Abstract
Avian beak diversity is a classic example of morphological evolution. Recently, we showed that localized cell proliferation mediated by bone morphogenetic protein 4 (BMP4) can explain the different shapes of chicken and duck beaks (Wu et al. [2004] Science 305:1465). Here, we compare further growth activities among chicken (conical and slightly curved), duck (straight and long), and cockatiel (highly curved) developing beak primordia. We found differential growth activities among different facial prominences and within one prominence. The duck has a wider frontal nasal mass (FNM), and more sustained fibroblast growth factor 8 activity. The cockatiel has a thicker FNM that grows more vertically and a relatively reduced mandibular prominence. In each prominence the number, size, and position of localized growth zones can vary: it is positioned more rostrally in the duck and more posteriorly in the cockatiel FNM, correlating with beak curvature. BMP4 is enriched in these localized growth zones. When BMP activity is experimentally altered in all prominences, beak size was enlarged or reduced proportionally. When only specific prominences were altered, the prototypic conical shaped chicken beaks were converted into an array of beak shapes mimicking those in nature. These results suggest that the size of beaks can be modulated by the overall activity of the BMP pathway, which mediates the growth. The shape of the beaks can be fine-tuned by localized BMP activity, which mediates the range, level, and duration of locally enhanced growth. Implications of topobiology vs. molecular blueprint concepts in the Evo–Devo of avian beak forms are discussed.
Keywords: Evo–Devo, craniofacial development, Darwin’s finches, Mesozoic birds, BMP4, FGF, Shh
INTRODUCTION
During the morphological transformation from reptiles to birds, new evolutionary challenges were imposed on the early avian-like species (Chiappe, 1995; Feduccia, 1999; Chuong et al., 2003). Accompanying the evolution of flight were novel eco-morphological opportunities that drove the evolution of diverse beak shapes (Zweers et al., 1997; Hou et al., 2003, 2004). The recruitment of forelimbs as wings allowed a newly found mobility resulting from flight and opened vast eco-morphological possibilities. However, this change came at a cost, because animals now needed to develop a new feeding mechanism without the use of forearms. This development exerted selection pressures on the morphology of the face; a strong, lightweight, and effective feeding apparatus had to evolve, leading to an amazing transformation of the snout into a large range of beak shapes adapted to different ecological niches. At the macro-scale, it involved a transformation of the reptile snout into a bird beak (Feduccia, 1999). At the micro-scale, it involved the fine tuning of the shapes of beaks in the Galapagos Island finches that inspired Darwin’s theory of evolution (Darwin, 1859; Grant, 1986). Although this work explained the basis of adaptive radiation of beaks, the mechanism that produced different shaped beaks at the developmental level is mostly unknown.
The beak is made of multiple facial prominences (Francis-West et al., 1998; Helms and Schneider, 2003; Richman and Lee, 2003). These prominences consist of a neural crest-derived and mesodermally derived mesenchymal core covered by an epithelial layer of ectoderm and endoderm. The frontal nasal mass (FNM), lateral nasal prominences (LNP), and maxillary prominences (MXP) comprise the upper beak. The mandibular prominence (MDP) forms the lower beak. Each prominence has a distinct shape, and all are coordinated with proportional sizes to compose a unique beak shape in different species (Fig. 1A). The identities of facial prominences are specified early in the neural crest stage (Noden, 1988; LaBonne and Bronner-Fraser, 1999; Le Douarin and Kalcheim, 1999) and may involve homeobox genes such as Hox (Couly et al., 1998), Dlx (Depew et al., 2002; Robledo et al., 2002), and so on. The identity of the MXP can be respecified to the FNM by a combination of noggin and retinoic acid (Lee et al., 2001). Chimerae between duck and quail cephalic crest cells suggested that the neural crest determines beak morphology (Schneider and Helms, 2003). Transplantation studies showed that a frontonasal ectoderm, marked by fibroblast growth factor 8 (FGF8) and Sonic hedgehog (Shh) expression, could pattern the mesenchyme (Hu et al., 2003). Further studies showed that Shh and FGF8 act synergistically to drive cartilage growth (Abzhanov and Tabin, 2004).
Fig. 1.
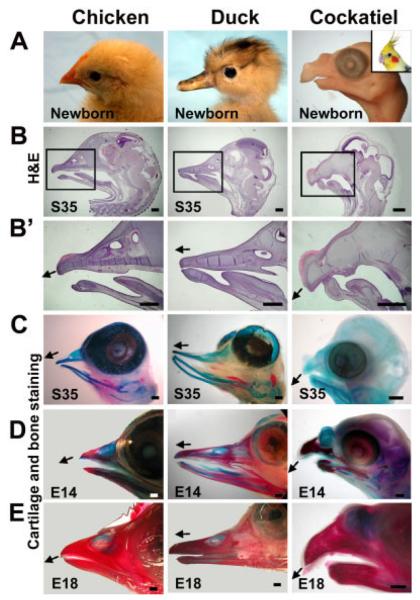
Diversity of developing chicken, duck, and cockatiel beaks. A: Comparison of beak from newborn or adults (inset). B,B’: Stage 35, hematoxylin and eosin (H&E) staining. Boxed region in B is shown enlarged in B’. Arrows indicate the angle of the upper beak curvature. C–E: Cartilage (blue) and bone (red) staining of stage 35, embryonic day (E) 14 and E18 beaks, respectively. At stage 35, cartilage staining prevails. At E14, the beak shows generous bone growth and beak shapes are similar to adult birds. At E18, most of the staining is bone. Scale bar = 1 mm.
Some early events have been studied (Francis-West et al., 1998; Helms and Schneider, 2003; Richman and Lee, 2003): from stage 20 to 29 these prominences grow, interact, fuse, and morph to form a beak with a species-specific shape. The morphogenetic principles operating in late stages though have not been studied thoroughly. Recently, we found that, starting at stage 26, there are localized mesenchymal zones with higher proliferative activity in the FNM. We define these zones as localized growth zones (LoGZ), meaning specific local regions where new cells are added at a significantly higher rate than in adjacent regions at a specific time. In a more broad sense, the growth could be mapped by subtracting the contour of one developing stage from the other. Mechanistically, differential growth can be caused by increasing cell proliferation, decreasing apoptosis, increasing cell immigration, or decreasing cell emigration (Wang et al., 1999a). Practically, we did not observe changes of apoptosis at this scale, and detailed data on cell flux/influx in these primordia are not yet available. We did observe multiple and dynamically changing localized niches of increased cell proliferation in developing facial primordia (Wu et al., 2004). Therefore, in this study, we used 1.5-hr 5-bromodeoxyuridine (BrdU) labeling to map the LoGZ.
Both chicken and duck embryos have two LoGZ in the lateral FNM at stage 26 (chicken Hamburger and Hamilton stage [H&H] or duck equivalent; Wu et al., 2004). The two LoGZ converged into one centrally localized LoGZ at stage 28 in the chicken but remained separated as two lateral LoGZ in the duck. We showed that these LoGZ regions are enriched with bone morphogenetic protein 4 (BMP4) and further showed that BMP4 is involved in mediating LoGZ activity (Wu et al., 2004). Dr. Tabin’s group investigated the molecular underpinnings of Galapagos Island finch beak evolution directly. They compared expression patterns of various growth factors and found that mesenchymal expression of Bmp4 in the upper beak strongly correlates with a robust beak morphology. They further went on to use chicken embryos as an experimental model to show that BMP4 is functionally involved (Abzhanov et al., 2004). Thus, the concept has emerged that a novelty for morphological evolution may not have to be based on the presence or absence of a signalling pathway but can derive from altering levels of signalling molecule activities or changing configurations of signalling molecule expressing cell clusters.
In this work, we try to evaluate this concept further by additional comparisons among three types of beaks: the conical and slightly curved chicken beaks, the straight and long duck beaks, and the highly curved cockatiel beaks. We ask how their developmental processes differ from each other. When do the developing beaks start to show different morphologies? Do all facial prominences maintain similar ratios in their growth activities? Can we change these ratios and obtain different beak morphologies? Is the curvature of beaks regulated by the position of the LoGZ? Can we make a beak curve by producing an ectopic LoGZ? Using the chicken beak as a model, we are able to learn several principles that address the above questions. We go on to show a working model explaining how multiple prominences can be integrated to form one functional unit with a large spectrum of possible variations in morphology.
RESULTS
Developing Morphology of Chicken, Duck, and Cockatiel Beaks
We first compared the distinct morphology of three avian beaks (Fig. 1A). Chicken beaks are conical in shape and moderately curved. Duck beaks are straight and wide. Cockatiel beaks are bigger, with more depth, and are highly curved (Fig. 1A). We collected several developmental stages for characterization. Comparisons of hematoxylin and eosin (H&E) staining from equivalent H&H (Hamburger and Hamilton, 1951) stage chicken, duck, and cockatiel embryos were used (see the Experimental Procedures section for further discussion on staging). Chickens have a narrow FNM, whereas ducks have a much wider and larger FNM at stage 29 (Fig. 2A). Cockatiels have a thicker FNM (dorsal–ventral dimension, brown line in Fig. 4B) than chickens and ducks. Cartilage staining (Fig. 1C) shows a similar pattern as H&E staining at stage 35 (Fig. 1B). Skeleton staining shows that the architecture is established in chicken, duck, and cockatiel embryos by embryonic day (E) 14 (Fig. 1D) and already resembles mature beaks. Among these three species, the major differences appear to be in the upper beak. Therefore, we will focus more on the FNM in the following experiments.
Fig. 2.
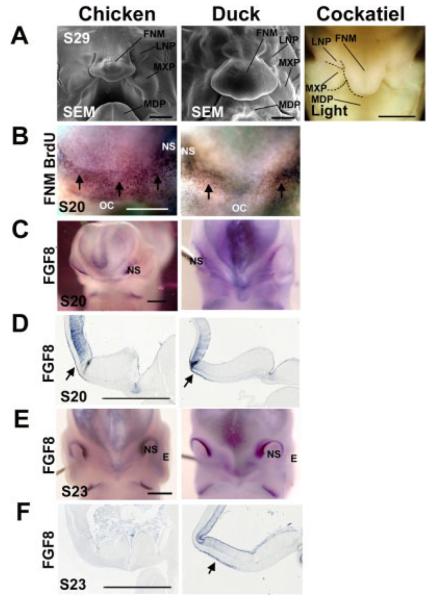
Comparative molecular expression in facial prominences during early chicken, duck, and cockatiel beak development. A: Stage 29 chicken, duck (scanning electron microscope), and cockatiel (light microscope) beak prominence configurations. B: Whole-mount BrdU staining of the epidermis growth zone in the FNM of stage 20 chickens and ducks. Arrows show regions of positive staining in the ectoderm. C–F: Whole-mount and section FGF8 in situ hybridization of chicken and duck FNM ectoderm at stage 20 (C,D) and 23 (E,F). D and F are mid-sagittal sections from the corresponding whole-mount samples depicted in C and E. BrdU, bromodeoxyuridine; E, eye; FNM, frontal nasal mass; NS, nasal slit; OC, oral cavity; SEM, scanning electron microscope. Scale bar = 0.5 mm.
Fig. 4.
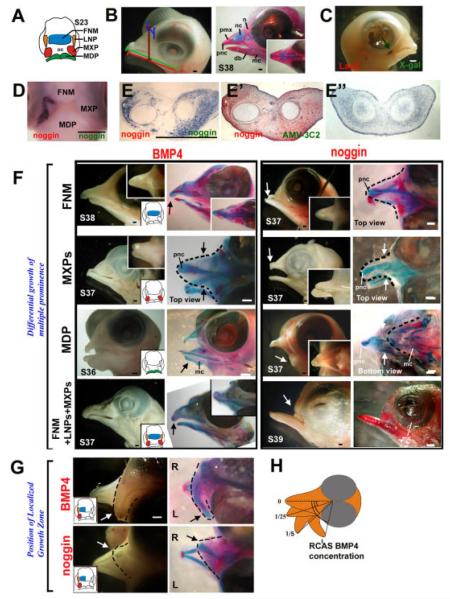
Modulation of beak shapes by perturbing the BMP pathway activity. Replication-competent avian sarcoma (RCAS) -BMP4 or noggin viruses were microinjected to either all or selective prominences. A: Schematic drawing of a stage 23 chicken beak. B: Normal embryonic day (E) 12 chicken head. Left panel, measurement of upper beak length (green), lower beak length (red), depth (brown), and width (blue) performed according to Wu et al. (2004). Right panel, Alcian blue (cartilage) and Alzarin red (bone) staining to show different skeletal elements. C: Limited viral spread when virus is microinjected into a single prominence as determined by X-gal staining of RCAS-LacZ injected beak prominences. D: Whole-mount in situ hybridization for noggin in RCAS-noggin injected LNP and MXP. E: Gene misexpression is verified by noggin expression on the coronal sections of the forming mandible. E’, distribution of virus detected by AMV-3C2 immunohistochemical staining. E”, endogenous noggin expression in a control chicken mandible. F,G: RCAS-mediated gene misexpression. Chicken embryos injected at stage 23 were harvested at the indicated stages. The injected prominences are colored in the diagram within the inset. Side (F) and top (G) views are presented. The left column is a whole-mount view, and the right column shows Alcian blue/Alzarin red staining. Resulting enlargements (black arrows) or reductions (white arrows) of beak sizes are indicated. F: Microinjection of BMP4 and noggin to selected maxillary prominences (FNM, MXP, and so on). G: Injection of RCAS BMP4 or RCAS noggin to the right maxilla. Arrows, curved beaks. H: Semiquantitative correlation of the degree of curvature and the titer of RCAS-BMP4 virus. BMP4, bone morphogenetic protein 4; db, dentary bone; FNM, frontal nasal mass; L, left; LNP, lateral nasal prominence; LoGZ, localized growth zones; mc, Meckel’s cartilage; MDP, Mandibular prominence; MXP, maxillary prominences; n, nasal bone; nc, nasal chonchae; pnc, prenasal cartilage; pmx, premaxilla bone. R, right. RCAS, replication-competent avian sarcoma. Scale bars = 0.5 mm.
LoGZ and Molecular Expression
We mapped differences in cell proliferation and molecular expression during the development of these different beaks by comparing equivalent staged (stage 20–29) chicken, ducks, and cockatiels. Scanning electron microscopy (SEM) showed that, at stage 29, chickens and ducks already have distinctly different beak shapes (Fig. 2A). Light microscopy showed that cockatiels have a narrow FNM at this stage (Fig. 2A). An initial study comparing the LoGZ of chicken and duck FNM was reported (Wu et al., 2004). Short (1.5-hr) BrdU pulse labeling of stage 26 to stage 28 chickens showed that cell proliferation in both FNM lateral edges shifted toward the rostral margin and gradually converged into one centrally localized zone. In ducks, the two bilaterally positioned growth zones persisted in the lateral edges. These findings were demonstrated in the three-dimensional reconstruction of the LoGZ (Wu et al., 2004). Here, we further compare the whole-mount BrdU staining between chickens and ducks at stage 20. We found that both chickens and ducks have an epidermal proliferation zone in the FNM (Fig. 2B). They are distributed below the middle horizontal line of the two nasal pits at this stage. The upper border of this epidermis proliferation zone may correlate with the position of the frontonasal ectodermal zone (FEZ) restricted by FGF8 and Shh (Hu et al., 2003). In chickens, this epidermis proliferation zone is diffusely distributed (Fig. 2B); however, this zone is restricted to the two lateral sides in the duck (Fig. 2B). The persistence of this restricted epidermis proliferation zone above the two bilaterally positioned mesenchymal growth zones (Wu et al., 2004) may contribute to the expanded width of the duck beak.
When we compared FGF8 expression in the FNM between chickens and ducks, we found that both chickens and ducks express FGF8 in the upper portion of the FNM ectoderm at stage 20 (Fig. 2C,D). However, whereas ducks still express FGF8 in the FNM ectoderm at stage 23, chickens do not (arrow, Fig. 2E,F). We propose that the persistent expression of FGF8 may contribute to the distinct morphogenesis of the duck beak, causing it to grow straight, longer, and to produce more cartilage.
We then compared the expression of signalling molecules and LoGZ among chicken, duck, and cockatiel beaks at stage 27 to stage 29 from mid-sagittal sections (Fig. 3). At stage 27, after FGF8 expression was diminished (data not shown), Shh expression was maintained. The Shh expression domain extended further anteriorly in ducks (Fig. 3E,E’) than in chickens (Fig. 3D,D’); whereas the Shh expression domain extended less anteriorly in cockatiels than in chickens (Fig. 3F,F’). BMP4 expression was enriched in the FNM mesenchyme in both chickens and ducks at this stage; however, the BMP4 expression domain was higher (more anterior) in ducks (Fig. 3H,H’) than in chickens (Fig. 3G,G’). Cockatiels have a lower BMP4 expression in the FNM at this stage (Fig. 3I,I’).
Fig. 3.
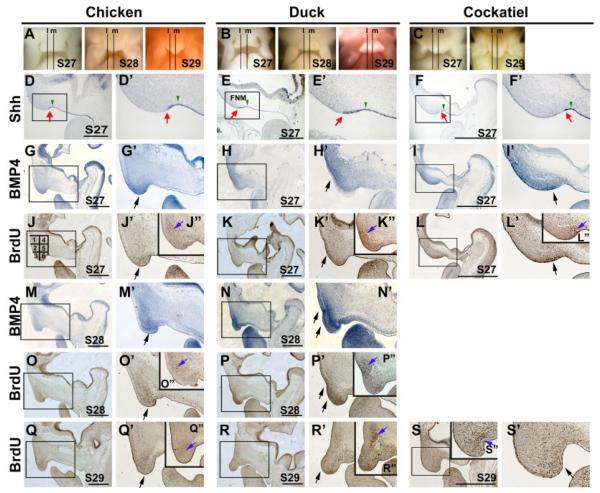
Comparative molecular expression and positioning of LoGZ during the development of chicken, duck, and cockatiel beaks. A–C: Frontal view of beak prominences of chickens, ducks, and cockatiels at stage 27–29. D–F’: Mid-sagittal sections showing expression of Shh in chicken, duck, and cockatiel beaks at stage 27. The regions boxed in D, E, and F are shown enlarged in D’, E’, F’. Red arrows indicate the anterior end of the Shh expression domain. Green arrows indicate a groove in the FNM as a reference point. G–I’; Mid-sagittal sections showing expression of BMP4 in chicken, duck, and cockatiel beaks at stage 27. Black arrows indicate the most-intense BMP4 expression. The regions boxed in G, H, and I are shown enlarged in G’, H’, I’. J–L’: Mid-sagittal sections showing bromodeoxyuridine (BrdU) staining patterns in stage 27 chickens, ducks, and cockatiel beaks. A grid with boxes 1–6, used to determine the data in Table 1, is indicated in J. Black arrows indicate the location of the most-intense proliferation staining. The regions boxed in J, K, and L are shown enlarged in J’, K’, L’. The panels in the upper right corner, J”, K” and L” are from the lateral sagittal sections. The blue arrows in J”, K”, and L” indicate the location of the most-intense proliferation staining in the lateral sagittal sections. M–N’: Mid-sagittal sections showing expression of BMP4 in chicken and duck beaks at stage 28. Black arrows indicate the most intense BMP4 expression. The regions boxed in M and N are shown enlarged in M’ and N’. O–P’: Mid-sagittal sections showing BrdU staining patterns in stage 28 chicken and duck beaks. Black arrows indicate the regions of most-intense proliferation staining. The regions boxed in O and P are shown enlarged in O’ and P’. O” and P” are from the lateral sagittal sections. The blue arrows in O” and P” indicate the location of the most-intense proliferation staining in the lateral sagittal sections. Q–S’: Mid-sagittal sections showing BrdU staining patterns in stage 29 chicken, duck, and cockatiel beaks. The black arrow indicates the proliferation center. The regions boxed in Q, R, and S are shown enlarged in Q’, R’, S’. Q”, R”, and S” are from the lateral sagittal sections. The blue arrows in Q”, R”, and S” indicate the location of the most-intense proliferation staining in the lateral sagittal sections. l, lateral sagittal section; m, mid-sagittal section.
We further examined the LoGZ of these three species at this stage (Fig. 3). We found that the distribution of concentrated BrdU-positive cells showed different patterns in these three species. The distributions followed a similar trend as that of the BMP4 expression pattern. The chicken LoGZ was positioned distally and ventrally in the FNM (Fig. 3J,J’), the duck LoGZ position was slightly higher (Fig. 3K,K”), whereas the cockatiel LoGZ was lower (posterior; Fig. 3L,L’, see arrows). To further analyze cell proliferation in the FNM, we studied the distribution of proliferating cells in sagittal sections of chicken, duck, and cockatiel beaks from stage 27–29 (Fig. 3J–L”,O–P”,Q–S”). For each animal, a lower power view on the left is used to show the overall distribution of growth zone activities and a high power view on the right is used to show proliferating cells. Both are sagittal sections along the middle plane. The right upper panel is from the lateral sagittal sections (see Fig. 3A–C, for the sites of these sections).
When we compared the LoGZ between chicken and duck beaks in mid-sagittal sections at stage 28, we found that the LoGZ in the duck is positioned higher than in the chicken and extends further rostrally (Fig. 3O–P’). The higher position of the LoGZ is also seen in the lateral section of the duck beak (Fig. 3P”) compared with the chicken beak at this stage (Fig. 3O”, also compare with Fig. 1C of Wu et al., 2004). BMP4 expression is enriched in the LoGZ (Fig. 3M–N’). Due to differences in the local growth activities of stage 29 FNM, chicken, duck, and cockatiel beaks start to show distinct shapes (Fig. 3Q–S’). The duck FNM shows a protruding process, which contains proliferating cells, before further elongation (Fig. 3R”). The cockatiel shows a more rectangular shaped FNM with its growth zone localized to the posterior side of the mid-sagittal section (Fig. 3S,S’). The lateral sagittal section shows a similar pattern as the mid-sagittal section, and the LoGZ is positioned further posteriorly (lingually; Fig. 3S”).
To quantify these differences in beak morphology, we made a grid template (as shown in Fig. 3J) to divide the FNM sagittal section into six regions. The percentage of BrdU-positive cells from three adjacent middle and lateral sagittal sections was calculated (Table 1). Overall, along the sagittal plane, we observed that a higher growth zone activity in the chicken was present at the 6 o’clock position of the FNM; for the duck, it is shifted to the 6–8 o’clock position; and for the cockatiel, it is present at the 4–5 o’clock position. In the lateral plane, we observed higher activity in the duck than in the chicken, consistent with that reported in Wu et al. (2004).
TABLE 1.
Comparison of the Percentage of BrdU-Positive Cells in Different FNM Regions of Chicken, Duck, and Cockatiel Beaksa,b
| Chicken |
Duck |
Cockatiel |
||||||
|---|---|---|---|---|---|---|---|---|
| Stage 27 | Stage 28 | Stage 29 | Stage 27 | Stage 28 | Stage 29 | Stage 27 | Stage 29 | |
| Middle | ||||||||
| Region1 | 7.5 ± 0.8 | 6.3 ± 1.7 | 4.0 ± 0.5 | 18.3 ± 4.3 | 7.0 ± 0.5 | 9.2 ± 1.2 | 7.5 ± 1.2 | 3.0 ± 1.0 |
| Region2 | 9.3 ± 0.6 | 16.5 ± 5.3 | 4.2 ± 1.2 | 25.5 ± 4.4 | 16.7 ± 3.1 | 14.1 ± 3.4 | 4.2 ± 0.8 | 1.6 ± 0.3 |
| Region3 | 16.0 ± 0.8 | 32.3 ± 4.8 | 11.5 ± 3.4 | 41.8 ± 5.8 | 36.9 ± 3.9 | 24.8 ± 2.9 | 7.9 ± 1.7 | 5.4 ± 0.3 |
| Region4 | 8.3 ± 0.8 | 5.7 ± 1.0 | 3.9 ± 1.0 | 11.3 ± 2.5 | 5.3 ± 0.3 | 1.5 ± 0.5 | 3.2 ± 0.8 | 3.2 ± 0.7 |
| Region5 | 12.1 ± 1.0 | 8.7 ± 2.3 | 1.8 ± 0.5 | 11.2 ± 1.8 | 6.5 ± 2.0 | 9.8 ± 1.1 | 7.3 ± 1.8 | 5.4 ± 1.1 |
| Region6 | 26.4 ± 4.1 | 28.0 ± 3.6 | 10.3 ± 1.2 | 48.2 ± 5.5 | 23.1 ± 3.6 | 19.3 ± 4.0 | 20.2 ± 3.4 | 19.9 ± 1.9 |
| Lateral | ||||||||
| Region1 | 8.0 ± 1.5 | 8.5 ± 1.1 | 2.7 ± 0.3 | 16.0 ± 3.7 | 18.2 ± 2.5 | 15.3 ± 1.4 | 5.2 ± 1.6 | 12.4 ± 0.8 |
| Region2 | 16.7 ± 3.8 | 20.6 ± 4.0 | 4.6 ± 0.9 | 21.2 ± 3.0 | 26.8 ± 3.0 | 25.1 ± 2.7 | 4.3 ± 0.2 | 11.1 ± 2.5 |
| Region3 | 29.0 ± 8.7 | 27.2 ± 2.4 | 3.6 ± 0.6 | 22.2 ± 3.8 | 29.8 ± 5.5 | 30.7 ± 2.6 | 10.5 ± 4.0 | 20.3 ± 2.9 |
| Region4 | 8.2 ± 1.0 | 7.5 ± 0.5 | 3.2 ± 0.3 | 7.5 ± 1.0 | 6.4 ± 0.9 | 2.3 ± 0.9 | 2.3 ± 0.2 | 7.6 ± 1.6 |
| Region5 | 14.3 ± 3.4 | 10.0 ± 1.1 | 0.9 ± 0.4 | 9.7 ± 1.9 | 8.3 ± 1.5 | 12.6 ± 2.7 | 9.0 ± 0.5 | 19.9 ± 5.5 |
| Region6 | 28.7 ± 8.4 | 20.5 ± 3.4 | 4.4 ± 0.4 | 17.8 ± 2.9 | 21.6 ± 3.9 | 25.6 ± 2.7 | 28.9 ± 7.8 | 33.1 ± 9.8 |
The numbers shown is the percentage of bromodeoxyuridine (BrdU) -positive cells. Average ± standard deviation (SD). Three adjacent sections from the same embryo were analyzed for each value shown here. Three independent embryos for chicken and duck were also analyzed which show a similar trend.
We can see that there is overall decreased proliferation in all regions when the embryo matures except in the specific LoGZ where proliferation remains high. These regions vary in different species of birds. Chickens have dramatically decreased proliferation in region 2 from stage 28 to stage 29 in both the middle and lateral sections. However, ducks maintain a high proliferation rate at stage 29 in region 1 and 2 in both the middle and lateral sections. At stage 27 and 29 in both the middle and lateral sections, cockatiels have high proliferation only in region 6.
Modulation of the Overall Growth of Multiple Prominences Leads to Changes of Beak Size
The avian beak is made of multiple prominences (Fig. 4A). We are interested to know how these prominences coordinate to form a distinct beak shape (Fig. 4B). We designed experiments to overexpress BMP4 in individual beak prominences, or overall throughout the beak, to test for morphogenetic changes. First, we tested the effect of the replication-competent avian sarcoma (RCAS) viral vector on beak growth. When RCAS-LacZ was injected in all of the beak prominences, there were no observable effects on beak growth (data not shown). Then, we tested the exogeneous gene expression in a single prominence. When we overexpressed certain genes in a single prominence, we hoped that the overexpressed exogenous gene would remain restricted to that prominence and not infect other prominence(s). To test this theory, we injected RCAS LacZ in one prominence (right MXP) at stage 23. After 5 days incubation, X-gal staining showed that the β-galactosidase–positive cells were restricted to the right edge of the upper mouth (Fig. 4C), which is derived from the right MXP. We then injected RCAS noggin only in the right LNP and right MXP. After 48-hr incubation, we examined noggin RNA distribution by whole-mount in situ hybridization. The result showed that exogenous noggin transcripts were only found in the right LNP and right MXP (Fig. 4D). When RCAS noggin was injected to the MDP, ectopic noggin expression was restricted to the lower beak (Fig. 4E). Compared with the endogenous noggin transcripts in the MDP, exogenous noggin is expressed to much higher levels in the mesenchyme (compare Fig. 4 E,E”). Furthermore, AMV-3C2 staining showed that the distribution of RCAS had a similar pattern as the exogenous noggin expression (compare Fig. 4E,E’). These results suggest that RCAS, being replication competent, spreads the exogenous gene within but not beyond the injected prominence.
It was suggested that beak size could be determined by the level of BMP pathway activity (Wu et al., 2004). Overexpression of BMP4 caused overgrowth of the beak when the virus was injected in all of the beak prominences (Wu et al., 2004). Enlarged premaxilla cartilage in the maxilla and Meckel’s cartilage in the mandible were observed after BMP4 overexpression. When a BMP antagonist, noggin, was used to neutralize endogenous BMP activity, “mini beaks” with reduced cartilage formation and a miniaturized skeleton formed (Wu et al., 2004). Analyses of tissue sections from these specimens show BMP4-enhanced cell proliferation and skeletal differentiation, whereas noggin reduced cell proliferation and reduced skeletal differentiation (supplement of Wu et al., 2004). Increases in beak width and length are mainly due to increases in skeletal mass as shown by beak cross-sections. Alcian blue and Alzarin red staining show that the skeletal elements increase or decrease proportionally. These results suggest that global misexpression of BMP4 or noggin in all beak prominences causes an overall increase or reduction of the beak size, without changing skeletal patterns. However, local delivery in selective prominences or in part of one prominence will lead to changes in beak shape, as will be shown below.
Modulation of Selective Prominences Leads to Differential Growth That Alters Beak Shapes
Beak shapes vary among species (e.g., some birds have relatively large upper or lower beaks). As the beak is made from multiple prominences, the differential growth of different prominences may contribute to the distinct morphologies. To identify the role of each component in beak formation, we microinjected RCAS virus to individual beak prominences. Misexpression of BMP4 in the FNM alone (Fig. 4F, n = 4) caused an elongated upper beak (15.5 ± 0.4 mm, average ± SD) compared with the control (14.5 ± 0.3 mm; P < 0.01). The lower beaks are of normal size. Injection to both MXPs formed a wider upper beak (Fig. 4F, n = 4, 5.7 ± 0.4 mm; control, 3.9 ± 0.3 mm; P < 0.001). If BMP was misexpressed only in the MDP, a larger lower beak formed (Fig. 4F, n = 8, P < 0.01, 13.7 ± 0.5 mm; control, 12.1 ± 0.4 mm), but the upper beak formed normally. Injection to all upper beak prominences (FNM, LNPs, and MXPs) produced a larger upper beak (Fig. 4F, n = 9, 16.0 ± 0.4 mm vs. control, 14.5 ± 0.3 mm; P < 0.001) and a normal lower beak.
In contrast, injection of Noggin to the FNM alone produced a shorter upper beak (Fig. 4F, n = 5, 10.9 ± 1.0 mm vs. control, 13.1 ± 0.4 mm; P < 0.001), but a normal lower beak. Introduction of RCAS noggin to both MXPs formed a narrower upper beak (Fig. 4F, n = 3, 3.4 ± 0.2 mm vs. control, 3.9 ± 0.3 mm; P < 0.01). Selective expression in the MDP suppressed lower beak formation (Fig. 4F, n = 4, 8.1 ± 1.2 mm vs. control, 12.1 ± 0.4 mm; P < 0.001). Expression in the FNM, LNP, and MXP almost completely inhibited the upper beak but left the lower beak intact (Fig. 4F, n = 10, P < 0.001). Therefore, differential growth in different prominences can alter beak shapes by changing different dimensions.
Position of LoGZ and Beak Curvature
In nature, some birds exhibit upper or lower beaks with different degrees of curvature in the dorsoventral or even left–right plane. We surmise that asymmetric growth can contribute to this shaping mechanism. To test the role of growth zone activity in the formation of beak curvature, we microinjected BMP/noggin to the lower FNM region. However, the resolution of this technique is limited, as RCAS spreads within prominences but not between prominences in the time frame of our experiments. Therefore, we used the upper beak composed of multiple prominences to test the role of the BMP pathway in beak curvature. We injected RCAS-BMP4 into the right upper beak (including the right side of the FNM, right LNP, and right MXP). The results showed that growth on the right side of the upper beak predominated, causing the beak to curve toward the left (Fig. 4G). Injection with RCAS-noggin had the opposite effect and the beak curved toward the right (Fig. 4G). When RCAS-BMP4 viral media was diluted (1, 1:5, 1:25), growth on the right side of the upper beak was predominant and the beak curved toward the left. There was a semiquantitative increase of deviating angles with higher titers (Fig. 4H). Therefore, asymmetric growth activity in a growing primordium can produce curvature in its final form.
MSX Genes Are Regulated by BMP4 in Beak Prominences
We have examined the change of cartilage differentiation (Wu et al., 2004). Collagen I expression increased after BMP4 was misexpressed in the cartilage. MSX genes are markers for regions of high proliferation during facial patterning (Brown et al., 1997). Msx1 and −2 have been shown to be expressed in the mesenchyme along the lateral edges of the FNM, MXP, and MDP up to stage 24 (Barlow and Francis-West, 1997). Here, we compared the expression pattern of Msx1 in chickens and ducks from stage 26 to 31. In stage 26 and 27 chickens, Msx1 was expressed at the two lateral edges of the FNM (Fig. 5A,B). From stage 28 to stage 31, the two Msx1 expression domains gradually shifted toward the middle (Fig. 5C–E). In ducks, the two bilaterally positioned Msx1 expression domains persisted at the lateral edges, from stage 26 to stage 31 (Fig. 5F–J). The changes in Msx1 expression pattern echoes the shift of localized growth zones between chickens and ducks (Wu et al., 2004).
Fig. 5.
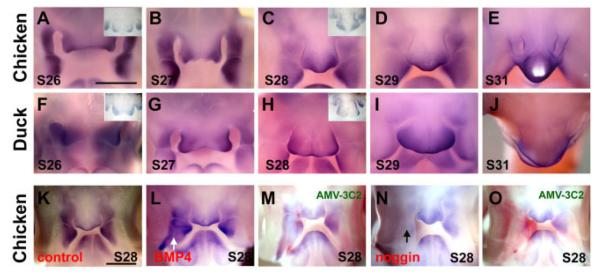
Expression of Msx1 in chicken and duck beaks. Whole-mount in situ hybridization. A–E: Msx1 expression in normal stage 26–31 chicken beaks. Inset showing the section in situ hybridization. F’–J: Msx1 expression in normal duck beaks from equivalent stages. Inset shows a section in situ hybridization. Note the Msx1-positive zones in chicken merge from two at stage 27 to one at stage 28; whereas that of duck remains as two at stage 28. The larger FNM in the duck is obvious at stage 29 and 30. K–O: Expression of Msx1 after Replication competent avian sarcoma (RCAS) -bone morphogenetic protein 4 (BMP4) and RCAS-noggin injection into the right MXP of chicken embryos. Stage 28 control (K), RCAS-BMP4 (L), RCAS-BMP4/virus staining (M), RCAS-noggin (N) and RCAS-noggin/virus staining (O). In situ hybridization is shown in blue. The presence of virus is revealed by antibody AMV-3C2 and shown in red. Scale bar = 1 mm.
To further test changes in downstream genes after overexpression of BMP4 or noggin, we injected the virus into the right LNP and right MXP at stage 23. After 48-hr incubation, whole-mount Msx1 in situ hybridization was performed. When BMP4 was overexpressed, we found an increased Msx1 expression domain (Fig. 5L). When we overexpressed noggin, we found a decreased Msx1 expression domain (Fig. 5N). Section in situ hybridization of Msx1 in the mandible indicated that BMP4 increased Msx1 expression, whereas noggin decreased it (data not shown). This result suggested that Msx1’s activity is regulated by the BMP pathway. The colocalization of the BMP downstream gene Msx1 and the proliferation activity in the FNM are consistent with the notion that BMP signalling is critical for beak morphogenesis. Our data are consistent with the previous studies that demonstrated that ectopic application of BMP protein-coated beads can activate MSXs gene expression in the developing facial primordia (Barlow and Francis-West, 1997; Wang et al., 1999b; Ashique et al., 2002a; Mina et al., 2002).
DISCUSSION
Multicomponent, Multi-LoGZ Morphogenesis of the Beak and the Generation of Diverse Beak Forms
All bird beaks are made of the same differentiation materials (e.g., bone, horny sheath, soft tissues), but they form diverse shapes in different species. The different shapes are based on different topobiologically arranged cellular activities. By varying the proportion of the width, depth, and length, the architecture of the beak is established. Here, we used a biological approach to examine how the varied distribution of cell proliferation activity during beak morphogenesis of different bird species contributes to different beak morphologies.
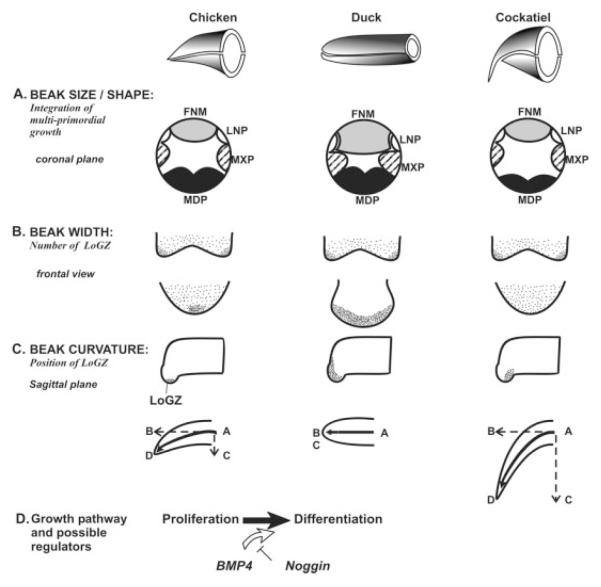
The beak is made from the coordinated growth of multiple facial prominences. We suggest that the following two categories of growth activities combine to alter beak shape: (1) Concerted overall growth activities that occur in all prominences are responsible for the global expansion and, therefore, the overall size of the beak; (2) Differential growth activities in some prominences or in part of a single prominence can contribute to altering the relative dimensions and, therefore, the shape of the beak. As these represent localized growth, we called these LoGZ (please see the introduction for further discussion on the definition of LoGZ). This concept can be applied to different levels of growth. One is at the interprominence level, i.e., the varied growth rates of multiple facial primordia in different species. For example, at stage 27, the ratio of the MXP/FNM is larger in the duck than in the chicken (Wu et al., 2004). At stage 29, the relative size of the FNM to MXP and MDP is larger in the duck. At E14, the ratio of the MDP to the maxilla is much smaller in the cockatiel than in the chicken and the duck. The other level is intraprominence. Strategically positioned LoGZ fine-tune the specific shapes of each prominence. In the working model (Fig. 6), each facial prominence can have its distinct distribution of LoGZ, and the number, size, and activity level of LoGZ within the same prominence in different birds can also be different. Thus, the diverse beak morphology of each bird is derived from a unique combination of the above three categories of growth activities acting at different hierarchical levels during prominence morphogenesis (Fig. 6A–C).
Fig. 6.
Working model for the shaping of the beak. Schematic chicken, duck, and cockatiel beaks are shown in the top row. A: Multiple prominences (coronal plane) have to be integrated to form one beak. The basic configurations of chicken, duck and cockatiel beaks are different. Ducks have bigger FNM and MXP. Cockatiels have a smaller MDP. B: Frontal views show different numbers and positions of LoGZ in chickens (one in the midline) and ducks (two bilateral LoGZ and a central LoGZ form a diffuse growth zone) in the FNM. The cockatiel growth zone is in the bottom. These differences lead to the wider duck beaks. C: Sagittal views show different dorso-ventral positions of LoGZ in the FNM. This could lead to vectorial differences in growth activity resulting in curved outgrowth. This asymmetric growth mechanism may be used to generate curved beaks as seen in the dorsal–ventral curved upper beaks (no curvature in the duck, less curvature in the chicken, more curvature in either the cockatiel or eagle), or left–right curved beaks. D, dorsal; L, left; R, right; V, ventral. D: Growth activity can be regulated by BMP and/or other pathways. Here we show BMP4 pathway activity enhances beak growth by increasing cell proliferation and differentiation, whereas noggin suppression of BMP activity decreased proliferation and differentiation.
To think about how specific organ forms, such as diverse beak shapes, are “designed” from the genome, one usually thinks about specific molecular coding. This process seems to be unrealistic when we face endless forms in nature. New concepts are needed. Weiss (2005) suggests that development uses a “phenogenetic” logic, using a few simple relational principles to build complex traits. The role of genes is in the process rather than the specifics of the trait, much as a variety of complex music can be made by playing different tones on the same instrument. Our recent work on the mapping of feather stem cells shows that the different relative positioning of stem cells and transient amplifying cells (TA cells in ectodermal organogenesis terminology; they are also defined by a short BrdU labeling and are equivalent to the LoGZ here) can lead to different feather morphologies. Specifically, stem cells are configured as a ring, positioned horizontally in the radially symmetric downy feathers but tilted anteriorly in bilaterally symmetric feathers (Yue et al., 2005). An anterior–posterior Wnt 3a gradient is critical for forming the bilateral but not radial symmetry of feathers (Yue et al., 2006). Thus, we further developed the original concept of topobiology (Edelman, 1988) to suggest that topologically positioned molecular activities and cell behavior can be used to generate diverse organ forms (Chuong et al., 2006). In this way, subtle differences in the stem cell ring configuration (e.g., by simple tilting) can lead to a continuum of radial to bilateral symmetry in feathers. Similarly, the timing and positioning of multiple localized growth activities at different hierarchical levels in facial primordia morphogenesis can have profound consequences in the making and fine-tuning of facial morphology. Thus, instead of searching for a molecular blueprint, the more flexible and pleiomorphic concept of phenogenetics and topobiology should be developed further between genomes and organ forms.
Because there are five beak prominences comprising the upper beak, the number of possible ways that beak shapes can be modulated are enormous. However, this complex morphogenesis process is also prone to errors as seen in the high incidence of cleft palate/lips due to a lack of coordination of cellular events (MacDonald et al., 2004). When all beak prominences increase their growth activities globally, a large beak formed: the beak size increased proportionally maintaining the overall shape. When only certain prominences enlarge, the beak assumes a new shape with altered relative dimensions (Fig. 6, top row, B). We also found that the curvature of upper beaks correlates with the position of the LoGZ within the FNM. Different degrees of curvature formed due to asymmetric growth that deviates from the original proximal–distal axis (Fig. 6C).
Molecular Basis of Growth Activities That Pattern Different Beak Shapes
What mediates the growth activities in beak morphogenesis? BMPs were shown to be expressed during avian craniofacial morphogenesis (Francis-West et al., 1998). At stage 20, BMP4 is expressed in the epithelium, but the expression is gradually switched to the mesenchyme from stage 24 to 28. BMP2 is more diffusely expressed in the epithelium from stage 20 to 28 (Francis-West et al., 1994). Ectopic delivery of BMP2 and BMP4 protein-coated beads cause a thickened or bifurcated cartilage to form, depending on the position of the beads (Barlow and Francis-West, 1997). The importance of the BMP pathway in craniofacial skeleton morphogenesis is further demonstrated by RCAS delivery of constitutive and dominant-negative BMP receptors, which lead to overgrowth or suppression of cartilage growth, respectively (Ashique et al., 2002b). BMP- and Noggin-coated bead studies show the homeostasis of BMP activity is important in epithelial– mesenchymal interactions in the FNM and can regulate mesenchymal growth and epithelial survival (Ashique et al., 2002a). These experimental results show that the BMP pathway plays a pivotal role in early stages of craniofacial morphogenesis.
Recently, it was found that the BMP pathway also plays an important role in shaping the beaks in late stage morphogenesis (Abzhanov et al., 2004; Wu et al., 2004). Mesenchymal BMP4 transcript expression is found to parallel that of the LoGZ (Wu et al., 2004). RCAS-mediated misexpression of BMP4 in the beak prominence mesenchyme causes proportional enlargement, whereas RCAS noggin causes proportional reduction of beak size (Abzhanov et al., 2004; Wu et al., 2004). Specific application of BMP4 protein-coated beads to the position of the LoGZ could increase beak width, therefore, changing the shape of the beak (Wu et al., 2004). That we obtained different altered skeletal phenotypes from those reported before (Barlow and Francis-West, 1997), by leaving BMP4 beads in different positions, further highlights the whole concept of LoGZ and topobiology (Chuong et al., 2006). Together these, results suggest that, in the late stages of craniofacial morphogenesis when the morphology of the beak is determined, the size of the beak can be modulated by the overall activity of the BMP pathway, which mediates its growth. Beak shape can be fine-tuned by localized BMP activity, which is involved in mediating the range, level, and duration of the LoGZ activity.
The key question to the generation of beak diversity is how LoGZ are induced and positioned. Whereas elegant transplantation experiments showed that the shapes of duck or quail beaks are determined by the cephalic crest (Schneider and Helms, 2003), the information residing in the crest mesenchyme still has to be executed. At stage 20, the interface of the FGF8 and Shh expression domains was shown to be the growth point of the FNM and was termed the frontonasal ectodermal zone (FEZ; Hu et al., 2003). Misexpression of Shh around stage 20 can duplicate the FNM and form duplicated beaks (Hu and Helms, 1999). This experiment may be explained by duplication of LoGZ, but more experiments will be required to test this hypothesis. Retinoic acid-coated and noggin-coated beads were shown to convert the MXP to the FNM (Lee et al., 2001). This finding may occur because the experimental conditions create differential growth activity and form an ectopic LoGZ in the MXP. It is possible that the early epithelial FEZ may directly or indirectly lead to the induction of the mesenchymal LoGZ in later stages (at and after stage 26). This finding needs to be studied further. The mechanism of how LoGZ are positioned is critical as it will link our understanding of earlier morphogenesis events to the late beak morphogenesis events.
In different birds, the ectodermal expression of FGF8 and Shh in the FNM show differences in their timing and locations. In the chicken, FGF8 was expressed at the upper part of the ectodermal frontonasal zone and may function to induce cartilage formation (Hu et al., 2003; Abzhanov and Tabin, 2004). Its expression disappeared in this region after stage 20. On the other hand, ducks still express FGF8 in the FNM at stage 23 (Fig. 2D). We also found that both chickens and ducks have an epidermis proliferation zone distributed below the middle horizontal line of the two nasal pits at stage 20 (Fig. 2B). This epidermis proliferation zone is distributed diffusely in chickens but restrictively to the two lateral sides in ducks (Fig. 2B). Thus, we suggest that the persistent expression of FGF8 may contribute to the distinct morphogenesis of the duck beak, causing it to grow longer, wider, and produce more cartilage.
We found that the anterior border of the Shh expression domain correlates with the position of the LoGZ. If we compare the position of the anterior point of Shh expression among ducks, chickens, and cockatiels at stage 27 (Fig. 3D–F’), we find the pattern is duck > (more anterior than) chicken > cockatiel. In comparing different birds, we noticed the position of the LoGZ in the mesenchyme is located beneath the anterior point of the Shh expression domain in the epidermis. These findings are consistent with the assumption that the position of the anterior border of the Shh expression domain may determine the position of the LoGZ and, therefore, the curvature of the beaks. Examination of the BMP4 expression domain also showed it to be higher (more anterior) in ducks than in chickens (Fig. 3G–I’, M–N’). Thus, specific growth activities in the FNM of different birds are likely to be defined by a unique combination of FGF, Shh, BMP4, and others. Obviously, more work is required to understand how these interactions function together to regulate growth activities.
Origin and Evolution of Avian Beaks
Beaks are the hardened horny sheaths formed on the snout. Morphogenesis of the beak consists of three major components: the outgrowth of beak primordial mesenchyme (skeleton), the integument inside the oral cavity (oral mucosa, teeth), and the integument covering the snout (horny sheath). Indeed, beak-like structures also existed in some ancient dinosaurs (e.g., Psittacosaurus) as well as in current turtles. It is also possible that beak-like structures may have evolved independently more than once. In birds, the beak became a unique feeding apparatus in Mesozoic times (many species are illustrated in Hou et al., 2003) and its diverse shapes are classic examples of evolution (Darwin, 1859; Grant, 1986). Accompanying the changes of beak shapes is the formation of the horny sheath and the loss of teeth. However, a latent response toward tooth formation remains and tooth-like appendages can be induced from the flat oral epithelia by altering the molecular signalling in the mesenchyme (Kollar and Fisher, 1980; Chen et al., 2000; Mitsiadis et al., 2003).
At the macro-scale, the appearance of novel beak shapes and accompanying new trophisms can be appreciated from the fossil record of Mesozoic birds. The approximately 150-million-year-old Archaeopteryx had jaws with both dentary and premaxillary teeth (reviewed in Fedducia, 1999). The recent discoveries of fossils from the 120- to 130-million-year-old Jehol Biota of Northeastern China have increased our knowledge of reptile/avian evolution enormously (Zhou et al., 2003). This finding is because these fossils are exceptionally well preserved, including integuments such as early feathers (Chuong et al., 2003). Confuciusornis is the first late Jurassic bird found in the Jehol Biota. It exhibited a toothless beak (Hou et al., 1995), more advanced than Archaeopteryx. It also has clawed wings and probably lived in an arboreal habitat, picking worms and fruits with its conical shaped beak. Recently, Longirostravis, the earliest known wading bird fossil, was found in the same area (Hou et al., 2004). The unique feature is that its beak is preferentially elongated, in comparison to other dimensions of the beak. In addition, there are small dentary and premaxillary teeth restricted to the distal end of the beak. This long toothed beak might have functioned well in capturing and holding worms and fishes, facilitating the evolution of probe feeding behavior and may have provided a new waterfront niche for Longirostravis. These are good examples of how modulation of beak shape can help the evolution of avian trophic diversification (Zweers et al., 1997). Other mesozoic birds in this region show even more diverse beak shapes, most with teeth (Hou et al., 2003). The rapid radiation of bird evolution is reflected in the diverse beaks shaped for probing, catching, slicing, browsing, and so on (Gill, 1994; Zweers et al., 1997). The diversity of beak shapes in the Mesozoic birds suggests that beaks of different sizes and shapes flourished to compete for the new niches during the late Cretacious–early Tertiary period.
At the micro-scale of beak morphological evolution, Darwin’s finches provide the most inspiring examples. A group of 14 species was first collected by Charles Darwin during his visit to the Galapagos Islands (Darwin, 1859). These finches proved to be a monophyletic group (Sato et al., 1999) and was considered to have originated from an ancestral species that reached the Galapagos Archipelago from Central or South America approximately 2.3 million years ago (Sato et al., 2001). The beak shape varied among these finches according to their diet (Grant, 1986). The width and depth of beaks fluctuate rapidly in response to environmental changes. A long-term study illustrated that natural-selection constantly affected beak shape in the medium ground finch (Geospiza fortis; Grant and Grant, 2002). This finding shows that random or directional selection affects the size of beaks (Grant and Grant, 2002). Although these works explained the reason Galapagos finches have diverse beak shapes and how they respond to the environment, we do not know which molecular pathways were involved and how these shapes were achieved.
Recently, Tabin’s group started to study the molecular differences in the developing beaks of different Galapagos finch species. Examinations of finch embryos showed a correlation between BMP4 expression levels and the thickness of the developing beaks (Abzhanov et al., 2004). From these and other results, we learn that BMP4 is involved in building beaks. Therefore, BMP pathway members, agonists and antagonists, may work as molecular candidates to alter prototypical molecular modules and generate a spectrum of morphologies for natural selection. Our experimental study with chickens showed that we could indeed produce beaks phenocopying those in nature by modulating different developmental steps (Wu et al., 2004).
In conclusion, multicomponent complex morphogenesis such as the beak requires more coordination during development, but also provides opportunities where developmental mechanisms can be easily modulated to generate beak shape diversity. By comparing beak morphogenesis processes in birds with characteristic beak shapes, we learn new perspectives on how nature fine-tunes morphogenesis. Regulation of this process remains the biggest challenge. We now know that the BMP4 pathway is a major player in beak morphogenesis and can start by studying molecules related to this pathway.
EXPERIMENTAL PROCEDURES
Animals
Pathogen-free fertilized chick eggs (White Leghorn) were purchased from SPAFAS (Preston, CT). Fertilized Peking duck eggs were from a local farm (AA Farms, Westminster, CA). Chicken embryos were staged according to Hamburger and Hamilton (1951). Duck embryos take longer (approximately 28 days) to hatch than chicken embryos (20 days), and staging was assessed by a combination of morphological criteria including the limbs, eyes, body folds, and flexures (Schneider and Helms, 2003). Cockatiel embryos (stage 27, n = 1; stage 29, n = 1; stage 35, n = 2; E14, n = 2) and newborns (n = 2) were obtained from a local colony. We tried to approximate duck and cockatiel stages as close to chicken H&H staging (Schneider and Helms, 2003; Wu et al., 2004) as possible. However, there are different levels of heterochrony among species. Chickens and ducks belong to precocious birds that are born with feathers and can feed by themselves. Cockatiels belong to altricial birds that are born with very few downy feathers on the body and no feathers at all on the head. They need their parents to feed them (Gill, 1994). Cockatiel embryos need 18–21 days to hatch (Harris, 1992). Because their staging system is not well established, we used embryonic day and morphological features in the developing head to choose embryos of similar developing stages for comparison.
Tissue Processing, Immunocytochemistry, and SEM
Paraffin sections were prepared from chicken, duck, and cockatiel samples. In situ hybridization and immunohistochemistry were performed as described (Jiang et al., 1998), and some were performed using the automated Ventana Discovery system. X-gal staining was performed according to Yu et al. (2002). Cartilage and bone were stained as described by Monsoro-Burq et al. (1994) using Alcian blue and Alizarin red, respectively. For SEM, heads were fixed in half-strength Karnovsky’s fixative overnight at 4°C and post-fixed in thiocarbohydrazide, followed by osmium fixation. The specimens were critical point-dried and coated with gold palladium. They were viewed with a Hitachi S-570 scanning microscope.
Mapping Cell Proliferative Zones
BrdU labeling was done by injecting 5 μl of 1% BrdU (Sigma) in DMEM into the vein of chicken embryos. Embryos were incubated for 1.5 hr before harvesting. After BrdU staining, slides were lightly counterstained with hematoxylin to view all cells. Antibodies to BrdU were from Chemicon. The whole-mount BrdU method was performed according to Chodankar et al. (2003). BrdU-positive cells are quantified in different regions of the FNM in both middle and lateral sections (as shown in Fig. 3A–C). The distance between the middle and lateral sections is approximately 160–200 μm, except for cockatiel stage 27 (approximately 100 μm). We divided the FNM into six regions. The grid overlay for these regions is shown in Figure 3J. A vertical line was first drawn from the groove inside the mouth until it touched the brain. A horizontal line was then drawn. This horizontal line between the intersection point and the facial ectoderm was separated into equal parts by drawing a second vertical line downward to the bottom of the FNM. This second vertical line was further divided into three equal parts by two horizontal lines. Thus, there are two columns and three rows. In this way, the FNM is divided into six regions. In each region, the percentage of BrdU-positive cells among all mesenchymal cells was calculated. For each number, three adjacent sections from the same embryo were counted and averaged with the standard deviation shown.
Virus Infection, In Situ Hybridization
RCAS retrovirus preparation and in situ hybridization were performed using protocols as described (Jiang et al., 1998). Virus was microinjected at E3.5–E4 (stage 22–23) into individual or multiple beak prominence(s). Embryo heads were lifted using a specially designed micro-spoon. Facial prominences were identified under a dissection microscope. Virus (2 μl) was injected to beak prominences in ovo. Embryos were harvested from stage 28 to 39. For detection of virus, samples after whole-mount in situ hybridization were subjected to whole-mount immunostaining with AMV-3C2 antibody (Potts et al., 1987).
Morphometry of the Beak
Beak width, depth, and length were measured as shown in Figure 4B. Width (blue line) and depth (brown line) of the beaks were measured at the plane along the frontal margin of the eyes. The length of the upper beak was measured from the distal tip of the maxilla to the end of the quadratojugal bone (green line). The length of the mandible was determined from the distal tip of the mandible to the end of the dentary bone (red line). A Student’s t-test was used to compare the variation between experimental and control beaks.
ACKNOWLEDGMENTS
We thank all Chuong lab members for discussions and technical help. We thank Dr. David Hinton of the Doheny Eye Institute for his support with SEM. We also thank the following investigators for their viral vectors and in situ hybridization probes: RCAS LacZ was originally constructed by Dr. Li Yi (Dunn et al., 2001) and provided to us by Dr. W.-P. Wang; RCASBMP4 and in situ hybridization probes were from P. Francis-West (Duprez et al., 1996); RCAS noggin was from R. Johnson (Capdevila and Johnson, 1998); and probes for Msx1 and 2 were from W.B. Upholt (Mina et al., 1995). Both C.M.C. and R.W. were funded by NIH.
Grant sponsor: NIH; Grant number: AR42177; Grant number: AR47364; Grant sponsor: NCI; Grant number: CA83716.
REFERENCES
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002a;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002b;46:243–253. [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ec-topic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Brown JM, Robertson KE, Wedden SE, Tickle C. Alterations in Msx 1 and Msx 2 expression correlate with inhibition of outgrowth of chick facial primordia induced by retinoic acid. Anat Embryol (Berl) 1997;195:203–207. doi: 10.1007/s004290050039. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci U S A. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe LM. The first 85 million years of avian evolution. Nature. 1995;378:349–355. [Google Scholar]
- Chodankar R, Chang CH, Yue ZC, Jiang TX, Suksaweang S, Burrus LW, Chuong CM, Widelitz RB. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/β-catenin pathway. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zoolog Part B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Wu P, Plikus MV, Jiang TX, Widelitz RB. Engineering Stem cells into organs: topobiological transformations demonstrated by beak, feather and other ectodermal organ morphogenesis. Curr Top Dev Biol. 2006;72:237–274. doi: 10.1016/S0070-2153(05)72005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jawdevelopment. Development. 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species: a facsimile of the first edition. Harvard University Press; Cambridge: 1859. 1975. [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Dunn KJ, Incao A, Watkins-Chow D, Li Y, Pavan WJ. In utero complementation of a neural crest-derived melanocyte defect using cell directed gene transfer. Genesis. 2001;30:70–76. doi: 10.1002/gene.1035. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Topobiology. Basic Books; New York: 1988. [Google Scholar]
- Feduccia A. The origin and evolution of birds. 2nd ed Yale University Press; New Haven: 1999. pp. 93–137. [Google Scholar]
- Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mech Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Gill FB. Ornithology. 2nd ed Freeman; New York: 1994. [Google Scholar]
- Grant PR. Ecology and evolution of Darwin’s finches. Princeton University Press; Princeton: 1986. pp. 1–492. [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chicken embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harris JC. Cockatiels: getting started. T.F.H. Publications, Inc.; Neptune City, NJ: 1992. pp. 1–96. [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Hou LH, Zhou ZH, Martin LD, Feduccia A. A beaked bird from the Jurassic of China. Nature. 1995;377:616–618. [Google Scholar]
- Hou LH, Chiappe L, Zhang FC, Chuong CM. New early cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91:22–25. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LH, Chuong CM, Yang A, Zeng XL, Hou JF. Fossil birds of China. Yunnan Science and Technology; China: 2003. [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Stott S, Widelitz RB, Chuong CM. Current methods in the study of avian skin appendages. In: Chuong CM, editor. Molecular basis of epithelial appendage morphology. Landes Co.; Austin: 1998. pp. 395–408. [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest for mation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The neural crest. 2nd ed Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Lee SH, Fu KK, Hui JN, Richman JM. Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001;414:909–912. doi: 10.1038/414909a. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Abbott UK, Richman JM. Upper beak truncation in chicken embryos with the cleft primary palate mutation is due to an epithelial defect in the frontonasal mass. Dev Dyn. 2004;230:335–349. doi: 10.1002/dvdy.20041. [DOI] [PubMed] [Google Scholar]
- Mina M, Gluhak J, Upholt WB, Kollar EJ, Rogers B. Experimental analysis of Msx-1 and Msx-2 gene expression during chick mandibular morphogenesis. Dev Dyn. 1995;202:195–214. doi: 10.1002/aja.1002020211. [DOI] [PubMed] [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–352. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc Natl Acad Sci U S A. 2003;100:6541–6545. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Bontoux M, Teillet MA, Le Douarin NM. Heterogeneity in the development of the vertebra. Proc Natl Acad Sci U S A. 1994;91:10435–10439. doi: 10.1073/pnas.91.22.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Potts WM, Olsen M, Boettiger D, Vogt VM. Epitope mapping of monoclonal antibodies to gag protein p19 of avian sarcoma and leukaemia viruses. J Gen Virol. 1987;68:3177–3182. doi: 10.1099/0022-1317-68-12-3177. [DOI] [PubMed] [Google Scholar]
- Richman JM, Lee SH. About face: signals and genes controlling jaw patterning and identity in vertebrates. Bioessays. 2003;25:554–568. doi: 10.1002/bies.10288. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, O’hUigin C, Figueroa F, Grant PR, Grant BR, Tichy H, Klein J. Phylogeny of Darwin’s finches as revealed by mtDNA sequences. Proc Natl Acad Sci U S A. 1999;96:5101–5106. doi: 10.1073/pnas.96.9.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Tichy H, O’hUigin C, Grant PR, Grant BR, Klein J. On the origin of Darwin’s finches. Mol Biol Evol. 2001;18:299–311. doi: 10.1093/oxfordjournals.molbev.a003806. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Wang W-P, Widelitz RB, Jiang T-X, Chuong C-M. Msx-2 and the regulation of organ size: epidermal thickness and hair length. J Invest Dermatol. 1999a;4:278–281. doi: 10.1038/sj.jidsp.5640229. [DOI] [PubMed] [Google Scholar]
- Wang YH, Rutherford B, Upholt WB, Mina M. Effects of BMP-7 on mouse tooth mesenchyme and chick mandibular mesenchyme. Dev Dyn. 1999b;216:320–335. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<320::AID-DVDY2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Weiss KM. The phenogenetic logic of life. Nat Rev Genet. 2005;6:36–45. doi: 10.1038/nrg1502. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1466–1467. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MK, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, Chuong CM. Wnt 3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci U S A. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421:807–814. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- Zweers GA, Vanden Berge JC, Berkhoudt H. Evolutionary pattern of avian trophic diversification. Zoology. 1997;100:25–57. [Google Scholar]