Abstract
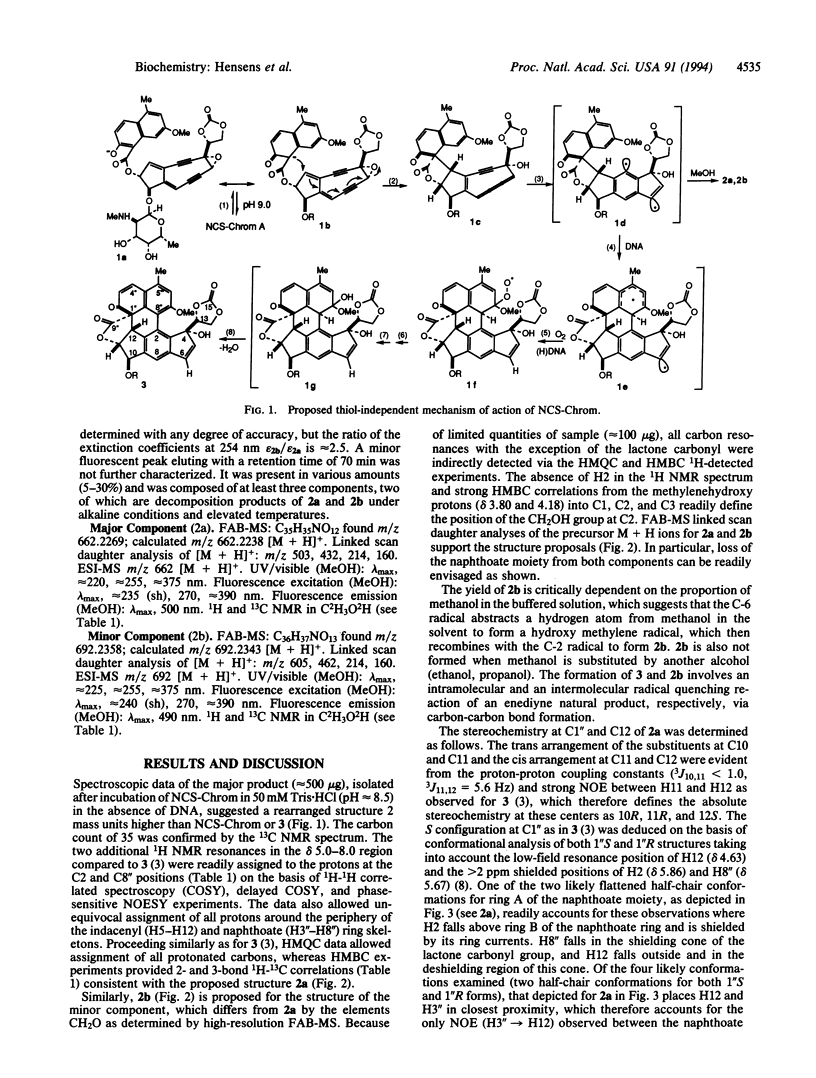
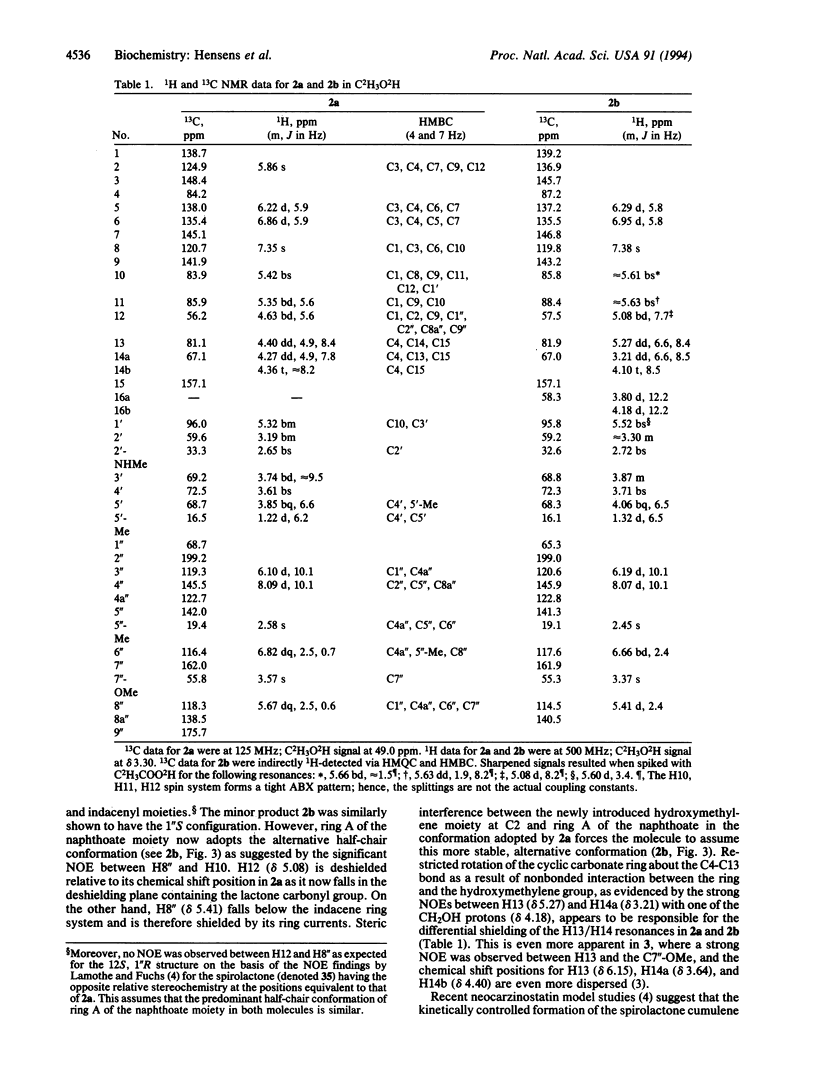
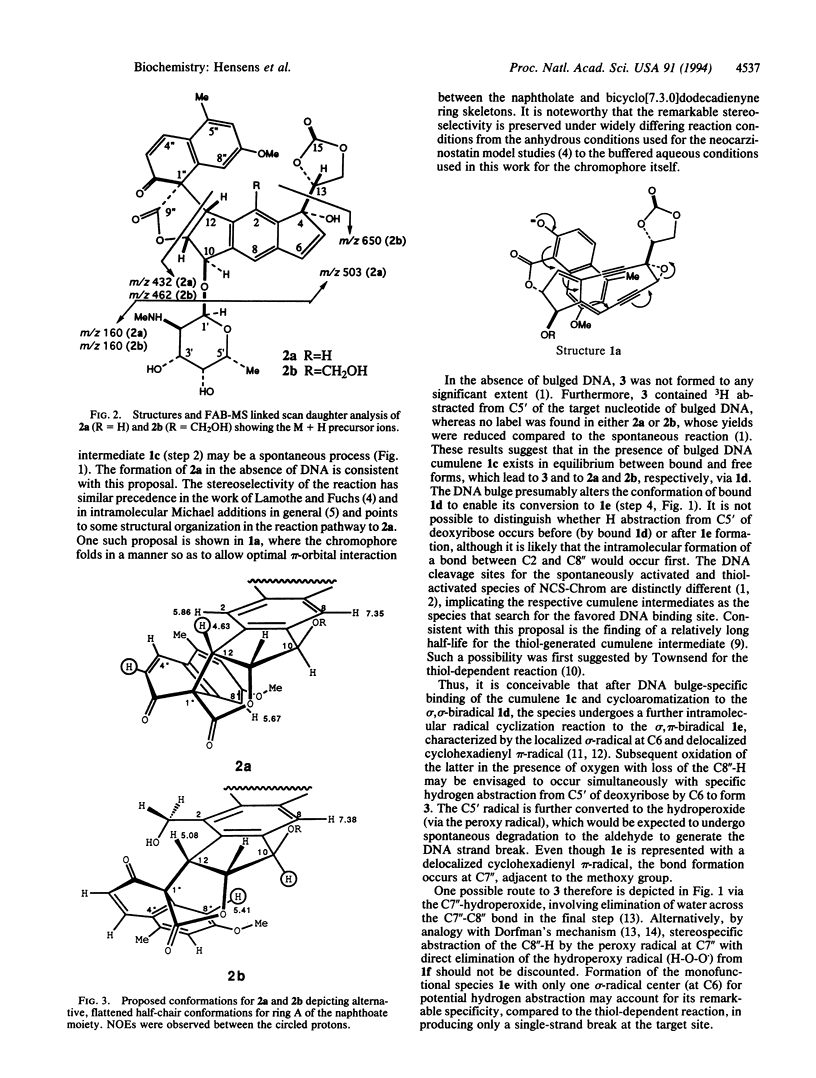
Detailed structure determination of the major and minor base-catalyzed degradation products of the chromophore of the enediyne anticancer antibiotic neocarzinostatin in the absence of DNA demonstrates that the enolate Michael addition reaction leading to a spirolactone cumulene intermediate is a spontaneous, stereoselective process. The implications of these findings for the mechanism of the thiol-independent, site-specific cleavage by the so-generated radical species of the drug at a DNA bulge are described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hensens O. D., Goldberg I. H. Mechanism of activation of the antitumor antibiotic neocarzinostatin by mercaptan and sodium borohydride. J Antibiot (Tokyo) 1989 May;42(5):761–768. doi: 10.7164/antibiotics.42.761. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. DNA conformation-induced activation of an enediyne for site-specific cleavage. Science. 1993 Sep 3;261(5126):1319–1321. doi: 10.1126/science.8362243. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Site-specific cleavage at a DNA bulge by neocarzinostatin chromophore via a novel mechanism. Biochemistry. 1993 Dec 7;32(48):13138–13145. doi: 10.1021/bi00211a024. [DOI] [PubMed] [Google Scholar]



