Human telomere length, as expressed in leukocytes, is strongly fashioned by heritability.1–5 A number of genes that explain some of the inter-individual variation in leukocyte telomere length have already been identified.6–9 Leukocyte telomere length is longer in women than in men3,10,11 and in African Americans than in whites of European descent.12,13 In addition, offspring of older fathers display a longer leukocyte telomere length, a finding that may reflect longer telomeres in sperm of older men.14–17 In contrast to the male germline, telomeres in replicative somatic tissues, including the hematopoietic system, display age-dependent shortening.
A shorter leukocyte telomere length is associated with aging-related diseases, principally atherosclerosis18,19 and reduced longevity.20,21 The prevailing interpretation is that a shorter leukocyte telomere length is the outcome of processes, mainly the age-dependent accruing burden of oxidative stress and inflammation22 that ultimately shorten the human lifespan. As such, leukocyte telomere length is considered to be a non-causal biomarker, ie, a telomeric “clock,” of human aging.
We propose that telomere length is not only associated with atherosclerosis and longevity but is also a causal determinant of both. This hypothesis is based on a model of leukocyte telomere length dynamics (leukocyte telomere length and its attrition rate) that incorporates two key features. First, the model differentiates the findings of studies of telomere length dynamics in vitro from those in vivo. Second, the model distinguishes two phases of human leukocyte telomere length dynamics in vivo: growth and adulthood.
THE TELOMERIC “CLOCK” BASED ON EXTRAPOLATIONS FROM IN VITRO STUDIES
The metaphor of telomeres as a biological clock is based on research showing that somatic cell replication in culture causes telomere attrition, ultimately leading to replicative senescence (or apoptosis).23 Although senescence might be triggered by mechanisms other than critically short telomeres,24–26 the evidence is strong that the telomeric clock is a major factor in replicative senescence in vitro. Studies in cultured cells also revealed that oxidative stress heightens the loss of telomere repeats per replication, presumably due to the sensitivity of telomeres to oxidative stress.27
Leukocytes are the most frequently used somatic cells to study telomere biology in epidemiological settings. The traditional model of leukocyte telomere length dynamics, extrapolated from cultured cells to the in vivo state, is based on the premise that oxidative stress augments the loss of telomere repeats per replication of all somatic cells. However, in hematopoietic stem cells (HSCs) not only oxidative stress but also inflammation increases telomere shortening, because HSCs undergo more replication to replace leukocytes consumed by the inflammatory response. Shorter leukocyte telomere length, which reflects HSC telomere length,28,29 has therefore been attributed to a faster HSC telomere-length attrition due to a higher cumulative burden of oxidative stress and inflammation—two biological processes that are considered the hallmarks of atherosclerosis and aging.22
The concept of leukocyte telomere length as a biomarker of aging entails the premise that at birth, if not at conception, all individuals have a biological age—a telomeric clock time—of zero. In newborns, however, we see great variation in leukocyte telomere length, with values ranging from 8 to 11 kb or more.30,31 This variation among newborns exceeds the total average leukocyte telomere length shortening throughout the adult life course (20–90 years) estimated at <2.0 kb, assuming an average leukocyte telomere length attrition rate of 25–30 bp/year.32 Thus, the use of leukocyte telomere length as a biomarker of aging in vivo should account for not only leukocyte telomere length attrition but also leukocyte telomere length at birth for each individual, an important point often overlooked.
TELOMERE DYNAMICS IN VIVO IN THE HEMATOPOIETIC SYSTEM
Cultured cells typically display exponential proliferation, whereby one cell divides into two daughter cells, ie, symmetric replication. However, in vivo, HSCs experience both symmetric replication to two daughter HSCs and asymmetric replication to a daughter hematopoietic progenitor cell (HPC) and a daughter HSC.33 Symmetric replication, as in cultured cells, serves to expand exponentially the HSC reservoir during growth. It takes, for example, four cycles of symmetric HSC replication to generate 16 HSCs [2-4-8-16]. In contrast, asymmetric HSC replication during growth, which linearly expands the HPC pool and ultimately the peripheral blood cell mass, takes 16 cycles to generate 16 HPCs. Therefore, during growth, expansion of the HPC pool in tandem with the growing soma requires far more replication and more telomere attrition than the expansion of the HSC reservoir.29,34 Accordingly, the expansion of the HPC pool is probably the key explanation for the rapid leukocyte telomere length attrition during growth, which amounts to 1.5–2 kb. The first 20 years of life comprise the full period of growth and cross- sectional analysis clearly shows rapid leukocyte telomere length attrition during this period.35 However, modeling suggests that most leukocyte telomere length attrition during growth occurs during early development (by age 5 years).29 Moreover, recent studies suggest that fetal and childhood exposures might influence leukocyte telomere length,36,37 but this effect might be small compared with the joint effect on leukocyte telomere length of heritability, sex, race, and paternal age.
In adulthood, there is still an ongoing need for symmetric and asymmetric HSC replication. Symmetric replication occurs to replace some HSCs that died or experienced senescence. Asymmetric replication accommodates the death and senescence of HPCs and needs for replenishment of the blood cell mass. Based on current evidence, the joint impact of both forms of replication on leukocyte telomere length during adult life is likely to be modest compared with the extensive interindividual variation in leukocyte telomere length at birth30,31 and the rapid leukocyte telomere length attrition required for expansion of the HPC pool during early development.29,34
Another important point not considered in the traditional model, which views leukocyte telomere length as a biomarker of human aging, is that the blood cell mass comprises erythrocytes as well as leukocytes. Epidemiologic studies have used leukocytes to gauge HSC telomere length dynamics and replicative kinetics because leukocytes have nuclei, required for measurement of telomere length, the proxy for HSC telomere length. This does not mean that changes in HSC telomere length, reflected in leukocyte telomere length, are primarily due to leukocyte turnover, as proposed by the traditional model of leukocyte telomere length dynamics. Given that the ratio between erythrocytes and leukocytes in the blood cell mass is ~800 to 1,38 and after accounting for the vastly different biological lifespan of blood cell lineages, it becomes evident that the principal driving force of HSC replication and attrition of leukocyte telomere length is not leukocyte turnover but erythrocyte turnover. Accordingly, the hallmark of catastrophic mutations that result in a critically short leukocyte telomere length is aplastic anemia.39 Processes such as inflammation might increase the turnover rate of erythrocytes as well as leukocytes. Our point, however, is that the impact of leukocyte turnover on HSC replication kinetics should be but a fraction of that of erythrocytes.
HSC TELOMERE DYNAMICS MIGHT PLAY A CAUSAL ROLE IN AGING AND LONGEVITY
The traditional view restricts leukocyte telomere length to a non-causal role, as a biomarker of aging. We, in contrast, hypothesize that HSC telomere length (as expressed in leukocyte telomere length) plays a causal role as a determinant of human aging. Support for a causal role of telomere biology in human aging is found in recent studies reporting that not only a short leukocyte telomere length but also variant genes associated with this phenotype predict increased risks for atherosclerosis9 and diminished longevity.40,41 A subset of these genes is directly engaged in leukocyte telomere length regulation, ie, they are determinants of the length of telomeres. A causal role of telomere biology is also compatible with the observation that much of the interindividual variation in leukocyte telomere length (and telomere length of other somatic cells) is established before adulthood,30,31,34 meaning that having a short (or a long) leukocyte telomere length precedes by many years the manifestations of aging-related diseases and death. Further support for this notion comes from observations of leukocyte telomere length “tracking” during adulthood, ie, adults with long/short leukocyte telomere length early in life display long/short leukocyte telomere length later in life.42 It is very likely that leukocyte telomere length “tracking” occurs throughout the course of human life.
Finally, although oxidative stress might augment leukocyte telomere length attrition to some extent, in genetically engineered mice with ablated telomerase, short telomeres impair mitochondrial biogenesis so as to increase the output of reactive oxygen species and compromise organ function.43 Thus, the cause-and-effect relation between oxidative stress and telomere length in the context of aging might be bidirectional. That said, it is important to distinguish telomere biology between laboratory mice, which live 2 to 3 years, and humans, the longest living terrestrial mammals. Domesticated mice typically display exceedingly long telomeres and active telomerase in somatic tissues44 and high susceptibility to cancer45 but resistance to atherosclerosis.46 Thus, findings in mice might not easily apply to humans.47 Although animal research has enabled insight into the fundamentals of telomere biology, epidemiologic research is necessary to understand the intertwined relation of telomere biology and aging-related diseases and longevity in humans
CONCLUSIONS AND TESTING THE HYPOTHESIS
Despite a growing body of evidence to the contrary, the prevailing conventional paradigm maintains that increased risks for atherosclerosis and premature death in individuals with short leukocyte telomere length largely reflect an accelerated rate of leukocyte telomere length attrition due to heightened oxidative stress and inflammation. In contrast, the centerpiece of our hypothesis is that individuals who are born with relatively short telomeres tend to enter adulthood with short leukocyte telomere length. These individuals are more likely to experience the consequences of replicative aging later in life (Figure). That is because short telomere length in somatic tissues ostensibly reflects less somatic cell reserves, expressed in diminished ability of stem cells, including HSCs, to maintain tissue repair.25,48,49 Thus, although the conventional model focuses on leukocyte telomere length as an index of tissue injury via the cumulative burden of oxidative stress and inflammation, our model emphasizes the potential role of telomeres as a causal determinant in somatic homeostasis.
FIGURE.
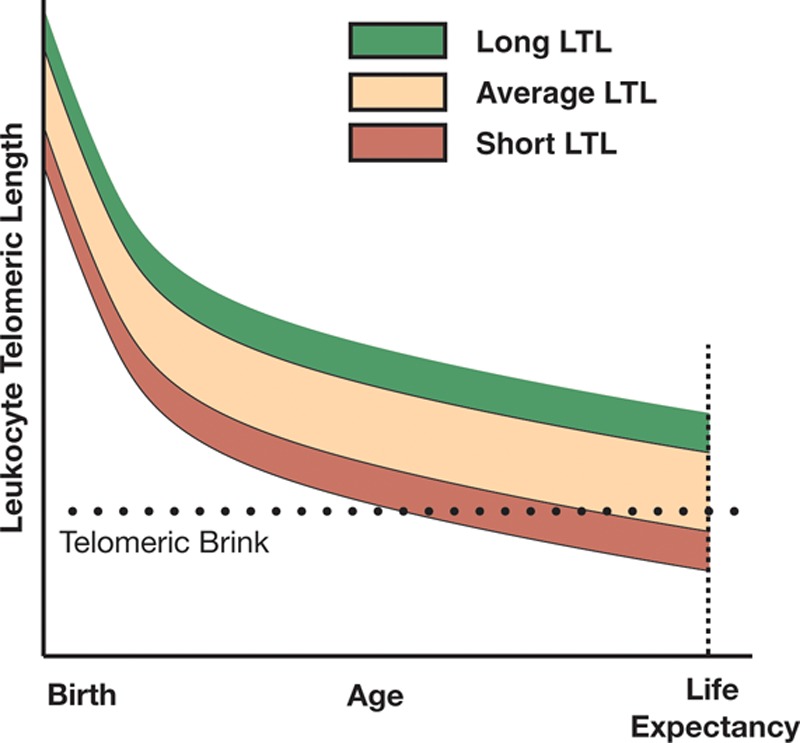
The roles of leukocyte telomere length at birth and its age-dependent attrition thereafter in human longevity. The key features of the model are as follows: (a) leukocyte telomere length is highly variable at birth and afterward; (b) a critical leukocyte telomere length, referred to as the telomeric brink, denotes compromised stem cell reserves, which impairs homeostasis; and (c) leukocyte telomere length displays tracking throughout the life course of individuals. The model solely reflects the connection of leukocyte telomere length with atherosclerosis (but not cancer). Moreover, the telomeric brink might reflect a range of short leukocyte telomere lengths, such that subset of individuals might survive with slightly shorter leukocyte telomere length than others. Thus, the telomeric brink might be viewed in terms of increased probability of atherosclerosis and premature death as the individual leukocyte telomere length converges toward a critical length beyond which survival drastically declines. Note that the variance of leukocyte telomere length early in life appears to be narrower than that in adulthood, but this is a visual illusion that stems from the steepness of the curves early in life.
The optimal way to test this hypothesis is by longitudinal studies that follow individuals from birth onward throughout the entire life course. At present, this design is not feasible. The model we propose instead is based on the following major premises: As telomere length is largely equivalent among tissues of the fetus,30,50 skeletal muscle telomere length at birth is probably the same as that of telomere length in leukocytes, which reflects telomere length in HSCs. However, during the first two decades of life, leukocyte telomere length experiences a much more rapid attrition than telomere length in skeletal muscle, because HSCs undergo massive proliferation to develop a vast HPC pool. Thereafter, the rates of attrition of leukocyte and skeletal muscle telomeres, which probably reflect “housekeeping” proliferative activities of stem cells in both systems, are similar.34 Therefore, skeletal-muscle telomere length minus leukocyte telomere length is primarily an index of HSC telomere shortening due to the expansion of the HPC pool. There might be some differences in “housekeeping” proliferative activities of stem cells in both systems due to altered turnover of blood cells and skeletal muscle cells, but these are likely to be modest.
We can in this way capture key features of leukocyte telomere length dynamics over the individual’s life course, from birth onward to the time of sample collection. For instance, the difference in skeletal muscle telomere length between a person with and one without atherosclerosis would reflect the difference between the two that was already present by age 20 years. Comparing skeletal muscle minus leukocyte telomere length between the two will provide information about whether HSC telomere length attrition during the first 20 years of life was on the average faster in subjects with atherosclerosis. Similarly, we can also test the potential role of telomere length at birth and its attrition during growth in exceptional human longevity. According to our model, if exceptional longevity partially stems from longer telomeres at birth, not only age-adjusted leukocyte telomere length but also skeletal muscle telomere length of exceptionally old people would be relatively longer than those of younger individuals who have a smaller probability of surviving to a very old age. In principle, this type of information might transform a cross-sectional study into a quasi-longitudinal study that enables backtracking the individual’s HSC telomere length dynamics from the time of sample collection to early adulthood and perhaps as early as birth. Notably, biopsies of skeletal muscle (and blood samples) can be obtained during surgical procedures for a variety of indications34 and post-mortem (provided that the DNA remains intact).
Our hypothesis and the proposed mode of testing it offer a new way of thinking about telomere biology and human aging not only in the context of the hematopoietic system but also with regard to all replicative somatic tissues.
Footnotes
This study was financially supported by R01HD071180; R01AG030678.
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 3.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci USA. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codd V, Mangino M, van der Harst P, et al. Wellcome Trust Case Control Consortium. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangino M, Hwang SJ, Spector TD, et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet. 2012;21:5385–5394. doi: 10.1093/hmg/dds382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427, 427e1. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbers CC, Garcia ME, Kimura M, et al. Comparison between southern blots and qPCR analysis of leukocyte telomere length in the health ABC study. J Gerontol A Biol Sci Med Sci. 2014;69:527–531. doi: 10.1093/gerona/glt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unryn BM, Cook LS, Riabowol KT. Paternal age is positively linked to telomere length of children. Aging Cell. 2005;4:97–101. doi: 10.1111/j.1474-9728.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 15.De Meyer T, Rietzschel ER, De Buyzere ML, et al. Asklepios investigators. Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet. 2007;16:3097–3102. doi: 10.1093/hmg/ddm271. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Cherkas LF, Kato BS, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aston KI, Hunt SC, Susser E, et al. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18:517–522. doi: 10.1093/molehr/gas028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura M, Hjelmborg JV, Gardner JP, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deelen J, Beekman M, Codd V, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43:878–886. doi: 10.1093/ije/dyt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 23.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 24.Fumagalli M, Rossiello F, Clerici M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 27.Von Zglinicki T. Replicative senescence and the art of counting. Exp Gerontol. 2003;38:1259–1264. doi: 10.1016/j.exger.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38:854–859. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37:514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG. 2006;113:318–323. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 32.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum–artifact or biology? Nucleic Acids Res. 2013;41:e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 34.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SH, Epel ES, Mellon SH, et al. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J Affect Disord. 2014;169:86–90. doi: 10.1016/j.jad.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Entringer S, Epel ES, Lin J, et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2013;208:134.e1–134.e7. doi: 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003642.htm. Accessed March 4, 2015.
- 39.Nelson ND, Bertuch AA. Dyskeratosis congenita as a disorder of telomere maintenance. Mutat Res. 2012;730:43–51. doi: 10.1016/j.mrfmmm.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnett-Hartman AN, Fitzpatrick AL, Kronmal RA, et al. Telomere-associated polymorphisms correlate with cardiovascular disease mortality in Caucasian women: the Cardiovascular Health Study. Mech Ageing Dev. 2012;133:275–281. doi: 10.1016/j.mad.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soerensen M, Thinggaard M, Nygaard M, et al. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross-sectional and longitudinal analysis. Aging Cell. 2012;11:223–227. doi: 10.1111/j.1474-9726.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes NM, Ryder OA, Houck ML, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 46.Kapourchali FR, Surendiran G, Chen L, Uitz E, Bahadori B, Moghadasian MH. Animal models of atherosclerosis. World J Clin Cases. 2014;2:126–132. doi: 10.12998/wjcc.v2.i5.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolker J. There is more to life than rats and flies. Nature. 2012;491:31–33. doi: 10.1038/491031a. [DOI] [PubMed] [Google Scholar]
- 48.Aviv A, Levy D. Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu Rev Med. 2012;63:293–301. doi: 10.1146/annurev-med-050311-104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell. 2013;12:330–332. doi: 10.1111/acel.12050. [DOI] [PubMed] [Google Scholar]
- 50.Youngren K, Jeanclos E, Aviv H, et al. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–643. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]


