Abstract
In this special issue of Evo-Devo of the amniote integument, Alibardi has discussed the adaptation of the integument to the land. Here we will discuss the adaptation to the sky. We first review a series of fossil discoveries representing intermediate forms of feathers or feather-like appendages from dinosaurs and Mesozoic birds from the Jehol Biota of China. We then discuss results from the molecular and developmental biological experiments using chicken integument as the model. Feather forms can be modulated using retrovirus mediated gene mis-expression that mimics those found in nature today and in the evolutionary past. The molecular conversions among different types of integument appendages (feather, scale, tooth) are discussed. From these evidences, we recognize that not all organisms with feathers are birds, and that not all skin appendages with hierarchical branches are feathers. We develop a set of criteria for true avian feathers: 1) possessing actively proliferating cells in the proximal follicle for a proximo – distal growth mode; 2) forming hierarchical branches of rachis, barbs and barbules, with barbs shaped by differential cell death into either bilaterally or radially symmetric structures; 3) having a follicle structure, with a mesenchyme core during development; 4) maturing into a structure consisting of epithelia without a mesenchyme core with two sides of the vane facing the previous basal and supra-basal layer, respectively; and 5) having stem cells and dermal papilla in the follicle and hence the ability to molt and regenerate. A model of feather evolution from feather bud → barbs → barbules → rachis is presented, which is opposite to the old view of scale plate → rachis → barbs → barbules.
Introduction
Among all organisms, birds have one of the most complex forms and physical structures that allow them to live in water, land and air. Birds today share fundamental features but show enormous diversity in order to adapt to different ecological environments (Bereiter-Hahn, 1986; Gill, 1994; Lucas and Stettenheim, 1972). While adaptation to these different environments required diverse morphological features of feathers, the basic functions of feathers are considered to be insulation, communication, and flight (Chatterjee, 1997; Chiappe, 1995; Feduccia, 1999). In this issue Alibardi’s article discusses the essential adaptation of the reptilian integument to land. In this article, we will discuss the adaptation of avian integuments to the sky. When did this evolutionary transformation begin? How did the feather evolve? Did feather-like appendages evolve only once or more than once in history (also see Prum and Brush, 2002; Homberger, 2003; Sawyer and Knapp, 2003)? To support flight, what feather forms and accessory structures had to evolve before birds were adapted to the sky (Homberger and de Silva, 2002, 2003)?
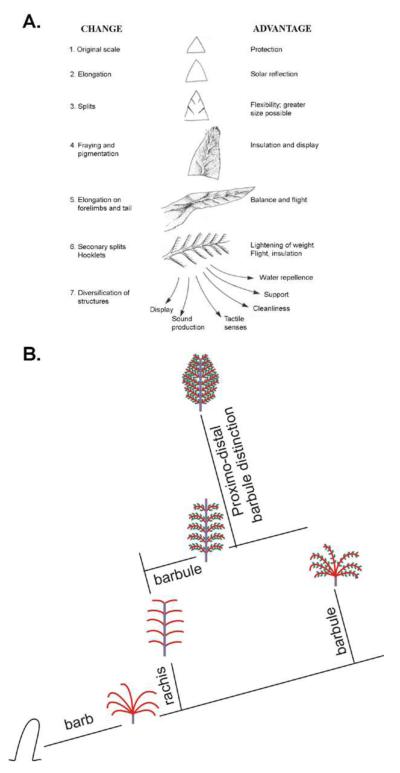
It is generally thought that birds evolved from reptiles, although when they evolved and from which lineage remains controversial (Chiappe and Witmer, 2002; Feduccia, 1999). The reptile integument is mainly made of scales (Landmann, 1984; Alibardi, 2003). In birds, there are scales on the foot and feathers on most of the rest of the body (Lucas and Stettenheim, 1972). A long held view is that avian feathers evolved from reptile scales; first through elongation of reptile scales, later through etching of the elongated scales to produce the branched feather vanes, and finally the inter-woven pennaceous feather barbs became plumulaceous (Regal, 1975, Fig. 5A). Two major events in the last decade have shaken this classical view and catalyzed a new level of understanding of the evolution of feathers. One comes from a series of discoveries of many intermediate forms of feather-like appendages from the Jehol Biota of China (also see Sawyer and Knapp, this issue), and the other comes from progress in molecular developmental biology and the ability to change one appendage form into another using today’s chickens as an experimental model (see Widelitz et al. in this issue).
Fig. 5. Models of scale / feather transformation.
Panel A is from Regal, 1975 that suggested the order being the scale like planes → partial pennaceous vanes with emerging rachis → bilaterally symmetric feather → plumulaceous barbs → radially symmetric downy feathers. We propose the order in panel B, favoring the order being cylindrical feather filaments splitting to form primitive barbs without barbules → radially symmetric downy feathers with plumulaceous barbs → bilaterally symmetric plumulaceous feathers → bilaterally symmetric pennaceous vanes → bilaterally asymmetric vanes.
There are likely to be some lineages not depicted here. Some lineages may have been lost through selection during Mesozoic time and become extinct while some lineages persisted and flourished till today. Some of the molecular pathways known to be involved under each new evolution process are indicated. Equipped with the new knowledge here and new technology (also see Widelitz et al., this issue), we are positioned to identify more molecular basis of evolutionary novelty using the feather Evo-Devo model.
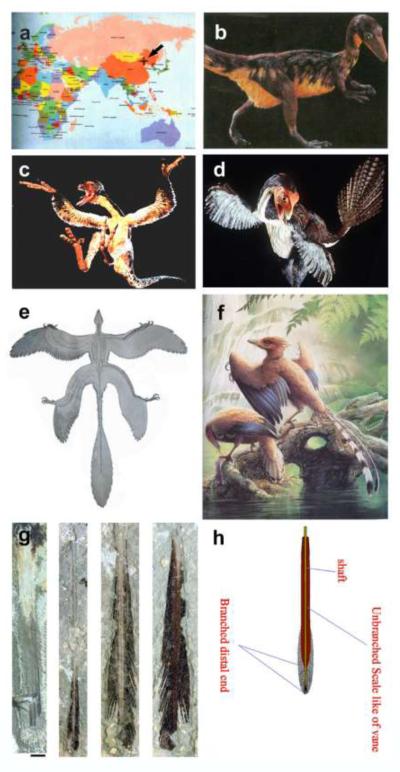
Special conditions in the Jehol Biota in China allowed for the excellent preservation of soft tissues. Hence many integuments of Mesozoic creatures evolving from reptiles to birds about 120 million years ago were well preserved in fossils (Chen et al., 1998; Zhou et al., 2003, Fig. 1). The most remarkable discoveries are the various dinosaur skeletons bearing diverse feather-like appendages. While birds and feathers used to be considered equivalent, the statement “All feathered animals are birds.” is no longer true. The statement “All branched skin appendages are feathers” is also challenged. When should a skin appendage be called a branched scale, a proto-feather (Chen et al., 1998), a non-avian feather (Jones et al., 2000) or a real feather (Xu et al., 2001)? Here we will review the integuments of major feathered dinosaur and Mesozoic bird fossils from China, but also include related fossils from other regions. We will evaluate the early integument appendages of these creatures and compare them with the characteristics of today’s feathers (Table 1).
Fig. 1. Representatives of feathered dinosaurs and Mesozoic birds from Jehol Biota.
a. Map showing the location of the excavated site. b. Sinosauropteryx. c. Sinornithosaurus. d. Caudipteryx. e. Microraptor gui. f. Confuciusornis. g, h. Tail feathers of Protopteryx and Confuciusornis that were used to support scale feather transformation (Zhang and Zhou, 2000) as suggested by Regal, 1975 (Fig. 5A). Panel b and d are from National Geographic. Panel e is from Xu et al., 2003. Panel f is from Hou, 1997.
Table 1.
Developing a definition for true avian feather*
|
The numbers do not indicate the order of appearance or prerequisite for subsequent characters.
All vertebrate skin appendages are made of epidermis and dermis, and are the result of epithelial - mesenchymal interactions. They all go through induction, morphogenesis and differentiation stages to achieve different phenotypes (Chuong, 1998; Widelitz and Chuong, 1999). Tissue interactions of feathers and scales have been analyzed in classical experiments that showed the reciprocal interactions between epidermis and dermis and their respective roles (reviewed in Sengel, 1976, Zeltinger and Sawyer, 1991; Dhouailly and Sawyer, 1984; Song and Sawyer, 1996). Since the 1990’s, RCAS retrovirus mediated gene mis-expression has allowed direct molecular analysis of early events of feather morphogenesis (reviewed in Chuong et al., 2000; Widelitz et al., this issue; Fig. 3A, B). With the advent of Evo-Devo research, developmental models have been proposed (Prum, 1999, Chuong et al., 2000; Alibardi and Sawyer 2002; Harris et al., 2002; Sawyer et al., 2003a). However, it has been difficult to do molecular analyses of later events in feather morphogenesis. The novel plucking / regeneration / molecular mis-expression feather model developed by Yu et al., (2002; and Fig. 3C, D) has opened a new way to analyze the forms of adult feathers. Through these experiments, we now are able to tackle molecular pathways underlying feather morphogenesis and alter feather forms (Fig. 5B). We can assume that some of these changes may reflect what happened in evolution. We will present several examples and discuss how they have influenced our thinking on feather evolution.
Fig. 3. Molecular biology technology used to mis-express genes and convert developmental pathways of skin appendages.
A. RCAS retroviral vectors used to mis-express genes in chicken. B. Demonstration of mis-expressed genes (in this case alkaline phosphatase, AP) in chicken embryos (The head is toward the left and not shown). Note the patchy staining. The insert in the lower right corner shows high expression of AP genes in the elongating feather buds.
C. Strategies to mis-express genes in feather follicles of hatched chickens. After feather plucking, stem cells regenerate a new feather. This is the opportunity to transduce exogenous genes to these regenerating epithelial stem cells (gray color, and panel D).
E. The balance of molecular pathways is perturbed and the forms of feathers are altered. Here we show noggin, a BMP antagonist, can split the rachis into multiples and enhance barbs to branch more. In contrast, BMP2 and BMP4 can produce a giant rachis and enhance barb fusion. Therefore, noggin favors barb formation while BMP favors rachis formation. Modified from Yu et al., 2002.
Jehol Biota, a Mesozoic landscape that preserved the integuments of intermediate species during the reptile – bird transition
The Jehol Biota spreads across the northern part of China and contains fossils of organisms that lived 120-145 million years ago (mya) as determined by isotope and plant dating methods. The Jehol Biota occupies a wide region including the Yixian Formation, Jiufotang Formation and other regions (Fig. 1a, Chen, 1998). It is a geological layer, in some parts 50-100 m thick, that represents the transition of the mid-Jurassic and early Cretaceous time. The landscape used to be rich in freshwater lakes with active volcanoes nearby and therefore contained many well-preserved biological specimens, including their soft tissues. Recent excavations and research on its plants, invertebrates, fishes, amphibians, reptiles, birds, and mammals, as well as on its geology and climate have established the Jehol Biota as one of the best preserved ecosystems (reviewed in Zhou et al, 2003). The Jehol Biota provided a valuable window to the biological diversity of the Mesozoic period. Each fossil specimen offers an opportunity to examine how the animal interacted with its environment and with other species.
Since birds were still evolving from reptiles during that period, their forms underwent intensive “re-engineering” to be adapted to the sky. They are in the conversion process from a tetrapod form, which mainly lived on land, to a smaller, winged bipedal animal, which mainly lived in trees and traveled through the air. The Jehol Biota in China provided a unique record in which the different integuments in transition were extraordinarily well-preserved. This is particularly valuable for the analyses of skin appendage evolution from dinosaurs to birds. It provides a rich source of new information for studying the origin and evolution of feathers and other integument related structures.
Feathered dinosaurs?
The major events during the evolution from reptiles to birds have been reviewed (Feduccia, 1999; Chatterjee, 1997; Chiappe, 1995; Chiappe and Witmer, 2002). The discovery of Archeopteryx (145 mya) in Germany and other fossils led to the compelling Dinosaur-bird hypothesis, suggesting that birds evolved from the dinosaur (Ostrom, 1974). Dinosaurs began flourishing in the Late Triassic, about 215 mya, and dominated the earth for the next 150 million years (Sereno, 1999). During the Triassic, the dinosaur clade split into two groups, the Ornithischia and the Saurischia. The latter gave rise to the hypothesized bird ancestors, the theropods. Before the Triassic ended, theropods had branched into the tetanurans from which the coelurosaurs arose. Coelurosaurs eventually evolved into the maniraptorans which, in the Late Jurassic, gave rise to Aves, the bird clade. Later, Aves gave rise to the Ornithothoraces that then branched off into the Enantiornithes (“opposite birds”) and Euornithes (“true birds”). The latter group radiated in the Late Cretaceous and gave rise to the Neornithes, modern birds.
There are some objections to the Dinosaur-bird hypothesis based on differences in skeletal structures. These scientists consider that the bird and dinosaur share common ancestors, such as the basal archosaur, an ancient reptile, and the “feathers” found on dinosaurs resulted from later convergent evolution (Feduccia, 1999; Chatterjee, 1997). In either case, one has to agree that feathers evolved from the reptile integument, and that there is a gradual transformation from the simple scale to the advanced forms of feathers (Prum, 1999; Chuong et al., 2000; Maderson and Homberger, 2000; Sawyer et al., 2003a, b), or through heterochrony of appendage morphogenetic events during embryogenesis (Sawyer and Knapp, this issue).
The skeleton of Archeopteryx suggests that it is an intermediate form between reptiles and birds. It already has different types of feathers over the body (e.g., tracts have already evolved), toothed jaws, claws on the wing, and a bony tail. The wing flight feathers have a closed pennaceous vane and are asymmetric, suggesting that it could fly (Feduccia and Tordoff, 1979), although it may not have been an excellent flyer. Therefore in the spectrum of the reptile-bird transition, Archeopteryx is closer to birds than to reptiles. For over a hundred years, scientists have been hoping to find new fossils representing the earlier forms that are closer to the reptiles and have a glimpse of the process of feather evolution.
The new findings from several sites in the Jehol Biota in China brought us new and exciting information. Theropods were a group of carnivorous, bipedal, terrestrial dinosaurs with small forelimbs and special predatory features, such as long hands with three digits for scratching and / or grasping prey (Sereno, 1999; Padian and Chiappe, 1998). Some non-avian theropod dinosaurs displayed a variety of skin appendage types, from simple filament-like proto-feathers to complex modern asymmetric feathers. Sinosauropteryx was among the first to have well-preserved skin appendages (Chen et al., 1998; Fig. 1b; about 120 mya). It has “fuzz fibers” on the body, especially along the dorsal midline. These filamentous “protofeathers” are about 20 mm long, ranging from 5 - 40 mm. They appear to be rather homogeneous over the body, without apparent signs of regional tract specificity. The filaments resemble down feathers, lacking aerodynamic properties. They may be hollow. They appear to have a short shaft with “barb” branches, but no further branching “barbules” were identified. These filaments may represent “proto-feathers” or some early branching skin appendages that may have provided insulation (Chen et al., 1998).
Two theropods, Beipiaosaurus and Sinornithosaurus, had large patches of filament-like integumentary structures preserved on the forelimbs, hindlimbs and body (Xu et al, 1999a, b). These filaments are arranged in parallel to each other and almost perpendicular to the bone. Some of the filaments seem to have branching distal ends. These primitive filaments appeared to be hollow, resembling the cylindrical feather filament. Further analyses (Xu et al, 2001) showed that skin appendages on Sinornithosaurus (Fig. 1c) have compound structures containing multiple filaments which are joined together. Two types of branched structures were identified. One is similar to avian downy feathers, and another one similar to avian pennaceous feathers, but lacking identifiable barbules. Another dinosaur, the smallest known non-avian theropod dinosaur, Microraptor zhaoianus (Xu et al., 2000), displayed a more advanced filament pattern near the femur. The filaments are long and contain a rachis. The fossil suggests that true feather structures may have already existed in these dinosaurs.
Feathers with close to modern feather shapes were first found in Caudipteryx and Protarchaeopteryx (Ji, et al., 1998). Caudipteryx (Fig. 1d) evolved different types of feathers over different body regions, indicating the establishment of feather tracts as an evolutionary novelty. This allowed the development of different types of feathers in different parts of the body, so specialized functions for each body part could evolve and enrich integument function. Caudipteryx formed spectacular pennaceous feathers in both the wing (remiges) and tail (retrices) with tapering shafts. The bilaterally symmetric pennacous structures in Caudipteryx and Protarchaeopteryx have been accepted as vaned feathers (Prum and Brush, 2002). However, the vanes lacked the asymmetric vane required for flight and were probably used for display to either attract or frighten others. It is unknown if they had yet to evolve asymmetric feathers or if the asymmetry was lost in adapting to life on land. There were plumulaceous feathers covering the body, most notably at the hips and the proximal region of the tail. Protarchaeopteryx also had bilaterally symmetric pennaceous feathers. The tail retrice feathers of Protarchaeopteryx were plumulaceous in the proximal part and pennaceous above the mid-shaft region (Ji et al., 1998). The vaned Protarchaeopteryx feathers appeared to be structurally transitional between the proto-feather-like structures of Sinosauropteryx and the modern feathers of Archaeopteryx.
Modern feathers were also detected in other non-avian theropod dinosaurs. Ji, et al (2001) reported a Dromaeosauridae covered with filamentous feather-like structures over its entire body. Three types of filamentous structures were identified in this specimen. The first type had single fibers. The second type had long plumulaceous fibers. The third type had symmetric pennaceous feathers which may have barbules. This type of pennaceous feather with a rachis and symmetric barbs were also found in a different species of Dromaeosauridae (Norell, et al. 2002).
The most interesting discovery to date among the feathered dinosaurs was the four-winged dinosaur recently reported by Xu et al (2003), Microraptor gui of Dromaeosauridae (Fig. 1e). Both fore and hind limbs were covered with pennaceous feathers arranged in a similar pattern. Feathers at the distal limb portion had asymmetric vanes. The remiges were preserved with the primary remiges longer than the secondary remiges. This arrangement may have been for improved aerodynamics as similar patterns are observed in modern birds. The body was covered with plumulaceous feathers. The “flight feathers” in the hind limb are not well designed for active flight. The creature may have adopted a gliding behavior in the flourishing Mesozoic jungles, gliding from one tree to another as seen in some mammals today.
The variety of skin appendages in non-avian theropod dinosaurs displayed a spectrum of integument appendages, from non-branched filaments to branched filaments to symmetric pennaceous vanes to asymmetric pennaceous vanes. Many of these skin appendages are considered to be possible homologues of avian feathers. The dinosaur integument coverings were probably very complex, including structures in addition to scales and feathers. The Mesozoic landscapes likely shaped many unusual appendages; some with branches and some not. Conceivably, there were many different occasions when there were adaptive advantages to have branched appendages. Branched skin appendages may have formed in different ways independently. One should not take it for granted, that all branching appendages are feathers.
Here let us look at some examples. A “non-avian feather” was reported for the Triassic archosaur, Longisquama (Jones et al., 2000). In the dorsal midline, Longisquama has a series of paired elongated integumentary appendages that form branches. However, the branches are perpendicular to the main axis and look like branched scales depicted by Regal, 1975 (Fig. 5B). The branches are atypical for those of feathers and have few resemblances to today’s avian feathers. Integument appendages that existed and vanished through evolutionary history are given names by modern scientists. Given our current understanding of skin appendage evolution is it appropriate to call them “non-avian feathers”, or would a different category of names be more appropriate?
Recently, a bristle-like, non-branched integumentary structure was found in the non-theropod dinosaur (Mayr et al., 2002). They are in the tail of the horned dinosaur (parrot-beaked dinosaur), Psittacosaurus. These bristle-liked structures are much longer and thicker than the proto-feathers in Sinosauropteryx and Sinornithosaurus, and were interpreted as cylindrical and possibly tubular epidermal structures. As it is cylindrical, a character considered very important in the first step of feather evolution (Prum and Brush, 2002), should we consider it as an early proto-feather, or as a modified feather like bristle on modern birds, or similar to the bristle of the beard in wild turkeys (Sawyer et al., 2003b)? On the other hand, they may not be homologous to those integument appendages on the theropods.
A tentative set of criteria is proposed in Table 1. Should we name only those that fit all proposed criteria as true avian feathers? More input and revision should be done to develop a consensus.
Another example of a potentially non-homologous skin appendage can be compared by analyzing integument appendages from animals not that remote: the turkey. Turkey beard bristles are distinct structures from feathers, although they express feather type beta keratin and show simple branching patterns (Sawyer et al., 2003b). These appendages are hollow at the distal end, and the branching may be due to partial separation (Lucas and Stettenheim, 1972). They lack follicles, yet grow continuously. Could these bristles resembling those found in Sinornithosaurus be homologues of dinosaur filamentous integument appendages or are they simply modified from modern avian feathers?
Paleontological research has shed new light on feather evolution. With the advent of EvoDevo research, the application of modern biochemical methods has begun to further enhance our understanding of this field. Using antibodies to beta keratin, Schweitzer et al., (1999) showed immunological cross reactivity with feather-like structures of the alvarezsaurid dinosaur, Shuvuuia deserti. Together with mass spectrometric data, they suggested that beta keratin existed in these dinosaurs. This type of molecular approach, when established, would be revolutionary to link paleontology research with molecular developmental research.
Feathers of Mesozoic birds
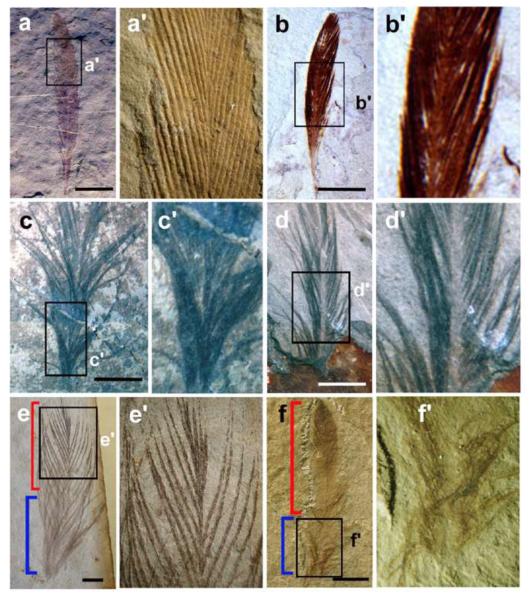
The continuous reptile-bird transition led to the formation of Mesozoic birds together with Archaeopteryx, described above, as the prototype. It already had evolved different types of feathers including down feathers, tail feathers arranged along the long bony tail, and asymmetric flight feathers. The crow sized Confucisornis from late Jurassic-early Cretaceous also had both down and flight feathers (Fig. 1f). They had evolved beaks with no teeth (Hou et al., 1995; 1996). The asymmetric flight feathers and toothless beak suggest it should have been a reasonably good flyer (Feduccia, 1999; Homberger, 2002). The fossils even show that they already had sexually dimorphic tail feathers and appear to have lived in groups (Fig. 1f, g).
Enantiornithes is the dominant group of Mesozoic birds (Chiappe and Witmer, 2002). Their skeletal characteristics suggest that they are different from modern birds. In China, the major species of this branch include Eoenantiornis, Cathayornis, Sinornis, Otogornis, Longipteryx, etc. The other branch, Ornithurine, represent the ancestors of the modern birds. Major members found in Mesozoic China include Liaoningornis, Chaoyangia, Gansus, etc. Most of these interesting and unusual Mesozoic birds already have true feathers, although they seem to have reached different levels of streamlined body shape for adaptation to the sky. One may speculate that the neuromuscular control, the coordinated motions in the wing, tail and trunk, and the different flight behaviors must also have been evolving (see Homberger, 2002; Homberger and de Silva, 2003). The Mesozoic birds in China are introduced in books by Hou (1997; 2003).
While Mesozoic feathers fit many criteria in Table 1, examination of feathers from Mesozoic birds show some unusual characteristics that are not seen in modern birds. For example, a primitive enantiornithine bird, Protopteryx fengningensis, has a thick, wide and flat rachis in the elongate central retrices (Zhang and Zhou, 2000, Fig. 1G). The retrices of Confucisornis also have a similarly dominant rachis, although not to the same extent. What is the purpose of this spectacular tail feather? Was it used for display, similar to peacock tail feathers, and evolved due to sexual selection? Was it used as a defensive weapon, like in some dinosaurs, to fend off predators? Presumably both Confucisornis and Protopeteryx lived an arboreal life, so these tail feathers which appear to be clumsy, may have been used to maintain balance while climbing trees as is true of modern squirrels. As the edge has branched barbs and the feathers look very much like that described in Regal (Regal, 1975; Fig. 1 and 5A), this feather was used as evidence to support the theory that feathers evolved from the elongated scales. However, Prum and Brush (2002) indicated that this evidence could not stand because the enlarged rachis was more likely to be formed later by the pronounced fusion of barbs. Also, its presence should have been wider (e.g., all over the body), should it be considered as a prototype of evolving feathers. In view of its existence only on the tail, it appears that these are specialized feathers and the giant shaft is more likely to be secondarily derived for specialized functions as discussed above. Recent molecular data (Yu et al., 2002) also offers an explanation through a developmental mechanism that such a giant shaft can be produced by over-production of BMP.
Developmental biology of the feather
These fossils show a sampling of the diversity and complexity of skin appendages found in the Jehol Biota in the Mesozoic landscape. Now let us look into their developmental biology to find a common theme and variations among different integument appendages. Reptilian integuments have scales which are periodic epithelial infoldings (See Alibardi, 2003). Their primary functions are to contain water and to provide protection. Most scales are short (Fig. 2A), although some reptiles can also grown long appendages as seen in today’s iguana. Chickens have three major types of scales, the reticulate, scutate and scutella scales (Sawyer et al., 1986). The radially symmetric reticulate scales are on the foot pad and express only alpha keratin. Scutate and scutella scales have anterior-posterior polarity, with an outer surface composed of beta keratin and an inner surface and a hinge region composed of alpha keratin (Sawyer and Knapp, 2003).
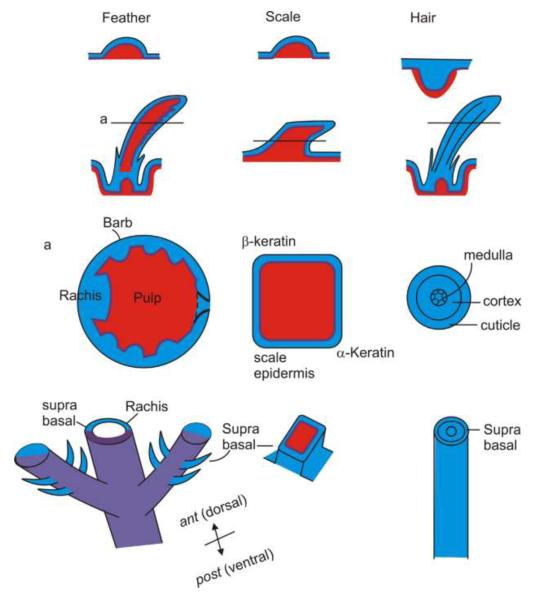
Fig. 2. Morphology and topological organizations of feathers, scales and hairs.
A, Avian foot scales and reptiles are very similar in morphology. However, their homology in evolution remains to be determined. Note the radial and bilateral symmetry of downy and flight feathers respectively. Also see Fig. 1 of Prum and Dyck in this issue for a contour feather. Panel A is modified from Lucas and Stettenheim, 1972 and Chuong et al., 2000.
B, Comparison of epithelium and mesenchyme composition of feathers, scales and hairs. Mature feathers are made of supra-basal epithelia only. The two sides of feather vanes originally face the suprabasal and basal side, respectively. The mesenchyme core only exists transiently during feather morphogenesis. Mature scales still have a mesenchymal core. All the scale surfaces are covered by the suprabasal layer. The mature hairs are made of suprabasal epidermal cells. Blue, epithelium, or suprbasal epithelia; purple, basal side of the epithelium (note in mature feather, the basal layer is gone, but still shown here to illustrate the topology of feather follicles); red, mesenchyme.
A typical feather in today’s birds consists of a shaft (rachis) from which barb branches are inserted. The barbs themselves are composed of a shaft (ramus) and numerous minute branches (barbules) (Lucas and Stettenheim, 1972; also see Prum and Dyck, 2003). Down feathers are mainly radially-symmetric (the rachis is absent or very short). Most contour feathers have bilateral symmetry (against the rachis axis). Flight feathers are bilaterally symmetric or asymmetric. Another level of complexity is within the barb. The barbules on the barbs can be bilaterally symmetric (across the ramus) and are therefore fluffy (plumulaceous) or the distal and proximal barbules can have different shapes. Distal barbules can form hooklets, enabling them to interlock with the proximal barbule of the next (more distal) barb in a Velcro-like mechanism to form a vane structure (pennaceous). In the base, feathers form follicles that protect the epithelial stem cells and dermal papilla and provide mechanical structures for muscle attachment and coordinated movement. New cell proliferation at the follicle base pushes the more differentiated portions of the feather to the distal end.
How do scales compare with feathers and hairs? Let us consider the basic configuration of these different skin appendages. They each are made from epithelial – mesenchymal interactions resulting in the formation of an epithelial placode and an underlying dermal condensation. Structurally, there is a basement membrane between the epithelium and mesenchyme, and the basal layer of the epidermis is on top of the basement membrane. However, there are some fundamental differences in the developmental processes that ensue. Scales do not form follicular structures. Proliferation is more diffuse (Tanaka and Kato, 1983), so the scales thicken and only elongate slightly. One major difference to feathers is that the mature scales are made of an epithelial shell and a mesenchymal core. The outside of the epithelial shell is the suprabasal layer. Compared to feathers, this is a different topological organization (Fig. 2).
The structure of avian foot scales and reptile scales are similar, although there are some reservations on whether avian foot scales are homologus to reptile scales or whether they are secondarily derived (see more discussion in Sawyer and Knapp, 2003). If the four winged dinosaur (Fig. 1E) is the prototype of an early dino-bird transition, not a special adaptation, one may say that avian foot scales are secondarily derived.
Feathers, on the other hand, initially start to proliferate from the tip of feather buds. Therefore, feather buds protrude out first (rather than hair pegs that invaginate first, see Botchkarev and Paus, this issue.). As the buds elongate, the localized proliferation zone gradually shifts through the shaft and localizes proximally to the base of the feather (Chondankar et al., 2003). In the mean time, epidermis surrounding the feathers starts to invaginate into the dermis to form a follicular wall. The dermal papilla is situated at the base of the follicle, inducing the epithelial collar above to continue proliferation. This allows for continual growth, and facilitates feather cycling as stem cells would be protected in the follicle. In adult feather morphogenesis, the feather filament is a cylindrical structure with the pulp inside (Fig. 2), facilitating branching formation (Prum and Brush, 2002). However, during development, feather branching can initiate in feather buds before the formation of follicles or feather filament cylinders (Sawyer and Knapp, 2003). The pulp contains loose mesenchyme made of blood vessels and nerves to support the growth of developing feathers. Toward the distal end, epithelial cells in the ramogenic zone start to differentiate into barb and rachidial ridges which form the feather backbone (rachis) and branches (barbs and barbules). Marginal plate cells between barb ridges, pulp epithelium and pulp in the filament center later degenerate to allow the opening of the feather filament cylinder into a two dimensional vane. This vane is made of epithelial cells only, with one side toward the original basal layer and the other side toward the supra-basal layer. However, the mature keratinized structure is made of only the supra-basal cells, as the basal layer has become the marginal plate and the pulp epithelium that disappear when the feather vane opens. Also see Prum and Dyck, and Bragulla and Hirschberg. in this issue for a more detailed discussion.
For comparison, we also examine the development of hairs. Hair placodes invaginate to form a hair peg. They then elongate with the dermal papilla (Fig. 2). Epithelial cells above the dermal papilla become the matrix, the localized growth zone of the hair. Epithelial cells between the matrix and inter-follicular epidermis become the outer root sheaths. Together with the dermal sheath, they form the follicular wall. Part of the distal follicle wall enlarges and forms two swellings. The upper swelling becomes the sebaceous gland, while the lower swelling forms the follicle bulge, the presumptive site housing stem cells (Cotsarelis et al., 1990). Above the matrix, epithelial cells differentiate into the hair filaments and inner root sheath. The most unique distinction from feather is that there is no mesenchymal component within the hair filament. Above the dermal papilla, it is all made of epithelial cells. For a more complete discussion of this process and the molecules involved, please see Botchkarev and Paus, this issue.
With this developmental understanding, we should know that not all epithelial appendages that form branches are feathers. The term, epithelial appendage, is a much broader name that includes all special derivatives of epithelial structures (Chuong, 1998). However, in terms of feathers, we may have to limit to those that share similar growth modes, most of the developmental processes, and many of the biochemical properties. Here we try to develop a set of criteria for feathers (Table 1). We try to include the major points discussed above, but there are also many other important characteristics of feathers we did not include at this stage. The numbers in the table do not indicate temporal order in evolution or evolution, or a pre-requisite for the subsequent criteria. Each component can be exaggerated or reduced to a minimum, thus allowing more shape possibilities (Bartels, 2003). Some feather variants today may not have barbules (e.g., some feathers in the lyre bird and egret), or have poorly developed barbs (e.g., penguins). Biochemically, they must contain beta keratin and share fundamental molecular pathways (Shames and Sawyer, 1987; Shames et al., 1991).
To search for the evolutionary origin of feathers, we have to deal with fossils. Unfortunately, some criteria in Table 1 are defined functionally and can not be measured in fossils. Based on morphological evidence, we can still do our best to look for tube like follicles, analyze the arrangement of branches, and evaluate the whole configuration of the appendages. For example, the branched scales of Longisquama do not fit the definition of true avian feathers and hence, were named “Non-avian feathers” (Jones et al., 2000). We can also evaluate unusual integument appendages today with these criteria. For example, turkey beard bristles grow continuously from finger-like outgrowths but do not assume the localized growth mode from the proximal to the distal end seen in feathers even though they show simple branching and express feather-type beta keratins (Sawyer et al., 2003b). Using the criteria in Table 1, turkey beard bristles would not be classified as feathers.
Evolution of other integumentary appendages: tooth, beak, and others
For the bird to adapt to a life in the sky, coordinated changes in structures other than feathers are also required. The loss of teeth and formation of a cornified beak was shown to have been driven by a selective regime favoring aerodynamically streamlined body contours, which are a fundamental characteristic for avian flight (Homberger, 2002). The non-avian theropod dinosaurs in the Jehol biota display a variety of tooth types. Sinosauropteryx has unserrated premaxillary but serrated maxillary teeth (Chen et al., 1998). There are four serrated premaxillary teeth preserved in the Protarchaeopteryx. Caudipteryx also had four preserved elongated, hooked premaxillary teeth (Ji et al., 1998). The smallest feathered dinosaur, Microraptor Zhaoianus, has developed posterior teeth that have a less compressed crown and a constriction “waist” beneath the crown. The heterodont dentition pattern represents the transition from the non-avian theropod type of dentition to that of Mesozoic birds (Xu et al., 2000). Interestingly, an Oviraptorosaurian, Incisivosaurus, had more diverse tooth types than other feathered dinosaurs. It had rodent incisor-like premaxillary teeth and tapered cheek teeth (Xu et al., 2002).
Archeopteryx had many teeth remaining in its snout. A long-tailed large basal bird, Jeholornis, had no teeth in its maxilla, but had only 3 small conical teeth on its mandible (Zhou and Zhang, 2002). Confucisornis, had a true beak and no teeth (Hou et al., 1995). While some of these Mesozoic birds had no teeth, some had a different number of teeth remaining with the beak, suggesting that the loss of teeth was a later event compared to the evolution of flight, and that a balance between the loss of teeth (facilitating flight) and tooth maintenance (facilitating catching prey). The fossil finds suggest that from feathered dinosaurs to Mesozoic birds, the trend is toward a reduction of tooth number. In modern times, all birds have lost their teeth.
The gradual diversification of integument appendages (beak, teeth, feathers, different types of claws) has allowed different trophisms to develop (Zweers et al., 1997). It allowed Mesozoic birds to reach different niches that were only possible after an adaptation toward flight, and contributed to the bio-diversity of the Mesozoic world. These are vividly introduced in Hou et al., 1997; 2003 (also Fig. 2).
Molecular conversion of one appendage phenotype into another
The changes of these integument appendages have to be based on available developmental pathways. “Evolutionary novelties” are added when new developmental pathways are produced. The evolution of novel developmental mechanisms is usually based on the alteration of homologous molecular modules that allow the connection or disconnection of existing molecular pathways (von Dassow and Munro, 1999; Chuong edit, 1998; Chuong and Noveen, 1999).
Can we explore the molecular basis underlying these changes through laboratory research? Many laboratories have investigated different molecular pathways to discern their expression patterns and roles in the complex developmental processes underlying epithelial appendage development. In general the order of appearance of these molecules is FGF4, BMP4 → SHH, Wnt-7a → Notch-1, Serrate-1 and Delta-1 → Msx-1, -2 → Hox, NCAM (Song et al., 1996; Jung et al., 1998; Ting-Berreth et al., 1996; Widelitz et al., 1999; Chen et al., 1997; Noveen et al., 1996; Chuong et al., 1990, Chuong and Edelman, 1985). These pathways affect feather induction, mesenchymal condensation, localized cell proliferation, etc. Our lab has used modern chickens as a research model (Fig. 3A-D). Here we will discuss some recent findings. The first example is about changing the balance between barb and rachis formation (Fig. 3E). The second example represents the gain of a pathway: growing feathers from scale epidermis (Fig. 4A). The third example represents the reactivation of a lost pathway: growing teeth from the chicken oral mucosa (Fig. 4B).
Fig. 4. Molecular conversion of feathers / scales and tooth / oral mucosa.
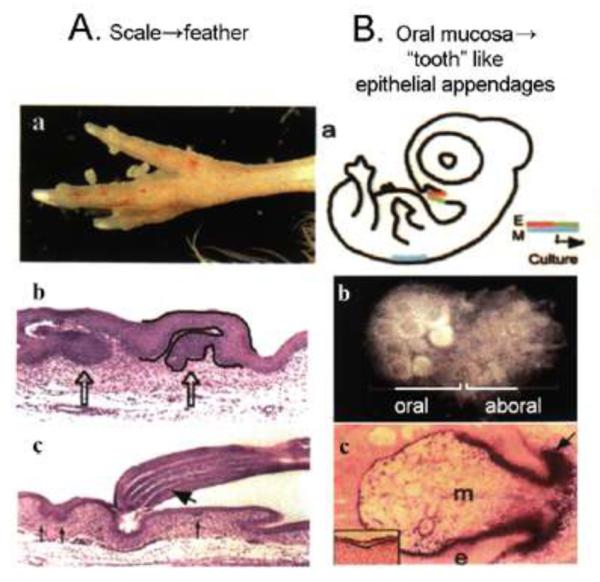
A. When RCAS beta catenin was used to infect embryonic chicken hind limb, scales are converted into feathers. a. feathers growing out from scale region. b, Follicles are seen to form from part of the scutate scale surface. c, Barb ridges form in these induced feathers. The newly induced feathers do have follicular structures and form barb ridges. Note it is part of the scale surface that is converted into feathers, not the transformation of the whole scales into feathers. It appears that some appendage stem cells may have remained in scale epidermis and are activated by beta catenin to form a feather. Modified from Widelitz et al., 2000.
B. Embryonic epithelia containing oral mucosa and chin epidermis were recombined with feather mesenchyma from the dorsal skin (a). The explant is put to develop in culture. Chin regions form feather buds while the oral mucosa regions form many tooth like appendages (left part of the explant, panel b), while the right part forms feather buds. Sections show follicular structures (panel c). Modified from Chen et al., 2000.
To investigate the molecules involved in feather branching, we looked for genes with expression patterns suggesting that they might be involved in this process. BMP4 was first expressed in the dermal papilla and overlying pulp, but later switched to the barb ridges in the ramogenic zone. BMP2 was expressed in the marginal plate but also moved to the barb plate. Noggin, a BMP antagonist, was expressed as a gradient in the pulp, with the highest expression levels found at the ramogenic zone. To further explore the role of this pathway in branching morphogenesis, we used the RCAS retrovirus to deliver noggin to regenerating feather follicles of modern chickens (Yu et al., 2002). The resulting rachidial ridges were severely fragmented. Retroviral mediated expression of BMP4 to the regenerating follicles produced feathers that lacked branches. The rachidial ridges were often fused. These data suggest that the BMP pathway is involved in feather branching (Fig. 3E; Yu et al., 2002).
Beta-catenin was first found to interact with APC in the colon (Rubinfeld et al., 1996). APC deletions or mutations of APC led to increased accumulation of beta-catenin and to the formation of colon polyps, which are epithelial growths. Thus, accumulation and activation of beta-catenin is able to stimulate epithelial cells towards growth. Transgenic mice expressing exogenous beta-catenin had new hairs and formed hair tumors (Gat et al., 1998). We characterized the expression of beta-catenin in chicken skin and found that it was first expressed throughout a feather tract and then, as the feather buds began to form, was up-regulated within the bud primordia and down-regulated in the interbud regions. To test its role in feather bud development, we mis-expressed a truncated, constitutively active form of beta-catenin from the replication competent avian sarcoma virus (RCAS). Increased beta-catenin expression in the scale primordia induced feathers to form from the leading edge of the scales (Fig. 4A; Widelitz et al., 2000). Manipulating other pathways also led to an induction of feathers from scale forming regions. Activation of the delta pathway and suppression of the BMP pathway in scales also induced some feathered scales (Crowe and Niswander, 1998; Viallet et al., 1998; Zhou and Niswander, 1996). These molecular pathways are likely to intersect and work in concert during the conversion of scales to feathers. We believe that similar, but not necessarily identical, molecular processes may have occurred during avian evolution to initiate the formation of ancestral feathers.
Mesozoic birds had teeth that were lost in the evolution of modern beaks. In the third example, we will examine whether latent molecular signals specifying tooth development were retained by modern birds but not expressed. The fact that the oral mucosa of modern chickens still forms a dental lamina, which soon degenerates, suggested that the ancestral molecular mechanisms might still exist. Classical experiments using recombination to form a chimera of mouse dental mesenchyme with chicken oral mucosa led to the expression of a “chicken enamel gene” (Kollar and Fisher, 1980), and to the formation of characteristic dental mesenchymal structures (Wang et al., 1998). What are the molecular signals involved? Some of these latent signas were revealed by in situ hybridization, which indicated that the chicken oral mucosa expressed Pitx2, Pax9, and FGF8, but not Bmp4, Msx1, and Msx2. All of these genes are expressed in the mouse oral mucosa and are considered to be essential for tooth formation. In fact, epithelial signaling to the mesenchyme involves a BMP4 → Msx1 → BMP4 pathway (Chen et al., 1996). Knockout mice lacking Msx-1 and Msx-2 fail to grow teeth. It is possible that during beak evolution a defect in the BMP4 → Msx1 → BMP4 pathway developed which led to the loss of teeth from modern birds. To test this theory, we tried to rescue tooth odontogenesis from the chicken oral mucosa by releasing BMP4 from beads. BMP4 did induce the expression of Msx1 and Msx2 from the chicken oral mesenchyme. FGF released from beads in a similarly designed experiment had an even greater effect. The effect was even greater still when applied to dorsal skin feather producing mesenchyme (Fig. 4B; Chen et al., 2000). It is difficult to be sure that these skin appendages were truly teeth since there is no chicken tooth marker. However, these experiments clearly show that the dental lamina has the ability to form follicular structures for integument appendages. Recently, the chicken oral mucosa was shown to be able to corroborate with mouse neural crest to form tooth like appendages in chimeric embryos (Mitsiadis et al., 2003). This is consistent with the above thesis that some signaling modules were lost in the chicken oral mucosa mesenchyme, and that it is possible to rescue, at least partially, the tooth forming pathway.
Models of feather evolution and conclusion
The feather is the centerpiece of bird flight and its origin and evolutionary history have long puzzled scientists. A long held view is that feathers evolved from an elongation of scales enlisted for protection. It was then subdivided over time to form pennaceous and then plumulaceous feather types (Regal, 1975). Therefore, the order of formation is from the scale like plates → partial pennaceous vanes with emerging rachis → bilaterally symmetric feather → plumulaceous barbs → radially symmetric downy feathers (Fig. 5A). Two major advances in the last decade have shaken this classical view: (1) a series of fossils discoveries representing intermediate forms of feathers or feather-like appendages from the Jehol Biota of China, and (2) molecular and developmental biological experiments using chickens as a model organism. Feather forms can be modulated using retrovirus mediated gene mis-expression that mimics those found in nature today and in the evolutionary past. Together the results favor an evolutionary sequence of feather filaments splitting to form primitive barbs without barbules → radially symmetric downy feathers with plumulaceous barbs→ bilaterally symmetric plumulaceous feathers → bilaterally symmetric pennaceous vanes → bilaterally asymmetric vanes (Fig. 5B).
This order occurs in development, and we feel that it should have occurred in evolution too, in a broad sense of ontogeny repeating phylogeny.). Each arrow probably represents one evolutionary novelty (Prum, 1999; Prum and Brush, 2002). Work in the molecular biology laboratories has allowed us to start to identify molecular pathways involved in each of these “evolutionary novelty” processes (Fig. 5B; Yu et al., 2002; Harris et al., 2002).
Integument and integument appendages are all made of ectodermal cells. They share common appendage stem cells. Indeed, all the diverse appendages can be viewed as variations on top of a common theme (Chuong, 1998). With experimental manipulation of molecular pathways, we now can modulate feather forms from one form to another, and we can also convert appendage phenotypes from one type to another. In the context of Evo-Devo, feather morphogenesis presents an excellent paradigm with rich fossil evidence, theoretical models (Prum and Dyck, 2003) and experimental possibilities (Sawyer and Knapp and Widelitz et al., 2003). We are positioned to identify more molecular basis of evolutionary novelties that eventually adapt the birds to the sky.
Acknowledgment
We thank Ms. Marijane Ramos for help in the manuscript preparation, and Drs. G. P. Wagner and D. Homberger for very helpful comments on the manuscript. For US authors, this work is supported by NIH RO1AR42177, RO1AR47364, NCI (R21CA094392), NSF IBN9808874 (C.M.C.), and NCI R01CA83716 (R.B.W.). For China authors, this work is supported by NSP co-sponsored project “Study of Jehol Biota” (4982020), China, and by the Institute of Vertebrate Paleontology and Paleoanthropology.
References
- Alibardi L. Adaptation to land: the skin of reptiles in comparison to that of amphibians and endotherm amniotes. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.24. *this issue. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Sawyer RH. Immunocytochemical analysis of beta keratins in the epidermis of chelonians, lepidosaurians, and archosaurians. J Exp Zool. 2002;293:27–38. doi: 10.1002/jez.10145. [DOI] [PubMed] [Google Scholar]
- Bartels T. Variations in the morphology, distribution and arrangement of feathers in domesticated birds. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.28. *this issue. [DOI] [PubMed] [Google Scholar]
- Vladimir B, Ralf P. Molecular biology of hair morphogenesis: Development and cycling. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.33. *this issue. [DOI] [PubMed] [Google Scholar]
- Bragulla H, Hirschberg RM. Horse hooves and bird feathers: Two model systems for studying the structure and development of highly adapted integumentary accessory organs-The role of the dermo-epidermal interface for the micro-architecture of complex epidermal structures. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.31. *this issue. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Biology of the integument. Springer Verlag; New York: 1986. [Google Scholar]
- Chatterjee S. The Rise of Birds. John Hopkins University Press; Baltimore, MD: 1997. [Google Scholar]
- Chen P, Dong Z, Zhen S. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–152. [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Chen CWJ, Jung HS, Jiang TX, Chuong CM. Asymmetric expression of Notch, Serrate and Delta is associated with the anterior posterior axis of feather buds. Dev Biol. 1997;188:181–187. doi: 10.1006/dbio.1997.8643. [DOI] [PubMed] [Google Scholar]
- Chen YP, Zhang Y, Jiang TX, Barlow A, Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathway in Aves. Proc Natl Acad Sci. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe LM. The First 85 million years of Avian Evolution. Nature. 1995;378:349–355. [Google Scholar]
- Chiappe LM, Witmer LM, editors. Mesozoic birds: Above the heads of dinosaurs. University of California Press; 2002. [Google Scholar]
- Chodankar R, Chang CH, Yue Z, Jiang TX, Suksaweang S, Burrus L, Chuong CM, Widelitz R. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol. 1985;101:1009–1026. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110:1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin, TX: 1998. [Google Scholar]
- Chuong CM, Noveen A. Phenotypic determination of epithelial appendages: Genes, developmental pathways and evolution. J Invest Dermatol Sym Proc. 1999;4:307–311. doi: 10.1038/sj.jidsp.5640235. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Chodankar R, Widelitz RB, Jiang T-X. Evo-Devo of Feathers and Scales: Building complex epithelial appendags. Curr Opin Dev Gene. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, Niswander Disruption of scale development by Delta-1 misexpression. Dev Biol. 1998;195:70–74. doi: 10.1006/dbio.1997.8844. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Sawyer RH. Avian scale development. XI. Initial appearance of the dermal defect in scaleless skin. Dev Biol. 1984;105:343–350. doi: 10.1016/0012-1606(84)90291-4. [DOI] [PubMed] [Google Scholar]
- Feduccia A, Tordoff HB. Feathers of Archaeopteryx: Asymmetric Vanes indicate Aerodynamic Function. Science. 1979;203:1021–1022. doi: 10.1126/science.203.4384.1021. [DOI] [PubMed] [Google Scholar]
- Feduccia A. The Origin and Evolution of Birds. 2nd Edition Yale University Press; New Havent, CT: 1999. [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gill FB. Ornithology. 2nd Edition Freeman; New York, NY: 1994. [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. (Mol. & Dev. Evol.) 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Homberger DG. The aerodynamically streamlined body shape of birds: Implications for the evolution of birds, feathers, and avian flight. In: Zhou Z, Zhang F, editors. Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution; Beijing. 1-4 June 2000; Beijing, China: Science Press; 2002. pp. 227–252. [Google Scholar]
- Homberger DG. Avian origins revisited. J Biosci. 2003;28:135–138. doi: 10.1007/BF02706209. [DOI] [PubMed] [Google Scholar]
- Homberger DG, de Silva KN. Functional microanatomy of the feather-bearing avian integument: Implications for the evolution of birds and avian flight. Amer Zool. 2000;40:553–574. [Google Scholar]
- Homberger DG, de Silva KN. The role of mechanical forces on the patterning of the avian feather-bearing skin: A biomechanical analysis of the integumentary musculature in birds. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.30. *this issue. [DOI] [PubMed] [Google Scholar]
- Hou LH, Zhou ZH, Martin LD, Feduccia A. A beaked bird from the Jurassic of China. Nature. 1995;377:616–618. [Google Scholar]
- Hou LH, Martin LD, Zhou ZH, Feduccia A. Early Adaptive Radiation of Birds: Evidence from Fossils from Northeastern China. Science. 1996;274:1164–1167. doi: 10.1126/science.274.5290.1164. [DOI] [PubMed] [Google Scholar]
- Hou LH. Chinese. Taiwan; Mutual Culture Co.: 1997. Mesozoic Birds of China. (English edition in prep) [Google Scholar]
- Hou LH, Chuong CM, Young, et al. Chinese Fossil Birds. Kum-Ming Pub.; China: 2003. In press. [Google Scholar]
- Ji Q, Currie PJ, Norell MA, Ji SA. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–761. [Google Scholar]
- Ji Q, Norell MA, Gao KQ, Ji SA, Ren D. The distribution of integumentary structures in a feathered dinosaur. Nature. 2001;410:1084–1088. doi: 10.1038/35074079. [DOI] [PubMed] [Google Scholar]
- Jones TD, Ruben JA, Martin LD, Kurochkin EN, Feduccia A, Maderson PFA, Hillenius WJ, Geist NR, Alifanov V. Non-avian feathers in a late Trissic Archosaur. Science. 2000;288:2202–2205. doi: 10.1126/science.288.5474.2202. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West F, Widelitz RB, Jiang TX, Ting S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: Implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- Landmann L. The Skin of Reptiles: Epidermis and Dermis. In: Bereiter-Hahn J, Matoltsy AG, Richards S, editors. Biology of the Integument. Springer-Verlag; New York, NY: 1984. pp. 150–187. [Google Scholar]
- Lucas AM, Sttetenheim PR. Agriculture Handbook 362. Agricultural Research Services. US Department of Agriculture; Washington DC: 1972. Avian Anatomy Integument. [Google Scholar]
- Maderson PFA, Homberger DG. The evolutionary origin of feathers: A problem demanding interdisciplinary communication. Amer. Zool. 2000;40:455–460. [Google Scholar]
- Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J. Development of teeth in chick embryos after mouse neural crest transplantation. Proc. Natl. Acad. Sci. 2003;100:6541–6545. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norell M, Ji Q, Gao K, Yuan C, Zhao Y, Wang L. ‘Modern’ feathers on a non-avian dinosaur. Nature. 2002;416:36–37. doi: 10.1038/416036a. [DOI] [PubMed] [Google Scholar]
- Noveen A, Jiang TX, Ting-Berreth SA, Chuong CM. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. J Invest Dermatol. 1995;104:711–719. doi: 10.1111/1523-1747.ep12606960. [DOI] [PubMed] [Google Scholar]
- Ostrom JH. Archaeopteryx and the Origin of Flight. Q Rev Biol. 1974;49:27–47. [Google Scholar]
- Padian K, Chiappe LM. The Origin of Birds and their Flight. Scientific American. 1998 Feb;:38–47. doi: 10.1038/scientificamerican0298-38. [DOI] [PubMed] [Google Scholar]
- Prum RO. Development and Evolutionary Origin of Feathers. J Exp Biol (Mol. & Dev. Evol.) 1999;285:291–306. [PubMed] [Google Scholar]
- Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Quart Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Prum RO, Dyck J. A hierarchical model of plumage: Morphology, Development, and Evolution. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.27. *this issue. [DOI] [PubMed] [Google Scholar]
- Regal PJ. The Evolutionary Origin of Feathers. Q Rev Biol. 1975;50:35–66. doi: 10.1086/408299. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.26. *this issue. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Knapp LW, O’Guin WM. The skin of birds: epidermis, dermis and appendages. In: Bereiter-Hahn J, editor. Biology of the integument. Vol. 2. Vertebrates. Springer Verlag; New York: 1986. p. 194. [Google Scholar]
- Sawyer RH, Salvatore BA, Potylicki TT, French JO, Glenn TC, Knapp LW. Origin of feathers: Feather beta (beta) keratins are expressed in discrete epidermal cell populations of embryonic scutate scales. J Exp Zoolog (Mol Dev Evol.) 2003a;295:12–24. doi: 10.1002/jez.b.5. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Washington LD, Salvatore BA, Glenn TC, Knapp LW. Origin of archosaurian integumentary appendages: The bristles of the wild turkey beard express feather-type beta keratins. J Exp Zool. 297B Mol. & Dev. Evol. 2003b:27–34. doi: 10.1002/jez.b.17. [DOI] [PubMed] [Google Scholar]
- Schweitzer MH, Watt J, Avci AR, Knapp L, Chiappe L, Norell M, Marshall M. Beta-keratin specific immunological reactivity in feather-like structures of the Cretaceous Alvarezsaurid, Shuvuuia deserti. J Exp Zool (Mol. & Dev. Evol.) 1999;285:146–157. [PubMed] [Google Scholar]
- Sengel P. Morphogenesis of skin. Cambridge Univ Press; Cambridge: 1976. [Google Scholar]
- Sereno PC. The Evolution of Dinosaurs. Science. 1999;284:2137–2147. doi: 10.1126/science.284.5423.2137. [DOI] [PubMed] [Google Scholar]
- Shames RB, Sawyer RH. Expression of beta-keratin genes during development of avian skin appendages. Curr Top Dev Biol. 1987;22:235–253. doi: 10.1016/s0070-2153(08)60106-4. [DOI] [PubMed] [Google Scholar]
- Shames RB, Jennings AG, Sawyer RH. Expression of the cell adhesion molecules, L-CAM and N-CAM during avian scale development. J Exp Zool. 1991;257:195–207. doi: 10.1002/jez.1402570208. [DOI] [PubMed] [Google Scholar]
- Song HK, Sawyer RH. Dorsal dermis of the scaleless (sc/sc) embryo directs normal feather pattern formation until day 8 of development. Dev Dyn. 1996;205:82–91. doi: 10.1002/(SICI)1097-0177(199601)205:1<82::AID-AJA8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Song H, Wang Y, Goetinck PF. Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proc Natl Acad Sci U S A. 1996;93:10246–10249. doi: 10.1073/pnas.93.19.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kato Y. Epigenesis in developing avian scales. II. Cell proliferation in relation to morphogenesis and differentiation in the epidermis. J Exp Zool. 1983;225:271–283. doi: 10.1002/jez.1402250210. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Viallet JP, Prin F, Olivera-Martinez I, Hirsinger E, Pourquie O, Dhouailly D. Chick Delta-1 gene expression and the formation of the feather primordia. Mech Dev. 1998;72:159–168. doi: 10.1016/s0925-4773(98)00027-6. [DOI] [PubMed] [Google Scholar]
- von Dassow G, Munro E. Modularity in animal development and evolution: elements of a conceptual framework for EvoDevo. J Exp Zool. 1999;285:307–325. [PubMed] [Google Scholar]
- Wang YH, Upholt WB, Sharpe PT, Kollar EJ, Mina M. Odontogenic epithelium induces similar molecular responses in chick and mouse mandibular mesenchyme. Dev Dyn. 1998;213:386–397. doi: 10.1002/(SICI)1097-0177(199812)213:4<386::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Chuong CM. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchymal cells. J Invest Dermatol. 1999;4:302–306. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Lu J, Chuong CM. beta catenin in Epithelial Morphogenesis: Conversion of Part of Avian Foot Scales into Feather Buds with a Mutated beta-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen J-Y, Wu P, Yu Z, Chuong C-M. Molecular biology of feather morphogenesis: A testable model for evo-devo research. J exp Zool (Mol Dev Evol) 2003 doi: 10.1002/jez.b.29. *this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Tang ZJ, Wang XJ. A therinzinosauroid dinosaur with integumentary structures from China. Nature. 1999a;399:350–354. [Google Scholar]
- Xu X, Wang XL, Wu XC. A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature. 1999b;401:262–266. [Google Scholar]
- Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–708. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–2004. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- Xu X, Cheng YN, Wang XL, Chang CH. An unusual oviraptorosaurian dinosaur from China. Nature. 2002;419:291–293. doi: 10.1038/nature00966. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltinger J, Sawyer RH. Avian scale development. XIII. Epidermal germinative cells are committed to appendage-specific differentiation and respond to patterned cues in the dermis. Dev Biol. 1991;144:335–352. doi: 10.1016/0012-1606(91)90426-4. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 2000;290:1955–1959. doi: 10.1126/science.290.5498.1955. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang F. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature. 2002;418:405–409. doi: 10.1038/nature00930. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421:807–14. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP Signaling in Interdigital Apoptosis and Scale Formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zweers GA, Vanden Berge JC, Berkhoudt H. Evolutionary pattern of avian trophic diversification. Zoology. 1997;100:25–57. [Google Scholar]