Abstract
Objective
Recent studies have shown, in asymptomatic concussed athletes, metabolic disruption in the primary motor cortex (M1) and abnormal intracortical inhibition lasting for more than six months. The present study aims to assess if these neurochemical and neurophysiological alterations are persistent and linked to M1 cortical thickness.
Methods
Sixteen active football players who sustained their last concussion, on average, three years prior to testing and 14 active football players who never sustained a concussion were recruited for a single session of proton magnetic resonance spectroscopy (1H-MRS) and transcranial magnetic stimulation (TMS). Measures of M1 and whole brain cortical thickness were acquired, and 1H-MRS data were acquired from left M1 using a MEGA-PRESS sequence. Cortical silent period (CSP) and long-interval intracortical inhibition (LICI) were measured with TMS applied over left M1.
Results
No significant group differences were observed for metabolic concentrations, TMS measures, and cortical thickness. However, whereas GABA and glutamate levels, and GABA levels and M1 mean thickness were positively correlated in control athletes, these relationships were absent in concussed athletes.
Conclusion
These data suggest the general absence of neurophysiologic, neurometabolic and neuroanatomical disruptions in M1 three years following the last concussive event. However, correlational analyses suggest the presence of a slight metabolic imbalance between GABA and glutamate concentrations in the primary motor cortex of concussed athletes.
Significance
The present study highlights the importance of multimodal assesments of the impacts of sport concussions.
Keywords: sport concussion, traumatic brain injury, magnetic resonance spectroscopy, transcranial magnetic stimulation, GABA, glutamate
Introduction
Over the past decades, interest in sport concussion research has increased considerably as the phenomenon evolved from being considered a minor injury to being considered a public health priority (Wiebe et al., 2011). In the United States of America, the Center for Disease Control and Prevention estimates that sport concussions affect about 1.6-3.8 million athletes annually (Rutland-Brown et al., 2006), most commonly in contact sports such as boxing and American football (Guskiewicz et al., 2003). However, this could be vastly underestimated because as many as 50% of sport concussions may go unreported (Harmon et al., 2013). This “silent epidemic” has recently gained general public and media attention following reported cases of chronic traumatic encephalopathy (CTE) in former athletes, a neurodegenerative disorder resembling tau-related dementias, parkinsonism, and amyotrophic lateral sclerosis (Chin et al., 2013).
In a recent position statement by the American Society of Sports Medicine, concussion has been defined as a traumatically induced transient disturbance of brain function involving a complex pathophysiological process (Harmon et al., 2013). Clinical symptoms of concussion include cognitive impairments such as memory and attention deficits, headaches, confusion, and behavioural changes (Barkhoudarian et al., 2011), which typically resolve completely within 2-3 weeks post-concussion (Lovell et al., 2003; McCrea et al., 2003). However, repeated concussions have been associated with greater symptom severity (Collins et al., 2002), longer recovery time (Guskiewicz et al., 2003), higher susceptibility to sustain a subsequent concussive event in both humans (Guskiewicz et al., 2003) and animals (Barkhoudarian et al., 2011), and a higher risk of developing dementia (Guskiewicz et al., 2005). Although standard imaging techniques such as CT-scan or magnetic resonance imaging (MRI) usually fail to show any gross structural damage following a concussive event, the consequences of multiple concussions suggest that the injury may induce “silent” pathophysiological or molecular changes to the brain. A particularly interesting mechanism explaining the susceptibility of the brain following a concussion is derived from animal models of traumatic brain injury (Blennow et al., 2012). Giza and Hovda (2001) first described the complex metabolic cascade of neurochemical and neurometabolic changes initiated by acceleration and deceleration forces induced by the concussive event. These events include massive depolarization, excessive release of glutamate (Glu), and decreased ATP production (Giza & Hovda, 2001; Barkhoudarian et al., 2011) leading to pathological cellular processes such as inflammation, oxidative stress, mitochondrial dysfunction, excitotoxicity, oedema and hypoxia (Harris et al., 2012).
Although there is currently no biomarker of these cellular dysfunctions, recent studies suggest that proton magnetic resonance spectroscopy (1H-MRS) could be a powerful approach to assess metabolic disruption following concussion (Harris et al., 2012). This technique allows sensitive in vivo detection and quantification of brain metabolites (Ashwal et al., 2004; Holshouser et al., 2006) including creatine/phoschocretaine (tCr), a general energy marker; phosphocholine (PCho), a marker of glial proliferation and membrane turnover; N-acetylaspartate + N-acetylaspartylglutamate (tNAA), a marker of neuronal integrity, bioenergetics and neuroprotection; Glu + glutamine (Gln) (Glx), a marker of excitatory neurotransmission; myo-inositol (mIns), a glial and oedema marker. Recent technological advances have allowed detection and quantification in humans of gamma-aminobutyric acid (GABA), a marker of inhibitory neurotransmission (Mescher et al., 1998). The assessment of metabolic disruption using 1H-MRS has been mostly studied in patients who sustained different severities of traumatic brain injuries (TBI). In these populations, studies have shown consistent decreases in NAA within a month following injury, which is usually considered the acute phase (Brooks et al., 2001; Macmillan et al., 2002; Govindaraju et al., 2004; Marino et al., 2011). Although less consistent (Xu et al., 2011), results for other brain metabolites showed altered Glx (Shutter et al., 2004; Babikian et al., 2006) and elevated lactate (lac), total choline (tCho), and mIns (Brooks et al., 2001; Marino et al., 2011) in the acute phase. Studies conducted with a population of concussed athletes showed a similar pattern of reduction in NAA (Cimatti, 2006; Vagnozzi et al., 2008; Vagnozzi et al., 2010; Henry et al., 2010; Johnson et al., 2012) and an increase in Glu (Henry et al., 2010) within a month post-concussion. Henry and collaborators (2011) also found chronic metabolic disruptions 6 months post injury in motor areas, where a decrease of NAA in premotor and primary motor (M1) cortices, and an increase of mIns in M1 were observed. Although motor function deficits are not included in the definition of a concussion, these results highlight the possibility of a specific vulnerability of motor areas following brain injury.
This hypothesis is consistent with recent literature showing persistent motor dysfunctions following concussion (De Beaumont et al., 2012). For example, postural stability is now increasingly used as part of post-concussion return-to-play protocols (Harmon et al., 2013) as a growing body of evidence suggests the presence of balance deficits following injury (Guskiewicz, 2001a,b; Cavanaugh et al., 2005; Parker et al., 2006). Neurophysiological motor alterations have also been reported in concussed athletes using transcranial magnetic stimulation (TMS). Long term abnormal intracortical inhibition was observed in young asymptomatic athletes who sustained multiple concussions, as revealed by increased duration of the cortical silent period (CSP: De Beaumont et al., 2007; Tremblay et al., 2011; De Beaumont et al., 2011a,b) and increased long interval intracortical inhibition (LICI; Tremblay et al., 2011; De Beaumont et al., 2011b). Increased CSP duration was also found in former athletes more than 3 decades after their last concussion, along with a significant slowness of movement resembling bradykinesia (De Beaumont et al., 2009). The physiological mechanisms underlying CSP and LICI have been suggested by pharmacological studies where both parameters have been found to be mediated by GABAB receptors (Ziemann, 2004; McDonnell et al., 2006), thus suggesting long lasting alterations in GABAergic transmission following concussion. Furthermore, De Beaumont and collaborators (2011a) have shown abnormal M1 long term potentiation (LTP)-like synaptic plasticity in asymptomatic athletes, as revealed by suppressed paired-associative stimulation (PAS), and reduced implicit motor learning. This result is consistent with the hypothesis of altered inhibitory mechanisms in M1 following concussion, as GABAergic transmission, more specifically GABAB receptors, is involved in LTP-like mechanisms (McDonnell et al., 2007). Although recent animal 1H-MRS studies have shown altered GABA concentrations in the hours and days following induced traumatic brain injury (Xu et al., 2011; Harris et al., 2012) no study has directly assessed the long-term effects of sport concussions on GABA levels in M1.
The main objective of the present study was to investigate, by combining multiple neuroimaging methods, the possible long-term effects of sport concussion on the primary motor cortex in asymptomatic, active university-level athletes. First, the integrity of M1 metabolism was assessed by 1H-MRS. Second, transcranial magnetic stimulation was used to assess GABAB transmission in M1 by CSP and LICI measurements. Finally, possible effects of concussions on whole brain and M1 neuronal integrity was assessed by standard cortical thickness analyses and anatomical connectivity analyses using the Mapping Anatomical Correlations Across Cerebral Cortex (MACCAC) method (Lerch et al., 2006).
Methods
Participants
All participants in the present study were active male football players from Canadian universities recruited with the help of team physicians and physiotherapists. Athletes were excluded if they had a history of psychiatric illness; alcohol and/or substance abuse; learning disability; neurological condition (i.e., seizures, brain tumor); TBI unrelated to sport; and medical conditions requiring daily medication. Concussed athletes were included in the study if they sustained their last concussion at least 10 months prior to the experimentation and were asymptomatic at the time of testing. The study was approved by the local ethics committee and all participants provided written informed consent prior to testing. Participants received a financial compensation of Can $80 for their participation in the study.
Participants were divided into two groups. The control group consisted of 14 university-level football athletes who never sustained a concussion and the experimental group consisted of 16 university-level football players who sustained their last sport concussion at least 10 months prior to testing. Both groups did not differ in age (t(28) = .25, p = .80) and level of education (t(28) = .35, p = .73; Table 1). All athletes were right-handed in the concussed group (right: 16, left: 0) whereas two athletes were left-handed in the control group (right: 12; left: 2). Athletes in the concussed group sustained 1 to 4 sport-related concussions (M = 1.88) and the time since the last concussion ranged from 10 to 96 months (M = 41.25 +/- 29.71). Information regarding concussions that occurred during university years was acquired from team medical records, whereas past concussions were self-reported. In order to obtain detailed information for any head injury that could have occurred prior to testing, a standardized concussion history questionnaire was administered to all participants in an interview setting. The questionnaire aimed to collect detailed information on the number of previous concussions (if any), approximate date(s) of each concussion(s), the description of the incident(s), the nature and duration of relevant post-concussion symptoms (i.e., loss of consciousness, confusion, retrograde and/or anterograde amnesia, disorientation). Concussion grade was assessed according to the American Academy of Neurology (1997) from grade 1 (confusion for less than 15 min without amnesia or loss of consciousness) to grade 3 (loss of consciousness, from few seconds to prolonged), with a mean of severity of grade 2 (SD = 0.89). All concussions were rated as mild (score of 13 to 15) on the Glasgow Coma Scale. Retrospective reports of past concussions by athletes may introduce a bias in the evaluation of the number of concussive events sustained by participants. This methodological caveat was compensated by a standardized evaluation of past concussive events.
Table 1. Between group comparisons of demographic and concussion history information.
| Variables | Group | Mean (SD) | t test | p value |
|---|---|---|---|---|
| Age (years, months) | Control | 22.03 (1.08) | 0.25 | 0.80 |
| Concussed | 22.00 (1.09) | |||
|
| ||||
| Education (years, months) | Control | 15.11 (1.09) | 0.35 | 0.73 |
| Concussed | 15.09 (1.08) | |||
|
| ||||
| Number of concussion | Control | N/A | N/A | |
| Concussed | 1.88 (0.89) | |||
|
| ||||
| Maximum severity (grade) | Control | N/A | N/A | |
| Concussed | 2.00 (0.89) | |||
| Time since the last concussion (years, months) | Control | N/A | N/A | |
| Concussed | 3.05 (2.06) | |||
Procedure
The experimental setting consisted of a single session of MRS of 1 h duration preceded by the administration of the concussion questionnaire. The TMS session was administered either immediately prior to the MRS testing or within 2 months after the MRS session (experimental group: 6 athletes post-MRS, 10 athletes pre-MRS; control group: 7 athletes pre-MRS, 7 athletes post-MRS) due to athlete and/or material availibility.
MR acquisition
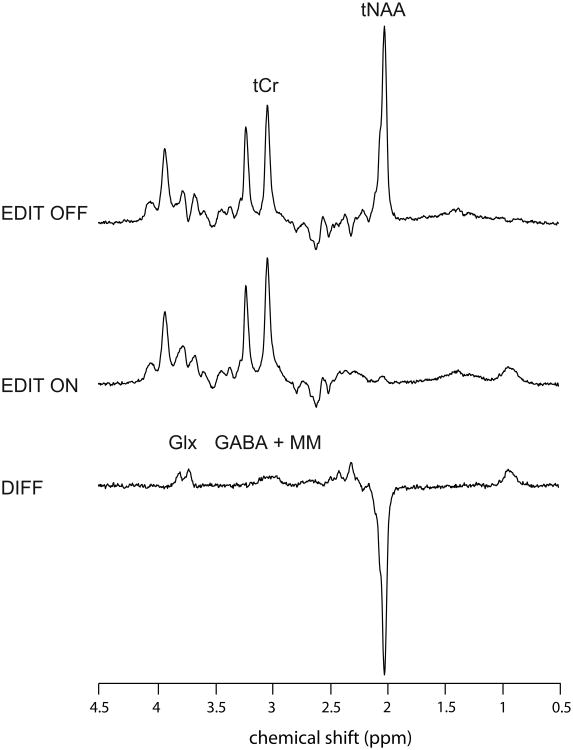
MR acquisitions were performed using the 3T whole-body system (MAGNETOM Trio, a TIM systems, Siemens, Erlangen, Germany) at the Unité de Neuroimagerie Fonctionnelle, Centre de recherche de l'Institut universitaire de gériatrie de Montréal. Radiofrequency transmission was performed with the built-in body coil, and signal was received with at 12-channel receive-only head coil. The prescription of M1 voxel and detection of potential structural abnormalities were performed using anatomical images of the brain obtained with a T1-weighted MPRAGE sequence (TR = 2300 ms; TE = 2.91 ms; FA: 9°; FOV = 256 × 256 mm2; 256 × 256 matrix; 160 axial slices of 1 mm; acquisition time: 9 min 50 s). The voxel of interest (27 × 24 × 32 mm3) was positioned over the left hand area of the primary motor cortex using two accepted anatomical landmarks (Yousry et al., 1997) (Figure 1a). These authors evaluated the location of the motor hand area within the precentral gyrus and described the region as a knob-like structure that can be identified using the two following landmarks: an omega shape in the axial plane and a hook-like shape in the sagittal plane (Yousry et al., 1997). MRS data were acquired using a MEGA-PRESS sequence (Mescher et al., 1998) with double-banded pulses used to simultaneously suppress water signal and edit the γ–CH2 resonance of GABA at 3 ppm. Additional water suppression, using variable power with optimized relaxation delays (VAPOR), and outer volume suppression (OVS) techniques (Tkác et al., 1999) were optimized for the human 3T system and incorporated prior to MEGA-PRESS. The final spectra were obtained by subtracting the signals from alternate scans with the selective double-banded pulse applied at 4.7 ppm and 7.5 ppm (‘EDIT OFF') and at 1.9 ppm and 4.7 ppm (‘EDIT ON’) (Figure 2). MEGA-PRESS data were acquired in four interleaved blocks of 32 (‘EDIT OFF’, ‘EDIT ON’) scans each with frequency drift correction between blocks. FIDs were stored separately in memory for individual frequency and phase correction using the tCr signal at 3.03 ppm, as well as correction for residual eddy-current using unsuppressed water signal obtained from the same voxel.
Figure 1.
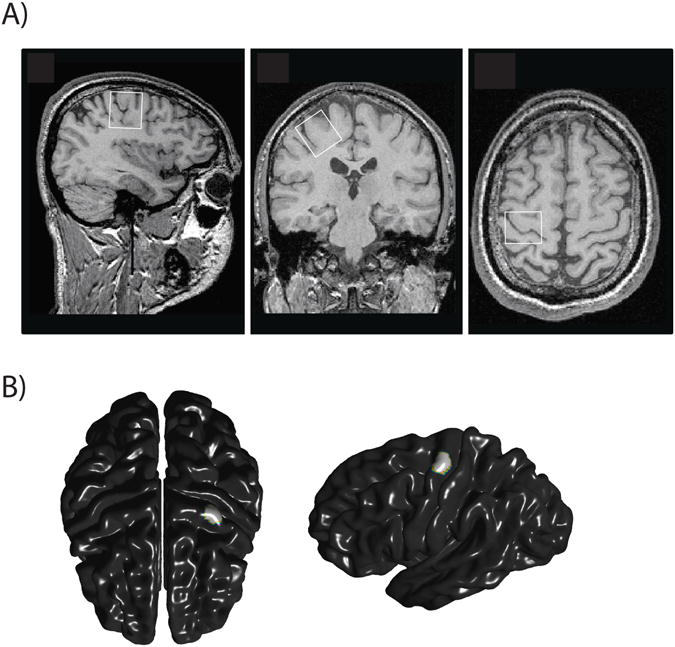
A) Position of the voxel of interest (27 × 24 × 32 mm3) over the left hand area of the primary motor cortex in (A) sagittal, (B) axial (C) and coronal slices.
B) M1 region used for cortical thickness analysis.
Figure 2.
Representative ‘EDIT OFF’, ‘EDIT ON’, and difference (‘DIFF’) spectra. tCr was obtained from ‘EDIT OFF’ spectrum, Glx, and GABA from difference spectrum, and tNAA from both. ‘EDIT OFF’ and ‘EDIT ON’ spectra are the average of 128 scans each.
Analysis of MRS data
Both ‘EDIT OFF’ and difference spectra were analyzed using LCModel 6.2-1A (Provencher, 1993; 2001) which calculated the best fit of the experimental spectrum as a linear combination of model spectra. The basis set for ‘EDIT OFF’ spectra included an experimentally measured metabolite-nulled macromolecular spectrum from the occipital region (average from 11 subjects) and metabolite spectra simulated with home-written software based on density matrix formalism (Henry et al., 2006) in MATLAB, using known chemical shifts and J couplings (Govindaraju et al., 2000). The simulated spectra of the following 20 brain metabolites were included in the basis set: acetyl moiety of NAA (sNAA), alanine (Ala), ascorbate (Asc), aspartate (Asp), aspartate moiety of NAA (mNAA), CH2 group of Cr (Cr-CH2), CH3 group of Cr (Cr-CH3), CH2 group of PCr (PCr-CH2), CH3 group of PCr (PCr-CH3), GABA, glucose (Glc), Glu, Gln, glycerophosphorylcholine (GPC), glycine (Gly), glutathione (GSH), lactate (Lac), mIns, N-acetylaspartylglutamate (NAAG), phosphorylcholine (PCho), phosphorylethanolamine (PE), scyllo-inositol (sIns), and taurine. From LCModel's default simulations of lipid and macromolecular resonances, only ‘Lip13a’ (modeling a broad peak at 1.28 ppm) was allowed during the LCModel fitting that was performed over the spectral range from 0.2 to 4.0 ppm, and modeling of the baseline was restricted to 6 spline knots (the minimum allowed by the program). The basis set for difference spectra included an experimentally measured metabolite-nulled macromolecular spectrum from the occipital region (average from 11 subjects) and the experimentally measured spectra from 100 mM phantoms of NAA, GABA, Glu and Gln at 37°C and with pH adjusted to 7.2. No LCModel's default simulations of lipid and macromolecular resonances were allowed during the LCModel fitting that was performed over the spectral range from 0.5 to 4.0 ppm, and modeling of the baseline was restricted to 9 spline knots. No baseline correction, zero-filling, or apodization functions were applied to the in vivo data prior to LCModel analysis. Visual inspection of the spectra led to exclusion of nine subjects (4 in the control group, 5 in the experimental group) because of contamination from subscapular lipid signal for a final cohort of 12 concussed athletes and 10 controls. Cramér-Rao lower bounds (CRLB) were < 40% for Glx, tNAA, mIns, and tCr (Cr-CH3 + PCr-CH3) and taurine. For six participants, Cramér-Rao lower bounds (CRLB) were > 40% for GABA. The six participants were therefore excluded from all analysis involving GABA, leading to a group of 8 control athletes and 8 concussed athletes. Because of the reduction in sample size, analysis was also performed with participants showing GABA concentrations with a CRLB < 60%, which eliminated one participant. Linewidth of water spectra were all < 10 Hz. A scaling factor between the simulated and measured basis sets was calculated using the group average of tNAA measured from ‘EDIT OFF’ spectra and the group average tNAA from difference spectra. tCr, mIns, taurine, and tNAA concentrations were obtained from ‘EDIT OFF’ spectra, and GABA and Glx concentrations were obtained from difference spectra. The concentration of metabolites was expressed as ratios to tCr.
Cortical thickness analysis
Cortical thickness was extracted from the T1-weighted images using the CIVET pipeline of the Brain-Imaging Centre of the Montreal Neurological Institute (McGill University, Montreal, Canada; Lyttelton et al., 2007). Mean cortical thickness of the whole brain was calculated and the hand representation over left M1 was calculated following the anatomical guidelines from Yousry and collaborators (1997; figure 1b). Statistical analyses were performed on the cortical thickness data using the SurfStat toolbox for Matlab© (http://www.math.mcgill.ca/keith/surfstat/), corrected for multiple comparisons across space using False discovery rate (FDR; Storey, 2002) Anatomical correlations between thickness of left M1 and all cortical vertices were computed using the MACACC method (Lerch et al., 2006), which allows the investigation of correlated changes in cortical thickness across and within diverse cortical networks.
Transcranial magnetic stimulation protocol
TMS was delivered through an 8 cm figure-of-eight coil connected to a MagPro stimulator (MagVenture, Farum, Denmark). The coil was positioned flat on the head of participants with an angle of 45° from the midline, with the handle pointing backwards. A biphasic current was induced with an anterior-posterior direction. The optimal site of stimulation was defined as the coil position from which TMS produced motor evoked potentials (MEPs) of maximum amplitude in the first dorsal interosseus (FDI) muscle of the contralateral hand. The optimal site was then marked down on a cap placed over the head of the participant prior to TMS. In order to measure muscle contractions, two self-adhesive electrodes were placed on the FDI muscle of the right hand and a ground electrode was positioned over the wrist. The EMG signal was filtered with a bandwidth of 20-1000 Hz and digitized at a sampling rate of 4 kHz using a Powerlab 4/30 system (ADInstruments, Colorado Springs, USA). MEPs were recorded using Scope v4.0 software (ADInstruments, Colorado Springs, USA) and stored for offline analysis. TMS pulses were delivered at a frequency of 0.1 to 0.2 Hz for all TMS protocols to avoid long lasting modulation of M1 excitability (Chen et al. 1997).
The resting motor threshold (RMT) was initially determined for each participant and defined as the minimum intensity used to elicit MEPs of 50 μV in 6 of 10 trials. For cortical silent period measurement, subjects were asked to maintain a voluntary isometric muscle contraction of the right FDI at approximately 20% of maximal strength while single pulse TMS was administered at intensities of 120% and 130% of RMT. To induce LICI, two pulses were applied at an intensity to produce test (TS) and conditioning (CS) stimulus amplitudes of approximately 1mV at an interstimulus interval of 100 ms. Ten MEPs were collected for each condition.
TMS analysis
The length of the CSP was manually evaluated and defined as the beginning of EMG activity suppression until the resumption of sustained EMG activity. For LICI, ratios of the conditioning stimulus over the TS were collected. Percentage of inhibition of the CS over the TS was then calculated.
Statistical Analysis
All values are expressed as means (SDs). A p value of < 0.05 was considered significant. Group differences on MRS-derived metabolite concentrations and TMS-derived CSP and LICI were tested with independent samples Student's t-tests. Pearson correlations were computed for each group to assess the relationship between TMS and MRS measures of intracortical inhibition/excitation. To assess the difference between both groups in correlation coefficients, a Fisher's exact test was applied. The impact of the number of concussions, the severity of concussions and the time elapsed since the last injury on TMS and MRS measures were also assessed with Pearson correlations. A Bonferonni correction for multiple comparisons was applied to multiple correlations.
Statistical analyses were performed on the cortical thickness data using the SurfStat toolbox for Matlab© (http://www.math.mcgill.ca/keith/surfstat/), corrected for multiple comparisons across space using False discovery rate (FDR; Storey, 2002) Anatomical correlations between thickness of left M1 and all cortical vertices were computed using the MACACC method (Lerch et al., 2006). Pearson correlations were also computed to assess the relationship between M1 mean thickness and MRS and TMS derived measures of intracortical inhibition. A Fisher's exact test was then computed to assess differences between both groups.
Results
MRS
Demographic data are shown in Table 1. Independent Student's t-tests showed no significant differences between groups for all metabolites of interest (see Table 2). For the control group, two-tailed Pearson correlations showed a significant correlation between GABA and Glx (r = .82, p = .01). However, concussed athletes showed no correlation between metabolites (r = -.04, p = .92). To assess the difference between coefficients, a Fisher's exact test was computed and showed a trend towards a significant difference between groups for the relationship between Glx and GABA (z = 1.87, p = .06). When participants with higher CRBL (< 60%) were included to enhance statistical power, the correlations obtained for both groups were similar (control athletes: r = .75, p = .01; concussed athletes: r = -.16, p = .63) and the difference between both coefficients was significant (z = 2.21, p = .03; Figure 3a).
Table 2. Between group comparisons of metabolites concentrations.
| Metabolite | Group | Mean (SD) | t test | p value |
|---|---|---|---|---|
| GABA/tCr | Control | 0.056 (0.022) | 0.222 | 0.827 |
| Concussed | 0.054 (0.017) | |||
|
| ||||
| Glx/tCr | Control | 0.855 (0.101)) | 0.783 | 0.443 |
| Concussed | 0.825 (0.079) | |||
|
| ||||
| tNAA/tCr | Control | 1.314 (0.110) | 0.753 | 0.446 |
| Concussed | 1.264 (0.184) | |||
|
| ||||
| Ins/tCr | Control | 0.611 (0.082) | 0.777 | 0.446 |
| Concussed | 0.637 (0.071) | |||
|
| ||||
| tau/tCr | Control | 0.186 (0.027) | 1.413 | 0.173 |
| Concussed | 0.204 (0.031) | |||
|
| ||||
| Asp/tCr | Control | 0.282 (0.040) | 1.041 | 0.310 |
| Concussed | 0.308 (0.070) | |||
|
| ||||
| Lac/tCr | Control | 0.034 (0.013) | 0.988 | 0.335 |
| Concussed | 0.039 (0.011) | |||
Figure 3.
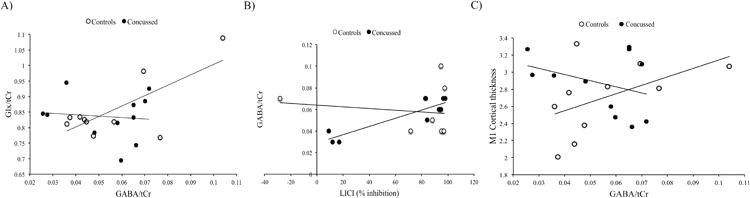
A) Pearson correlations for Glx/tCr and GABA/tCr levels for both groups. Concussed group: r = -.16, p = .63; Control group r = .75, p = .01.
B) Pearson correlations for GABA/tCr levels and LICI percentage of inhibition for both groups. Concussed group r = .92, p = .0001; Control group: r = -.12, p = .72.
C) Pearson correlations for M1 mean thickness and GABA/tCr levels for both groups. Concussed group: r = -.34, p = .30; Control group: r = .50, p = .14
TMS
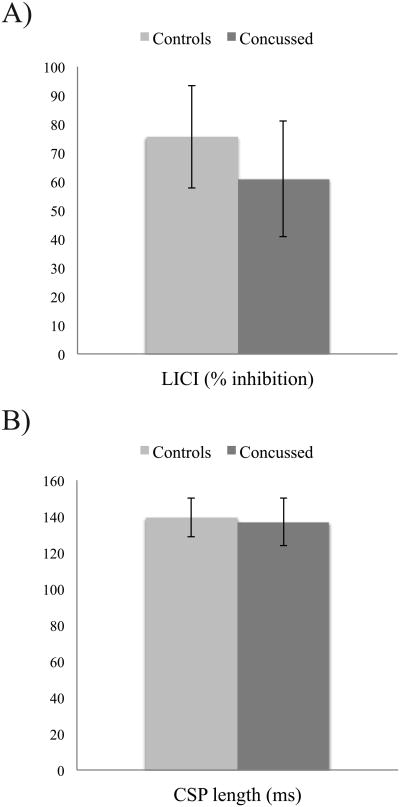
Because both CSP conditions (120-130%) were highly correlated (r = 86, p = .0001), they were used as a compound to reduce the number of comparisons. Independent Student's t-tests showed no significant differences between groups for LICI (t(26) = 1.00, p = .32; figure 4a) and CSP duration (t(28) = .29, p = .77; Figure 4b). Two-tailed Pearson correlations were computed to verify the relationship between TMS and MRS-derived measures of intracortical inhibition/excitation for both groups individually. Controls participants showed no significant relationship between GABA and LICI (r = -.12, p = .71) or CSP (r = .253, p = .18). They also showed no significant relationship between Glx and LICI (r = .12, p = .74) or CSP (r = .34, p = .34). Concussed athletes also showed no significant correlation between GABA and LICI (r = .42, p = .30) and CSP (r = .35, p = .39). No significant correlation was observed between Glx and LICI (r = -.12, p = .71) or CSP (r = .30, p = .35). Further exploratory analyses were computed including GABA concentrations with CRBL < 60%. With this larger group, concussed athletes showed a significant correlation between LICI and GABA (r = .92, p = .0001), which was absent in control athletes (r = -.12, p = .72). To assess the difference between both coefficients, a Fisher's exact test was computed and showed a significant difference between groups for the relationship between LICI and GABA (z = 3.25, p = .001; Figure 3b).
Figure 4.
A) Mean LICI percentage inhibition for both groups.
B) Mean CSP duration for both groups
Cortical thickness
No significant differences were observed for whole brain cortical thickness and left M1 thickness between groups. MACCAC anatomical correlations between M1 thickness and whole brain vertices also showed no significant differences between groups. Two-tailed Pearson correlations were also computed in order to assess the correlation between MRS/TMS-derived measures of intracortical inhibition/excitation and M1 mean thickness. No significant correlations were observed for both controls (r = .40, p = .33) and concussed athletes (r = .003, p = .99). Further exploratory analyses were computed including GABA concentrations with CRBL < 60%. In this case, although correlations were not significant, both groups showed opposite relationships between GABA and M1 thickness. Control athletes showed a positive correlation between both measures (r = .50, p = .14), whereas concussed athletes showed a negative correlation (r = -.34, p = .30). Fisher's exact test showed a trend towards a significant difference between both coefficients (z = 1.76, p = .08; Figure 3c).
Discussion
In the present study, the long-term impact of sport concussions on M1 metabolism, anatomy and physiology was investigated in a sample of asymptomatic athletes who sustained their last concussion on average 3 years prior to testing. The study revealed four main findings: 1) no significant alteration in intracortical inhibition, as measured by CSP and LICI, was observed in the concussed group; 2) no significant metabolic alteration was observed in the M1 of concussed athletes; 3) concussed athletes showed no significant cortical thickness abnormalities in M1 or the whole brain, as well as no abnormalities in M1-whole brain connectivity; and 4) group differences were observed in the relationship between GABA and glutamate and GABA and LICI, suggesting the presence of subtle alterations in M1 inhibition/excitability balance in concussed athletes.
TMS data revealed no significant alterations of the magnitude of M1 GABAB-related intracortical inhibition in concussed athletes, as shown by CSP and LICI measurements. In contrast, previous studies have shown long term alterations in M1 intracortical inhibition in samples of both young asymptomatic and retired athletes. These studies revealed 1) increased CSP duration in asymptomatic university-level football players who sustained multiple concussions from an average of 13 months (De Beaumont et al., 2011a) to 31 months prior to testing (De Beaumont et al., 2007); 2) increased LICI and CSP duration in asymptomatic active university-level football players who sustained multiple concussions from an average of 19 months (De Beaumont et al., 2011b) to an average of 24 months prior to testing (Tremblay et al., 2011b); and 3) increased CSP duration in former athletes who sustained multiple concussions more than 30 years prior to testing.(De Beaumont et al., 2009) Several factors could account for these divergent results. First, the number of concussions suffered in our sample ranged from 1 to 4 with an average of less than two concussions; in contrast, all previous studies consisted of samples of athletes who sustained at least two concussions. The lower number of concussions suffered in our sample did not allow us to look at differences in TMS measures between single and multiple concussions. However, a negative relationship (non-significant) was found between the number of concussions and both TMS measures suggesting the absence of an impact of this factor on our results. Second, the time elapsed since the last concussive event in our study differed considerably with previous studies conducted with young asymptomatic athletes. Athletes from our study sustained their last concussion an average 3 years prior to testing, suggesting a possible recovery of inhibitory dysfunction. Finally, alterations in M1 intracortical inhibition may not be a widespread and stable feature of the neurophysiological response to concussion.
Results from the present study also suggest the absence of long-term disruptions in the concentration of metabolites in primary motor cortex after sport concussion. Recent studies have shown effects of sport concussions on brain metabolism in the acute and chronic phases (Vagnozzi et al., 2008; 2010; Henry et al., 2011). However, no study has investigated metabolic alterations in athletes beyond the establishment of chronicity phase (that is, more than 6 months post-concussion). Moreover, there is currently no consensus on the acute or chronic metabolic effects of sport concussions; results from Henry and collaborators have shown the presence of chronic NAA/Cr and M-I disruption 6 months post-injury in M1 (Henry et al., 2011), whereas other studies have shown complete recovery of NAA/Cr levels within 45 days in the frontal lobe (Vagnozzi et al., 2008; 2010). Given the present data, we can hypothesize that alterations seen in M1 during the chronic phase eventually recover 3 years after the concussive event. Additionally, results from most 1H-MRS studies do not include the measurement of GABA and consequently, use a smaller voxel of interest. In the present study, the VOI included some contamination from somatosensory regions, which could also explain the discrepancy between our data and previous studies. Finally, we cannot conclude on metabolic disruptions that could be seen in other brain regions such as the corpus callosum or the hippocampus, which have been shown to display some vulnerability to concussion in moderate to severe TBI (Babikian et al., 2010; Harris et al., 2012). As a result, variability in the regions of interest used to assess neurometabolic alterations in concussed athletes could contribute to the lack of consensus in the literature.
Since no alterations were found in the concussed groups using highly sensitive measures such TMS and 1H-MRS, it is not surprising that group differences were not found in the cortical thickness analysis, a less direct measure of neuronal function. Furthermore, results using the MACCAC method (Lerch et al., 2006) also suggest that sport concussion does not affect anatomical connectivity, as cortical thickness correlations between M1 and multiple cortical regions were not different between groups. In the current TBI literature, there are very few studies that have assessed cortical thickness integrity in adults as most studies have assessed populations of children and adolescents, or animals models of pediatric TBI (Fineman et al., 2000; Merkley et al., 2008; Turken et al., 2009; Hanten et al., 2011; Palacios et al., 2012). One recent study reported measures of cortical thickness following concussion in healthy aging adults and found no difference in cortical thickness between groups, but a link between regions of cortical thinning and episodic memory deficits (Tremblay et al., 2012). However, no study has looked at the effect of cortical thinning in relation to concussion in younger athletes. Results from the present study suggest that acute and chronic metabolic or neurophysiological dysfunctions in M1, as revealed in more recent studies (De Beaumont et al., 2012), have no long-term impact on cortical thickness.
Since multiple studies have shown long term alterations in M1 intracortical inhibition mediated by GABAergic transmission after sport concussion (De Beaumont et al., 2012), we hypothesized altered GABA concentrations in the M1 of concussed athletes. Although no alterations were seen in measures of the GABAergic system by TMS and 1H-MRS, further correlational analysis suggest the presence of subtle changes in inhibitory M1 mechanisms in concussed athletes. Control athletes showed a significant positive correlation between GABA and Glx, whereas concussed athletes displayed no correlation between both metabolites. Data in control athletes are in line with recent studies showing a positive correlation between these inhibitory and excitatory neurometabolites in healthy individuals using 1H-MRS (Stagg et al., 2011; Tremblay et al., 2012; Prescot et al., 2013). Therefore, the non-existent relationship between GABA and Glx in concussed athletes suggests that sport concussions could cause an imbalance between excitability and inhibition in M1. A trend towards a differential correlation between GABA and M1 cortical thickness was observed as controls showed a positive non-significant correlation between the two variables whereas concussed athletes showed a negative non-significant relationship. Although these results are exploratory, they indicate that subtle alterations in GABA transmission and organization in M1 could be present in the brain of concussed athletes even though the absolute metabolite concentrations do not differ from non-concussed athletes.
The exact mechanism underlying this possible slight metabolic imbalance is unknown. Based on recent studies, it could be hypothesized that increases in intracortical inhibition revealed by increased CSP duration and LICI (De Beaumont et al., 2012) and the abnormally high glutamate concentration in M1 in the chronic phase (Henry et al., 2011) could trigger a long-lasting disruption in the interaction between GABA and glutamate. In the even longer term, this subtle imbalance could make the brain more susceptible to a subsequent concussion and partly explain recent findings suggesting a link between sport concussion and abnormal aging (Broglio et al., 2012). Normal aging is typically associated with structural and chemical changes together with a functional impairment of neurons. However, the decline in cognitive function associated with these brain changes could be related to the amount of “cognitive reserve” available (Broglio et al., 2012), which may be influenced by concussive and sub-concussive hits. Therefore, we can hypothesize that, if the differential relationships seen in the present study are related to alterations in metabolic interactions, these dysfunctions in brain metabolism could accelerate or accentuate the neurodegenerative process of aging and increase the odds of developing an abnormal aging trajectory.
Moreover, concussed athletes also showed a differential relationship between GABA and LICI, where concussed athletes showed a positive significant correlation while no correlation was observed in the control group. The absence of a relationship between GABA levels as measured by 1H-MRS and TMS GABA-mediated inhibitory measures in the control group is surprising. However, this result is in line with two recent studies that showed no correlation between MRS and multiple TMS-derived GABA measures in healthy controls (Stagg et al., 2011; Tremblay et al., 2011). This finding suggests that H-MRS GABA does not precisely reflect GABAergic synaptic activity (Stagg et al., 2011), and more specifically that involving GABAB receptors. Surprisingly, however, LICI and GABA levels were correlated solely in concussed athletes. Although difficult to interpret, this result could reflect the presence of subtle inhibitory dysfunctions in M1. Moreover, the present data suggest that LICI and CSP, both measures of the GABAergic system, are likely to tap into different mechanisms underlying GABAB-related inhibition in the primary motor cortex as they did not correlate in the control group. Physiological studies support this hypothesis as CSP was found to be linked to spinal inhibition (Inghilleri et al., 1993), whereas LICI was found to rely on cortical inhibition (Werhahn et al., 1999).
It should be noted that several factors can limit the generalization of the present results. First, sub-concussive blows to the head throughout an athlete's career may have resulted in subtle brain alterations. This is coumpounded by the fact that many real concussions go undiagnosed. A recent study conducted in a sample of high-school football players revealed a very high average of impacts to the head, and of markedly high rotational and linear acceleration forces (Broglio et al., 2011). Indeed, Chamard and collaborators (2012) reported the presence of metabolic disruptions in non-concussed hockey players throughout a season that was hypothesized to be caused by cumulative effects of sub-concussive events. As a result, metabolic disruptions in primary motor cortex of concussed athletes may have been underestimated. To control for this, further studies should include an additional control group comprising high-level athletes who do not participate in contact sports, such as track and field. Nevertheless, direct comparisons between athletes that play the same contact sport can reveal important information. Most notably, since it can be assumed that the prevalence of sub-concussive blows is similar between athletes of both groups (concussed and non-concussed), something specific about diagnosed concussions may emerge. This appears to be the case indeed, since most concussion studies using a similar recruiting approach to the one sued in the present study have revealed wide-ranging brain abnormalities. Second, there is increasing evidence suggesting cumulative and deleterious effects of repeated concussions (Blennow et al., 2012). For instance, Guskiewicz and collaborators (2005) showed that football players with a history of multiple concussions have a strikingly increased risk of developing long lasting cognitive impairments. Unfortunately, the size of the present sample did not allow a direct comparison between athletes that had suffered one or many concussive events throughout their career. It should also be noted that there exists a possibility that the TMS measures, which were taken before MR acquisition in a portion of the sample of athletes, may have altered glutamate and GABA concentration in M1. However, this appears unlikely since a low frequency of stimulation was used (between 0.1 and 0.2 Hz), which has been shown not to modify cortical excitability (Chen et al. 1997) and only a limited number of pulses were applied for the measurement of LICI and CSP.
In conclusion, the present data suggest the general absence of neurophysiologic, neurometabolic and neuroanatomical disruptions in M1, three years after the last concussion in a sample of active university-level football players. However, correlational analyses suggest the presence of a slight metabolic imbalance between GABA and glutamate concentrations in the primary motor cortex of concussed athletes. Even though this abnormality appears relatively modest, it highlights the need for multimodal evaluations of the impact of concussions that may not be seen using neuropsychological or standard functional evaluations. The present data also stress the importance of assessing the long-term impact of sport concussions on brain function and evaluating the role of subtle disruptions in metabolic balance that may contribute to abnormal aging (Tremblay et al. 2012).
Highlights.
- Absence of M1 disruptions following concussion, as assessed by transcranial magnetic stimulation and anatomic measures.
- Normal concentration of GABA, glutamate and NAA in M1 of concussed athletes.
- Abnormal correlation between GABA and glutamate in concussed athletes suggesting a slight metabolic imbalance in M1.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Fonds de Recheche du Québec – Santé to HT and ML. MM acknowledges the support from Biotechnology Research Center (BTRC) grant P41 RR008079 and P41 EB015894 (NIBIB), and NCC P30 NS076408.The authors would like to thank Edward J. Auerbach, Ph.D. (Center for Magnetic Resonance Research, University of Minnesota) for implementing MEGA-PRESS sequence on Siemens, and Romain Valabregue, Ph.D. (Centre de NeuroImagerie de Recherche, Paris, France) and Brice Tiret, Ing (Unité de neuroimagerie fonctionnelle, Montréal) for developing processing tools.
References
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, et al. Proton MR Spectroscopy Detected Glutamate/Glutamine Is Increased in Children with Traumatic Brain Injury. J Neurotrauma. 2004;21:1539–1552. doi: 10.1089/neu.2004.21.1539. [DOI] [PubMed] [Google Scholar]
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Babikian T, Marion SD, Copeland S, Alger JR, O'Neill J, Cazalis F, et al. Metabolic levels in the corpus callosum and their structural and behavioral correlates after moderate to severe pediatric TBI. J Neurotrauma. 2010;27:473–481. doi: 10.1089/neu.2009.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The Molecular Pathophysiology of Concussive Brain Injury. Clin Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The Neuropathology and Neurobiology of Traumatic Brain Injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, Randolph C. Cummulative head impact burden in high school football. J Neurotrauma. 2011;28:2069–2078. doi: 10.1089/neu.2011.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc Sport Sci Rev. 2012;40:138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 2001;16:149–164. doi: 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer V, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39:805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamard E, Théoret H, Skopelja EN, Forwell LA, Johnson AM, Echlin PS. A prospective study of physician-observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. Neurosurg Focus. 2012;33:1–7. doi: 10.3171/2012.10.FOCUS12305. E4. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chin LS, Montenegro P, Cantu RC. Chronic Traumatic Encephalopathy. Curr sports med. 2013;47:15–26. doi: 10.1249/JSR.0b013e31827ec9e3. [DOI] [PubMed] [Google Scholar]
- Cimatti M. Assessment of metabolic cerebral damage using proton magnetic resonance spectroscopy in mild traumatic brain injury. J Neurosurg Sci. 2006;50:83–88. [PubMed] [Google Scholar]
- Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–1179. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Davidson T, Tremblay F. Hemispheric differences in corticospinal excitability and transcollosal inhibition in relation to degree of handedness. Plos one. 2013;8:1–9. doi: 10.1371/journal.pone.0070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Henry LC, Gosselin N. Long-term functional alterations in sports concussion. Neurosurg Focus. 2012;33:1–7. doi: 10.3171/2012.9.FOCUS12278. E8. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61:329–336. doi: 10.1227/01.NEU.0000280000.03578.B6. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011b;46:234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered Bidirectional Plasticity and Reduced Implicit Motor Learning in Concussed Athletes. Cereb Cortex. 2011a;22:12–21. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- Fineman I, Giza CC, Nahed BV, Lee SM, Hodva DA. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. J Athl Train. 2001;36:228–35. [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR Am J Neuroradiol. 2004;25:730–737. [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM. Postural stability assessment following concussion: one piece of the puzzle. Clin J Sport Med. 2001a;11:182–189. doi: 10.1097/00042752-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Ross SE, Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train. 2001b;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- Hanten G, Cook L, Orsten K, Chapman SB, Li X, Wilde EA, et al. Effects of traumatic brain injury on a virtual reality social problem solving task and relations to cortical thickness in adolescence. Neuropsychologia. 2011;49:486–497. doi: 10.1016/j.neuropsychologia.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, Herring SA, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J sports med. 2013;47:15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, et al. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32:2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010;27:65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, et al. Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 2011;11:105. doi: 10.1186/1471-2377-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Res Med. 2006;55:250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, Ashwal S, Oyoyo U, Ghamsary M, Saunders D, et al. Prospective longitudinal proton magnetic resonance spectroscopic imaging in adult traumatic brain injury. J Magn Reson Imaging. 2006;24:33–40. doi: 10.1002/jmri.20607. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Gay M, Zhang K, Neuberger T, Horovitz SG, Hallett M, et al. The Use of Magnetic Resonance Spectroscopy in the Subacute Evaluation of Athletes Recovering from Single and Multiple Mild Traumatic Brain Injury. J Neurotrauma. 2012;29:2297–2304. doi: 10.1089/neu.2011.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Collins MW, Iverson GL, Field M, Maroon JC, Cantu R, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98:296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–44. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Macmillan CSA, Wild JM, Wardlaw JM, Andrews PJD, Marshall I, Easton VJ. Traumatic brain injury and subarachnoid hemorrhage: in vivo occult pathology demonstrated by magnetic resonance spectroscopy may not be “ischaemic.” A primary study and review of the literature. Acta Neurochir. 2002;144:853–862. doi: 10.1007/s00701-002-0966-x. [DOI] [PubMed] [Google Scholar]
- Marino S, Ciurleo R, Bramanti P, Federico A, De Stefano N. 1H-MR spectroscopy in traumatic brain injury. Neurocrit Care. 2011;14:127–133. doi: 10.1007/s12028-010-9406-6. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180(1):181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- Merkley TL, Bigler ED, Wilde EA, McCauley SR, Hunter JV, Levin HS. Diffuse Changes in Cortical Thickness in Pediatric Moderate-to-Severe Traumatic Brain Injury. J Neurotrauma. 2008;25:1343–1145. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Neurology AAO. Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997:581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Fernández-Espejo D, Roig T, Tormos JM, et al. Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex. 2012;49:646–57. doi: 10.1016/j.cortex.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Parker TM, Osternig LR, van Donkelaar P, Chou LS. Gait stability following concussion. Med Sci Sports Exerc. 2006;38:1032–1040. doi: 10.1249/01.mss.0000222828.56982.a4. [DOI] [PubMed] [Google Scholar]
- Prescot AP, Renshaw PF, Yurgelun-Todd DA. γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013;129:232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21:1693–1705. doi: 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589:5845–55. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A Direct Approach to False Discovery Rates. JRSSB. 2002;64:479–98. [Google Scholar]
- Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Lassonde M, Théoret H. Evidence for the Specificity of Intracortical Inhibitory Dysfunction in Asymptomatic Concussed Athletes. J Neurotrauma. 2011;4:493–502. doi: 10.1089/neu.2010.1615. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, De Beaumont L, Marjanska M, Doyon J, et al. The relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J Neurophysiol. 2012;109:1343–1349. doi: 10.1152/jn.00704.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Henry LC, Boulanger Y, Evans AC, Bourgouin P, et al. Sports Concussions and Aging: A Neuroimaging Investigation. Cereb Cortex. 2012;23:1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- Turken AU, Herron TJ, Kang X, O'Connor LE, Sorenson DJ, Baldo JV, et al. Multimodal surface-based morphometry reveals diffuse cortical atrophy in traumatic brain injury. BMC Med Imaging. 2009;9:20. doi: 10.1186/1471-2342-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgrò E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe DJ, Comstock RD, Nance ML. Concussion research: a public health priority. Injury Prev. 2011;17:69–70. doi: 10.1136/ip.2010.031211. [DOI] [PubMed] [Google Scholar]
- Xu S, Zhuo J, Racz J, Shi D, Roys S, Fiskum G, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J Neurotrauma. 2011;28:2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]