Abstract
Nuclear receptor subfamily 1, group I, member 3 (NR1I3) is reported to be a possible novel therapeutic target for some cancers, including lung, brain and hematopoietic tumors. Here, we characterized expression of NR1I3 in a mouse model of lung carcinogenesis induced by 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone (NNK), the most potent tobacco carcinogen. Lung tumors were collected from mice treated with NNK (400 mg/kg) and euthanized after 52 weeks. Benign and malignant lesions were formalin-fixed and paraffin-embedded for histology and immunohistochemistry, with samples snap-frozen for mRNA analysis. Immunohistochemically, we found that most macrophages and type I and II pneumocytes expressed NR1I3, whereas fibroblasts and endothelial cells were NR1I3−. Compared with benign lesions, malignant lesions had less NR1I3+ tumor cells. Gene expression analysis also showed an inverse correlation between NR1I3 mRNA expression and tumor size (P=0.0061), suggesting that bigger tumors expressed less NR1I3 transcripts, in accordance with our immunohistochemical NR1I3 tests. Our results indicate that NR1I3 expression decreased during progression of malignant lung tumors induced by NNK in mice.
Keywords: NR1I3, CAR, NNK, Cyclin D1, Lung carcinogenesis
Introduction
Nuclear hormone receptors comprise a family of more than 50 different transcriptional factors that regulate a diverse range of functions in living cells. One of these receptors, the nuclear receptor subfamily 1, group I, member 3 (NR1I3, also known as the Constitutive Androstane Receptor, CAR), is an orphan nuclear receptor that was first characterized as a cell “xenosensor,” and considered a master regulator of drug metabolism and excretion (1). NR1I3 is also associated with energy and lipid metabolism (2) and hormonal regulation (3). Several chemical substances that could act as agonists or antagonists can activate NR1I3. After activation, NR1I3 translocates to the nucleus and dimerizes with NR2B1 (retinoid X-receptor alpha, RXR-alpha); the resulting heterodimer recognizes NR1I3-responsive elements through the genome, thus regulating expression of several genes (1).
The importance of NR1I3 in cancer became evident when promotion by phenobarbital of liver tumors in mice was shown to require it (4). NR1I3 was also considered a novel therapeutic target for brain and hematopoietic tumors (5,6). Our group recently found specific NR1I3 ligands to modulate antineoplastic effects of paclitaxel in non-small-cell lung cancer cell lines (7), which suggests that NR1I3 has a profound effect in cancer. Here, we aimed to characterize its presence and expression in lung tumors induced by the tobacco-specific nitrosamine 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone (NNK).
Material and Methods
Mouse model of lung cancer and tumor samples
This experiment was performed with samples from a previous published experiment (8), using only the wild-type phenotype. Briefly, male CD1/AJ mice were injected with NNK (2×200 mg/kg) at days 15 and 18 of life and were euthanized 54 weeks later with pentobarbital (250 mg/kg, ip). Lungs were excised for macroscopic evaluation; nodules were counted and measured (mm), carefully removed, and snap-frozen in liquid nitrogen. Additionally, representative sections of all lung lobes were fixed in formalin for histopathological and immunohistochemical (IHC) analysis. Lung sections were classified using the recommendations of the Mouse Models of Human Cancer Consortium (9). The current study used 20 frozen lesions and histological sections containing a total of 25 different neoplastic lesions (Table 1). All procedures using animals followed the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised in 1985), and were approved by the Bioethics Committee of Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo (#953/2006).
Mouse NR1I3 IHC
Lung sections were deparaffinized and re-hydrated in consecutive baths of xylene and ethanol. Endogenous peroxidase was blocked with 3% H2O2 in phosphate-buffered saline. Non-specific protein binding sites were blocked with 5% skim milk; slides were incubated with anti-NR1I3 (clone AB1, AV37028, Sigma-Aldrich, USA) overnight at 4°C, with IgG in the place of primary antibodies as the negative control. Slides were washed and exposed to streptavidin-biotin-peroxidase complex (LSAB, Dako, USA) according to the manufacturer's instructions, developed with 3,3-diaminobenzidine (DAB) and counterstained with hematoxylin. We used whole neoplasms to analyze histologic NR1I3 staining patterns. Cells were considered NR1I3+ when their nuclei and/or cytoplasm were stained. We counted ≥ 250 cells in each lesion (range: 279-3649 cells), depending on size.
Gene expression of NR1I3 and cyclin-D1
Gene expression analysis was performed as previously described by our group (8). Briefly, total RNA was isolated from frozen lung tumors (Perfect RNA, Eukaryotic, Mini, Eppendorf, Germany) following the manufacturer's instructions, and then quantified; 260/280 ratios were observed. Only samples presenting 1.7-2.0 and good quality (not degraded) after the electrophoresis analysis in an agarose gel (1.5%, Tris-buffered saline) were used. Thus, 1 µg of total RNA was reverse transcribed with oligo-DT primers and superscript II into cDNA. Cyclin-D1 and 18s primers were the same as previously used (8). Primers for NR1I3 were designed with Primer-3 software (10) and were run in BLAST (11) to verify the absence of local alignments with DNA and other mouse RNA transcript sequences. Power SYBR¯ Green (Life Technologies, USA) was used for real-time PCR with primers for NR1I3 (NM_009803.5; F: 5′-GGGCCTCTTTGCTACAAGAT-3′, R: 5′-AGGTTTTTATGGAAGTGGAGGA-3′); cyclin-D1 (F: 5′-CGTGGCCTCTAAGATGAAGG-3′, R: 5′-TTGAGCTTGTTCACCAGAAGC-3′) and 18s (F: 5′-CCTGCGGCTTAATTTGACTC-3′, R: 5′-CTGTCAATCCTGTCCGTGTC-3′) as the housekeeping gene. The reactions were carried out in an ABI Prism 7500 thermocycler (Life Technologies). Analysis of relative gene expression data used the ΔΔCT method (12).
Statistical analysis
We used Graphpad Prism 6 for Windows (Graphpad Software, USA) for statistical analysis, and the unpaired t-test and Spearman's tests for correlations. P<0.05 was considered significant.
Results
IHC detection of NR1I3 in normal and neoplastic lung tissue
We randomly selected 25 lung neoplastic lesions from lung histological sections of a previous experiment in which we determined the role of Gap Junction Alpha 1 in lung cancers induced by NNK (8). Histological classifications and numbers of these lesions are described in Table 1. We next used IHC to analyze NR1I3 expression in non-neoplastic and neoplastic tissue. In normal tissue, most macrophages and type I and II epithelial cells showed staining for NR1I3 (Figure 1). However, in epithelial lung neoplastic cells, NR1I3 staining varied from almost none in the cytoplasm to cytoplasm and nucleus positive staining (Figure 1). Interestingly, malignant lesions had significantly less NR1I3+ tumor cells than did benign lesions (P<0.001; Figure 2).
Figure 1. Immunohistochemical localization of NR1I3 in lung tumors of mice treated with 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone (NNK). A and B: Representative solid-type lung adenomas with intense staining for NR1I3 in cytoplasm and most nuclei. C: Solid-type lung adenocarcinoma with bronchiolar invasion. D: Higher magnification of invasive component. Note NR1I3 expression mostly in cytoplasm, and in nuclei of a few cells. E, Papillary lung adenocarcinoma. F, Higher magnification of malignant lesion showing few positive nuclei staining. Red bar: 250 μm; black bar: 25 μm.
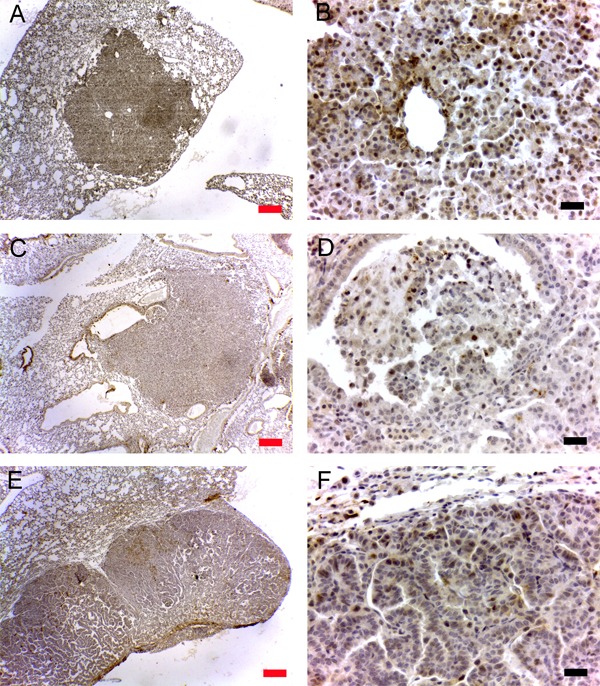
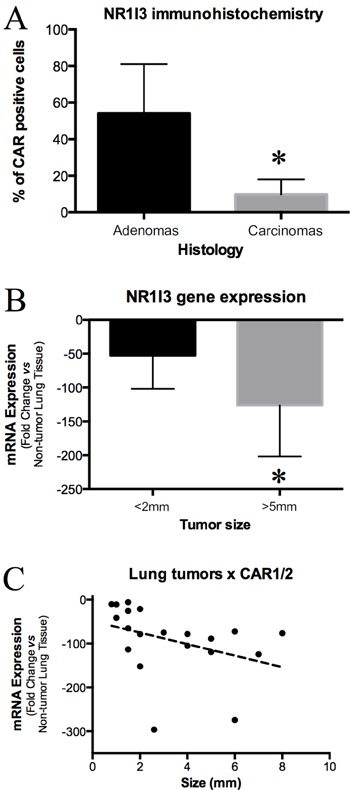
Figure 2. Detection of NR1I3 in lung tumors induced by 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone (NNK) in mice. A: Immunohistochemical detection of NR1I3 in lung adenomas and carcinomas. Note that carcinomas showed significantly less NR1I3 expression than did adenomas. B: NR1I3 mRNA expression showed an inverse correlation with tumor size. C: NR1I3 mRNA expression compared between lesions <2 mm and lesions >5 mm. Tumors >5 mm are all carcinomas and lesions <2 mm are all adenomas (Ref. 9). Note that lesions >5 mm expressed significantly less NR1I3 mRNA than did lesions <2 mm. *P<0.05 (unpaired t-test).
Gene expression of cyclin-D1 and NR1I3 in lung tumors
We evaluated NR1I3 and cyclin-D1 mRNA in 20 frozen lung tumors of different sizes, and found tumor size and cyclin-D1 expression to be significantly associated (P<0.01, not shown), indicating that larger tumors harbored more proliferating cells. Conversely, NR1I3 expression was inversely correlated with tumor size (P<0.01, Figure 2), suggesting that more aggressive tumors expressed less NR1I3. We also analyzed lesions by size, using the recommendations of the Mouse Models of Human Cancers Consortium (9), which found that macroscopic mouse lung lesions <2 mm are always adenomas and lesions >5 mm are always carcinomas (9). Thus, we excluded lesions of 2-5 mm and sorted the others into two groups: 10 lesions <2 mm and 6 lesions >5 mm. Although the carcinoma group expressed significantly more cyclin-D1 than did the adenoma group (P<0.01, not shown), the opposite was true for NR1I3 gene expression: malignant neoplasms expressed less NR1I3 than did benign lesions (P<0.05; Figure 2).
Discussion
The orphan nuclear receptor NR1I3 is mostly known for its effect in endo- and xenobiotic metabolism, typically by regulating expression of specific genes in the liver and other organs (13). However, its role in cancer has only started to be unraveled; as far as we know, this work is the first investigation into the presence and localization of NR1I3 in mouse lung tumors. Here, IHC showed lower NR1I3 expression in carcinomas than in adenomas, and NR1I3 mRNA expression was inversely associated with tumor size, indicating that NR1I3 expression decreases during malignant transformation in this mouse model.
As an intriguing option, Chen et al. (14) recently suggested that activation of NR1I3 and NR1I2 (pregnane-X receptor, PXR) by specific ligands, combined with chemotherapeutics might attenuate, or even overcome, multi-drug resistance in cancer cells. NR1I2 is another nuclear receptor that closely resembles NR1I3 in function and homology; it has a controversial role in cancer pathogenesis and multi-drug resistance (15,16). NR1I2 has been shown to suppress proliferation and tumorigenicity of colon cancer cells by regulating the E2F/RB and p21 pathways (17). Although NR1I2 activation has been shown to be anti-proliferative in p53 wild-type breast cancer cells (18), another study found NR1I2 activation to enhance neoplastic characteristics of both human colon tumor cell lines and primary human colon cancer tissue xenografted into immunodeficient mice (19).
Until recently, only two published studies found NR1I3 to be a possible molecular target for cancer therapy. Wang et al. (5) showed that targeting NR1I3 could improve cyclophosphamide (CPA)-based treatment of hematopoietic malignancies, by selectively promoting CPA bioactivation. Chakraborty et al. (6) found that CITCO - a human NR1I3 agonist - could target brain cancer stem cells, inhibiting their growth and expansion. Most recently, our group showed that specific NR1I3 ligands modulate the antineoplastic effect of paclitaxel in lung cancer cell lines (7). This effect was modulatory and probably not synergistic, as the ligands alone showed no cytotoxic effect on cancer cells in the concentrations tested. Taken together, these three studies indicate that NR1I3 is a molecular target for cancer therapy.
In this mouse model, IHC detection of NR1I3 in lung tissue sections showed specificity for epithelial cells and macrophages, but no clear labeling for fibroblasts and endothelial cells. A similar pattern was seen in lung neoplastic lesions as parenchymal cells showed varying degrees of NR1I3 labeling and fibroblasts and endothelial cells were NR1I3 −. This effect was more evident when we examined NR1I3 in several human cancer samples from different histological types, where the stromal tissue was generally NR1I3 − (data not shown). Neoplastic cells from our samples were generally NR1I3 −, with only cytoplasmic or cytoplasmic and nuclear labeling, possibly owing to the expected function of NR1I3 in cells: it is an orphan receptor that stays in the cytoplasm conjugated with the HSP90 and translocates to the nucleus when activated by protein phosphatase 2A (20). Thus, it is possible to have NR1I3 labeling in cytoplasm and in the nucleus.
Notably, carcinomas had a lower percentage of NR1I3 + neoplastic cells than did adenomas in our mouse model - a result that was confirmed by the inverse correlation between NR1I3 mRNA expression and tumor size. Additionally, when we selected lesions <2 mm (all adenomas) or >5 mm (all carcinomas), according to the histology recommendations of Nikitin et al. (9), the larger lesions showed significantly less NR1I3 expression. These results are particularly interesting in light of our recent findings that NR1I3 expression can be increased in human and mouse lung cancer cell lines by use of specific NR1I3 ligands (7). We also showed that NR1I3-specific ligands increase paclitaxel cytotoxicity in those cell lines (7). The present research found that the more malignant lesions produced less NR1I3 protein and mRNA, which implies that malignant lesions could increase their expression of NR1I3 with use of its specific ligands, and this effect could increase the sensitivity of tumor cells to paclitaxel, as shown in vitro (7).
To our knowledge, this is the first time that NR1I3 has been detected in mouse lung tumors and associated with malignancy and cell proliferation. Ongoing studies in our laboratory aim to characterize the effect of NR1I3 on prognosis and its potential as a therapeutic target.
Acknowledgements
This research was supported by FAPESP (#2008/56584-2, #2009/11081-6, #2010/00535-3, #2010/05650-5, and #2011/05690-0).
Footnotes
First published online.
References
- 1.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 2.Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A. 2003;100:4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146:995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Li L, Yang H, Ferguson SS, Baer MR, Gartenhaus RB, et al. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 2013;121:329–338. doi: 10.1182/blood-2012-06-436691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty S, Kanakasabai S, Bright JJ. Constitutive androstane receptor agonist CITCO inhibits growth and expansion of brain tumour stem cells. Br J Cancer. 2011;104:448–459. doi: 10.1038/sj.bjc.6606064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukumasu H, Rochetti AL, Pires PR, Silva ER, Mesquita LG, Strefezzi RF, et al. Constitutive androstane receptor ligands modulate the anti-tumor efficacy of paclitaxel in non-small cell lung cancer cells. PLoS One. 2014;9: doi: 10.1371/journal.pone.0099484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumasu H, Avanzo JL, Sanches DS, Mennecier G, Mori CM, Dagli ML. Higher susceptibility of spontaneous and NNK-induced lung neoplasms in connexin 43 deficient CD1 × AJ F1 mice: paradoxical expression of connexin 43 during lung carcinogenesis. Mol Carcinog. 2013;52:497–506. doi: 10.1002/mc.21884. [DOI] [PubMed] [Google Scholar]
- 9.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–2316. doi: 10.1158/0008-5472.CAN-03-3376. [DOI] [PubMed] [Google Scholar]
- 10.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 11.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Swales K, Negishi M. CAR, driving into the future. Mol Endocrinol. 2004;18:1589–1598. doi: 10.1210/me.2003-0397. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem Pharmacol. 2012;83:1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pondugula SR, Mani S. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer Lett. 2013;328:1–9. doi: 10.1016/j.canlet.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao E, Ji M, Wu J, Ma R, Zhang X, He Y, et al. Expression of the PXR gene in various types of cancer and drug resistance. Oncol Lett. 2013;5:1093–1100. doi: 10.3892/ol.2013.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, Laffins B, et al. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br J Cancer. 2010;102:1753–1761. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC Cancer. 2009;9:3. doi: 10.1186/1471-2407-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M. Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett. 2003;548:17–20. doi: 10.1016/S0014-5793(03)00720-8. [DOI] [PubMed] [Google Scholar]


