Abstract
It may appear counter-intuitive to compare feathers and mammary glands. However, through this Evo–Devo analysis, we appreciate how species interact with the environment, requiring different ectodermal organs. Novel ectodermal organs help define evolutionary directions, leading to new organism classes as exemplified by feathers for Aves and mammary glands for Mammals. Here, we review their structure, function, morphogenesis and regenerative cycling. Interestingly, both organs undergo extensive branching for different reasons; feather branching is driven by mechanical advantage while mammary glands nourish young. Besides natural selection, both are regulated by sex hormones and acquired a secondary function for attracting mates, contributing to sexual selection.
Keywords: Development, Ectodermal organ, Evolutionary novelty, Branching morphogenesis, Breast cancer
1. Introduction
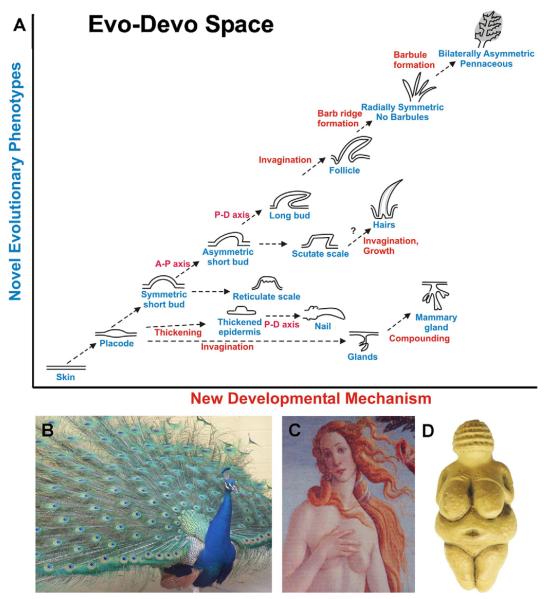
Feathers and mammary glands, and other ectodermal organs such as hairs, teeth and nails, derive from skin and result from novel epithelial–stromal interactions. In general, stromal signals suffice to induce dermal condensation and formation of an epithelial placode [1] (a localized area of epithelium destined for a new developmental fate), and subsequent morphogenesis and differentiation [2,3]. Unique patterns of morphogenesis produce variations in the shape of ectodermal organs that enable animals to adapt to different niches, contributing tremendously to animal diversity [3]. It is truly amazing how a flat piece of epithelium is transformed into elaborate structures with specialized forms and functions (Fig. 1A) [3,4].
Fig. 1.
Evo-Devo of ectodermal organs with emphasis on feathers and mammary glands. (A) Evolutionarily novel developmental mechanisms (x axis) result in new phenotypes (y axis) upon which natural selection acts. The arrows are in broken lines since they are hypothetical. (B) When a male peacock reaches sexual maturity, tail coverts are replaced by the colorful giant feathers. Also note the different feather types on different body regions. (C) Venus by Botticelli shows the standard of female beauty today with hairs and breasts. This is in contrast to the Venus of Willendorf (D) which probably shows the standard of beauty for the ancient humans who lived 24,000 years ago with enlarged breasts, probably due to the needs at that time. Panel B, C are photos by Chuong. Panel D is from (www.northernsun.com).
Here, we make a bold comparison of mammary glands and feathers. By searching for their evolutionary path, we can appreciate how species acquire new forms of ectodermal organs, among other changes, to best fit into ecological niches. Changing environments select for or against them. Feathers allow endothermy and flight, thus opening the sky as a new niche, enabling the evolution of the Avian class. Mammary glands provide an effective strategy for fostering the young, increasing the success of breeding and allowing further development outside the egg or uterus, which ultimately allowed the evolution of a large-sized human brain.
We compare the structure, function, morphogenesis and regenerative cycling of feathers and mammary glands (Table 1). Several aspects of these ectodermal organs are parallel, although they may result from different developmental mechanisms. For example, both undergo extensive branching morphogenesis. In feathers, driven by mechanical advantage, branches are patterned in a hierarchical order, involving a process of differential apoptosis [1]. In mammary glands, driven by the advantage of more and better secretions to nourish the young [5,6], new branches form in a random pattern through bifurcation or sprouting of existing ducts and subsequent elongation [7], increasing the milk producing surface area. In addition, the growth of some feathers and all mammary glands is regulated by sex hormones. Interestingly, both organs have acquired the secondary function of attracting mates. A peacock and human with extraordinary skin appendages are shown in Fig. 1B and C. The Venus by Botticelli shows today’s standard of female beauty, in contrast to the Venus of Willendorf (Fig. 1D) which presumably shows the standard of beauty 24,000 years ago, illustrating how human brains have remarkably changed in perception of beauty.
Table 1.
Comparison of mammary glands and feathers
| Appendage Define class in evolution |
Mammary gland Mammals |
Feather Aves |
|---|---|---|
| Basic structures | Requires both epithelium and mesenchyme. Invaginates into dermis and branches |
Requires both epithelium and mesenchyme. Protrudes out of skin surface and branches |
| Function | Chemical secretion (developed with endothermy) to moisten eggs and feed offspring. Display |
Mechanical properties for endothermy, display, and flight |
| Developmental origins | Skin | Skin |
| Inductive events and inducers | Ectoderm–mesenchymal interactions, mammary gland stroma |
Ectoderm–mesenchymal interactions, dermal papilla |
| Number of appendages | 2–18 (hundreds in platypus) | 20,000–100,000 |
| Molecular regulator of induction and morphogenesis |
FGF-, BMP- and Wnt-signaling, etc., but not Hh signaling |
FGF-, BMP-, Wnt, Hh-signaling, etc. |
| Mechanisms of branching morphogenesis |
Branches made by budding and elongation | Branches made by differential apoptosis from multi-layered epithelia |
| Regional specificity | Morphologically: yes. Functionally: none | Both morphological and functional specificity in flight, downy and contour feathers |
| Differentiation markers | B-casein, whey acidic protein | Feather beta keratin |
| Regenerative cycling | Partially. Do go through lactation and involution phases. Next phase starts morphologically equivalent to pubertal stage |
Yes, go through growth, resting, molting and regenerative phases |
| Stem cells | Cap and other places | Follicle bulge |
| Hormonal regulation | Yes, for expanding milk production | Yes, for sexually dimorphic feathers |
The saga started more than 310 million years ago. Diverging from their common ancestors, the early Amniotes gave rise to the Synapsida and Sauropsida. Synapsida started to evolve into the Mammals around 220–230 million years ago. Sauropsida started to evolve into the Aves around 170–190 million years ago. We first discuss the evolution of feathers and mammary glands, which, unavoidably, includes some speculation. The changes are based on the evolutionary novelty generated from changes in DNA or developmental processes. We discuss the recent insights in molecular and cellular events during organogenesis of feathers and mammary glands. As both feathers and mammary glands share an ability to molt or involute periodically followed by regeneration, we then compare post-natal cycling of feathers and mammary glands and the effects of sex hormones on cycling. At the end, we return to compare the salient features of these two ectodermal organs, and hope the Evo–Devo perspective will give readers a fresh appreciation of both feather and mammary gland biology.
1.1. Evolution of the feather
Reptilian scales, avian scales, and avian feathers result from epithelial–mesenchymal interactions, and may evolve from homologous structures [8,9]. Scales, composed of thickened epithelia, have a round or rectangular shape. Some scales can overlap, producing an inner hinge-like membrane [3,8,10]. In contrast, feathers have a more complex topological organization, with three-leveled hierarchical branches (rachis, barbs and barbules) arranged with different symmetries and relative lengths. They also have a follicular structure at the base, allowing them to grow continuously and regenerate after molting [1,9,11]. How is the scale topologically transformed into the feather in development and in evolution?
The salient features of feathers were built step by step, selected and refined over about 50 million years of evolution (approximately from 190 to 140 million years ago) [3,13]. There have been debates about whether feather-like appendages found on dinosaurs are true feathers. We need to specify distinct criteria by which to define feathers so we can judge the nature of those Mesozoic protofeathers [1,13]. Here, we will compare feathers and scales based on the following criteria: (1) Proliferation in avian scales and reptilian scales is diffuse [10,14]. In contrast, feather buds proliferate in a localized growth zone, first in the distal bud. As buds elongate, the localized proliferation zone gradually shifts to the proximal end at the base of the feather [3]. This topological arrangement allows for continuous proximal-distal feather growth. (2) Scales do not form follicular structures, while epidermis surrounding the feather bud invaginates into the dermis to form a follicular wall. (3) The mature scales are made of an epithelial shell and a mesenchymal core. The outside of the epithelial shell is the suprabasal layer. Feather bud mesenchyme loosens to become the pulp surrounded by a multi-layered cylindrical feather filament epithelium. (4) Scales do not form branches, while feathers do. (5) Scales do not grow or regenerate post-natally but feathers contain stem cells protected within the follicle [15] which can be induced to initiate the growth of a new feather. Only those skin appendages that fulfill all five criteria for feathers will be considered true feathers.
The discovery of feathered dinosaurs provides evidence of the many intermediate forms of evolving feathers [3,12,13,16 and references therein]. The initial driving force for feather evolution is likely endothermy. This only requires skin appendages to trap the air. The skin appendages found in Sinosauropteryx probably belong to this category. There is no definite follicle structure and all appendages covering the body surface appear the same. The second driving force may be display and communication. For this purpose, regional specific feather tracts have evolved, as well as a central backbone of the feather, called the rachis, which converts radial to bilateral symmetry, and gives the feather a more rigid structure. The symmetric, pennaceous vaned feathers with interlinked barbs, found in the tail region of Caudipteryx are in this category. The Caudipteryx had longer legs than wings and was apparently not able to fly. Finally, bilaterally asymmetric feathers evolved in conjunction with other body modifications, allowing the most intriguing function of flight. There are still many unsolved issues. For example, how did the feathers covering all four legs of the microraptor work? Were the avian foot scales remnants of reptilian scales or the result of convergent evolution?
1.2. Evolution of the mammary glands
In the context of this review, it may seem coincidental that mammary glands seem to have evolved from a shared feature of birds and primitive mammals (monotremes): oviparity. However, based on an extensive study of literature in a wide variety of disciplines, Oftedal convincingly proposed that indeed eggs were at the cradle of the evolution of the mammary glands [5,6].
When the Amniotes, the earliest terrestrial vertebrates, explored the land, they had to prevent themselves and their eggs from dehydration. For although the Amniotes laid eggs with outer membranes and layers that permitted survival outside an aquatic environment, the egg shells were still permeable. While Sauropsida evolved a highly calcified impermeable egg shell to prevent evaporation of fluids from their eggs, the earliest Synapsids, instead, presumably used the “parchment” shell’s permeability to replenish evaporated fluids with liquid secretions from their glandular skin, similar to existent amphibians with terrestrial eggs, such as salamanders.
The observation that monotreme mammary glands are fully secretory prior to egg-laying and during egg incubation [17] may support an egg-moisturizing function for these glands. The presence of a little fat and a variety of whey proteins suggests a nutritive function of these secretions that predominantly contain water, if transferred through the egg shell similar to the uptake of external salts and minerals by eggs of extant species. This suggests that synapsid skin secretions, although different in composition from milk, could be considered a primitive form of lactation. In that scenario, the evolution of lactation preceded the evolution of a defined mammary gland by about 150 million years.
How then did mammary glands evolve as a consequence of, rather than simultaneous with lactation? Oftedal’s reviews [5,6] may provide an answer: Supposedly, eggs of the earliest synapsids had a large yolk to feed the embryo until it hatched fairly precocial from the egg. Thus, initially “lactation” had a moisturizing rather than nutritive function, and a glandular skin provided an advantageously larger surface than localized glands to moisten many eggs. But when synapsids became endothermic, contact with the warm body of the egg-tending parent would promote egg dehydration. Dehydration was prevented by the evolution of a pouch, as inferred from fossil records of epipubic bones in some Mammaliaformes (the last pre-mammalians in the line of Synapsids). Among extant vertebrates, epipubic bones are only present in monotremes and marsupials, and are therefore believed to be associated with the existence of a pouch or the support of young hanging from nipples. The pouch served as a closed “incubatorium” for the eggs from which evaporation is prevented, and provided direct contact with the mother’s skin-secretions. With the evolution of pouches, the diffusely glandular skin could be replaced by large secretory glands localized exclusively in the pouch skin.
In monotremes, the mammary glands open at the skin in mammary patches; areolae without nipples and covered with hairs from which secretions ooze. The hairs in the areola may be advantageous to the “moisturizer-function” in mediating the distribution of the secretions over the egg shell. The hairs are also compatible with the “feeder-function”, as the hatchlings can suckle the milk-like secretions containing lipids and proteins from those structures. Interestingly, the approximately 300 specialized “mammary hairs” in the platypus are located on two “mammary” lines that run symmetrically in the anterior–posterior direction along the belly, lateral to the ventral midline. In higher mammals, a pair of such lines still exists during embryonic life. Dependent on the species, one or several pairs (e.g. up to nine in domestic pigs) of mammary glands develop on these lines. The number of glands relate to the number of young per clutch [18]. The evolutionary determinants that position the mammary line and individual mammary glands on this line in various species remain elusive and intriguing.
Viviparity of higher mammals made the egg-moisturizing function for their mammary glands obsolete. However, the altricial newborn depended more than oviparous hatchlings on the maternally derived nutrients. A hairless areola with a nipple allows for targeted delivery of larger volumes of a richer milk with less effort for the young. Therefore, we speculate that the evolution of this novel epidermal appendage may be associated with viviparity of mammals. The relationship between hair and nipples can be observed among other mammalian orders. In some marsupials (opposums), the mammary glands grow out from the sebaceous glands of hair follicles, which are lost during the formation of the nipple during late pregnancy [19]. Also, the mouse permanently loses the hair that borders the nipple late in its first pregnancy and subsequent lactation [20].
Therefore, we can speculate that the multiple steps of mammary gland evolution progressed as follows: (1) Diffuse secretions from all over the skin to moisten eggs. (2) Clustering of these glands to a localized region. (3) Modifications of the secretions to contain fat, whey proteins and to have a higher nutritional value. (4) Increased efficiency in the secretion delivery apparatus. This includes the evolution of the areola and nipple and may be accompanied with myoepithelial cell mediated contraction. (5) The mammary gland becomes a display signal to attract a mate.
To come back to the bird, the transient loss of feathers is a feature of avian incubation patches, to optimize heat transfer to impermeable calcified eggs [21]. Thus, the hormonal underpinnings of lactation may have originated from the creation of specialized epidermal appendages required to tend to the young from the generalized skin appendages that cover the vertebrate integument.
2. Feather
2.1. Embryonic development of the feather
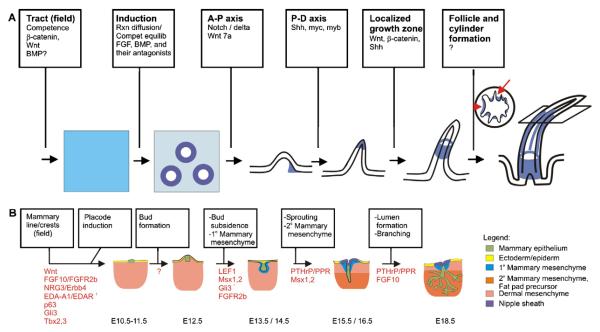
Embryonic feather formation has been reviewed recently [1] and is depicted schematically in Fig. 2A. Chicken skin begins to form at about embryonic day 6.5 (E6.5), when the skin is a single layer of epithelium overlying a single cell layer of mesenchyme. Shortly thereafter, the skin is divided into about 20 different feather tracts [11]. Each tract will contain feathers of a similar type and orientation although there can be size variation along the length of the tract. The dorsal tract, which is best characterized, expresses moderate levels of Wnt1, 3a, 5a and 11 at this time. After tract formation, cells begin to aggregate along the midline of the dorsal tract to form dermal condensations followed by the formation of epithelial placodes [22], induced by FGFs [reviewed in 1]. BMP4 is expressed in the dermal condensations during early feather bud formation and later acts to inhibit feather bud formation in the interbud region. As feather placodes begin to form, the expression of Wnt1 and 3a become restricted to the placode epithelium while Wnt5a and 11 are elevated in the inter-tract and inter-placode regions [1]. The signal directing feather formation is thought to originate from the mesenchyme, while continuous epithelial–mesenchymal interactions are required for feather bud development. A wave of sequential feather placode formation progresses along the midline and then radiates bilaterally toward neighboring tracts. This process can generate 20,000–100,000 feathers distributed across the surface of a bird.
Fig. 2.
Formation of appendages. (A) Embryonic feather morphogenesis. Molecules which affect major events along the progression to successive stages of feather development are shown. Proliferative cells are indicated in blue with darker shades indicating areas of higher proliferation. Proliferation starts at the feather tip but recedes down toward the feather base as morphogenesis proceeds. The plane on the right most feather shows the height from which the adjacent cross section was taken to show barb ridge (red arrow) and rachis (red arrowhead) formation. AP-axis: anterior–posterior axis; P-D axis: proximal-distal axis. (B) Embryonic mammary gland morphogenesis. Key stages in mammary gland development are shown. Known molecules which regulate each morphogenetic step are presented. The legend identifies the tissues shown in the schematic. Modified from [32].
Subsequently, the placodes elongate and protrude from the skin surface to form short symmetric buds. Already at this stage molecular anterior–posterior asymmetries arise. Msx2 and tenascin C are expressed in the anterior bud epithelium, Sonic hedgehog (Shh) in the distal epithelium, while Wnt7a and β-catenin become restricted to the posterior bud epithelium. HoxC6 and NCAM are in the anterior mesenchyme, Notch is in the central mesenchyme while mRNAs encoding its ligands, Delta and Serrate are in the posterior mesenchyme. This asymmetric molecular expression is crucial for later bud elongation to form asymmetric long buds [reviewed in 3].
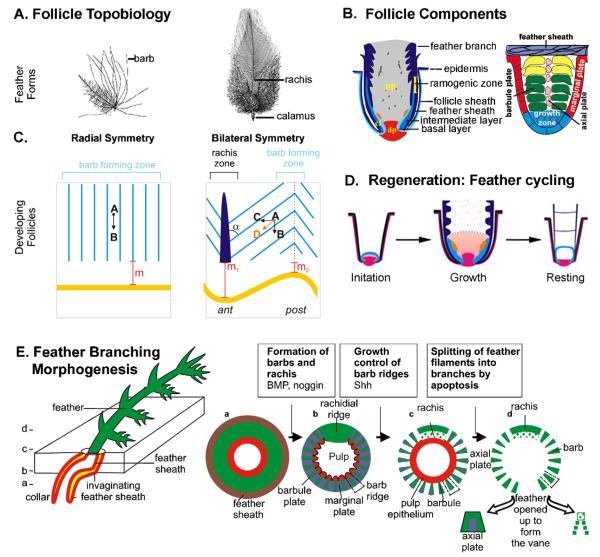
Next, the buds invaginate into the underlying mesenchyme to form feather follicles, aided by the expression of tenascin in the mesenchyme directly adjacent to epithelium [1]. The feather follicle epithelium contains an outer follicle sheath, within which reside a feather sheath, an intermediate layer and an inner-most basal layer. The dermis contains the dermal papilla at the base of the feather and central mesenchyme that forms the feather core above the dermal papilla (Fig. 3B). The latter mesenchyme loosens to form the pulp as the feather continues to proliferate and elongate outward from the skin as small cone shaped extensions. The distal tip is the oldest part of the feather and differentiates first. Epithelial cells in the ramogenic zone invaginate to form barb ridges containing centrally aligned cells of the axial plate, surrounded by centripetally oriented cells of the barb plate. This is enwrapped by cells of the marginal plate [1,23]. Shh becomes expressed in the marginal plate of the barb ridges, while barb plate and barbule plate cells express feather keratins [24]. Apoptosis in the feather sheath enables the feather to emerge [1]. Apoptosis of the axial plate cells, central pulp and Shh expressing marginal plate cells enables branching morphogenesis, releasing the barbs and barbules which give feathers their wispy appearance that enables them to provide warmth, and structure in the form of a feather vane to allow flight (Fig. 3E). Hence, branching morphogenesis within feathers occurs by virtue of localized cell death in the multi-layered epithelial cylinder.
Fig. 3.
Adult feather follicle cycling. (A) Representative feather forms. (B) Components of feather follicle (left). Barb ridge morphogenesis. cl, collar; dp, dermal papilla; pp, pulp. (C) Barb ridge orientation in an open follicle preparation. Barb ridges are oriented parallel to the feather follicle in radially symmetric feathers (left). In bilaterally symmetric feathers (right) the barb ridges form an angle, α, with the rachis. The barbs slant (A–D) because they are made from a horizontal component (A–C) and a vertical component (A–B) due to feather growth. Asymmetric feathers form in response to a Wnt3a gradient, forming an angled stem cell ring (yellow line). Cells at the anterior end (rachis) have to move further than cells at the posterior end (compare m1 and m2) to be at the same height. Light blue; rachis, dark blue; ant, anterior, post; posterior. (D) Regenerative feather cycle. The initiation, growth and resting phases are depicted. Red, dermal papilla at the base and dermal sheath rising along the side; light blue, collar epithelia; dark blue, feather sheath; pink, collar mesenchyme; orange, stem cells; speckled brown, pulp. (E) Spatial distribution of apoptotic cells (purple) during successive stages of feather branching morphogenesis. Cross sections at different levels from proximal to distal are depicted.
2.2. Post-natal cycling of the feather
Feathers cycle throughout the adult life of birds. The whole feather organ is replaced in each cycle. The feather cycle is marked by phases of growth, resting and initiation of a new cycle (Fig. 3D) [11,15]. The length of the growth phase differs among the different tracts. The growth phase comes to an end when the blood vessels that feed the growing feathers dry up. The feathers then enter a resting phase during which they remain in place and undergo maturation, but do not elongate. Ultimately feathers become fully keratinized down to their base. This permits the feathers to shed away from the surrounding tissue through a molt [11].
New feather follicle initiation depends on interactions between feather epithelial stem cells and the dermal papilla. Where are these epithelial stem cells located? Long-term label retention studies identified a small population of slow cycling cells located on the inner bulge of the feather collar epithelium just above the dermal papilla (Fig. 3B, shown in orange) [15]. Cell division of these stem cells yields faster cycling cells known as transient amplifying cells, which migrate up away from their site of origin and further proliferate or differentiate as needed during feather growth. Thus, placing the growth center near the feather base enables the regeneration of lost feathers.
Feathers of the first generation emerge prior to hatching. They are downy feathers that show radial symmetry and are plumulaceous (the barbs are not linked together by barbules). The first molt, about two weeks after hatching, replaces the radially symmetric feathers in a tract dependent fashion by bilaterally symmetric (e.g. body) or bilaterally asymmetric feathers (e.g. wing; Fig. 3A). The upper portion of many of these feather barbs becomes pennaceous, which means their barbules link between adjacent barbs to form a feather vane, while the lower section of the feather often retains a plumulaceous character to continue to provide warmth [25].
In radially symmetric downy feathers, the branches essentially grow vertically from the base of the feather; their distal ends droop toward the sides when branches lengthen. In contrast, the bilaterally symmetric and bilaterally asymmetric feathers branch out diagonally from the central backbone of the feather, called the rachis. An anterior–posterior Wnt3a gradient is necessary for the formation of bilaterally symmetric feathers and a rachis (Fig. 3C). Uniform misexpression of Wnt3a causes radially symmetric feathers to form. A localized spot of exogenous Wnt3a produces an ectopic rachis [26]. BMPs function in post-natal feathers to promote the fusion of feather branches to make a rachis, while Noggin, a BMP antagonist, promotes more branch formation as determined by RCAS gene misexpression studies [23–27].
Similar to the growth of mammary glands, feather growth is regulated by sex hormones. The tail feathers of roosters are longer and wider than those of hens [28]. A few anecdotal studies addressed the role of sex hormones in feather development: Capons (castrated roosters) grow longer tail feathers than intact roosters, suggesting that testosterone is not responsible for the increased length. In contrast, in unilaterally ovariectomized young female chicks the remaining ovary often produces androgens. These chickens develop long feathers as seen in roosters [29]. Birds with constitutively active aromatase activity convert androgen to estrogen in the skin and have female feathering [30]. So far, these studies have not been able to clarify whether androgen or estradiol determines gender-specific feather length. However, androgens seem to extend the tail feather growth period in roosters while the growth rate is essentially identical to that in hens [31]. In general, male plumage is more ornate than female plumage, but a possible androgen or estrogen involvement remains to be elucidated.
3. Mammary gland
3.1. Embryonic development of the mammary gland
Mammogenesis in the mouse occurs around E10.5–11.0, when the maturing embryonic skin is one or two cell layers thick, overlaying one or two layers of mesodermal cells [22,32,33]. On both flanks it starts with streaks converging into a mammary line: a band of multilayered ectoderm specifically expressing Wnt6 and Wnt10b [34]. The lines are partially induced by somitic (“mesodermal”) FGF10 [33] and laterally inhibited by BMP4 [35]. Multilayered Lef1-expressing placodes (Fig. 2B) form at five precisely reproducible and paired symmetric positions along these lines. Formation of the individual placodes involves different signals, as demonstrated by their different genetic requirements [32,33,36,37]. However, all inductive signals seem to activate or cooperate with p63 and Wnt signaling, as inactivation of p63 or Wnt signaling abolishes the formation of all placodes [38,39]. The differentially impaired induction of distinct placode pairs in Lef1−/− mice [40], suggests that individual placode pairs transduce Wnt signaling differently. We have previously suggested that repressing Hedgehog signaling may be a critical determinant of mammary versus other appendage fate in ectodermal placodes [41,42]. Indeed, Gli3-mediated transcriptional repression inhibiting Hedgehog signalling, was recently shown to be required for the formation of mammary placodes 3 and 5 [43].
Like the feather buds, each mammary placode quickly becomes a spherical bud elevated above the surrounding skin at E12.5, but then invaginates into the dermal mesenchyme. This process requires Gli3 (Veltmaat et al., in preparation) and co-expression of Msx1 and Msx2 in the epithelium [44], and coincides with the condensation of the adjacent layers of dermal mesenchyme that become the primary mammary mesenchyme (Fig. 2B). Type I Receptor for PTH and PTHrP (PPR), as well as mesenchymal Wnt-signaling are required for induction of mammary mesenchyme and maintenance of mammary bud epithelium [40,45,46]. The buds subsequently regress in males through apoptosis induced by androgen receptor activation [45,47]. In females, the buds enter a growth arrest around E14.5 [48]. Meanwhile, the deeper dermal mesenchyme condenses and differentiates into the secondary mammary mesenchyme or fat pad precursor in both sexes. Around E15.5, the buds in females resume growth and sprout into the fat pad precursor, depending on Msx2 expression [44,49] and PTHrP/PPR signaling with downstream β-catenin activation in the primary mammary mesenchyme [46]. At E16.5, resulting from PTHrP/PPR induced differentiation of the mesenchyme, the epidermis overlying the sprout forms the nipple sheath characterized by a thickened patch of epidermis delimited by a bat wing-like invagination of the basal layer [46]. Whereas the structure of the nipple changes very little throughout prenatal life [50] several intercellular cavities are formed in the sprout, which coalesce to form a primary lumen, i.e. milk canal, that opens at the nipple [51]. The distal end of the sprout further elongates into the fat pad precursor and bifurcates. Before birth, these new branches undergo several rounds of elongation and bifurcation, giving rise to a rudimentary mammary tree [32,52]. The ducts are short and consist of a single layer of luminal epithelial cells, which produce and secrete milk, and ultimately are ensheathed by an outer layer of myoepithelial cells around birth [53]. Myoepithelial cells express smooth muscle actin, enabling them to contract, facilitating the release of milk from the luminal cells [54]. Thus, although lactation is not needed until after pregnancy, the mammary gland is in principle capable of producing milk and delivering it to the nipple at birth (a.k.a. witch’s milk in humans).
What regulates these early elongation and branching events? Heterochronic and heterotypic recombinations of mouse embryonic mammary epithelium with primary mammary mesenchyme or mesenchyme from other branching organs, reveal that elongation and branching in a pattern typical for mammary glands, is instructed by soluble factors from the mammary fat pad precursor [55,56]. Production of such factors may depend on mammary epithelial PTHrP, activating the PPR in the fat pad precursor, which in turn is required for normal budding, branching and elongation of the epithelium [57]. To date, FGF10 is the only identified soluble stromal factor, required for epithelial branching. In the absence of FGF10, the fat pad precursor remains underdeveloped and contains only a poorly elongated and branched sprout [58]. As FGF10 is required for adipocyte differentiation in culture [59], epithelial branching may depend on FGF10-induced adipocyte differentiation. In contrast to feather branching, mammary branching is not stereotypical: At birth, the size and branching pattern differ for each of the mammary trees. Moreover, branching pattern and size of mammary glands are not reproducible among individual pups, and even two glands of a single pair are not mirror images, suggesting that non-intrinsic factors likely play an additional role in epithelial branching morphogenesis [32].
3.2. Post-natal development and cycling of the mammary gland
At birth, the primary duct of the mammary gland is surrounded by a thick layer of adipocytes, interspersed with fibroblasts. This stroma is separated from the epithelium by a basement membrane secreted by the myoepithelial cells [60,61]. The growth of the mammary glands is proportional to body growth until puberty. In post-natal life prior to puberty, the nipple is barely distinguishable from the surrounding epidermis but gradually acquires the expression of specialized keratins [50,62,63]. As the nipple matures, multiple suprabasal keratinocyte layers develop over a unique connective tissue [50].
During further post-natal life, mammary gland development (Fig. 4) occurs primarily in response to hormones, which act in concert with or upstream of other signaling pathways, too numerous to address here. Briefly, during puberty, in response to rising estrogen levels, the epithelial ducts grow and branch to fill the fat pad [64]. Some branches terminate in secretory alveoli, while others form extended lateral buds. The club-shaped distal ends (terminal end buds, TEBs) of the ducts are sites of highly proliferative cap cells that give rise to both luminal and myoepithelial cells. The TEBs are responsible for ductal elongation and branching, and thus considered the growth centers of the mammary gland [60]. In contrast to the proximally located growth center of the feather bud, the growth center in the mammary gland is thus located distally. Once the TEBs run into connective tissue surrounding other ducts or the capsule at the edge of the fat pad, they differentiate into terminal ducts. Within the center of the ducts, cells undergo apoptosis to form the lumen [65].
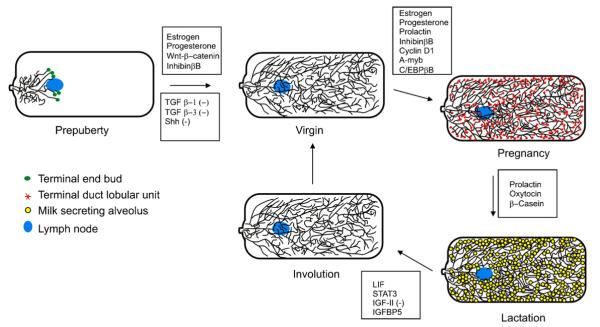
Fig. 4.
Adult mammary gland cycling. Glands are shown in a ventral (left) to dorsal (right) orientation. (−) indicates the molecules are inhibitory to this step in morphogenetic progression. Lists of molecules are not all-inclusive. The legend identifies the tissues shown–in the schematic.
Most mammary gland differentiation occurs during pregnancy. Progesterone increases the proliferation rate of the epithelium, and permits expansion of the ductal network and alveolar formation [66]. In later stages of pregnancy, alveolar buds differentiate further into alveoli and milk secreting lobules, expanding to populate most of the fat pad [67]. During pregnancy, the connective tissue surrounding the ducts is replaced by adipocytes. At this stage the myoepithelial cells form a discontinuous border adjacent to the alveoli, allowing luminal epithelial cells to make contact with the basement membrane in close proximity to the adipocytes across the narrow strip of connective tissue. Association with adipocytes may be essential for the complete differentiation of the luminal epithelia into milk secreting cells [68]. Near term, the epithelial cells gain secretory vesicles for the secretion of milk [69]. Prolactin receptor activity mediated by STAT5 induces transcription of β-casein. This induction is suppressed by progesterone receptor activity [70]. Upon delivery, oxytocin induces contraction of the myoepithelial cells, squeezing the alveoli to release their milk proteins into the ducts during lactation [71]. In mice, this continues for approximately 3 weeks until the pups are weaned.
The nipple also changes with puberty and pregnancy. After puberty, the nipple grows out above the surrounding epidermis and is characterized with a distinctive thick cornified covering [50]. During pregnancy it triples in size under the influence of estrogen and relaxin [72]. This growth is accompanied by increased epidermal proliferation, expansion of the smooth muscle lining the primary duct and base of the nipple as well as a reorganization of collagen bundles and extracellular matrix components [50,73]. Such pregnancy induced connective tissue reorganization fails to occur in the nipples of a mouse with reduced PPR expression [73]. The defective nipples of this mouse are unable to deliver milk, which is similar to the phenotype of the relaxin-null mouse [73,74].
The gland regresses during involution following lactation. Leukocyte Inhibitory Factor (LIF1) stimulates STAT3 activity which induces apoptosis during involution [75], leading to loss of alveoli [76]. This process is balanced by the apoptosis-inhibiting effects of type I Transforming Growth Factor (TGF) β receptor activity [77] and Insulin-like Growth Factor II (IGF-II) [78], the latter antagonized by Insulin-like Growth Factor Binding Protein 5 (IGFBP5) [79]. Simultaneously, the myoepithelium and luminal epithelium are pared back, and adipocytes fill in the empty space created by the loss of alveoli [80]. Ultimately, involution remodels the gland close to its virgin structure; however, some alveoli persist. The gland is now ready to resume the estrus cycle or ready for pregnancy.
Where are the stem or progenitor cells located from which the gland can regenerate during the next pregnancy? The TEBs that function as growth centers during puberty disappear after puberty, yet the regeneration of the gland with multiple pregnancies indicates the presence of stem cells in the post-pubertal mammary gland. Smith and Medina [81] first identified regenerative capacity in any portion of the mammary epithelium, indicating dispersed distribution of stem cells in ducts, ductules, and alveolar and lobular structures. They then proposed microscopically discernable large pale cells, mainly located in the basal layer of the epithelium, as stem cells [81]. Recently, FACS-sorting of mouse mammary epithelial cells identified an enrichment in cells capable of generating ducts with lumens, spheroid bodies, alveolar structures and myoepithelial cells, in a cell population with high β1-integrin expression. Some single cells from this population show properties of progenitor cells or self-renewal as they can regenerate a complete and normal mammary gland upon serial transplantation [82,83]. Previously, Comma-Dβ mouse mammary cells were shown to have great mammary gland differentiation potential [84]. These cells express high levels of stem cell antigen 1 (sca-1) and have characteristics of basal (myoepithelial) cells, but can convert to a luminal cell fate upon stimulaton by EGF [85], and show properties of self-renewal as they can regenerate a normal gland upon transplantation [86]. The dispersion of stem cells throughout the mammary gland thus contrasts with the concentration of stem cells at one site in the feather, corresponding with dispersed growth of the gland versus unidirectional growth from a growth zone in the feather. Although much still remains unknown, roles for Wnt and Delta-Notch signaling seem involved in “stemness” as well [87,88].
4. Conclusion
We propose that various epithelial appendages result from epithelial–mesenchymal interactions and become specialized structures to confer specialized functions to help animal species adapt to different ecological environments [2]. Epithelial appendages derived from ectoderm are called ectodermal organs. Here, we compared the Evo–Devo of feathers and mammary glands, two highly successful ectodermal organs which became the cardinal characteristics of Class Aves and Mammalia, respectively (Table 1). This implies that species possessing these ectodermal organs were so advantaged that their progenies expanded and diversified to form a whole class.
The highlights of the common and different features in these ectodermal organs are: (1) Both are derivatives of the skin. The specified mesenchyma and epithelia form ectodermal organs to exert special functions which help the species compete better. (2) For feather, the driving force is selection for mechanical structures which are versatile to serve thermoregulation, display and flight functions. (3) For mammary gland, the driving force is the selection for chemical secretions which can best nourish the offspring. (4) In the evolutionary path of both ectodermal organs, the complex features are made through multiple steps. The intermediate forms did not function as well. Species possessing them were gradually competed out by selective pressures, making the bridge between these two successful ectodermal organs (e.g. reptilian scales and avian feathers) disappear. (5) At the molecular level, they share many signaling molecules, but there are also differences, which may contribute to differences in placodal fate. For example, Hh signaling is repressed during mammary placode formation, but active during feather placode formation. For each step toward evolutionary novelty, new molecular/cellular events were built upon the old, existent forms. (6) Both ectodermal organs contain stem cells and cycle. (7) The growth of mammary glands and some feathers are regulated by sex hormones. Both also acquired a secondary signaling function and became part of the platform for sexual selection.
This comparison is a useful and interesting exercise to appreciate the basic biology and diversity of ectodermal organs. Now let us speculate on the evolution of ectodermal organs and the formation of classes in vertebrates. In the Precambrian explosion, approximately 530 million years ago, metazoans acquired morphogenetic abilities to organize themselves into different body plans, forming many phyla [89]. For further evolution within a phylum, reorganizing the basic body plan or early organogenesis is usually fatal as witnessed by the severity of these types of congenital anomalies. Molecular signaling modules capable of generating evolutionary novelty are added in later developmental stages so the survival of the fetus is not jeopardized. Ectodermal organs sometimes are considered less essential because they are numerous and dispensable for basic survival. However, these traits and post-natal cycling of these organs, allow animal species to “safely” play with appendage structure to achieve diversity and fitness through natural and sexual selection. Species successful in forming novel ectodermal organs can venture into new niches and expand their numbers. These organs have become the cardinal characteristics of novel classes as described here. Of course, changes to the integument alone are only skin deep and less consequential. To have a broader impact the newly formed ectodermal organs must be integrated with the rest of the body. In the feather, this involves the re-arrangement of muscle, skeleton and nerves for flight [90]. In the mammary gland, hormones generally mediate communication with other organ systems. Finally, for sexual selection to take effect, ectodermal organs must appropriately stimulate the brains of prospective mates. Thus, ectodermal organs can be excellent models for Evo–Devo research [3] because of their accessibility, visibility, testability, and connection to other organs at the level of system biology.
Acknowledgements
This work is supported by grants from NIAMS, AR052397 (R.B.W.) AR2177, AR47364 (C.M.C.), AR45585 (J.F.), the CBCRP 10-FB-0116, and CHLA intramural funding (J.M.V.). J.A.M. was partially supported by the USC/Norris Breast Cancer Research Program Training Fellowship and partially by grant BC 044808 to Dr. MC Pike from the Congressionally Directed Breast Cancer Research Program administered by the Department of Defense US Army Medical Research and Materiel Command. C.M.C. thanks Dr. P. Cowin (NYU) for discussion on the evolution of mammary glands. We regret that due to space constraints, we were not able to cite all original publications.
Appendix A. Annotated references
-
Feather evolution
Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, et al. Adaptation to the sky: defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zoolog B Mol Dev Evol 2003;298:42–56.
A review that covers recent findings of feathered dinosaurs/Mesozoic birds in China and laboratory findings of scale/feather phenotypes by molecular perturbation. It then develops a definition for feathers.
-
Mammary evolution
Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia 2002b;7:253–66.
This paper and a companion paper in the same journal issue by the same author combines insights from a wide range of disciplines (dietetics, cell and molecular biology, zoology), to propose a new hypothesis about the evolution of the mammary gland and lactation.
-
Feather development
Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr Opin Cell Biol 2006;18:730–41.
A most recent review that covers the molecular biology of feather development in five phases: macropatterning, micropatterning, intra-bud morphogenesis, follicle morphogenesis and regenerative cycling.
-
Feather cycle
Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci USA 2006;103:951–5.
The paper shows how topological arrangements of stem cells in the feather follicle can be used to set up bilateral or radial symmetry.
-
Mammary development
Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala, FG, van Veelen W. et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 2006;133:2325–35.
This paper identifies the somites as the signaling-source that induces some but not all mammary glands and determines their dorso-ventral position on the flank. It also unravels a hierarchical order of several signaling pathways in the induction process.
-
Mammary cycling
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006;439:993–7.
This paper identifies characteristics of pluripotent mammary stem cells that show properties of self-renewal which is essential for mammary cycling.
-
Nipple development
Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development 2001;128:512–25.
This manuscript established that the unique hairless ventral skin phenotype of the K14-PTHrP was actually due to the conversion of this region to nipple epidermis.
References
- [1].Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr Opin Cell Biol. 2006;18:730–41. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chuong CM, editor. Molecular basis of epithelial appendage morphogenesis. Landes Bioscience; Austin: 1998. pp. 3–14. [Google Scholar]
- [3].Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–70. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chuong CM, Wu P, Plikus M, Jiang TX, Widelitz RB. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr Top Dev Biol. 2006:237–74. doi: 10.1016/S0070-2153(05)72005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7:225–52. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- [6].Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. 2002;7:253–66. doi: 10.1023/a:1022848632125. [DOI] [PubMed] [Google Scholar]
- [7].Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–9. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alibardi L. Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J Exp Zoolog B Mol Dev Evol. 2003;298:12–41. doi: 10.1002/jez.b.24. [DOI] [PubMed] [Google Scholar]
- [9].Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- [10].Alibardi L. Scale morphogenesis during embryonic development in the lizard Anolis lineatopus. J Anat. 1996;188:713–25. [PMC free article] [PubMed] [Google Scholar]
- [11].Lucas AM, Stettenheim PR. Avian Anatomy—Integument. Agricultural Handbook 362: Agricultural Research Services. US Department of Agriculture; Washington DC: 1972. [Google Scholar]
- [12].Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zoolog B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- [13].Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, et al. Adaptation to the sky: defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zoolog B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tanaka S, Kato Y. Epigenesis in developing avian scales. II. Cell proliferation in relation to morphogenesis and differentiation in the epidermis. J Exp Zool. 1983;225:271–83. doi: 10.1002/jez.1402250210. [DOI] [PubMed] [Google Scholar]
- [15].Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–9. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–95. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- [17].Griffiths M, McIntosh DL, Coles REA. The mammary gland of the echidna, Tachyglossus aculeatus, with observations on the incubation of the egg and on the newly-hatched young. J Zool, London. 1969;158:371–86. [Google Scholar]
- [18].Diamond JM. Evolutionary adaptations. Aristotle’s theory of mammalian teat number is confirmed. Nature. 1987;325:200. doi: 10.1038/325200a0. [DOI] [PubMed] [Google Scholar]
- [19].Mayer G, Klein M. Histology and cytology of the mammary gland. In: Kon SK, editor. Milk: the mammary gland and its secretion. Vol. 1. Academic Press; New York: 1961. pp. 47–126. [Google Scholar]
- [20].Franz E, Bosse K. Effect of pregnancy and lactation on hair growth in mice. Arch Dermatol Res. 1975;254:149–57. doi: 10.1007/BF00586890. [DOI] [PubMed] [Google Scholar]
- [21].Jones RE. Hormonal control of incubation patch development in the California quail Lophortyx californicus. Gen Comp Endocrinol. 1969;13:1–13. doi: 10.1016/0016-6480(69)90215-9. [DOI] [PubMed] [Google Scholar]
- [22].Sengel P. Morphogenesis of skin. Cambridge University Press; Cambridge: 1976. [Google Scholar]
- [23].Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci USA. 2005;102:11734–9. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–12. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alibardi L. Fine structure of juvenile feathers of the zebrafinch in relation to the evolution and diversification of pennaceous feathers. J Submicrosc Cytol Pathol. 2005;37:323–43. [PubMed] [Google Scholar]
- [26].Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci USA. 2006;103:951–5. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chuong CM, Hou L, Chen PJ, Wu P, Patel N, Chen Y. Dinosaur’s feather and chicken’s tooth? Tissue engineering of the integument. John Ebbling lecture. Eur J Dermatol. 2001;11:286–92. [PMC free article] [PubMed] [Google Scholar]
- [28].Aparicio JM, Bonal R, Cordero PJ. Evolution of the structure of tail feathers: implications for the theory of sexual selection. Evol Int J Org Evol. 2003;57:397–405. doi: 10.1111/j.0014-3820.2003.tb00273.x. [DOI] [PubMed] [Google Scholar]
- [29].Frankenhuis MT, Kappert HJ. Experimental transformation of right gonads of female fowl in to fertile testes. Biol Reprod. 1980;23:526–9. doi: 10.1095/biolreprod23.3.526. [DOI] [PubMed] [Google Scholar]
- [30].Somes RG, Jr, George FW, Baron J, Noble JF, Wilson JD. Inheritance of the henny-feathering trait of the Sebright bantam chicken. J Hered. 1984;75:99–102. doi: 10.1093/oxfordjournals.jhered.a109902. [DOI] [PubMed] [Google Scholar]
- [31].Mayer JA, Chuong CM, Widelitz RB. Rooster feathering, androgenic alopecia, and hormone dependent tumor growth: what is in common? Differentiation. 2004;72:474–88. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- [33].Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala FG, van Veelen W, et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development. 2006;133:2325–35. doi: 10.1242/dev.02394. [DOI] [PubMed] [Google Scholar]
- [34].Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn. 2004;229:349–56. doi: 10.1002/dvdy.10441. [DOI] [PubMed] [Google Scholar]
- [35].Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, Kim HJ, et al. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc Natl Acad Sci USA. 2006;103:16788–93. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005;19:2078–90. doi: 10.1101/gad.338505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jerome-Majewska LA, Jenkins GP, Ernstoff E, Zindy F, Sherr CJ, Papaioannou VE. Tbx3, the ulnar-mammary syndrome gene, and Tbx2 interact in mammary gland development through a p19(Arf)/p53 independent pathway. Dev Dyn. 2005;234:922–33. doi: 10.1002/dvdy.20575. [DOI] [PubMed] [Google Scholar]
- [38].Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2005;131:4819–29. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- [39].Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- [40].Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295:219–31. doi: 10.1016/j.ydbio.2006.03.030. [DOI] [PubMed] [Google Scholar]
- [41].Chuong CM, Patel N, Lin J, Jung HS, Widelitz RB. Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol Life Sci. 2000;57:1672–81. doi: 10.1007/PL00000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lewis MT, Veltmaat JM. Next stop, the twilight zone: hedgehog network regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:165–81. doi: 10.1023/B:JOMG.0000037160.24731.35. [DOI] [PubMed] [Google Scholar]
- [43].Hatsell SJ, Cowin P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133:3661–70. doi: 10.1242/dev.02542. [DOI] [PubMed] [Google Scholar]
- [44].Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- [45].Dunbar ME, Dann PR, Robinson GW, Hennighausen L, Zhang JP, Wysolmerski JJ. Parathyroid-hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–93. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- [46].Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:512–25. doi: 10.1242/dev.128.4.513. [DOI] [PubMed] [Google Scholar]
- [47].Kratochwil K, Schwartz P. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci USA. 1976;73:4041–4. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robinson GW, Karpf AB, Kratochwil K. Regulation of mammary gland development by tissue interations. J Mammary Gland Biol Neoplasia. 1999;4:9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- [49].Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–37. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- [50].Abdalkhani A, Seller R, Gent J, Wulitich H, Childress S, Stein B, et al. Nipple connective tissue and its development: insights from the K14-PTHrP mouse. Mech Dev. 2002;115:63–77. doi: 10.1016/s0925-4773(02)00092-8. [DOI] [PubMed] [Google Scholar]
- [51].Sakakura T. Mammary embryogenesis. In: Neville MC, Daniel CW, editors. The mammary gland: development, regulation, and function. Plenum Press; New York: 1987. pp. 37–66. [Google Scholar]
- [52].Russo IH, Medado J, Russo J. Endocrine influences on the mammary gland. In: Jones T, Mohr U, Hunts R, editors. Integument and Mammary Glands. Springer Verlag; New York: 1989. pp. 252–66. [Google Scholar]
- [53].Deugnier MA, Moiseyeva EP, Thiery JP, Glukhova M. Myoepithelial cell differentiation in the developing mammary gland: progressive acquisition of smooth muscle phenotype. Dev Dyn. 1995;204:107–17. doi: 10.1002/aja.1002040202. [DOI] [PubMed] [Google Scholar]
- [54].Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, et al. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–32. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91:202–7. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- [56].Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- [57].Dunbar ME, Young P, Zhang JP, McCaughern-Carucci J, Lanske B, Orloff JJ, et al. Stromal cells are critical targets in the regulation of mammary ductal morphogenesis by parathyroid hormone-related protein. Dev Biol. 1998;203:75–89. doi: 10.1006/dbio.1998.9029. [DOI] [PubMed] [Google Scholar]
- [58].Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- [59].Sakaue H, Konishi M, Ogawa W, Asaki T, Mori T, Yamasaki M, et al. Requirement of fibroblast growth factor 10 in development of white adipose tissue. Genes Dev. 2002;16:908–12. doi: 10.1101/gad.983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–90. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- [61].Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–98. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- [62].Mahler B, Gocken T, Brojan M, Childress S, Spandau DF, Foley J. Keratin 2e: a marker for murine nipple epidermis. Cells Tissues Organs. 2004;176:169–77. doi: 10.1159/000077033. [DOI] [PubMed] [Google Scholar]
- [63].Eastwood J, Offutt CD, Menon K, Keel M, Hrncirova P, Novotny MV, et al. Identification of markers for nipple epidermis: changes during pregnancy and lactation. Differentiation. 2006;74:1–9. doi: 10.1111/j.1432-0436.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- [64].Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- [65].Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–22. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- [66].Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–81. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–41. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- [68].Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- [69].Mather IH, Keenan TW. Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia. 1998;3:259–73. doi: 10.1023/a:1018711410270. [DOI] [PubMed] [Google Scholar]
- [70].Buser AC, Gass Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, et al. Progesterone receptor repression of Prolactin/Stat5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol Endocrinol. 2007;21:106–25. doi: 10.1210/me.2006-0297. [DOI] [PubMed] [Google Scholar]
- [71].Wagner KU, Young WS, 3rd, Liu X, Ginns EI, Li M, Furth PA, et al. Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct. 1997;1:233–44. doi: 10.1046/j.1365-4624.1997.00024.x. [DOI] [PubMed] [Google Scholar]
- [72].Sherwood OD. Purification and characterization of rat relaxin. Endocrinology. 1979;104:886–92. doi: 10.1210/endo-104-4-886. [DOI] [PubMed] [Google Scholar]
- [73].Kobayashi T, Kronenberg HM, Foley J. Reduced expression of the PTH/PTHrP receptor during development of the mammary gland influences the function of the nipple during lactation. Dev Dyn. 2005;233:794–803. doi: 10.1002/dvdy.20406. [DOI] [PubMed] [Google Scholar]
- [74].Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, et al. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinol. 1999;140:445–53. doi: 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- [75].Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, et al. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development. 2003;130:3459–68. doi: 10.1242/dev.00578. [DOI] [PubMed] [Google Scholar]
- [76].Quarrie LH, Addey CV, Wilde CJ. Apoptosis in lactating and involuting mouse mammary tissue demonstrated by nick-end DNA labelling. Cell Tissue Res. 1995;281:413–9. doi: 10.1007/BF00417859. [DOI] [PubMed] [Google Scholar]
- [77].Muraoka-Cook RS, Shin I, Yi JY, Easterly E, Barcellos-Hoff MH, Yingling JM, et al. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene. 2006;25:3408–23. doi: 10.1038/sj.onc.1208964. [DOI] [PubMed] [Google Scholar]
- [78].Moorehead RA, Fata JE, Johnson MB, Khokha R. Inhibition of mammary epithelial apoptosis and sustained phosphorylation of Akt/PKB in MMTVIGF-II transgenic mice. Cell Death Differ. 2001;8:16–29. doi: 10.1038/sj.cdd.4400762. [DOI] [PubMed] [Google Scholar]
- [79].Flint DJ, Boutinaud M, Tonner E, Wilde CJ, Hurley W, Accorsi PA, et al. Insulin-like growth factor binding proteins initiate cell death and extracellular matrix remodeling in the mammary gland. Domest Anim Endocrinol. 2005;29:274–82. doi: 10.1016/j.domaniend.2005.02.021. [DOI] [PubMed] [Google Scholar]
- [80].Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- [81].Smith GH, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988;89:173–83. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- [82].Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- [83].Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- [84].Medina D, Oborn CJ, Kittrell FS, Ullrich RL. Properties of mouse mammary epithelial cell lines characterized by in vivo transplantation and in vitro immunocytochemical methods. J Natl Cancer Inst. 1986;76:1143–56. [PubMed] [Google Scholar]
- [85].Deugnier MA, Faraldo MM, Janji B, Rousselle P, Thiery JP, Glukhova MA. EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J Cell Biol. 2002;159:453–63. doi: 10.1083/jcb.200207138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Deugnier MA, Faraldo MM, Teuliere J, Thiery JP, Medina D, Glukhova MA. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dbeta cell line. Dev Biol. 2006;293:414–25. doi: 10.1016/j.ydbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [87].Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA. 2004;101:4158–63. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Valentine JW, Jablonski D, Erwin DH. Fossils, molecules and embryos: new perspectives on the Cambrian explosion. Development. 1999;126:851–9. doi: 10.1242/dev.126.5.851. [DOI] [PubMed] [Google Scholar]
- [90].Lindhe Norberg UM. Structure, form, and function of flight in engineering and the living world. J Morphol. 2002;252:52–81. doi: 10.1002/jmor.10013. [DOI] [PubMed] [Google Scholar]