Abstract
The purpose of this study was to analyze the relationship between the anaerobic components of the maximal accumulated oxygen deficit (MAOD) and of the 30-second Wingate anaerobic test (30-WAnT). Nine male physical education students performed: a) a maximal incremental exercise test; b) a supramaximal constant workload test to determine the anaerobic components of the MAOD; and c) a 30-WAnT to measure the peak power (PP) and mean power (MP). The fast component of the excess post-exercise oxygen consumption and blood lactate accumulation were measured after the supramaximal constant workload test in order to determine the contributions made by alactic (ALMET) and lactic (LAMET) metabolism. Significant correlations were found between PP and ALMET (r=0.71; P=0.033) and between MP and LAMET (r=0.72; P=0.030). The study results suggested that the anaerobic components of the MAOD and of the 30-WAnT are similarly applicable in the assessment of ALMET and LAMET during high-intensity exercise.
Keywords: Oxygen uptake, Blood lactate, Anaerobic metabolism, Energy metabolism, Excess post-exercise oxygen consumption, High-intensity exercise
Introduction
During short, continuous high-intensity exercises (lasting ∼120 s), ATP is maximally resynthesized through aerobic and anaerobic metabolism in order to maintain the required power output (1). Thus, several tests have been proposed in the literature to assess the energy metabolism profile during exhaustive exercise (2). In particular, it is believed that measurement of muscle metabolites could provide relevant information regarding anaerobic metabolism (3). However, determination of the maximum amount of ATP that can be resynthesized by breakdown of phosphocreatine and intramuscular glycogen, using small muscle biopsy samples, can result in a limited representation of anaerobic metabolism activation in all the muscles recruited during exercise (4). Consequently, alternative methods based on oxygen uptake (3,5) and power output (6) measurements have been proposed to estimate the contribution of anaerobic metabolism during high-intensity exercise.
Medbo et al. (5) proposed measuring the maximal accumulated oxygen deficit (MAOD) to determine anaerobic capacity based on oxygen uptake. According to this method, the MAOD represents the difference between the predicted oxygen demand and the accumulated oxygen uptake measured during a supramaximal exhaustive test. Previous studies have reported that the MAOD is sensitive in responding to anaerobic training (7), does not change during acute exposure to hypoxia (8), and is significantly correlated with muscle phosphocreatine and lactate concentrations (3). Although these findings suggest that the MAOD might be a valid physiological measurement of anaerobic metabolism, it does not provide separate estimates of the contributions made by alactic (ALMET) and lactic (LAMET) metabolism.
Recently, an alternative method was proposed to estimate the anaerobic components of the MAOD (9), based on evidence that the resynthesis of high-energy phosphate stores and the glycolytic energy cost can be assessed by the fast component of excess post-exercise oxygen consumption (10-13) and the blood lactate accumulation [(La−)] O2 equivalent (14). It was reported that this new method was able to accurately assess the total energy that can be supplied by anaerobic metabolism (9). An interesting advantage of this new approach is that the contributions of ALMET and LAMET to the total anaerobic energy expenditure during a supramaximal test can also be assessed (9).
While there is evidence that ALMET and LAMET can be satisfactorily measured during high-intensity exercise (9), they have not yet been compared with alactic and lactic parameters derived from more traditional testing methods. In the absence of a universally accepted gold standard method to measure anaerobic metabolism (2), some new anaerobic tests have concurrently been validated with the 30-second Wingate anaerobic test (30-WAnT) (15,16). It is largely recognized that the peak power (PP) and mean power (MP) output measured during a 30-WAnT must represent the breakdown of phosphocreatine and muscle glycogen depletion, respectively (6,17). Thus, it is plausible to assume that ALMET and LAMET could be assessed either via physiological (i.e., estimated anaerobic components of the MAOD) or mechanical (i.e., PP and MP derived from the 30-WAnT) measurements. Nevertheless, the relationship between these physiological and mechanical tests is still not fully elucidated.
Therefore, the aim of this study was to analyze the relationship between the ALMET and LAMET of the MAOD with the PP and MP parameters derived from the 30-WAnT. We hypothesized that the ALMET and the LAMET would be positively correlated with the PP and the MP, respectively.
Subjects and Methods
Participants
Nine male physical education students (23±4 years of age; height 175.0±5.5 cm; body weight 71.7±8.4 kg; maximal heart rate 179±9 bpm; maximal oxygen uptake 41.3±6.0 mL·kg−1·min−1), who were familiar with exhaustive exercises, volunteered to participate in this study. At the time of enrollment, participants had been training 3-4 times per week in recreational sports (jogging, soccer, and tennis) for at least 1 year. Subjects were informed of the experimental risks and signed an informed consent form prior to participation. The investigation was approved by an Institutional Review Board of the Escola de Educação Física e Esporte, Universidade de São Paulo for use of human subjects.
Exercise testing procedures
Participants performed the following tests: a) a maximal incremental exercise test to measure the maximal oxygen uptake (VO2max) and power output corresponding to VO2max (Wmax); b) a supramaximal constant workload test at 110% Wmax; and c) a 30-WAnT. The supramaximal constant workload test and the 30-WAnT were carried out in a randomized order. Each test was performed on different occasions at least 48 h apart, and at the same time of the day to avoid circadian interference. The participants were instructed to refrain from exhaustive exercises during the 24 h preceding the testing sessions and to avoid caffeine and food intake for 2 h before the tests. Subjects were also instructed to maintain the same dietary habits throughout the study.
Maximal incremental exercise test
The maximal incremental exercise test was carried out on an electromagnetically braked cycle ergometer (Standart Lannoy Ergometer, Godart-Statham, Holland). The seat height was individually adjusted for the subject's comfort, with the legs being nearly fully extended during each pedal revolution. After a 3-min warm-up using only the inertial resistance of the equipment, participants exercised at a pedal frequency of 60 rpm with power output increments of 30 W/min until exhaustion. Exhaustion was defined as the incapacity to maintain a minimum pedal cadence of 50 rpm. In order to attain maximal values, participants received strong verbal encouragement to continue for as long as possible. Oxygen uptake (VO2) was measured breath-by-breath throughout the test using a portable gas analyzer (K4b2, Cosmed, Italy) and was subsequently averaged over 30-s intervals. The device was calibrated according to the manufacturer's specifications given in the instruction manual. The VO2max was recorded as the oxygen uptake measured during the last 30 s of the test.
Supramaximal constant workload test
The cycle ergometer and all procedures used in the maximal incremental exercise test were also used in the supramaximal constant workload test. Before the test, the participants rested quietly on the cycle ergometer for 5 min for resting VO2 values (VO2rest) to be measured. They then performed the supramaximal test using a workload corresponding to 110% Wmax. Peak oxygen uptake (VO2peak) was defined as the average of the last 30 s of the supramaximal test. The recovery time used to obtain the fast component of the excess post-exercise oxygen consumption was 10 min (9). Heart rate was measured during the test with a heart rate transmitter (Polar Electro Oy, Finland) coupled to the gas analyzer. Blood samples (25 µL) were collected from the ear lobe at rest, immediately, 3, and 5 min after the exercise for [La−]peak determination (1500 Sport, Yellow Springs Instruments, USA).
30-WAnT
The 30-WAnT was performed as described by Inbar et al. (18). Briefly, the test was preceded by a 5-min warm-up period at the inertial resistance of the equipment, including 2 bouts of 4 s performed in the final seconds of the second and fourth minutes. After a 10-min rest, the participants were instructed to pedal “all-out” for 30 s against a resistance of 0.09 kg/kg body mass. Previous studies used this load setting because it seems to be adequate for physically active adults (19,20). Verbal encouragement was provided throughout the test. The external power output was calculated every 1 s (Ergometric 6.0, Cefise, Brazil). The highest external power output in the first 5 s of the test was used to represent the PP, whereas the average power generated over 30 s corresponded to the MP (18). In addition, the fatigue index (FI) is reported as the percentage decline from PP to the lowest power produced at the end of the test [(PP-lowest power)/PP×100)].
Calculations
The ALMET and LAMET contributions during the supramaximal constant workload test were calculated as previously described (9,21). Firstly, the breath-by-breath VO2 off-transient response was fitted using a bi-exponential model (equation 1) (Origin 6.0, Microcal, USA), and equation 2 was applied to obtain the contribution of the ALMET (9). Secondly, the O2 equivalent from blood lactate accumulation was determined as the difference between [La−]peak and resting blood lactate concentration [La−]rest, reported as a delta value [La−]net. A value of 1 mM [La−]net was considered to be equivalent to 3 mL O2/kg body mass, and was used to obtain the contribution of LAMET (14). MAOD was determined by the sum of the ALMET and LAMET contributions (9).
| (Eq 1) |
| (Eq 2) |
VO2(t) is the oxygen uptake at time t and VO2baseline is the oxygen uptake at baseline. A is the amplitude, td is the time delay, t is a time constant, and 1 and 2 denote the fast and slow components.
Statistical analysis
The distribution of the data was analyzed by the Shapiro-Wilk test and the results showed a normal Gaussian distribution. Data are reported as means±SD. Pearson's product-moment coefficients were used to determine the relationships between ALMET and PP and between LAMET and MP. Statistical significance was set at P≤0.05. All analyses were performed using the SPSS software (version 13.0, USA).
Results
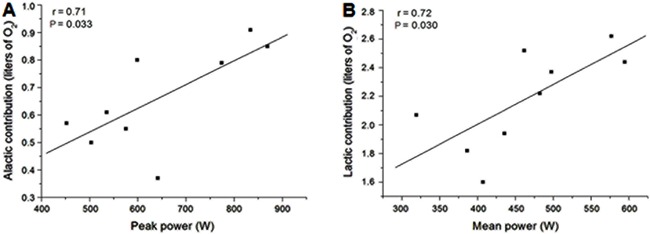
The workload employed during the supramaximal test was 272±44 W and the time-to-exhaustion was 154±38 s. The mean values of VO2peak, HRpeak, and [La−]peak measured during the supramaximal test were 36.6±5.3 mL·kg−1·min−1, 173±14 bpm, and 10.9±1.2 mM, respectively. The mean values of ALMET, LAMET, and the MAOD were 0.98±0.25, 2.20±0.34, and 3.18±0.11 LO2, respectively. Data obtained during the 30-WAnT are shown in Table 1. Figure 1 shows that significant correlations were observed between PP and ALMET (r=0.71) and between MP and LAMET (r=0.72; P<0.05). Significant correlations were also observed between MAOD and the absolute values of PP (r=0.78; P<0.05) and MP (r=0.79; P=0.05). In contrast, no significant correlations were observed between the fatigue index and ALMET (r=0.53; P=0.14), LAMET (r=-0.26; P=0.51) or MAOD (r=0.06; P=0.88).
Figure 1. Relationships between peak power and alactic contribution (panel A) and between mean peak and lactic contribution (panel B). The Pearson's product-moment coefficient test was used for statistical analysis.
Discussion
It was recently shown that an alternative method of estimating MAOD can provide estimates of the contributions of ALMET and LAMET during continuous, high-intensity exercise (9). However, studies relating ALMET and LAMET to alactic and lactic parameters measured by traditional anaerobic tests are scarce. Thus, the main objective of the present study was to analyze the relationships between ALMET and LAMET and the PP and MP derived from 30-WAnT. Our findings demonstrated that ALMET and LAMET were positively correlated with PP and MP, respectively.
ALMET was significantly correlated with the PP. To the best of our knowledge, this is the first study to show a relationship between these physiological and mechanical variables. The method used to determine ALMET provides an estimate of the amount of ATP resynthesized by phosphocreatine breakdown and therefore indicates anaerobic alactic capacity. Although the PP is assumed to be a measure of anaerobic alactic power, previous findings showed that PP during the 30-WAnT can be reached in the first 3-5 s (6) and that anaerobic alactic capacity can be depleted within ∼10 s during an all-out test similar to a 30 s-Wingate (4). This suggests that about 50% of the anaerobic alactic capacity could be used during this initial phase, when the rate of ATP resynthesized by phosphocreatine breakdown is highest (i.e., anaerobic alactic power). In addition, it was demonstrated that phosphocreatine breakdown was closely related to PP (17), suggesting that the contribution of alactic metabolism during exhaustive exercise can be estimated by PP. Therefore, considering that anaerobic alactic capacity might be highly dependent on the anaerobic alactic power, these findings suggested that individuals with higher peak power values might also have higher ALMET values.
Our results also showed that the contribution of LAMET was correlated with MP. Previous findings have shown that performance in short-duration tests was positively associated with blood lactate accumulation (22), which was used in the present study to determine LAMET. It was also reported that muscle glycogen depletion was closely associated with MP (17), which suggests a relationship between MP and the contribution of glycolytic metabolism during an exhaustive exercise bout. Several studies reported that MP is positively correlated with the percentage and cross-sectional area of muscle fast twitch fibers (23-25). These findings suggest there is a close relationship between blood lactate accumulation and the maintenance of high values of mechanical power output during the 30-WAnT. Thus, it is plausible to consider that LAMET and MP have a similar ability to estimate the glycolytic metabolism activation during high-intensity exercise.
On the other hand, the fatigue index was not correlated with LAMET, ALMET, or MAOD. It is important to note that the fatigue index is determined by calculating the percentage of power drop of two data points (i.e., highest and lowest power output). Therefore, calculation of the fatigue index would be influenced by PP values and does not necessarily represent tolerance to fatigue (6). For example, in the present study, a decrease in fatigue index was accompanied by a decrease of PP, meaning that the participant who had these characteristics had a lower alactic anaerobic metabolism and not necessarily a high ability to tolerate the fatigue (i.e., high muscle buffer capacity). In this respect, it has been pointed out that the ability of the fatigue index to represent the fatigue process remains unclear (6).
The results of the current study indicated that the MAOD was significantly correlated with both PP and MP. These findings are in contrast to a study by Minahan and Wood (26) that reported that the MAOD was not related to PP, and that the correlation observed between the MAOD and MP was not exclusively determined by the metabolic events in skeletal muscle. At least two distinct explanations based on methodological aspects could account for these differences. First, the classical method used to determine the MAOD assumes that the efficiency during submaximal exercise is unchanged as the intensity increases. However, it may be difficult to achieve a true oxygen uptake steady state at intensities above the anaerobic threshold because of the appearance of an additional slow component of the oxygen uptake kinetics (27). Consequently, the energy demand during supramaximal exercises might have been overestimated, affecting the traditional calculation of the MAOD (28). Secondly, the traditional MAOD does not distinguish between the alactic and lactic anaerobic components. Thus, if anaerobic power and anaerobic capacity are two different entities (26), they could interfere with the relationship between the MAOD and PP and MP. Conversely, the alternative method used to determine the MAOD in the current study does not present these methodological limitations because this method is independent of energy efficiency and provides separate estimates of alactic and lactic anaerobic metabolism.
It is important to acknowledge some limitations of the present study. It has been reported that there are some restrictions on the use of the excess post-exercise oxygen consumption, blood lactate accumulation and WAnT (2,29). In fact, the determination of the metabolic demand of active muscles through the assessment of whole-body physiological variables or power output during high-intensity exercise could be limited (30). For example, the contribution of LAMET could be underestimated because a portion of the lactate that is released into the blood may be oxidized in other tissues (e.g., heart and inactive muscles) during exercise. In addition, the O2 equivalent from blood lactate accumulation used in the present study does not represent the exact stoichiometric relationship between lactate formation and resynthesis of ATP. Lastly, active subjects may also have a lower anaerobic capacity compared with trained subjects (31) and therefore some caution should be exercised in extrapolating these findings to anaerobically trained athletes.
In conclusion, the results of the present study indicated that the anaerobic components of MAOD (ALMET and LAMET) were positively correlated with mechanical parameters (PP and MP, respectively) obtained during the 30-WAnT. These findings suggest that both methods are similarly applicable in the assessment of the ALMET and LAMET in physically active subjects.
Acknowledgments
M.V. Damasceno is supported by a Master scholarship from FAPESP (#2013/00371-9).
Footnotes
First published online.
References
- 1.Medbo JI, Tabata I. Relative importance of aerobic and anaerobic energy release during short-lasting exhausting bicycle exercise. J Appl Physiol. 1989;67:1881–1886. doi: 10.1152/jappl.1989.67.5.1881. [DOI] [PubMed] [Google Scholar]
- 2.Green S, Dawson B. Measurement of anaerobic capacities in humans. Definitions, limitations and unsolved problems. Sports Med. 1993;15:312–327. doi: 10.2165/00007256-199315050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Medbo JI, Tabata I. Anaerobic energy release in working muscle during 30 s to 3 min of exhausting bicycling. J Appl Physiol. 1993;75:1654–1660. doi: 10.1152/jappl.1993.75.4.1654. [DOI] [PubMed] [Google Scholar]
- 4.Gastin PB. Quantification of anaerobic capacity. Scand J Med Sci Sports. 1994;4:91–112. doi: 10.1111/j.1600-0838.1994.tb00411.x. [DOI] [Google Scholar]
- 5.Medbo JI, Mohn AC, Tabata I, Bahr R, Vaage O, Sejersted OM. Anaerobic capacity determined by maximal accumulated O2 deficit. J Appl Physiol. 1988;64:50–60. doi: 10.1152/jappl.1988.64.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Or O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987;4:381–394. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Weber CL, Schneider DA. Increases in maximal accumulated oxygen deficit after high-intensity interval training are not gender dependent. J Appl Physiol. 2002;92:1795–1801. doi: 10.1152/japplphysiol.00546.2001. [DOI] [PubMed] [Google Scholar]
- 8.Friedmann B, Frese F, Menold E, Bartsch P. Effects of acute moderate hypoxia on anaerobic capacity in endurance-trained runners. Eur J Appl Physiol. 2007;101:67–73. doi: 10.1007/s00421-007-0473-0. [DOI] [PubMed] [Google Scholar]
- 9.Bertuzzi RC, Franchini E, Ugrinowitsch C, Kokubun E, Lima-Silva AE, Pires FO, et al. Predicting MAOD using only a supramaximal exhaustive test. Int J Sports Med. 2010;31:477–481. doi: 10.1055/s-0030-1253375. [DOI] [PubMed] [Google Scholar]
- 10.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- 11.Margaria R, Edwards HT, Dill DB. The possible mechanisms of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol. 1933;106:689–715. [Google Scholar]
- 12.Idstrom JP, Subramanian VH, Chance B, Schersten T, Bylund-Fellenius AC. Oxygen dependence of energy metabolism in contracting and recovering rat skeletal muscle. Am J Physiol. 1985;248:H40–H48. doi: 10.1152/ajpheart.1985.248.1.H40. [DOI] [PubMed] [Google Scholar]
- 13.Piiper J, Spiller P. Repayment of O2 debt and resynthesis of high-energy phosphates in gastrocnemius muscle of the dog. J Appl Physiol. 1970;28:657–662. doi: 10.1152/jappl.1970.28.5.657. [DOI] [PubMed] [Google Scholar]
- 14.di Prampero PE, Ferretti G. The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respir Physiol. 1999;118:103–115. doi: 10.1016/S0034-5687(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 15.Zagatto AM, Beck WR, Gobatto CA. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J Strength Cond Res. 2009;23:1820–1827. doi: 10.1519/JSC.0b013e3181b3df32. [DOI] [PubMed] [Google Scholar]
- 16.Bulbulian R, Jeong JW, Murphy M. Comparison of anaerobic components of the Wingate and Critical Power tests in males and females. Med Sci Sports Exerc. 1996;28:1336–1341. doi: 10.1097/00005768-199610000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol. 1996;80:876–884. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 18.Inbar O, Bar-Or O, Skinner JS. The Wingate anaerobic test. Champaign: Human Kinetics; 1996. [Google Scholar]
- 19.Souissi N, Bessot N, Chamari K, Gauthier A, Sesboue B, Davenne D. Effect of time of day on aerobic contribution to the 30-s Wingate test performance. Chronobiol Int. 2007;24:739–748. doi: 10.1080/07420520701535811. [DOI] [PubMed] [Google Scholar]
- 20.Souissi N, Driss T, Chamari K, Vandewalle H, Davenne D, Gam A, et al. Diurnal variation in Wingate test performances: influence of active warm-up. Chronobiol Int. 2010;27:640–652. doi: 10.3109/07420528.2010.483157. [DOI] [PubMed] [Google Scholar]
- 21.Bertuzzi RC, Franchini E, Kokubun E, Kiss MA. Energy system contributions in indoor rock climbing. Eur J Appl Physiol. 2007;101:293–300. doi: 10.1007/s00421-007-0501-0. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs I. Blood lactate. Implications for training and sports performance. Sports Med. 1986;3:10–25. doi: 10.2165/00007256-198603010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Jansson E, Dudley GA, Norman B, Tesch PA. Relationship of recovery from intensive exercise to the oxidative potential of skeletal muscle. Acta Physiol Scand. 1990;139:147–152. doi: 10.1111/j.1748-1716.1990.tb08907.x. [DOI] [PubMed] [Google Scholar]
- 24.Froese EA, Houston ME. Performance during the Wingate anaerobic test and muscle morphology in males and females. Int J Sports Med. 1987;8:35–39. doi: 10.1055/s-2008-1025637. [DOI] [PubMed] [Google Scholar]
- 25.Esbjornsson M, Sylven C, Holm I, Jansson E. Fast twitch fibres may predict anaerobic performance in both females and males. Int J Sports Med. 1993;14:257–263. doi: 10.1055/s-2007-1021174. [DOI] [PubMed] [Google Scholar]
- 26.Minahan C, Wood C. Strength training improves supramaximal cycling but not anaerobic capacity. Eur J Appl Physiol. 2008;102:659–666. doi: 10.1007/s00421-007-0641-2. [DOI] [PubMed] [Google Scholar]
- 27.Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev. 1996;24:35–71. doi: 10.1249/00003677-199600240-00004. [DOI] [PubMed] [Google Scholar]
- 28.Bangsbo J. Oxygen deficit: a measure of the anaerobic energy production during intense exercise? Can J Appl Physiol. 1996;21:350–363. doi: 10.1139/h96-031. [DOI] [PubMed] [Google Scholar]
- 29.Gastin PB, Lawson DL. Influence of training status on maximal accumulated oxygen deficit during all-out cycle exercise. Eur J Appl Physiol Occup Physiol. 1994;69:321–330. doi: 10.1007/BF00392038. [DOI] [PubMed] [Google Scholar]
- 30.Graham TE. Oxygen deficit: introduction to the assumptions and the skepticism. Can J Appl Physiol. 1996;21:347–349. doi: 10.1139/h96-030. [DOI] [PubMed] [Google Scholar]
- 31.Pizza FX, Naglieri TA, Holtz RW, Mitchell JB, Starling RD, Phillips MD, et al. Maximal accumulated oxygen deficit of resistance-trained men. Can J Appl Physiol. 1996;21:391–402. doi: 10.1139/h96-036. [DOI] [PubMed] [Google Scholar]


