Abstract
The present study aimed to investigate visceral adipose tissue-specific serpin (vaspin) concentrations in serum and term placentas and relate these values to insulin resistance and lipid parameters in women with gestational diabetes mellitus (GDM). A total of 30 GDM subjects and 27 age-matched pregnant women with normal glucose tolerance (NGT, control) were included. Serum glucose, glycated hemoglobin (HbA1c), lipid profile, insulin, and vaspin were measured at the end of pregnancy, and homeostasis model of assessment-insulin resistance (HOMA-IR) values were calculated. Vaspin mRNA and protein levels in placentas were measured by real-time fluorescence quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blotting, respectively. Serum vaspin levels were significantly lower in the GDM group than in controls (0.49±0.24 vs 0.83±0.27 ng/mL, respectively; P<0.01). Three days after delivery, serum vaspin levels were significantly decreased in subjects with GDM (0.36±0.13 vs 0.49±0.24 ng/mL, P<0.01). However, in the GDM group, serum vaspin levels were not correlated with the parameters evaluated. In contrast, in the control group, serum vaspin levels were positively correlated with triglycerides (TG; r=0.45, P=0.02) and very low-density lipoprotein cholesterol (VLDL-C; r=0.42, P=0.03). Placental mRNA vaspin (0.60±0.32 vs 0.68±0.32, P=0.46) and protein (0.30±0.08 vs 0.39±0.26; P=0.33) levels in the GDM group did not differ significantly from those in the control group, but were negatively correlated with neonatal birth weight in the GDM group (r=-0.48, P=0.03; r=-0.88; P<0.01). Our findings indicated that vaspin may be an important adipokine involved in carbohydrate and lipid metabolism and may also play a role in fetal development.
Keywords: Vaspin, Gestational diabetes, Placenta, Pregnancy, Insulin resistance
Introduction
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition of pregnancy (1,2), is a state of chronic insulin resistance (IR) and occurs in 1-14% of pregnant women (2). The reported prevalence of GDM varies between 0.6 and 20% of pregnancies, depending on the screening method used, gestational age, and the population studied (3). Recently, several cytokines, including leptin (3), C-reactive protein (CRP) (4), tumor necrosis factor (TNF)-α (4), adiponectin (3), and visfatin (3), have been associated with GDM.
Visceral adipose tissue-specific serpin (vaspin) is a newly discovered adipocytokine secreted mainly by visceral adipose tissue that has insulin-sensitizing effects (5). It was identified as a member of a serine protease inhibitor family, which was originally discovered in the visceral adipose tissue of an animal model of type 2 diabetes mellitus (T2DM) (5). In rats, vaspin protein expression and its serum levels increase at the peak of obesity and IR, and decrease with the exacerbation of diabetes (5). Administration of vaspin to obese mice has been shown to improve glucose tolerance and insulin sensitivity and reduce food intake (5,6). Thus, vaspin may become an attractive candidate as a drug for the treatment of obesity and hyperglycemia (7). The human vaspin protein is 415 amino acids long, and homology analysis has indicated that vaspin shares 40.4% identity with α1-antitrypsin (5). Human serum vaspin concentrations correlate positively with age, body mass index (BMI), and IR, which are also associated with T2DM (8). However, the association between circulating vaspin and insulin sensitivity is controversial in T2DM (9-11).
In normal pregnancy, vaspin is expressed in the placenta and increases throughout gestation, reaching its highest level at the end of the third trimester (12). It has been postulated that vaspin expression in the placenta may be associated with fetal development (10). At 24-30 weeks of gestation, normal pregnant subjects have significantly lower vaspin serum levels than non-pregnant controls (13). In subjects with GDM, however, vaspin levels slowly decrease from the 2nd trimester until delivery. In the 3rd trimester of pregnancy, it has been reported that vaspin levels are positively correlated with insulin levels, homeostasis model of assessment-insulin resistance (HOMA-IR), and triglycerides in subjects with GDM (14). During pregnancy, IR in women with GDM is most severe in the third trimester. Therefore, it was hypothesized that vaspin may play a role in GDM and fetal development.
To the best of the authors' knowledge, this is the first study to investigate serum vaspin concentrations in pregnant Chinese women and vaspin expression in GDM placentas at term. Our primary aim was to investigate maternal serum vaspin levels at term and postpartum and their relationship to IR, lipid parameters, and HbA1c. To determine changes in vaspin expression in the placenta and its association with neonatal birth weight, we also compared findings in subjects with GDM to those in pregnant women with normal glucose tolerance (NGT).
Material and Methods
Subjects and tissue collection
Fifty-seven pregnant women were enrolled at the Obstetrics and Gynecology Department of Hebei General Hospital. The group consisted of 30 women with GDM aged 29.23±2.80 years and 27 age-matched pregnant women with normal oral glucose tolerance tests (OGTTs) who served as controls (27.85±2.41 years). All pregnancies were singletons and required elective cesarean section owing to breech presentation and/or previous cesarean section. GDM was diagnosed between 24 and 28 weeks gestation when subjects had one or more values above the threshold for a 75-g 2-h OGTT according to the diagnostic criteria of the International Association of Diabetic Pregnancy Study Group (IADPSG), in which the values of the 75-g 2-h OGTT considered normal are: fasting glucose <5.1 mM (92 mg/dL), 1 h <10.0 mM (180 mg/dL), and 2 h <8.5 mM (153 mg/dL) between 24 and 28 weeks gestation (15).
All subjects were non-smokers and had not taken any drugs known to affect carbohydrate metabolism. All GDM patients were treated by diet alone. Patients with premature rupture of membranes, pregnancy-induced hypertension, preeclampsia, or other pregnancy complications were excluded. All participants were in good health, without any known disease.
Venous blood samples were collected before cesarean section and 3 days after surgery after an 8-h overnight fast. Blood samples were collected in tubes without anticoagulants and centrifuged (999 g, 10 min) immediately after clotting. The serum samples were stored at -80°C until analysis. Placental tissues were obtained during cesarean sections from the maternal side near the umbilical cord insertion site within 10 min of delivery, frozen in liquid nitrogen, and stored at -80°C until analysis.
The protocol was approved by the Human Ethics Committee of Hebei General Hospital, and all patients participating in the study gave written informed consent.
Laboratory assays
Blood glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C) were analyzed at the biochemical laboratory of Hebei General Hospital. Plasma glucose and lipids were determined by routine methods with the Hitachi 7600-110 Automatic Analyzer (Japan). Plasma insulin was measured using chemiluminescence assays (Cobas e 601, China). HbA1c was measured by chromatography (TOSOH Corporation, G8-90sl, China). Serum vaspin was determined by enzyme-linked immunosorbent assay (ELISA, Bluegene, China; sensitivity 0.1 ng/mL, intra-assay coefficient of variance [CV] <10%, inter-assay CV <10%). Insulin sensitivity was determined using the HOMA index with the formula HOMA-IR = fasting insulin (FINS, µU/mL) × fasting plasma glucose (FPG, mM) / 22.5.
Total RNA extraction and RT-qPCR
Total RNA was extracted from the placenta using TRIzol (Transgen, China) according to the manufacturer's instructions, and 1 μg total RNA was reverse transcribed with a cDNA synthesis kit (Transgen). Two microliters of each RT reaction were amplified in a 20-μL PCR mix using UltraSYBR Mixture (cwbiotech, China). The real-time fluorescence quantitative reverse transcription polymerase chain reaction (RT-qPCR) was detected on an ABI PRISM 7300 sequence detector (Applied Biosystems, USA). Samples were incubated in the ABI 7300 sequence detector for initial denaturation at 95°C for 10 min, followed by 40 PCR cycles each consisting of 95°C for 15 s, 58°C for 20 s, and 72°C for 27 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal standard. The primer sequences used for this study are shown in Table 1 (all the primers were synthesized by Sangon, China).
Western blot analysis
Placental tissue was weighed and then processed with a total protein extraction kit (Sangon Biotech, China) according to the manufacturer's instructions. Protein samples were resolved by polyacrylamide gel electrophoresis on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred to polyvinylidene fluoride (PVDF) membranes. After blocking with Tris-buffered saline with Tween (TBS-T) with 5% non-fat milk for 2 h at room temperature, membranes were incubated with the vaspin polyclonal antibody (1:1000, GeneTex, USA) overnight at 4°C. The immunoreactive bands were detected by a chemiluminescence detection kit (Tiangen, China) after incubation with horseradish peroxidase-labeled rabbit IgG antibody (1:10,000, Proteintech, USA). The membrane was re-probed with β-actin (1:5000, Proteintech) as a protein loading control. The results were analyzed with a White/Ultraviolet Transilluminator (UV-II, Gene Company Limited, China).
Statistical analysis
For clinical and anthropometric variables, normally distributed data are reported as means±SE. For variables with a non-Gaussian distribution, values are reported as medians (25-75%). Parametric methods were used for normally distributed parameters, and comparisons between groups were performed using one-way analysis of variance (ANOVA). Non-parametric analyses were performed for non-normally distributed parameters, and comparisons between groups performed using Kruskal-Wallis tests with post hoc Mann-Whitney U tests. Correlation analyses were performed with Spearman's or Pearson's correlation coefficients, depending on the distribution of the variables. Statistical significance was accepted at P<0.05. All statistical analyses were performed using SPSS version 13.0 for Windows (SPSS, USA).
Results
Clinical and demographic characteristics of the subjects
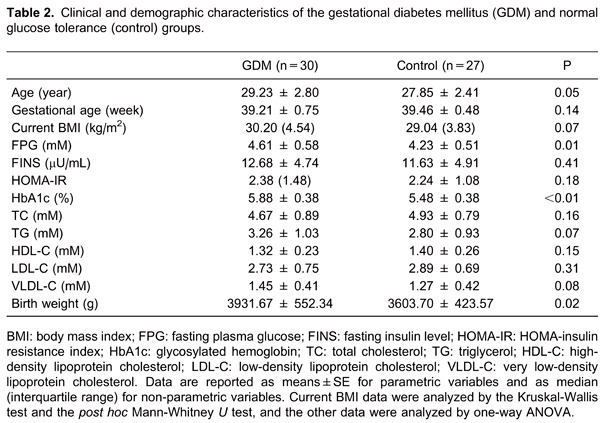
The characteristics of all participants are summarized in Table 2. The two groups of pregnant women were similar in terms of age, gestational age, pre-pregnancy BMI, and current BMI. The subjects with GDM had significantly higher fasting glucose values (4.61±0.58 vs 4.23±0.51, P<0.05) and significantly higher HbA1c (5.88±0.38±0.36 vs 5.48±0.38%, P<0.01) than the NGT group. There were, however, no significant differences in plasma lipids, FINS, or HOMA-IR between the two groups. Current BMI data were analyzed by the Kruskal-Wallis test and the post hoc Mann-Whitney U test, and the other data were analyzed by one-way ANOVA.
Serum vaspin concentrations before and 3 days after delivery
Serum vaspin concentrations in the two groups are shown in Figure 1. Prior to delivery, fasting serum vaspin concentrations at term were significantly lower in the GDM group than in the control group (0.49±0.24 vs 0.83±0.27 ng/mL, respectively, P<0.01). Three days after delivery, serum vaspin concentrations were significantly decreased in the GDM group compared to before delivery (0.36±0.13 vs 0.49±0.24 ng/mL, P<0.01). Serum vaspin concentrations prior to and 3 days after delivery were not significantly different in the pregnant control group (0.83±0.27 vs 0.74±0.31 ng/mL, P>0.05). Data were analyzed by one-way ANOVA.
Figure 1. Median maternal serum vaspin concentrations in women with normal glucose tolerance (control) and gestational diabetes mellitus (GDM) at term (before delivery) and 3 days after delivery. *P<0.01, compared to control before delivery; **P<0.01, compared to before delivery (one-way ANOVA).
Vaspin mRNA and protein expression in placenta
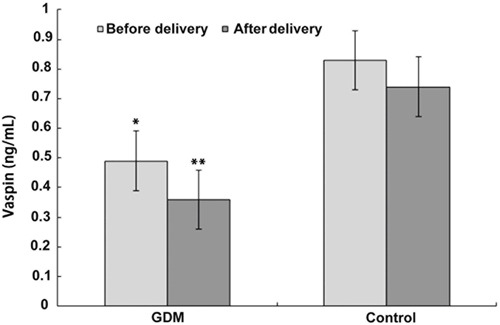
Relative placental mRNA levels of vaspin (0.60±0.32 vs 0.68±0.32, P>0.05; Figure 2A) and protein expression (0.30±0.08 vs 0.39±0.26, P>0.05; Figure 2B and C) were not significantly different in the GDM group compared to the control group. Data were analyzed by one-way ANOVA.
Figure 2. Real-time RT-qPCR and Western blotting were performed on gestational diabetes mellitus (GDM) and normal glucose tolerance (NGT) term placentas. A, Quantification of differences in vaspin mRNA expression between the two groups; B and C, quantification of differences in vaspin protein expression between the two groups. β-actin served as an internal control. One-way ANOVA was used for statistical analysis.
Correlation between serum vaspin levels and clinical and demographic characteristics
In the healthy control group, vaspin concentrations were positively correlated with TG and VLDL-C. There was no correlation between vaspin and other metabolic parameters in either group (Table 3). Correlations were determined with Pearson's correlation coefficients.
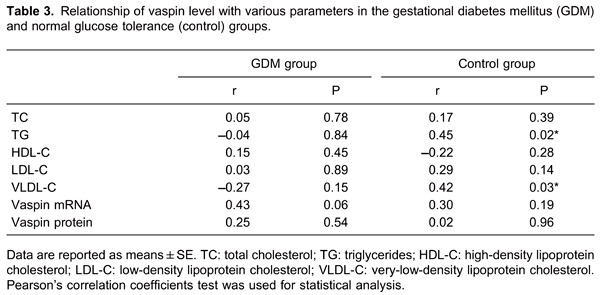
Correlation between serum vaspin levels and placental vaspin expression
Maternal serum vaspin levels were not associated with placental vaspin mRNA levels or vaspin protein expression in the two groups (Table 3). Interestingly, in the GDM group placental vaspin mRNA and protein expression were negatively correlated with neonatal birth weight (r=-0.48, P=0.03; r=-0.88, P<0.01). Correlations were determined with Pearson's correlation coefficients.
Discussion
In the current study, serum vaspin concentrations were significantly decreased at term in GDM subjects compared to NGT subjects. In contrast, Gkiomisi et al. (14) and Stepan et al. (16) reported that circulating vaspin levels were not significantly different between GDM and healthy pregnant controls in the 2nd or 3rd trimesters of pregnancy. Previous studies reporting serum vaspin levels in T2DM patients and obese patients were also controversial. One study described increased serum vaspin levels in T2DM patients compared to control subjects (17), whereas another found that serum vaspin levels were lower in women with T2DM than in NGT controls (8). The discrepancies between the current study and previous investigations may be due to differences in the ethnicities or ages of the sample group, sample size, or GDM severity. In the current study, the HOMA-IR values were similar in GDM patients and healthy controls. Previous studies (16,18,19) also reported similar HOMA-IR levels in GDM and NGT controls. We assume that similar HOMA-IR levels in GDM and NGT controls might be the reason that serum vaspin concentrations were lower in GDM subjects in the current study.
The underlying mechanisms by which circulating vaspin levels are decreased in GDM women at term may be associated with unknown factors, and it remains to be clarified how vaspin may be linked to the deterioration of glucose metabolism and insulin sensitivity. Bluher (7) postulated that vaspin inhibits a protease that has a role in the degradation of a hormone or molecule with direct or indirect glucose-lowering effects. Hida et al. (5) found that in Otsuka Long Evans Tokushima Fatty (OLETF) rats serum vaspin levels decreased with the amelioration of diabetes and concurrent body weight loss, whereas vaspin serum levels could be normalized by insulin or pioglitazone treatment. This suggests that the increase in serum vaspin may protect against IR (5). Administration of recombinant vaspin to obese mice has been shown to improve glucose tolerance and insulin sensitivity (5). Giomisi et al. (13) reported that vaspin was positively correlated to adiponectin in both pregnant and non-pregnant women, and postulated that vaspin may act as a protective cytokine in GDM.
In the present study, subjects with GDM at term had significantly increased serum FPG and HbA1c levels compared to the control group. In contrast to previous studies (13,16), circulating vaspin levels were not significantly correlated with markers of adiposity, including BMI, IR (fasting blood glucose), HOMA-IR, and lipid metabolism (cholesterol, triglycerides).
Interestingly, in the normal pregnant control group circulating vaspin was only positively correlated with TG and VLDL-C. Contrary to the findings of the present study, Giomisi et al. (13) found a negative correlation between vaspin and TG in normal pregnancy, and hypothesized that vaspin may be a marker of lipid metabolism in pregnancy. The findings of the current study support this hypothesis; however, the relationship between vaspin and lipid metabolism requires further investigation.
The results of our study show that maternal vaspin levels in GDM subjects declined significantly 3 days after delivery, indicating that maternal serum vaspin may be partly derived from the placenta. The placenta is considered an active endocrine organ (20,21) that produces many cytokines (22,23), including leptin, resistin, visfatin, and TNFα, which are also produced by adipose cells. Cytokines are secreted by the placenta into the maternal circulation and have an effect on maternal metabolism to fulfill fetal growth energy demand. Some of these adipokines are key players in the regulation of insulin action (23). Caminos (12) found that vaspin was expressed in cytotrophoblasts and syncytiotrophoblasts in first-trimester placentas, whereas in the third trimester it was expressed only in syncytiotrophoblasts. In addition, vaspin expression in the placenta gradually increased during pregnancy, reaching its highest level at the end of gestation. Furthermore, placental vaspin mRNA levels were significantly elevated in pregnant rats with intrauterine growth restriction compared to control pregnant rats. Finally, a previous study reported that cord vaspin levels are significantly higher in small-for-gestational age neonates than in neonates appropriate for gestational age or large for gestational age (24). Consequently, it is postulated that vaspin may have a catabolic function during pregnancy, and that its levels are modulated by placental energy status.
This is the first report investigating the expression of vaspin mRNA and protein in term GDM placentas. Compared with NGT placentas, vaspin mRNA levels and protein expression were downregulated in the GDM placentas; however, this difference was not significant. Interestingly, in the GDM group placental vaspin mRNA levels and protein expression were negatively correlated with neonatal birth weight. The underlying mechanism of the observation needs further investigation.
We found that maternal serum vaspin levels in GDM subjects were mismatched with the changes of vaspin protein expression in the placenta. Interestingly, the maternal serum vaspin levels in GDM subjects declined markedly 3 days after delivery. This suggests that vaspin in the maternal blood may be partly derived from the placenta. It is hypothesized that vaspin may act locally in the placenta as a paracrine/autocrine agent and thereby play a major role in fetal development. As maternal blood vaspin is not primarily produced by the placenta, future studies investigating the relationship between vaspin and fetal development should be performed.
There are two major limitations of the current study. First, all subjects in the GDM group were on a controlled diet, so that this group had similar HOMA-IR levels to those of controls. Second, the sample size was limited.
In conclusion, the results of this study suggested that serum vaspin levels are lower in GDM subjects than in normal control subjects at term. However, no differences in vaspin mRNA levels or protein expression were observed in term placentas. Vaspin mRNA levels and protein expression in the placentas were, however, negatively correlated with neonatal birth weight in the GDM group. Therefore, vaspin may act locally in the placenta as a paracrine/autocrine agent and may play a major role in fetal development. The study showed that vaspin may be an important adipokine involved in carbohydrate and lipid metabolism, hence it could potentially be developed as a candidate drug to treat GDM. Further research is required to establish the exact role of vaspin in the pathogenesis of GDM and fetal development.
Acknowledgments
This research was supported by grants from the Science and Technology Department of Hebei Province.
Footnotes
First published online.
References
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21((Suppl 2)):B161–B167. [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34((Suppl 1)):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol. 2012;76:2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 4.Bo S, Signorile A, Menato G, Gambino R, Bardelli C, Gallo ML, et al. C-reactive protein and tumor necrosis factor-alpha in gestational hyperglycemia. J Endocrinol Invest. 2005;28:779–786. doi: 10.1007/BF03347566. [DOI] [PubMed] [Google Scholar]
- 5.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada J. [Vaspin and insulin resistance] Rinsho Byori. 2008;56:705–711. [PubMed] [Google Scholar]
- 7.Bluher M. Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine. 2012;41:176–182. doi: 10.1007/s12020-011-9572-0. [DOI] [PubMed] [Google Scholar]
- 8.Youn BS, Kloting N, Kratzsch J, Lee N, Park JW, Song ES, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. doi: 10.2337/db07-1045. [DOI] [PubMed] [Google Scholar]
- 9.Korner A, Neef M, Friebe D, Erbs S, Kratzsch J, Dittrich K, et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int J Obes. 2011;35:578–586. doi: 10.1038/ijo.2010.196. [DOI] [PubMed] [Google Scholar]
- 10.von Loeffelholz C, Mohlig M, Arafat AM, Isken F, Spranger J, Mai K, et al. Circulating vaspin is unrelated to insulin sensitivity in a cohort of nondiabetic humans. Eur J Endocrinol. 2010;162:507–513. doi: 10.1530/EJE-09-0737. [DOI] [PubMed] [Google Scholar]
- 11.Akbarzadeh S, Nabipour I, Jafari SM, Movahed A, Motamed N, Assadi M, et al. Serum visfatin and vaspin levels in normoglycemic first-degree relatives of Iranian patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012;95:132–138. doi: 10.1016/j.diabres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Caminos JE, Bravo SB, Garces MF, Gonzalez CR, Cepeda LA, Gonzalez AC, et al. Vaspin and amylin are expressed in human and rat placenta and regulated by nutritional status. Histol Histopathol. 2009;24:979–990. doi: 10.14670/HH-24.979. [DOI] [PubMed] [Google Scholar]
- 13.Giomisi A, Kourtis A, Toulis KA, Anastasilakis AD, Makedou KG, Mouzaki M, et al. Serum vaspin levels in normal pregnancy in comparison with non-pregnant women. Eur J Endocrinol. 2011;164:579–583. doi: 10.1530/EJE-10-1020. [DOI] [PubMed] [Google Scholar]
- 14.Gkiomisi A, Makedou KG, Anastasilakis AD, Polyzos SA, Kourtis A, Gerou S, et al. Serum vaspin levels in women with and without gestational diabetes mellitus during pregnancy and postpartum. Cytokine. 2013;61:127–132. doi: 10.1016/j.cyto.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc10-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepan H, Kralisch S, Klostermann K, Schrey S, Reisenbuchler C, Verlohren M, et al. Preliminary report: circulating levels of the adipokine vaspin in gestational diabetes mellitus and preeclampsia. Metabolism. 2010;59:1054–1056. doi: 10.1016/j.metabol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 17.El-Mesallamy HO, Kassem DH, El-Demerdash E, Amin AI. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism. 2011;60:63–70. doi: 10.1016/j.metabol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, et al. Visfatin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Diabetes Res Clin Pract. 2009;84:68–75. doi: 10.1016/j.diabres.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Gok DE, Yazici M, Uckaya G, Bolu SE, Basaran Y, Ozgurtas T, et al. The role of visfatin in the pathogenesis of gestational diabetes mellitus. J Endocrinol Invest. 2011;34:3–7. doi: 10.1007/BF03346687. [DOI] [PubMed] [Google Scholar]
- 20.Skalba P, Dabkowska-Huc A, Chelmicki Z. [The endocrinology of the human placenta] Wiad Lek. 2003;56:475–480. [PubMed] [Google Scholar]
- 21.Lacroix MC, Guibourdenche J, Frendo JL, Pidoux G, Evain-Brion D. Placental growth hormones. Endocrine. 2002;19:73–79. doi: 10.1385/ENDO:19:1:73. [DOI] [PubMed] [Google Scholar]
- 22.Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obes. 2008;32:223–231. doi: 10.1038/sj.ijo.0803719. [DOI] [PubMed] [Google Scholar]
- 23.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30((Suppl 2)):S120–S126. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]
- 24.Akcay A, Akar M, Demirel G, Canpolat FE, Erdeve O, Dilmen U. Umbilical cord and fifth-day serum vaspin concentrations in small-, appropriate-, and large-for-gestational age neonates. J Pediatr Endocrinol Metab. 2013;26:635–638. doi: 10.1515/jpem-2012-0111. [DOI] [PubMed] [Google Scholar]


