Annu Rev Psychol. 1994;45:229–259. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- Akdeniz C, Tost H, Streit F, Haddad L, Wüst S, Schäfer A, Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority associated environmental risk. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychol Sci. 2011;22(4):498–506. doi: 10.1177/0956797611400918. This paper examines brain regions involved in self-control that are associated with smoking, craving, and the attempt to quit smoking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. HYPERTENSIONAHA.109.146621 [pii] [DOI] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14(4):426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD. Biological pathways linking mindfulness with health. In: Brown KW, Creswell JD, Ryan R, editors. Handbook on Mindfulness Science. New York, NY: Guilford Publications; 2014. [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. Social ties and health: a social neuroscience perspective. Curr Opin Neurobiol. 2013;23(3):407–413. doi: 10.1016/j.conb.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. This paper describes the results of an exercise intervention on hippocampal volume in older adults. One year of exercise increased the size of the hippocampus relative to the control group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Lieberman MD. From neural responses to population behavior: neural focus group predicts population-level media effects. Psychol Sci. 2012;23(5):439–445. doi: 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010;30(25):8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, Schulenberg J. What is a representative brain? Neuroscience meets population science. Proc Natl Acad Sci U S A. 2013;110(44):17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, Manuck SB. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013;23(9):2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31(30):11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Mayer EA. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33(29):11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22(1):447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. NYAS5331 [pii] This paper reviews human and non-human research on the impact of stress and physical health factors on brain pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Ann Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Sung NS, Gordon JI, Rose GD, Getzoff ED, Kron SJ, Mumford D, Kopell NJ. Science education. Educating future scientists. Science. 2003;301(5639):1485. doi: 10.1126/science.1086133. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, Sheu LK, Marsland AL, Gianaros PJ. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. Neuroimage. 2013;79:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74(7):682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek JB, Voytek B. Automated cognome construction and semi-automated hypothesis generation. J Neurosci Methods. 2012;208(1):92–100. doi: 10.1016/j.jneumeth.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist MA, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat, Part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]