Abstract
Vaccination via skin often induces stronger immune responses than via muscle. This, in line with potential needle-free, painless delivery, makes skin a very attractive site for immunization. Yet, despite decades of effort, effective skin delivery is still in its infant stage and safe and potent adjuvants for skin vaccination remain largely undefined. We have shown that laser technologies including both fractional and non-fractional lasers can greatly augment vaccine-induced immune response without incurring any significant local and systemic side effects. Laser illumination at specific settings can accelerate the motility of antigen-presenting cells or trigger release of ‘danger’ signals stimulating the immune system. Moreover, several other groups including the authors explore laser technologies for needle-free transcutaneous vaccine delivery. As these laser-mediated resurfacing technologies are convenient, safe and cost-effective, their new applications in vaccination warrant clinical studies in the very near future.
Keywords: adjuvant, intradermal injection, laser, skin vaccination, stratum corneum, transcutaneous delivery, vaccine
Vaccination is one of the most effective and economical means to control infectious diseases and has led to the global eradication of smallpox, near-eradication of polio and tremendous reduction of deaths resulting from vaccine-preventable diseases like measles, diphtheria and influenza [1]. Vaccines are regarded as one of the greatest achievements in the modern medicine. The first vaccination practice with a written record can be chased back to approximately 1550 when ancient Chinese practitioners blew up powdered smallpox scabs into noses of the healthy people to generate immunity against smallpox [2]. Later in 1700s, skin scarification was used to deliver cowpox for smallpox vaccination [3], which contributes greatly to the global control and eventual eradication of smallpox. Yet, scarification causes severe skin lesion, risks infection and lacks control and reproducibility, and it is thus not used in modern era [4].
Nowadays, vaccines are mostly delivered into the muscular tissue by a hypodermic needle as it is convenient and sufficient, but it causes pain and generates bio-hazardous sharp wastes [5]. Moreover, about 10% of the population has needle phobia and it is difficult for them to be vaccinated by this procedure [6]. In the past few years, there is a renewed interest in skin vaccination as the skin contains large amounts of antigen-presenting cells (APCs) [7]. Yet, there are two major hurdles for skin vaccination to be practical in the clinic. Firstly, adjuvants that are suitable for skin vaccination are critically lacking despite the fact that a number of adjuvants have been developed in preclinical studies, but mainly for intramuscular (IM) vaccination [8]. Secondly, new technologies are urgently needed for needle-free, convenient and safe skin vaccination [7,9]. The current review discusses some of these advances with a specific focus on the use of laser technology to enhance vaccine immunogenicity and to deliver vaccines in a needle-free, painless manner.
Vaccine adjuvant for skin vaccination
Currently, there are only three clinic vaccines delivered by intradermal (ID) injection and all three of them contain no adjuvants. The most popular Bacille Calmette–Guerin (BCG) vaccine is best delivered by ID injection [4]. It consists of live-attenuated Mycobacterium bovis, that express multiple Toll-like receptor (TLR) agonists, like TLR2, TLR4 and TLR9 [10], and thus no adjuvant is needed for the vaccine. Another ID vaccine is seasonal influenza vaccine that was approved for ID injection 2 years ago by a newly developed ID microinjection system in both EU and USA [11,12]. Again, no adjuvant is incorporated in this delivery route despite that MF59 and AS03 adjuvants have been approved for IM injection of this vaccine in EU [13]. Likewise, rabies vaccine was based on killed rabies virus and recently recommended for ID injection by WHO because ID injection induced a comparable or better seroconversion than IM injection, yet with a less vaccine dose in the absence of adjuvant [14]. The fact that no adjuvant is currently approved for skin vaccination by US FDA raises an urgent need of developing such adjuvants, if we are to explore this route of vaccination into adjuvanted vaccines. Moreover, delivery of adjuvanted vaccines into the epidermis of the skin may be also safer than delivery of the vaccine into the muscle because the adjuvant would be more restricted in the epidermis where no nerves reside. This is of particular clinical significance because of renewed concerns about the long-term side effects of adjuvants related to narcolepsy. Several recent studies reported a possible association of an AS03-adjuvanted 2009 pandemic H1N1 influenza vaccine (Pandemrix) with the rising cases of narcolepsy, an incurable chronic neurological disease with disturbed sleep-wake cycles, in EU [15–17]. In contrast to the rising cases of narcolepsy in EU, no such an increase was observed in USA where no adjuvant was used in its pandemic 2009 H1N1 vaccine in the same period of time. If the causative link is confirmed, it is clear that it is the adjuvant, not the antigen contributes to narcolepsy, although the underlying mechanism is not known. ‘Molecular mimicry’ has been proposed as a common mechanism underlying adjuvant-induced autoimmune and neurodegenerative diseases due to the modulation of self-antigen and production of self-reactive anti-bodies and/or T cells [18–20]. Although the causative link of these chronic diseases to the use of adjuvants is not yet confirmed, adjuvant safety will continue to be the center of debates among scientific communities, health authorities and the general public.
Similar to development of adjuvants for IM immunization, potential systemic and long-term side effects are also a major concern for development of adjuvants for skin vaccination. It will be difficult for any new adjuvants to be approved by FDA without sufficient proof of long-term adjuvant safety. Yet, development of adjuvants for skin vaccination faces additional challenges. Skin is very sensitive to inflammation in general compared to muscle. We recently evaluated local reactions following ID injection of Alum, TLR agonists, emulsion adjuvants and combinatorial adjuvants and found that Alum might be too toxic to be used in skin vaccination because Alum caused severe and persistent local reactions in the skin for weeks, concurrent with heavy infiltration of inflammatory cells into adjuvant-treated skin [8,21]. All the other adjuvants tested, except for TLR4 agonist monophosphoryl lipid A (MPL) and TLR9 agonist CpG, induced severe local reactions including swelling, ulceration and long-term deposition, which jeopardized skin barrier function and increased local infection risk [8,21]. The high sensitivity of the skin to inflammation prevents the use of the majority of traditional adjuvants for skin vaccination. Moreover, emulsion adjuvants may not be applicable to the skin. High viscosity emulsion adjuvants are difficult for administration with newly approved ID microinjection systems and almost all emulsion adjuvants are unable for delivery by microneedles due to the biological incompatibility with microneedle fabrication processes. Emulsion adjuvants also tend to stay in the skin for a prolonged period of time, causing itching, irritation and discomfort compared to IM immunization.
Laser-vaccine adjuvant
We recently developed laser-based vaccine adjuvant (LVA) in attempt to augment vaccine-induced immune responses without injection of any foreign or self-materials into the body [8,22]. Adjuvantation by a physical means is a completely novel concept by which the major concern on the long-term side effects of adjuvants can be largely eased. We explored two types of laser illumination (non-fractional and micro-fractional) to enhance immune responses induced by several vaccines.
Non-fractional laser adjuvant
Non-fractional laser adjuvant is laser illumination of a small area (<1 cm2) of the skin followed by ID injection of the vaccine into laser-illuminated site to enhance motility and antigen-uptake ability of APCs (Figure 1, left panel).
Figure 1. Illustration of non- and micro-fractional laser-based vaccine adjuvant.
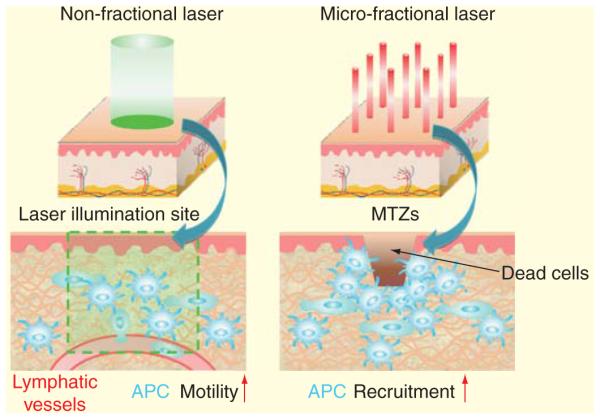
Non-fractional laser shines a light beam onto the skin. The laser energy is low enough not to hurt cells, but high enough to disrupt dermal tissue scaffolds and increase motility, migration and entry of antigen-presenting cells into lymphatic vessels. Micro-fractional laser shines multiple tiny laser beams onto the skin. The laser energy is sufficient to kill cells that are directly exposed to the laser, and the dead cells release endogenous danger signals leading to active recruitment of antigen-presenting cells around each MTZ.
MTZ: Micro-thermal zone.
Our investigation showed that a brief (1–2 min) illumination of the inoculation site with a 532 nm Nd:YAG laser was able to enhance vaccine-induced immune response [22]. The brief illumination did not raise the skin temperature higher than 41°C as measured by an infrared camera or cause alteration in the skin visibly or histologically [22]. Laser illumination significantly enhanced ovalbumin (OVA)-induced serum antibody titer by approximately fourfold as compared to OVA immunization alone [22]. The laser adjuvant effect appears not to be limited to 532 nm Nd:YAG laser. Lasers at other wavelengths in the visible and near-infrared ranges show a comparable adjuvant effect (data not shown), hinting a common mechanism irrelevant to laser wavelengths, peak energy levels and working modes.
Indeed, intravital confocol imaging showed that the brief laser illumination significantly enhanced motility and migration of APCs in the dermal tissue [22]. The motility and migration could be further enhanced by antigen injection [22]. Enhanced APC motility and migration enable them to survey a larger area and facilitate antigen sampling as previously described as dendrite surveillance extension and retraction cycling habitude (dSEARCH) [23–26]. In accordance to this, more OVA was taken up by APCs in laser-treated skin as compared to control skin following ID injection [22]. In addition, the interstitial space of the dermal tissue consists of a complex microarchitecture comprising fibrillar proteins and proteoglycans, and APCs are anchored within the matrix, limiting cell migration through the interstitium [27]. The dense dermal tissue scaffolds are also altered considerably by brief laser illumination as shown by transmission electron microscopy (TEM) [8,28]. The dermal collagen fibers were dissociated and the interaction between DCs and surrounding tissue scaffolds was disrupted at the site of laser illumination, while leaving the surrounding APCs alive [8,28]. Conceivably, loose interstitial microarchitecture can enhance the tissue permeability and permit the relatively free movement of APCs in laser-treated skin (Figure 1). Besides the increased APC migratory ability, we observed ‘dermal cord’ formation right after laser illumination, indicative of migration of a large number of APCs into lymphatic vessels (LVs) [28]. Moreover, laser illumination appeared to enlarge preformed portals in perilymphatic membrane to facilitate DC entry into LVs [28]. The diameter of these portals was significantly increased after laser illumination and further increased by DC occupancy, in line with previous report [29].
To validate a potential of the novel LVA for clinical use, we explored its immune-enhancing effects on an experimental nicotine vaccine (Nic-KLH) and mouse-adapted PR8 influenza vaccine in murine models. Incorporation of LVA into the primary immunization of a three-dose ID immunization regimen (LVA/ID) significantly increased serum anti-nicotine antibody (NicAb) titer by approximately threefold as compared to three-dose ID immunization alone. Serum NicAb titer in LVA/ID group was also much higher than that induced by four doses of Alum-incorporated IM immunization (Alum/IM) [21]. Multiple Alum/IM immunizations are currently used for anti-nicotine immunotherapy against nicotine addiction in the clinic [30,31]. Besides the significantly increased serum NicAb titer, LVA/ID immunization also prolonged the peak NicAb titer as compared to ID immunization alone or Alum/IM immunization [21]. The High NicAb titer in LVA/ID group translated into more significant inhibition on nicotine entry into the brain following intravenous nicotine challenge [21]. Brain nicotine level in LVA/ID group was reduced by 34% as compared to ID immunization alone [21]. LVA/ID also significantly enhanced PR8 vaccine-induced hemagglutination inhibition (HAI) titer by 50% as compared to ID immunization or 100% as compared to IM immunization (Figure 2A). After viral challenge, LVA/ID immunization significantly reduced lung viral titer from 11 × 104 in IM group and 5 × 104 in ID group to only 6 × 102, a 2-log reduction as compared to IM and ID group (Figure 2B).
Figure 2. Laser adjuvant effects on PR8 vaccine.
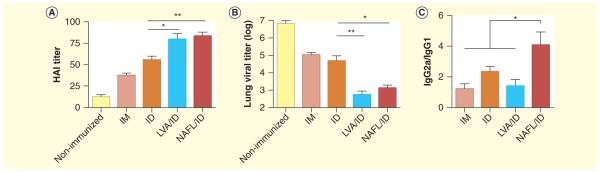
BALB/c mice (6–8 weeks) were intradermally immunized with mouse-adapted formalin-inactivated PR8 vaccine equivalent to 2ng hemagglutin per mouse in the presence of non-fractional (LVA/ID) or micro-fractional laser illumination (NAFL/ID) or absence of adjuvants (ID). Naive mice (non-immunized) and IM of the same amount of PR8 vaccine served as controls. Four weeks later, serum HAI titers and a ratio of IgG2a to IgG1 were quantified (A & C). Mice were then challenged with 10 × 50% lethal dose (LD50) of PR8 virus and 4 days later lung viral titers were quantified (B).
*, p<0.05; **, p<0.01, n = 4–6.
ID: Intradermal; IM: Intramuscular; LVA: Laser-based vaccine adjuvant; NAFL: Non-ablative fractional laser.
The effect of LVA is similar to that of Alum and considered to be moderate. We are thus pursuing combination of LVA with other adjuvants to further boost vaccine-induced immune response. Because laser illumination did not induce DC maturation [22], we explored whether a combination of LVA with a stimulator of DC maturation could synergistically enhance vaccine-induced immune responses. To this end, MPL was chosen since it induces only a mild skin reaction following ID injection and it has been approved for human use [8,21]. Combinatorial LVA/MPL was found to enhance OVA-induced immune response by 21-fold, while LVA or MPL alone only enhanced immune response by 4- or 10-fold, respectively [8,21]. As for Nic-KLH vaccine, LVA/MPL significantly enhanced serum NicAb titer by 33-fold, while LVA and MPL alone only enhanced serum NicAb titer by 4- and 13-fold, respectively [21]. Importantly, combination of LVA with MPL didn’t increase local side effects as compared to either adjuvant alone [8,21].
Given that laser treatment can enhance in situ DC migration and entrance into LVs, we employed this technology to augment DC-based immunotherapy [28,32]. We used a slightly invasive laser illumination in an assumption that a mild local inflammation might further boost DC migration to the draining lymph nodes [28,32]. It was found that such laser illumination followed by ID injection of tumor lysate-pulsed DCs induced more vigorous expansion of tumor-specific IFN-γ-secreting CD8+ T lymphocytes, completely abrogated early growth of 4T1 breast cancer and B16F10 melanoma in prophylactic models and significantly extended the survival of 4T1-resected mice in a therapeutic model [28,32]. This simple, convenient laser-based approach merits further investigation for improving DC-based immunotherapy in clinical settings.
Micro-fractional laser adjuvant
Micro-fractional laser adjuvant under the current development is non-ablative fractional laser illumination of the inoculation site. While keeping the stratum corneum in place, high-precision laser microbeams coagulate an array of narrow and deep columns in the epidermis and dermis with a desirable density (Figure 1, right panel). It is well known that skin injury is healed by expansion of surrounding healthy epithelial cells to close the injury. The larger the injured skin area is, the longer the closure takes. On the contrary, if many micropores are generated in the skin with the total area equivalent to the injury, it would be healed much quickly since it takes only a day or two for each micropore to be fully closed [33]. This well-known phenomenon leads to the development of ablative fractional laser (AFL) and non-ablative fractional laser (NAFL) for skin resurfacing. These fractional laser resurfacing technologies become a gold standard in today’s skin rejuvenation industry [34–37]. While these micro-coagulated tissues present no visible lesion in the skin, they serve as endogenous adjuvants by release ‘danger signals’ to stimulate the immune system. In this regard, recent mechanism study of alum revealed that alum could cause cell deaths and release of endogenous danger signals, such as uric acid [38], dsDNA [39,40] and possible RNA, heat shock proteins, purine metabolites, ATP and others. These endogenous danger signals are recognized by damage-associated molecular patterns (DAMPs) in or on the APCs, including TLRs, NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), C-type lectin receptors (CLRs) and a DNA-binding receptor [41]. Binding of the DAMPs with the endogenous danger signals activates NF-κB transcriptional factors and inflammasome, leading to the production of chemokines and inflammatory cytokines to augment specific and adaptive immune responses [41,42]. In mimicking the action of alum, laser-damaged cells in the microthermal zones (MTZs) would also release DAMPs resulting in recruitment of a large number of APCs into each MTZ and enhancement of their antigen-uptake and activation (Figure 1).
In accordance to this, treatment of the inoculation site with NAFL prior to ID injection of OVA elevated OVA-specific antibody production by 4.5-fold. NAFL also significantly enhanced PR8 flu vaccine-induced HAI titer by 50% as compared to ID injection and 120% as compared to IM injection (Figure 2A). After viral challenge, lung viral titer in NAFL/ID group was reduced significantly compared to ID or IM group (Figure 2B). No significant difference was found in HAI and lung viral titers between the non-fractional and fractional laser groups (Figure 2A & B), indicating a comparable adjuvant effect between the two laser adjuvants. However, by analysis of subtype antibodies, we found that NAFL induced primarily a Th1-biased immune response as evidenced by a significant increase in IgG2a/IgG1 ratio in NAFL/ID group over ID or LVA/ID group (Figure 2C). Another advantage of NAFL is that its adjuvant effect is independent on skin colors, as the laser energy is mainly absorbed by water, in contrast to the non-fractional laser [43]. Finally, micro-fractional laser has a potential to further augment vaccine-induced immunity by delivery of vaccines into or through the MTZs generated. Recently, a handheld non-ablative fractional laser device was approved by FDA for wrinkle removal at home, which emits a tiny laser beam of 1410 nm with 0.5 μm in diameter and generates a 5 × 8 array of MTZs in a 7 × 10 mm2 skin area within 1–2 sec [44]. Approval of this device for cosmetics at home confirms super safety of the device and the laser treatment. The safety is further warranted by the fact that only a tiny skin area on the arm is exposed to laser for vaccination, whereas a large area of the skin on the face is treated by the laser for cosmetics. Remarkably, this super safe laser device is found to greatly boost various vaccines ID administered (manuscript in preparation). Mechanistically, NAFL leads to enhanced expression of proinflammatory cytokines such as TNF-α, IL-6, IL-12, CCL-2 and the like, in contrast to the non-inflammatory LVA described earlier. Presumably, laser-induced tissue damage triggers release of DAMPs that then actively recruit APCs, resulting in sufficient antigen-uptake and activation of APCs (Figure 1).
Advantages of LVA
LVA is completely novel for immune-enhancement of ID vaccines. In contrast to conventional adjuvants that intend to improve immunogenicity of vaccines, LVA primes our body to better respond to a given vaccine and it has the following advantages. Firstly, LVA doesn’t require any modification of vaccine formulation or an increase in injection volume. This is not trivial in that any modification of vaccine manufacturing faces new regulatory approval and that skin tissue can only accommodate a limited volume of injection [45]. Secondly, LVA can be a standalone adjuvant or combined with ID microinjection systems or microneedle array patches to enhance vaccine-induced immune response [12,46]. Thirdly, LVA induces little skin reactions, whereas a majority of traditional adjuvants induce long-term deposition and ulceration that can potentially jeopardize skin barrier function [8,21]. LVA also has little concern of long-term side effects as no additive is injected besides antigen itself, which are the major concern on the use of traditional adjuvants [15–17,47–51]. Fourthly, LVA enhances skin vaccination independent of specific vaccine/adjuvant formulations. In contrast, traditional adjuvants, like alum, emulsion adjuvants, liposomes, depend on the right vaccine/adjuvant formulations to be effective [52–55]. Finally, LVA can be conveniently combined with other adjuvants with good safety profiles to further boost skin vaccination because of its unique way to enhance the activity of APCs [56–60].
Advanced technologies for vaccine delivery via the skin
ID injection of vaccines into the thin skin via hypodermic needles requires specially trained personnel, which hampers its broad use in the clinic. To eliminate pain and biohazardous wastes, needle-free vaccine delivery technologies have been actively pursued in the last two decades [7,9,12,46,61]. These technologies aim to breach the barrier function of stratum corneum (SC) located on superficial skin with 10–20 μm in thickness. SC is composed of a densely packed lipid structure impermeable to most of the environmental pathogens and it presents a major barrier for transcutaneous immunization [62]. A variety of physical, mechanical and chemical strategies have been explored to breach SC and enhance transcutaneous drug or vaccine delivery. For example, microneedles use ultra-fine and -short needles to physically penetrate the SC layer while tape stripping can mechanically remove SC to facilitate transcutaneous delivery [46,61,63]. The best example of this line of investigation is recent approval of using an ID microinjection system to deliver seasonal influenza vaccine in USA and EU [12]. The ID micro-injection system precisely delivers vaccines into the dermal tissue and results in dose-sparing of seasonal influenza vaccine as compared to IM immunization [11]. Ultrasound, electrical and thermal effects have been also evaluated for increasing SC permeability and transcutaneous vaccine delivery [5,64–66]. Some of these technologies under the development have a rather limit in delivery efficiency, while others like microneedle-based delivery must deal with biological incompatibility of microneedle fabrication processes in spite of their high delivery efficiency. The latter becomes an outstanding issue for adjuvanted vaccines because most of adjuvants are not compatible to the process.
Laser-facilitated transcutaneous immunization
Laser-facilitated skin vaccination may serve as an alternative for delivery of adjuvanted vaccines via skin. The technology is able to retain the high delivery efficiency as microneedles do, but without need of any needles. The earliest investigation showing increased entrance of vaccines into the skin by full-surface laser illumination (Figure 3A) was taken more than 20 years ago. Nelson et al., used 250 μs Er:YSGG laser (2790 nm) to reliably and precisely remove SC and enhance penetration of large molecules into the skin, but the efficacy was only half of that induced by tape stripping [67]. Subsequently, Lee et al., found that treatment of the skin with low-fluence Er:YAG laser (2940 nm) partly ablated the SC and significantly enhanced transdermal delivery and immunization of lysozyme [68]. Erbium:YAG laser also significantly increased permeability of macromolecule dextran and insulin, as well as small molecules such as 5-aminolaevulinic acid, 5-fluorouracil, vitamin C, methotrexate, narcotic analgesics, DNA, small interfering RNA and peptides [69–75]. The laser treatment is capable of selectively ablating SC layer, generating photomechanical stress between intercellular regions and altering the morphology and arrangement of corneocytes [66,71]. Unfortunately, the degree of laser enhancement is relatively low, and laser-affected skin displays a slow recovery in general [76].
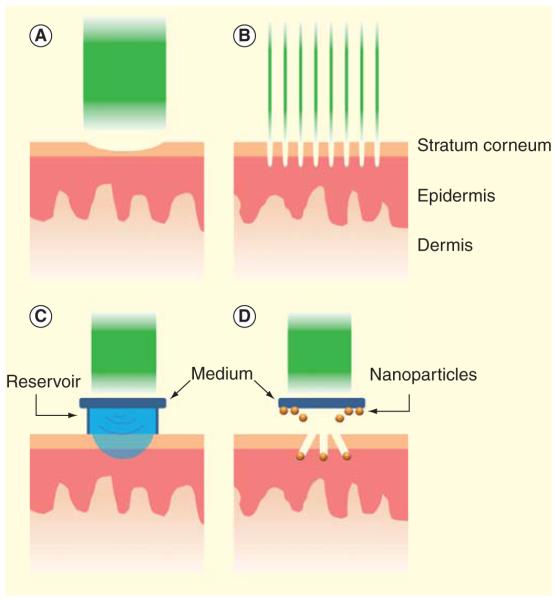
Figure 3. Laser technologies in vaccine delivery.
(A) Full-surface laser illumination ablates part or entire light-exposed SC layer to facilitate transcutaneous vaccine delivery. (B) Ablative fractional laser generates multiple microchannels in the skin. (C & D) Laser can also hit on specific ‘medium’ generating photoacoustic effects that impel the liquid drug or vaccine reservoir (C) or coated gold nanoparticles (D) into the skin.
A different laser platform called ablative fractional laser AFL) (Figure 3B) has been evaluated for its ability to enhance transcutaneous drug/vaccine delivery in recent years [34,76–81]. AFL generates an array of self-renewable microchannels in the skin and each spans from skin surface to deep epidermal or dermal tissue depending on laser energy and epidermal thickness [76,77]. These microchannels provide ‘fast lanes’ for topically applied drugs or vaccines to enter the dermis [77]. The microchannels also permit drugs or vaccines to radically or 3D diffuse into the surrounding tissue [77]. Besides efficient delivery, AFL-based transcutaneous vaccine delivery is extremely safe due to a quick recovery of laser-affected skin within 1–2 days by growing healthy epithelial cells surrounding each microchannel [76,77]. Although the AFL-based vaccine delivery has not been advanced to clinical stages, AFL-based drug delivery has been practiced in the clinic by dermatologists to deliver photo-sensitizers for photodynamic therapy (PDT) [82,83]. Brief laser illumination of the inoculation site can sufficiently boost transcutaneous vaccine delivery while allowing a quick skin recovery within the same day, provided that laser energy and percent coverage are appropriately justified. Such a laser platform is expected to have a great impact on needle-free, painless transcutaneous immunization.
In accordance to this, Lee et al., showed that a fractional Er:YAG laser (2940 nm) greatly increased permeability of murine and porcine skins to dextran of at least 150 kDa in size, or to small molecules imiquimod and 5-aminolevulinic acid, with a quick skin recovery within the same day [76,84]. Our group evaluated a clinical UltraPulse fractional CO2 laser (Lumenis Inc.) for its enhancement of transcutaneous delivery of OVA [77]. We found AFL was much more efficient than tape stripping as reflected by approximately 100-times higher serum anti-OVA antibody titer in AFL group than in tape stripping group [77]. Another group of investigators took advantages of a commercial P.L.E.A.S.E Device (Pantec Biosolutions AG) to generate micropores in the skin and to assess enhanced transcutaneous delivery of OVA, β-galactosidase and a grass pollen allergen Phl p 5 [85–87]. Their study showed that laser-based delivery induced a Th2-biased immune response, which could be modulated by incorporation of Th1-inducing adjuvants, like R848, poly I:C and CpG [85,86]. Moreover, CpG-adjuvanted allergen immunization through laser-generated micropores gave rise to a therapeutic efficacy equivalent to that of subcutaneous injection [86]. Details of laser parameters, drug or vaccine, model system as well as the major findings in the above and the following laser-facilitated transcutaneous drug/vaccine delivery are summarized in Table 1.
Table1.
Laser technology for drug/vaccine delivery.
| Study (year) | Laser (wavelength, pulse) |
Drugs/vaccines | Model system | Results | Ref. |
|---|---|---|---|---|---|
| Nelson et al. (1991) | Er:YSGG (2790nm, 250μs) |
Hydrocortisone IFN | Swine | Increase permeability by 2.8- and 2.1-times, respectively |
[67] |
| Lee et al. (2008) | Er:YAG (2940nm, 250μs) |
Peptides Hen egg lysozyme |
Mice | Enhance peptide delivery by three to 140-times and antibody titer to lysozyme by three-fold |
[68] |
| Chen et al. (2012) | CO2 laser (1060nm) | OVA | Mice | Enhance antibody titer to OVA by 100-times as compared to tape stripping |
[77] |
| Weiss et al. (2012) | P.L.E.A.S.E. | Recombinant Phl p 5with CpG |
Mice | Generate equal humoral immune response as subcutaneous injection |
[85] |
| Shangguan et al. (1996) | Flash-lamp-pumped dye laser (504nm, 1.3μs) |
Hydrophobic dye | gelatin gel | Drive the dye a few millimeters into the gels in both axial and radial directions |
[88] |
| Park et al. (2012) | Er:YAG (2940nm, 250μs) |
FITC | Guinea pig | Deliver drug over the epidermis and dermis within 280–452 μm from the skin |
[89] |
| Doukas et al. (2001) | Q-switched ruby laser (694nm, 28ns) |
Rhodamin-B dextran, dinitrochlorobenzene, insulin |
Guinea pig | Enhance the transdermal delivery of small molecules and the systemic delivery of insulin |
[90] |
| Zeira et al. (2007) | Argon ion laser (780nm, 200fs) |
DNA encoding HBsAg | Mice | A single ID injection induced high titer and long-lasting humoral response and protective cellular response |
[93] |
| Tsen et al. (2009) | Yb:glass fiber laser (1043nm, 500fs) |
DNA encoding luciferase | Mice | Enhance transfection efficiency of the ID administrated plasmids |
[94] |
| Chakravarty et al. (2010) | Ti:sapphire laser (800nm, 100fs) |
Carbon black nanoparticles with calcein, BSA or DNA |
Cell lines | Nanoparticles activated by laser could facilitate the delivery of molecule, protein and DNA |
[95] |
| Menezes et al. (2012) | Nd:YAG (1064nm, 5.5ns) |
Gold microparticles with DNA |
Allium cepa | Laser ablates aluminum foil to successfully deliver deposited microparitcles into plant cells |
[96] |
ID: Intradermal; IFN: Interferon; OVA: Ovalbumin.
Besides direct SC ablation (Figure 3A & B), laser energy can also be absorbed by specific medium to generate pressure driving drugs/vaccines into the tissue through the effects known as ‘laser-induced cavitation bubble expansion and collapse’ (Figure 3C & D). In this regard, a microsecond pulsed dye laser (Palomer, 504 nm) was used to direct a hydrophobic dye into clear gelatine about 20 years ago [88]. In that system, the dye was delivered into the gelatine gel in axial and radial directions from a distance of as much as 500 μm [88]. Very recently, Park et al., used a 250μs pulsed Er:YAG laser at 2940 nm to induce photoacoustic effects in water to inject drug through a nozzle of only 100 μm in diameter into gelatine gel or skin of guinea pigs [89]. In their model, a fine liquid stream of fluorescein isothiocyanate (FITC) was successfully ejected vertically into the target surface after a relatively low level laser treatment (1-2J) [89]. Doukas et al., irradiated a black polystyrene with a nanosecond pulsed Q-switched ruby laser (694 nm) to generate photomechanical waves, which then propagate to the underneath rhodamine-B dextran solution and finally impinge into the skin [90]. The advantage of this treatment is to transiently increase the permeability of the SC layer and the barrier function of SC can be fully recovered within minutes, ensuring the safety of this technology [90]. The above laser-based driving system by shining laser onto specific laser-absorption medium is very promising for needle-free transcutaneous vaccine delivery.
Laser-enhanced DNA immunization
DNA vaccines are regarded as the third generation of vaccines and have the advantage to induce Th1-biased immune response and cellular immunity to attack intracellular pathogens [91]. Yet, the major challenge of DNA immunization is to efficiently deliver DNA into cells and achieve high expression efficiency [92]. Several groups evaluated laser-based strategies for their ability to enhance DNA entry into cells. Zeira et al., explored a femtosecond (fs) laser beam gene transduction system (SG-LBGT) that could enhance non-viral DNA immunization by laser-induced cell permeability [93]. They exposed the inoculation site for 30 sec to a pulsed fs laser (200 fs argon ion pump laser, 76 MHz, 780 nm) with the focal plane only 1.5 mm in diameter, after ID injection of HBsAg DNA vaccine. It was found that the treatment significantly increased and prolonged HBsAg gene expression, induced higher serum anti-HBsAg antibody titer and retarded HBsAg-expressing synergistic tumor growth in mice compared to the same vaccination in the absence of laser treatment [93]. Tsen et al., utilized a different fs laser (500 fs fiber laser, 200 kHz, 1043 nm) to enhance DNA immunization, in which an unfocused laser beam with 4 mm in diameter was used to illuminate ID or IM DNA injection site [94]. The unfocused laser illumination significantly enhanced DNA immunization by increasing transfected area, minimizing the damage and simplifying the implementation [94]. Fs laser was also used to illuminate carbon black nanoparticles to induce localized shock waves and transient holes on the cell membrane facilitating efficient entry of DNA into the cells and gene expression while maintaining a good cell viability [95]. Moreover, when a thin layer of DNA-coated gold microparticles was deposited onto an aluminium foil, ablation of the aluminium foil with a Nd:YAG laser (1064 nm) generates a plasma jet to carry the accelerated particles with a high velocity (>1000 m/s) to reach the target tissue without undergoing a severe deceleration and achieves a better penetration and distribution of particles in the target tissue [96].
Expert commentary
The renewed interesting in skin vaccination is highlighted by the recent approval of ID microinjection system for flu vaccines and the extensive evaluation of other skin delivery technologies. However, many needle-free vaccine delivery technologies under development are still in their infant stages. Potential benefits of significant improvement in vaccine efficacy, patient-compliance and cost–effectiveness in a long term warrant accelerated investigation and advance of skin vaccination to the clinics, despite the fact that any significant alteration of current vaccination practice is rather costly due to a relatively large population required for clinic evaluation. Novel adjuvants, especially those with new features in safety and delivery are urgently needed for boosting skin vaccination. We are focused on the development of a physical type LVA by brief illumination of the inoculation site to enhance ID vaccine-induced immune response. The biggest advantage of LVA is its superior safety without the risk of long-term side effects, which are the major concern on the use of traditional adjuvants. Besides LVA, the use of laser to enhance transcutaneous vaccine delivery and DNA immunization has also been actively pursued in the last two decades and significant progress has been made in this field of research in the past few years. These technologies take advantages of endogenous tissue absorbers or exogenous medium to facilitate transcutaneous vaccine delivery or DNA transfection, concomitant with little skin damage. AFL-based delivery of photosensitizers and fertility hormones has been evaluated in the clinic with excellent safety profile and efficiency. Laser technology for vaccine delivery is expected to be advanced to the clinic evaluation in the near future. A laser technology that integrates the effect of laser adjuvant and needle-free delivery will be the next focus of our investigation. Such a technology will offer safe, cost-effective, convenient vaccine delivery and adjuvantation in the clinic.
Five-year view
Non-fractional and micro-fractional LVA alone or in combination with other suitable skin adjuvants are being validated and optimized in large animal models in preparation for clinical trials. We anticipate the first clinic trial in the coming years in which non-ablative fractional laser will be assessed for its ability to boost seasonal flu vaccine in the elderly. Moreover, LVA has been investigated in association with nicotine, influenza and DC-based cancer vaccines so far. The investigation should be extended to other vaccines, like hepatitis B, rabies, polio and measles vaccines. Concurrent with these efforts, an integrated system that combines laser-mediated vaccine adjuvant and needle-free, painless transcutaneous immunization will be targeted in the next 5 years. Special focus needs to be placed on developing a convenient vaccine deploying system that can accurately deliver vaccines right into laser-generated microchannels. Ideally, the laser device is small as a pen or flashlight that costs only tens of dollars. With this device, laser-directed vaccination can be self-applicable or requires little trained personnel, significantly reducing the cost of vaccine delivery and distribution. The device may also result in dose-sparing of various vaccines, further lowering the cost.
Key issues.
Novel adjuvants with minimal reactogenicity and potent adjuvanticity are highly demanded.
A short-term laser illumination is able to safely and efficiently enhance vaccine-induced immune response and serves as a novel physical type vaccine adjuvant.
Non-fractional laser vaccine adjuvant (LVA) enhances immune response by modification of dermal tissue scaffolds and enhancement of APC migration and antigen uptake, without incurring damage of the skin cells.
Fractional LVA enhances immune response by damage of a well-defined number of skin cells that provoke sterile inflammation transiently.
Needle-free, painless transcutaneous vaccine delivery technologies are fast emerging.
Various laser technologies have been explored to facilitate transcutaneous vaccine delivery and DNA immunization with distinct advantages.
Ablative fractional laser has been explored by several groups to safely and efficiently enhance transcutaneous vaccine delivery.
Fabrication of a cost-effective, handheld laser device that can integrate vaccine delivery and adjuvantation is highly anticipated for its clinical application.
Acknowledgments
This work is supported in part by the National Institutes of Health grants AI070785 and RC1 DA028378 (to MX Wu), Bullock-Wellman Fellowship, and A Ward Ford Memorial Research Grant #NSP0511 from American Society for Laser Medicine and Surgery (to X Chen). X Chen, D Shah, J Wang. designs and performs the experiments, analyzes the data. X Chen and J Wang prepare the manuscript. MX Wu participates in data analysis and experimental design and writes the paper.
Footnotes
Financial & competing interest disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Reference
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Schlipkoter U, Flahault A. Communicable diseases: achievements and challenges for public health. Public Health Rev. 2010;32:90–119. doi: 10.1007/BF03391594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham J. Science and Civilization in China: Volume 6, Biology and Biological Technology. Vol. 134. Cambridge University Press; Cambridge, UK: 1999. 2013. Part 6, Medicine. [Google Scholar]
- 3.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc.(Bayl. Univ Med. Cent.) 2005;18(1):21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton JG. Needle phobia: a neglected diagnosis. J. Fam. Pract. 1995;41(2):169–175. [PubMed] [Google Scholar]
- 7.Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev. Vaccines. 2008;7(8):1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Wu MX. Laser vaccine adjuvant for cutaneous immunization. Expert Rev. Vaccines. 2011;10(10):1397–1403. doi: 10.1586/erv.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• Summary of laser and traditional adjuvants for skin vaccination.
- 9.Combadiere B, Liard C. Transcutaneous and intradermal vaccination. Hum. Vaccin. 2011;7(8):811–827. doi: 10.4161/hv.7.8.16274. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 11.Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia licensed micro injection system. Hum. Vaccin. Immunother. 2012;8(1):67–75. doi: 10.4161/hv.8.1.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent PE, Bonnet S, Alchas P, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25(52):8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- • Clinical evaluation of an ID microinjection system later approved for seasonal influenza vaccine delivery.
- 13.Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev. Vaccines. 2013;12(7):747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 14.Mills DJ, Lau CL, Fearnley EJ, Weinstein P. The immunogenicity of a modified intradermal pre-exposure rabies vaccination schedule–a case series of 420 travelers. J. Travel. Med. 2011;18(5):327–332. doi: 10.1111/j.1708-8305.2011.00540.x. [DOI] [PubMed] [Google Scholar]
- 15.Kothare SV, Wiznitzer M. Association between H1N1 vaccination and narcolepsy-cataplexy: flu to sleep. Neurology. 2013;80(14):1276–1277. doi: 10.1212/WNL.0b013e31828ab382. [DOI] [PubMed] [Google Scholar]
- 16.Miller E, Andrews N, Stellitano L, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 17.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoSOne. 3;7:e33536–2012. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12(13):1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waisbren BA., Sr. Acquired autoimmunity after viral vaccination is caused by molecular mimicry and antigen complimentarity in the presence of an immunologic adjuvant and specific HLA patterns. Med. Hypotheses. 2008;70(2):346–348. doi: 10.1016/j.mehy.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Spratt A, Key T, Vivian AJ. Chronic anterior uveitis following bacille Calmette-Guerin vaccination: molecular mimicry in action? J. Pediatr. Ophthalmol. Strabismus. 2008;45(4):252–253. doi: 10.3928/01913913-20080701-15. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31(1):159–164. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Kim P, Farinelli B, et al. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS. One. 2010;5(10):e13776. doi: 10.1371/journal.pone.0013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• First report describing laser-based vaccine adjuvant.
- 23.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest Dermatol. 2006;126(4):787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 24.Flacher V, Tripp CH, Stoitzner P, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J. Invest Dermatol. 2010;130(3):755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liard C, Munier S, Joulin-Giet A, et al. Intradermal immunization triggers epidermal Langerhans cell mobilization required for CD8 T-cell immune responses. J. Invest Dermatol. 2012;132(3):615–625. doi: 10.1038/jid.2011.346. Pt 1. [DOI] [PubMed] [Google Scholar]
- 26.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006;7(3):265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangaletti S, Gioiosa L, Guiducci C, et al. Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J. Cell Sci. 2005;118:3685–3694. doi: 10.1242/jcs.02474. Pt 16. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zeng Q, Wu MX. Improved efficacy of dendritic cell-based immunotherapy by cutaneous laser illumination. Clin. Cancer Res. 2012;18(8):2240–2249. doi: 10.1158/1078-0432.CCR-11-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009;206(13):2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornuz J, Zwahlen S, Jungi WF, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoogsteder PH, Kotz D, van Spiegel PI, et al. The efficacy and safety of a nicotine conjugate vaccine (NicVAX(R)) or placebo co-administered with varenicline (Champix (R)) for smoking cessation: study protocol of a phase IIb, double blind, randomized, placebo controlled trial. BMC. Public Health. 2012;12:1052. doi: 10.1186/1471-2458-12-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Wu MX. How effective could laser-based approaches be in assisting dendritic cell immunotherapy? Immunotherapy. 2012;4(10):983–985. doi: 10.2217/imt.12.101. [DOI] [PubMed] [Google Scholar]
- 33.Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg. Med. 2006;38(2):142–9. doi: 10.1002/lsm.20254. [DOI] [PubMed] [Google Scholar]
- 34.Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004;34(5):426–438. doi: 10.1002/lsm.20048. [DOI] [PubMed] [Google Scholar]
- •• Describing the concept of fractional photothermolysis.
- 35.Lin JY, Warger WC, Izikson L, Anderson RR, Tannous Z. A prospective, randomized controlled trial on the efficacy of fractional photothermolysis on scar remodeling. Lasers Surg. Med. 2011;43(4):265–72. doi: 10.1002/lsm.21061. [DOI] [PubMed] [Google Scholar]
- 36.Saedi N, Petelin A, Zachary C. Fractionation: a new era in laser resurfacing. Clin. Plast. Surg. 2011;38(3):449–61. doi: 10.1016/j.cps.2011.02.008. vii. [DOI] [PubMed] [Google Scholar]
- 37.Waibel J, Wulkan AJ, Lupo M, Beer K, Anderson RR. Treatment of burn scars with the 1,550 nm nonablative fractional Erbium Laser. Lasers Surg. Med. 2012;44(6):441–446. doi: 10.1002/lsm.22038. [DOI] [PubMed] [Google Scholar]
- 38.Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flach TL, Ng G, Hari A, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 2011;17(4):479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 40.Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 41.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Update on fractional laser technology. J. Clin. Aesthet. Dermatol. 2010;3(1):42–50. [PMC free article] [PubMed] [Google Scholar]
- 44.Brown AS. At-home laser and light-based devices. Curr. Probl. Dermatol. 2011;42:160–165. doi: 10.1159/000328319. [DOI] [PubMed] [Google Scholar]
- 45.Zehrung D, Jarrahian C, Wales A. Intradermal delivery for vaccine dose sparing: Overview of current issues. Vaccine. 2012;31(34):3392–3395. doi: 10.1016/j.vaccine.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SP, Koutsonanos DG, Del Pilar MM, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Macrophagic myofaciitis a vaccine (alum) autoimmune-related disease. Clin. Rev. Allergy Immunol. 2011;41(2):163–168. doi: 10.1007/s12016-010-8212-4. [DOI] [PubMed] [Google Scholar]
- 48.Gherardi RK, Authier FJ. Macrophagic myofasciitis: characterization and pathophysiology. Lupus. 2012;21(2):184–189. doi: 10.1177/0961203311429557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War syndrome. Exp. Mol. Pathol. 2000;68(1):55–64. doi: 10.1006/exmp.1999.2295. [DOI] [PubMed] [Google Scholar]
- 50.Tomljenovic L, Shaw CA. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? J. Inorg. Biochem. 2011;105(11):1489–1499. doi: 10.1016/j.jinorgbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Phillips CJ, Matyas GR, Hansen CJ, Alving CR, Smith TC, Ryan MA. Antibodies to squalene in US Navy Persian Gulf War veterans with chronic multisymptom illness. Vaccine. 2009;27(29):3921–6. doi: 10.1016/j.vaccine.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 52.Guy B. The perfect mix recent progress in adjuvant research. Nat. Rev. Microbiol. 2007;5(7):505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 53.Clapp T, Siebert P, Chen D, Jones BL. Vaccines with aluminum-containing adjuvants: optimizing vaccine efficacy and thermal stability. J. Pharm. Sci. 2011;100(2):388–401. doi: 10.1002/jps.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collin N, Dubois PM. The Vaccine Formulation Laboratory: a platform for access to adjuvants. Vaccine. 2011;29(Suppl. 1):A37–A39. doi: 10.1016/j.vaccine.2011.04.125. [DOI] [PubMed] [Google Scholar]
- 56.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines. 2003;2(2):253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 57.Roukens AH, Vossen AC, Boland GJ, Verduyn W, van Dissel JT, Visser LG. Intradermal hepatitis B vaccination in non-responders after topical application of imiquimod (Aldara) Vaccine. 2010;28(26):4288–4293. doi: 10.1016/j.vaccine.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 58.Othoro C, Johnston D, Lee R, Soverow J, Bystryn JC, Nardin E. Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod. Infect. Immun. 2009;77(2):739–48. doi: 10.1128/IAI.00974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnston D, Bystryn JC. Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine. 2006;24(11):1958–1965. doi: 10.1016/j.vaccine.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 60.Scharton-Kersten T, Glenn GM, Vassell R, Yu J, Walwender D, Alving CR. Principles of transcutaneous immunization using cholera toxin as an adjuvant. Vaccine. 1999;17(Suppl. 2):S37–S43. doi: 10.1016/s0264-410x(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 61.Frerichs DM, Ellingsworth LR, Frech SA, et al. Controlled, single-step, stratum corneum disruption as a pretreatment for immunization via a patch. Vaccine. 2008;26(22):2782–2787. doi: 10.1016/j.vaccine.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 62.Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int. J. Pharm. 2012;435(1):3–9. doi: 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JW, Gadiraju P, Park JH, Allen MG, Prausnitz MR. Microsecond thermal ablation of skin for transdermal drug delivery. J. Control Release. 2011;154(1):58–68. doi: 10.1016/j.jconrel.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J. Control Release. 2011;152(3):330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 67.Nelson JS, McCullough JL, Glenn TC, Wright WH, Liaw LH, Jacques SL. Mid-infrared laser ablation of stratum corneum enhances in vitro percutaneous transport of drugs. J. Invest Dermatol. 1991;97(5):874–879. doi: 10.1111/1523-1747.ep12491600. [DOI] [PubMed] [Google Scholar]
- • Among the early reports to describe non-fractional laser to enhance transcutaneous drug delivery.
- 68.Lee WR, Pan TL, Wang PW, Zhuo RZ, Huang CM, Fang JY. Erbium:YAG laser enhances transdermal peptide delivery and skin vaccination. J. Control Release. 2008;128(3):200–208. doi: 10.1016/j.jconrel.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Wang KH, Fang JY, Hu CH, Lee WR. Erbium:YAG laser pretreatment accelerates the response of Bowen’s disease treated by topical 5-fluorouracil. Dermatol. Surg. 2004;30(3):441–445. doi: 10.1111/j.1524-4725.2004.30122.x. [DOI] [PubMed] [Google Scholar]
- 70.Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium: YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br. J. Dermatol. 2004;151(1):132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- 71.Fang JY, Lee WR, Shen SC, Wang HY, Fang CL, Hu CH. Transdermal delivery of macromolecules by erbium:YAG laser. J. Control Release. 2004;100(1):75–85. doi: 10.1016/j.jconrel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 72.Lee WR, Shen SC, Liu CR, Fang CL, Hu CH, Fang JY. Erbium:YAG laser-mediated oligonucleotide and DNA delivery via the skin: an animal study. J. Control Release. 2006;115(3):344–353. doi: 10.1016/j.jconrel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Lee WR, Shen SC, Fang CL, Liu CR, Fang JY. Skin pretreatment with an Er:YAG laser promotes the transdermal delivery of three narcotic analgesics. Lasers Med. Sci. 2007;22(4):271–278. doi: 10.1007/s10103-007-0452-z. [DOI] [PubMed] [Google Scholar]
- 74.Lee WR, Shen SC, Fang CL, Zhuo RZ, Fang JY. Topical delivery of methotrexate via skin pretreated with physical enhancement techniques: low-fluence erbium:YAG laser and electroporation. Lasers Surg. Med. 2008;40(7):468–476. doi: 10.1002/lsm.20655. [DOI] [PubMed] [Google Scholar]
- 75.Lee WR, Shen SC, Zhuo RZ, Wang KC, Fang JY. Enhancement of topical small interfering RNA delivery and expression by low-fluence erbium:YAG laser pretreatment of skin. Hum. Gene Ther. 2009;20(6):580–588. doi: 10.1089/hum.2008.156. [DOI] [PubMed] [Google Scholar]
- 76.Lee WR, Shen SC, Pai MH, Yang HH, Yuan CY, Fang JY. Fractional laser as a tool to enhance the skin permeation of 5-aminolevulinic acid with minimal skin disruption: a comparison with conventional erbium:YAG laser. J. Control Release. 2010;145(2):124–33. doi: 10.1016/j.jconrel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, Shah D, Kositratna G, Manstein D, Anderson RR, Wu MX. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J. Control Release. 2012;159(1):43–51. doi: 10.1016/j.jconrel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • • First study of ablative fractional laser to enhance transcutaneous immunization.
- 78.Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR. Fractional CO(2) laser-assisted drug delivery. Lasers Surg. Med. 2010;42(2):113–122. doi: 10.1002/lsm.20860. [DOI] [PubMed] [Google Scholar]
- • First report of ablative fractional laser to enhance transcutaneous drug delivery.
- 79.Paasch U, Haedersdal M. Laser systems for ablative fractional resurfacing. Expert Rev. Med. Devices. 2011;8(1):67–83. doi: 10.1586/erd.10.74. [DOI] [PubMed] [Google Scholar]
- 80.Tajirian AL, Goldberg DJ. Fractional ablative laser skin resurfacing: a review. J. Cosmet Laser Ther. 2011;13(6):262–264. doi: 10.3109/14764172.2011.630083. [DOI] [PubMed] [Google Scholar]
- 81.Bloom BS, Brauer JA, Geronemus RG. Ablative fractional resurfacing in topical drug delivery: an update and outlook. Dermatol. Surg. 2013;39(6):839–848. doi: 10.1111/dsu.12111. [DOI] [PubMed] [Google Scholar]
- 82.Togsverd-Bo K, Haak CS, Thaysen-Petersen D, Wulf HC, Anderson RR, Haedersdal M. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br. J. Dermatol. 2012;166(6):1262–1269. doi: 10.1111/j.1365-2133.2012.10893.x. [DOI] [PubMed] [Google Scholar]
- 83.Ruiz-Rodriguez R, Lopez L, Candelas D, Zelickson B. Enhanced efficacy of photodynamic therapy after fractional resurfacing: fractional photodynamic rejuvenation. J. Drugs Dermatol. 2007;6(8):818–820. [PubMed] [Google Scholar]
- 84.Lee WR, Shen SC, Al Suwayeh SA, Yang HH, Yuan CY, Fang JY. Laser-assisted topical drug delivery by using a low-fluence fractional laser: imiquimod and macromolecules. J. Control Release. 2011;153(3):240–248. doi: 10.1016/j.jconrel.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 85.Weiss R, Hessenberger M, Kitzmuller S, et al. Transcutaneous vaccination via laser microporation. J. Control Release. 2012;162(2):391–399. doi: 10.1016/j.jconrel.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hessenberger M, Weiss R, Weinberger EE, Boehler C, Thalhamer J, Scheiblhofer S. Transcutaneous delivery of CpG-adjuvanted allergen via laser-generated micropores. Vaccine. 2012;31(34):3427–3434. doi: 10.1016/j.vaccine.2012.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheiblhofer S, Thalhamer J, Weiss R. Laser microporation of the skin: prospects for painless application of protective and therapeutic vaccines. Expert Opin. Drug Deliv. 2013;10(6):761–773. doi: 10.1517/17425247.2013.773970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shangguan H, Casperson LW, Shearin A, Gregory KW, Prahl SA. Drug delivery with microsecond laser pulses into gelatin. Appl. Opt. 1996;35(19):3347–3357. doi: 10.1364/AO.35.003347. [DOI] [PubMed] [Google Scholar]
- 89.Park MA, Jang HJ, Sirotkin FV, Yoh JJ. Er: YAG laser pulse for small-dose splashback-free microjet transdermal drug delivery. Opt. Lett. 2012;37(18):3894–3896. doi: 10.1364/ol.37.003894. [DOI] [PubMed] [Google Scholar]
- 90.Lee S, McAuliffe DJ, Flotte TJ, Kollias N, Doukas AG. Photomechanical transdermal delivery: the effect of laser confinement. Lasers Surg. Med. 2001;28(4):344–347. doi: 10.1002/lsm.1060. [DOI] [PubMed] [Google Scholar]
- 91.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9(10):776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang G, Pan L, Zhang Y. Approaches to improved targeting of DNA vaccines. Hum. Vaccin. 2011;7(12):1271–1281. doi: 10.4161/hv.7.12.17983. [DOI] [PubMed] [Google Scholar]
- 93.Zeira E, Manevitch A, Manevitch Z, et al. Femtosecond laser: a new intradermal DNA delivery method for efficient, long-term gene expression and genetic immunization. FASEB J. 2007;21(13):3522–33. doi: 10.1096/fj.06-7528com. [DOI] [PubMed] [Google Scholar]
- • Exploring femtosecond laser to enhance DNA immunization.
- 94.Tsen SW, Wu CY, Meneshian A, Pai SI, Hung CF, Wu TC. Femtosecond laser treatment enhances DNA transfection efficiency in vivo. J. Biomed. Sci. 2009;16:36. doi: 10.1186/1423-0127-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chakravarty P, Qian W, El Sayed MA, Prausnitz MR. Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses. Nat. Nanotechnol. 2010;5(8):607–611. doi: 10.1038/nnano.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Menezes V, Mathew Y, Takayama K, Kanno A, Hosseini H. Laser plasma jet driven microparticles for DNA/drug delivery. PLoS One. 2012;7(11):e50823. doi: 10.1371/journal.pone.0050823. [DOI] [PMC free article] [PubMed] [Google Scholar]