Abstract
We previously identified peptide Lv, a novel bioactive peptide that enhances the activity of L-type voltage-gated calcium channels (L-VGCCs) in cone photoreceptors. In this study, we verified that peptide Lv was able to augment L-VGCC currents in cardiomyocytes, as well as promote proliferation of endothelial cells. We used a proteomics approach to determine the specific receptors and binding partners of peptide Lv and found that vascular endothelial growth factor receptor 2 (VEGFR2) interacted with peptide Lv. Peptide Lv treatment in embryonic cardiomyocytes stimulated tyrosine autophosphorylation of VEGFR2 and activated its downstream signaling. Peptide Lv activity was blocked by DMH4, a VEGFR2 specific blocker, but not by SCH202676, an allosteric inhibitor of G protein-coupled receptors, suggesting that the activity of peptide Lv was mediated through VEGFR2 signaling. Inhibition of VEGFR tyrosine kinase or its downstream signaling molecules abolished the augmentation of L-VGCCs elicited by peptide Lv in cardiomyocytes. In addition, peptide Lv promoted cell proliferation of cultured human endothelial cells. Calcium entry through L-VGCCs is essential for excitation-contraction coupling in cardiomyocytes. Since peptide Lv was able to augment L-VGCCs through activation of VEGF signaling in cardiomyocytes and promote proliferation of endothelial cells, peptide Lv may play an important role in regulating the cardiovascular system.
Keywords: Peptide, calcium channel, vascular endothelial growth factor receptor, cardiomyocyte
1. Introduction
Peptide Lv, a novel bioactive peptide, was discovered by screening the full length human and mouse cDNA databases with an improved bioinformatics strategy modified from the Hidden Markov model [1]. “Peptide Lv” was named according to its ability to enhance L-type voltage-gated calcium channel (L-VGCC) currents in retinal photoreceptors [1]. The genomic sequence of peptide Lv is embedded in the mouse E130203B14Rik gene (Gene ID: 320736), which is predicted to encode a hypothetical immunoglobulin-like protein. The corresponding gene in humans is known as the V-set and transmembrane domain-containing protein 4 (VSTM4, also known as C10orf72; Gene ID: 196740), which is located on chromosome 10 and may be associated with late-onset Alzheimer disease [2]. Chromosome 10 also contains genes encoding chemokines, cadherins, excision repair proteins, early growth response factors, and fibroblast growth receptors. Mutations of genes on chromosome 10 are associated with Charcot-Marie Tooth disease, Jackson-Weiss syndrome, Usher syndrome, nonsyndromatic deafness, Wolman’s syndrome, Cowden syndrome, multiple endocrine neoplasia type 2, and porphyria [3–9]. Down regulation of C10orf72 in cultured breast cancer cells using RNAi enhances the sensitivity to tamoxifen and suppresses the growth of these cells [10]. Even though the identities of the biological proteins translated from E130203B14Rik/C10orf72 are not completely known, based on the evidence mentioned above, identification and characterization of proteins translated from this gene, including peptide Lv, will enable us to fully understand the functions of this gene.
The main peptide Lv coding region contains 49 amino acids in mice and 40 in human, which is conserved across human, mouse, rat, and chicken [1]. The peptide Lv mRNA is widely expressed in various organs including the lung, spleen, intestine, retina, and various regions in the brain. However, the binding partners and receptors of peptide Lv are unknown, which completely cloud the physiological functions of peptide Lv. Since the brain expresses peptide Lv, in this study we applied a proteomics approach using a mouse whole brain preparation, and identified that vascular endothelial growth factor receptor 2 (VEGFR2) is a binding partner of peptide Lv. VEGFR2 (KDR/FLK-1) belongs to a tyrosine kinase receptor family that is subjected to autophosphorylation of tyrosine residues after the receptor is activated via ligand binding [11]. VEGFR signaling is essential for the development of the cardiovascular system during embryonic stages, and it is critical for angiogenesis and wound healing throughout adulthood [11]. There are four major tyrosine autophosphorylation sites (951, 1054/1059, 1175, and 1212/1214) located in the intracellular domain of VEGFR2 [11]. These four phosphorylation sites may elicit different signaling pathways upon activation, thus prompting different functions. Since VEGFR2 is important in the developing heart, we further verified the interaction between peptide Lv and VEGFR2 in the embryonic chicken heart. In this report, we examined the physiological effects of peptide Lv in embryonic chicken cardiomyocytes and human endothelial cells and established a potential functional link between peptide Lv and VEGFR2.
We previously showed that exogenous peptide Lv increases L-VGCC currents in cultured retinal photoreceptors [1]. The augmentation of L-VGCC currents is mainly through the increase of mRNA and protein expressions of L-VGCCα1 subunits. The L-VGCCs are highly expressed in the nervous and cardiovascular systems [12, 13]. The voltage-dependent calcium entry through L-VGCCs promotes various cellular processes including regulating intracellular calcium homeostasis, modulating activities of calcium-dependent enzymes, and further regulating gene expressions [14]. In particular, L-VGCCs are essential for neurotransmitter release from non-spiking neurons, such as retinal photoreceptors and bipolar cells [15]. In cardiomyocytes, the L-VGCCs contribute to the action potential and excitation-contraction coupling [12, 13], and dysregulation of L-VGCCs in the cardiovascular system causes cardiac arrhythmias and heart failure [16, 17]. Since L-VGCCs are important for the function of cardiomyocytes, in this study we further explored whether peptide Lv regulated cardiomyocyte physiology and examined the signaling molecules that mediate the action of peptide Lv.
2. Materials and Methods
2.1. Chicken embryonic cardiomyocyte culture and peptide Lv treatment
All experimental procedures complied with the regulations of Texas A&M University. Fertilized eggs (Gallus gallus, Single Comb White Leghorns) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX, USA). All chicken embryos were maintained at 39°C ± 0.5°C. Chicken hearts were harvested at embryonic day 12 (E12) and ventricular cardiomyocytes were dissociated and cultured on poly-d-lysine/collagen double-coated dishes (for biochemical and molecular biological assays) or coverslips (for electrophysiological recordings) as described previously [18]. The culture medium contained Dulbecco’s modified Eagle’s medium (DMEM; Biowhittaker, Walkersville, MD, USA), 5% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 10% heat-inactivated horse serum (Biowhittaker), 2 mM GlutaMAX (Gibco/Invitrogen, Carlsbad, CA, USA), 50 U/mL penicillin (Sigma, St. Louis, MO, USA), 50 μg/mL streptomycin (Sigma), and 5 μg/mL retinol (Sigma). All cultures were maintained at 39°C ± 0.5°C with 5% CO2. Cells were cultured for 2–3 days and subjected to treatments with vehicle, peptide Lv, or pharmaceutical blockers prior to Western blotting or electrophysiological recordings. SCH202676, DMH4, PD98059, and chelerythrine chloride were purchased from Tocris Bioscience (Minneapolis, MN, USA). Pertussis toxin (PTX) was from Calbiochem/EMD (San Diego, CA, USA). The endogenous ligand for VEGF receptor, VEGFa, and the VEGFa antibody were from Bioss Antibodies (Woburn, MA, USA). Synthesized peptide Lv was obtained from Genscript (Piscataway, NJ, USA) and dissolved in 40% acetic acid/water to reach a stock concentration of 2 mg/ml.
2.2. Reverse transcription PCR (RT-PCR)
Total RNA from mouse eyes, spleen, intestine, lung, and heart were isolated (Qiagen, Valencia, CA, USA). One step RT-PCR amplification (Applied Biosystems/Life Technologies, Grand island, NY, USA) was used for detection of Peptide Lv precursor and GAPDH mRNA expression as described previously [1]. The primers for peptide Lv: sense primer was 5′-GAATTCATGCGGCTCCTAGCGCTGG CGGCGG-3′; antisense primer was 5′-CTCGAGCTACAGCTGGTTCTCCTCGAAGAGGA-3′. For mouse GAPDH, the forward primer was 5′-CATTGTGGAAGGGCTCATGACCA-3′, and reverse primer was 5′-TGGGATGACCTT GCCCACAGCCTTG-3′.
2.3. Western blots and trichloroic acid (TCA) precipitation
Treated or control cells were washed, lysed in radioimmunoprecipitation assay (RIPA) buffer, and proteins were denatured by mixing with 2X Lamelli sample buffer for 5 min at 95°C. Samples were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes. The primary antibodies used in this study were pan L-VGCCα1 (Chemicon/Millipore, Temecula, CA, USA), ERK1/2 (Santa Cruz Biochemicals, Santa Cruz, CA, USA), di-phosphorylated ERK1/2 (pERK1/2, Sigma), actin (Cell Signaling Technology, Danvers, MA, USA), AKT (Cell Signaling Technology), phosphorylated AKT at thr308 (pAKT308; Cell Signaling Technology), phosphorylated PKC α/βII subunit (pPKC α/β; Cell Signaling Technology), phosphorylated tyrosine (pTyr-100; Cell Signaling Technology), VEGFR2 (Cell Signaling Technology), and phosphorylated VEGFR2 at tyr1054/1059 (pVEGFR2 at tyr1054/1059; Cell Signaling Technology). Chickens appear to express a single form of ERK (on the basis of molecular weight), so the antibodies against ERK1/2 and pERK1/2 only label a single band on Western blots, while both antibodies label two bands in samples prepared from mammalian origins [19, 20]. Blots were visualized by the appropriate secondary antibodies and an ECL detection system (Pierce/Thermo Scientific, Rockford, IL, USA). For TCA precipitation, 100% (g/ml) TCA was added to each sample to achieve a final concentration of 20%. The samples were kept on ice for 4 hours then the precipitate was collected by centrifugation. Pellets were washed twice with acetone and completely air dried before addition of SDS sample buffer.
2.4. Electrophysiological recordings and statistical analysis
Ventricular cardiomyocytes were cultured for two days. Whole-cell patch-clamp recordings of L-VGCC currents were carried out from spontaneously pulsing cardiomyocytes [21]. The external solution was (in mM): 145 TEACl, 9 BaCl2, 0.5 MgCl2, 5.5 glucose, 0.1 NiCl2, and 5 HEPES, pH 7.4 with CsOH or TEAOH. The pipette solution was (in mM): 125 Cs acetate, 20 CsCl, 3 MgCl2, 10 EGTA, and 5 HEPES, pH 7.4 adjusted with CsOH. Currents were recorded at room temperature using an A-M Systems model 2400 patch-clamp amplifier (Sequim, WA, USA). Signals were low-pass filtered at 2 kHz and digitized at 5 kHz with Digidata 1440A interface and pCLAMP 10.0 software (Molecular Devices, Sunnyvale, CA, USA). Cardiomyocytes were held at −40 mV, and Ba2+ currents were recorded immediately after whole-cell patches were formed by gentle suction. Current-voltage (I–V) relationships were elicited from a holding potential at −40 mV using 200 ms steps (5 s between steps) over a range from −60 to +60 mV in 10 mV increments. The current densities were calculated by dividing the current amplitudes (pA) by membrane capacitances (pF).
2.5. Co-immunoprecipitation and proteomics with mouse brains
Adult mouse whole brains were collected and lysed in 8 ml immunoprecipitaion buffer (Thermo Scientific) containing a protease inhibitor cocktail (Sigma). The cell lysate was cleaned with 1ml sepharose 4B resin prior to incubation with peptide Lv antibody conjugated sepharose 4B. 10 μg custom-made rabbit polyclonal antibody (Biomatik, Ontario, Canada) was immobilized in 40 μl AminoLink Plus Coupling Resin by following the manufacturer’s protocol (Thermo Scientific). The lysate and antibody were incubated for 5 h at 4°C. The resins were washed three times with immunoprecipitation buffer and twice with PBS. Samples were resolved on 12% SDS-PAGE gels and stained with Coomassie brilliant blue R-250. Four major bands ranging from 40–200 kD were carefully excised and subjected to in-gel digestion and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. Mass spectrometry and database analysis were performed in the Laboratory of Biological Mass Spectrometry, Texas A&M University.
2.6. Co-immunoprecipitation (co-IP)
Eight chicken embryonic hearts (E18) were collected and homogenized in 4 ml of immunoprecipitation buffer (Thermo Scientific). Samples were rotated at 4°C for 2 h to solubilize membrane proteins. Samples were then centrifuged to remove cell debris, and a small portion of the supernatant was taken for protein quantification analyses and for a SDS-PAGE gel subsequently stained with Coomassie brilliant blue R-250. The rest of the supernatant was cleaned with Protein A-agarose beads (GBiosciences, Maryland Heights, MO, USA) followed by incubating the beads with 10 μl of the antibody (anti-peptide Lv or anti-VEGFR2) for 3 h. Because both anti-peptide Lv and anti-VEGFR2 antibodies were derived from the rabbit, we used rabbit IgG in our study as our negative control. Any protein fractions that could co-IP with rabbit IgG, as well as anti-peptide Lv or anti-VEGFR2 antibody, would be considered to be non-specific protein targets of peptide Lv. After incubation, 20 μl of Protein A-agarose was added to each tube and incubated for another 1.5 h. The beads were collected, washed, and processed for Western blotting analysis of peptide Lv and VEGFR2. Western blots were visualized as described previously. For some IPs, antibody preparatory and immunoprecipitation kits (Thermo Scientific) were used to remove heavy and light chain interference. All co-IPs were repeated three times.
2.7. Tetrazolium dye (MTT) colorimetric assay for cell proliferation
Human Umbilical Vein Endothelial Cells (HUVECs) were seeded onto 24-well plates in endothelial cell growth medium (Cell Application, San Diego, CA, USA) and allowed to adhere overnight. The culture media were exchanged to opti-MEM (Cell Application) for 45 min. Peptide Lv (200 or 500 ng/ml) was added to the cells, and the cells were continuously incubated for another 48 h. Opti-MEM with 20% FBS alone acted as the negative control. The proliferation of HUVECs was determined by the MTT assay following the manufacturer’s protocol (Life Technology). In brief, cells were incubated with the MTT solution (1.2 mM final concentration) for 4h at 37°C. Then DMSO was added to a final concentration of 50% in order to break the plasma membrane, and the absorbance at 540 nm was measured by a spectrophotometer.
2.8. Statistics
All data are presented as mean ± standard error of the mean (SEM). Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test for unbalanced n was used for statistical analyses. Throughout, p < 0.05 was regarded as significant.
3. Results
3.1. Peptide Lv enhanced L-VGCC activities in time- and dose-dependent manners in cardiomyocytes
Previously, we demonstrated that peptide Lv enhances the L-VGCC currents in retinal photoreceptors in time- and dose-dependent fashions [1]. Since the L-VGCCs are essential in the excitation-contraction coupling of cardiomyocytes [12, 13], we postulated that peptide Lv might also regulate the L-VGCCs in cardiomyocytes similar to its action in the photoreceptors. To test our hypothesis, cultured embryonic cardiomyocytes were treated with a synthetic peptide Lv for 4 h at 500 ng/ml or 1000 ng/ml followed by the patch-clamp electrophysiological recordings of L-VGCC currents. At 1000 ng/ml, peptide Lv elicited significantly higher L-VGCC currents (Figure 1A). Treatment with peptide Lv for only 30 or 60 min quickly elicited an increase of L-VGCC currents (Figure 1B), which was in part through an increase of protein expression of the pore-forming L-VGCCα1 subunits (Figure 1C). Since the mRNA of peptide Lv was present in various tissues, including the heart (Figure 1D), eye, and various brain areas [1], we next verified the existence of a functional peptide Lv expressed endogenously. If a functional peptide Lv is expressed in the heart, application of an antibody specifically against peptide Lv might affect L-VGCCs by antagonizing the action of endogenous peptide Lv in embryonic cardiomyocytes. Cultured embryonic cardiomyocytes were treated with a specific antibody against peptide Lv for 18–22 h prior to patch-clamp recordings. Cardiomyocytes treated with the anti-peptide Lv antibody (α-peptide Lv) showed decreased L-VGCC currents, while in contrast, cardiomyocytes treated with a denatured anti-peptide Lv antibody did not have diminished L-VGCC currents (Figure 1D). These results demonstrated that exogenous peptide Lv augmented the L-VGCCs by enhancing the expression of L-VGCCα1 subunits, and blocking the endogenous peptide Lv dampened the L-VGCC currents, hereafter indicating a functional role of peptide Lv in cardiomyocytes during embryonic development.
Figure 1. Peptide Lv enhances L-VGCC currents and protein expression in cultured embryonic cardiomyocytes.
(A) The augmentation of L-VGCC currents by peptide Lv was dose-dependent. Cardiomyocytes were dissociated and cultured at E12, and L-VGCC currents were recorded at E14. Cultures were treated with 0, 500, 1000 ng/ml of synthetic peptide Lv for 4 h prior to electrophysiological recordings. There was a stepwise increase in the L-VGCC current densities with 1000 ng/ml peptide Lv being significantly higher. (B) The effect of peptide Lv on L-VGCCs in cardiomyocytes was time-dependent. Cardiomyocytes were treated with synthetic peptide Lv (500 ng/ml) for 0, 30, or 60 min prior to electrophysiological recordings. (C) Treatment with synthetic peptide Lv (500 ng/ml) for 4 h in cultured cardiomyocytes (E12+2) elicited a significant increase of L-VGCCα1 expression (1.98 ± 0.29 folds; * indicates a significant difference at p < 0.05). (D1): The mRNA of the precursor peptide of peptide Lv was detected in the mouse eye, spleen, intestine, lung, and heart. (D2 and D3) A specific antibody against peptide Lv (α-peptide Lv) decreased L-VGCC currents. Cultures were treated with peptide Lv antibody or heat-inactivated peptide Lv antibody (5 μg/ml; denatured antibody) for 24 h prior to electrophysiological recordings. Treatment with the peptide Lv specific antibody decreased the maximal current density of L-VGCCs compared to the L-VGCCs recorded from the control or cardiomyocytes treated with denatured antibody.
3.2. Identification of VEGFR2 (KDR/FLK-1) as a binding partner for peptide Lv
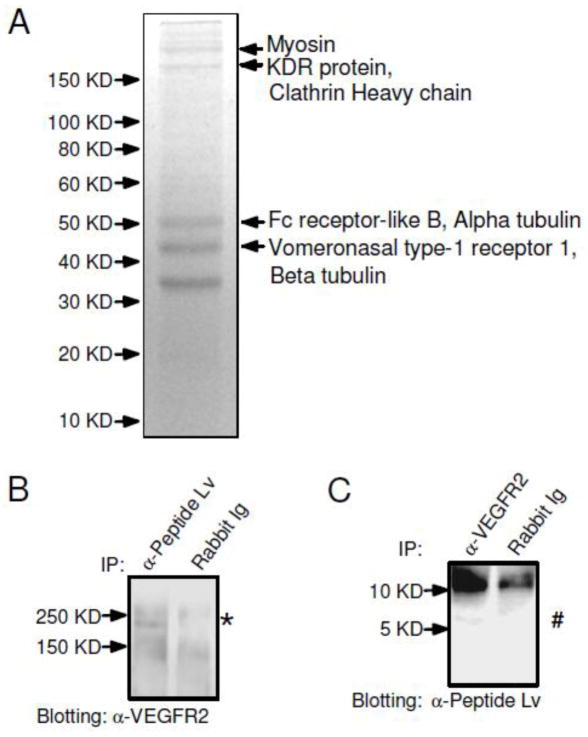
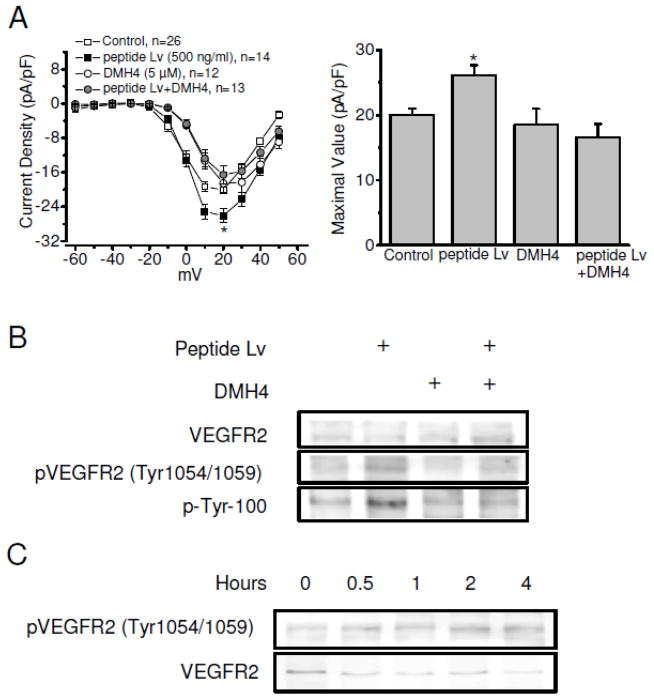
We utilized a proteomics approach to identify potential receptors or binding partners in order to determine the underlying molecular mechanisms of peptide Lv on L-VGCCs in both photoreceptors and cardiomyocytes. Since the mouse brain also expresses peptide Lv abundantly [1] and yields more tissue than the heart, we used a mouse whole brain preparation with co-immunoprecipitation (co-IP) followed by a SDS-PAGE and mass spectrometry analysis to narrow down the potential receptor candidates for peptide Lv. The co-IP samples (using the anti-peptide Lv antibody) were resolved by SDS-PAGE and visualized by Coomassie blue staining (Figure 2A). There were five major bands visible on the SDS-PAGE, but only the top four bands with molecular weights ranging from 40 kD – 200 kD were excised and subjected to MALDI-TOF MS analyses (Figure 2A). The lowest band was not selected for the MS analysis because this protein fraction was also present in the co-IP with a rabbit IgG (as our control), indicating that its interaction with anti-peptide Lv antibody is non-specific. Hence, only the top four major bands were further subjected for the MS-proteomics analyses. Excluding the cytoskeleton chaperone proteins (myosin, clathrin heavy chain, and tubulin), there were three potential receptor-like candidates, including KDR protein (VEGFR2/KDR/FLK-1) (Access No. EDL37891), Fc receptor-like B (Access No. NP_001025155), and vomeronasal type-1 receptor (Access No. NP_035814). The Vomeronasal type-1 receptor is a G-protein coupled pheromone receptor mainly located in the olfactory bulb [22]. The Fc receptor-like B is a member of the Fc receptor family that is involved in phagocytosis, antibody-dependent cell cytotoxicity, and transcytosis [23]. The KDR/FLK1 protein, also known as the vascular endothelial growth factor receptor 2 (VEGFR2), belongs to the tyrosine kinase (TK) receptor family [11]. Further proteomics analysis indicated that VEGFR2 (KDR/FLK1) and vomeronasal type-1 receptor were the possible candidate receptors for peptide Lv. To determine which receptor interacted with peptide Lv, we employed co-IP assays (Figure 2B, C) and found an interaction between peptide Lv and VEGFR2 in the chick hearts. Using the anti-peptide Lv antibody, we were able to co-immunoprecipitate a protein near 250 KD (*) from the E18 chicken hearts that was detected with the anti-VEGFR2 antibody (Figure 2B). While we used the anti-VEGFR2 antibody to co-immunoprecipitate proteins, a protein near 6 KD (#) was detected with the anti-peptide Lv antibody (Figure 2C). In both cases, we did not detect the presence of VEGFR2 or peptide Lv using the rabbit IgG for co-IP (Figures 2B and 2C). In cultured cardiomyocytes, treatment with a selective VEGFR2 inhibitor, DMH4 (5 μM) [24], was able to block the augmentation effect of peptide Lv on L-VGCC currents (Figure 3A), but DMH4 by itself did not affect the L-VGCCs, indicating that the action of peptide Lv on L-VGCCs was in part through the VEGFR2. Hence, VEGFR2 (KDR/FLK1) was a potential receptor for peptide Lv.
Figure 2. VEGFR2 interacts with peptide Lv.
(A) Mouse whole brain lysate samples were incubated with the anti-peptide Lv antibody conjugated with sepharose 4B to determine potential receptors for peptide Lv. Four major bands indicated by arrows with molecular weights ranging from 40 kD to 200 kD were excised for MALDI-TOF analyses. (B, C) Verification of the interaction between peptide Lv and VEGFR2 was carried out in chicken embryonic heart lysate using co-immunoprecipitation (co-IP). (B) Anti-peptide Lv antibody was able to pull down VEGFR2(*). (C) Likewise, anti-VEGFR2 antibody was able to pull down peptide Lv (#). The rabbit IgG (Rabbit Ig) was used as a control.
Figure 3. Peptide Lv stimulates tyrosine phosphorylation of VEGFR2 in cardiomyocytes.
Cultured cardiomyocytes (E12+2) were treated with synthetic peptide Lv (500 ng/ml) or DMH4 (5 μM) in the presence or absence of peptide Lv for 4 h prior to electrophysiological recordings for L-VGCCs (A) or harvest for Western blots (B and C). (A) The average current-voltage (I–V) relationships of L-VGCCs recorded from the control, peptide Lv treated, DMH4, or peptide Lv+DMH4 are shown in the left panel. Treatment with DMH4 (peptide Lv+DMH4) significantly blocked the effect of peptide Lv on L-VGCCs. The maximal values of the L-VGCC current densities (in pA/pF) are: control, 20.86 ± 1.19; peptide Lv treated, 26.11 ± 1.57; DMH4 alone, 18.52 ± 2.46, peptide Lv+DMH4, 16.57 ± 2.06. *p < 0.05. (B) Treatment with peptide Lv increases the tyrosine phosphorylation at tyr1054/1059 of VEGFR2 (pVEGFR2-tyr1054/1059), overall tyrosine phosphorylation (p-tyr-100), and pERK. Treatment with DMH4 blocks these effects. (C) Peptide Lv elicits the phosphorylation of VEGFR2 in a time-dependent manner. Cultured cardiomyocytes were treated with synthetic peptide Lv (500 ng/ml) for 0.5, 1, 2, and 4 h before harvest for Western blots. Phosphorylation of VEGFR2 (pVEGFR2-tyr1054/1059) is upregulated after 0.5 h of peptide Lv treatment and reached maximal levels after 2 hr.
3.3. Peptide Lv stimulated VEGFR2 autophosphorylation in cardiomyocytes
Since VEGFR2 belongs to a tyrosine kinase receptor family that is subjected to autophosphorylation of tyrosine residues after the receptor is activated via ligand binding [11], we next examined whether peptide Lv would elicit tyrosine phosphorylation of VEGFR2. Among the four tyrosine phosphorylation sites on VEGFR2, tyr1054/1059 phosphorylation is required for maximal kinase activation and is considered a prerequisite for activating VEGFR2 signaling [25]. Because the amino acid sequences near tyr1054/1059 of VEGFR2 are highly conserved across the chicken, mouse, and human, the specific antibody against phosphorylated VEGFR2 at tyr1054/1059 (pVEGFR2-tyr1054/1059) was used to detect VEGFR2 autophosphorylation. We also used a p-tyr-100 antibody, an antibody used to detect phosphorylated tyrosine residues, to verify the activation of tyrosine kinases. Cultured chicken embryonic cardiomyocytes were treated with peptide Lv in the presence or absence of DMH4. Peptide Lv elicited enhanced phosphorylation of VEGFR2 (pVEGFR2-tyr1054/1059) and phosphorylated tyrosine, but DMH4 blocked these effects (Figure 3B). We also found that treatment with peptide Lv was able to elicit tyrosine autophosphorylation of VEGFR2 (pVEGFR2-tyr1054/1059) within 30 min, and this phosphorylation was time-dependent with a maximal activation at 2 h (Figure 3C). Hence, VEGFR2 is a candidate receptor for peptide Lv.
3.4. Peptide Lv activated downstream signaling molecules of VEGFR2 in cardiomyocytes
Activation of VEGFR2 triggers several downstream signaling cascades, including phosphoinositide phospholipase C (PLCγ), phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) pathways [26, 27]. PLC participates in phosphatidylinositol 4,5-bisphosphate (PIP2) metabolism and generates inositol triphosphate (IP3) and diacylglycerol (DAG), in which DAG further serves as an activator of protein kinase C (PKC) [28]. In both porcine aortic endothelial cells and cultured cardiomyocytes, activation of VEGFR2 causes phosphorylation and activation of ERK1/2 [29]. Since PKC and ERK are downstream of VEGFR2, we next examined whether these signaling molecules would respond to peptide Lv treatments. In cultured cardiomyocytes, treatment of peptide Lv for 4 h increased the phosphorylation levels of ERK (pERK) and PKC (pPKCα/β, Figure 4A and 4B). While the general G-protein coupled receptor (GPCR) inhibitor SCH202676 did not inhibit the effects of peptide Lv on pERK and pPKCα/β, the VEGFR2 inhibitor DMH4 did (Figure 4B), indicating that peptide Lv was able to elicit activation/phosphorylation of the downstream signaling targets of VEGFR2, the ERK and PKC. Treatment with PD98059 (50 μM), an inhibitor of MEK1 upstream of ERK, decreased the peptide Lv augmentation of L-VGCC currents (Figure 4C), as did the PKC inhibitor chelerythrine (2 μM, Figure 4D). Inhibition of PKC or MEK1-ERK signaling blocked the effect of peptide Lv on L-VGCCs. Hence, peptide Lv’s action of L-VGCC augmentation first went through VEGFR2 binding, which further activated downstream signaling, including PKC and ERK.
Figure 4. Peptide Lv activates downstream signaling molecules of VEGFR2.
(A) Treatment with synthetic peptide Lv (500 ng/ml) for 4 h in cultured cardiomyocytes (E12+2) elicited a significant increase of phosphorylated ERK (2.32 ± 0.34 folds; * indicates a significant difference at p < 0.05). (B) Cultured cardiomyocytes (E12+2) were treated with synthetic peptide Lv (500 μg/ml) in the presence or absence of SCH202676 (2 μM) or DMH4 (5 μM) for 4 h before harvest for Western blots to detect VEGFR2 downstream signaling molecules, phosphorylated ERK (pERK) and phosphorylated PKCα/βII (pPKCα/β). Total ERK and actin served as loading controls. (B) Both pERK and pPKCα/β levels are enhanced after peptide Lv treatments. Treatment with DMH4 blocked these effects. Treatment with SCH202676 (2 μM) slightly decreased the effect of peptide Lv on pERK and pPKCα/β. (C) The average current-voltage (I–V) relationships of L-VGCCs recorded from treatments with the control, peptide Lv, PD98059 (MEK1 inhibitor, 50 μM), or peptide Lv+PD98059 are shown in the left panel. Treatment with PD98059 in the presence or absence of peptide Lv significantly decreased the L-VGCCs (shown as #). The maximal values of the L-VGCC current densities (in pA/pF) are: control, 20.86 ± 1.19; peptide Lv treated, 26.11 ± 1.57; PD98059 alone, 13.06 ± 1.70, peptide Lv+PD98059, 14.27 ± 1.51; *, # indicate p < 0.05. (D) The average current-voltage (I–V) relationships of the L-VGCCs recorded from treatments with the control, peptide Lv, chelerythrine (PKC inhibitor, 2 μM), or peptide Lv+chelerythrine are shown in the left panel. Treatment with chelerythrine in the presence or the absence of peptide Lv significantly decreased the L-VGCCs (shown as #). The maximal values of the L-VGCC current densities (in pA/pF) are: control, 20.86 ± 1.19; peptide Lv treated, 26.11 ± 1.57; chelerythrine alone, 10.27 ± 1.77, peptide Lv+ chelerythrine, 14.13 ± 1.30; *, # indicate p < 0.05.
3.5. The VEGF signaling pathway increases L-VGCC activities in the cardiomyocytes
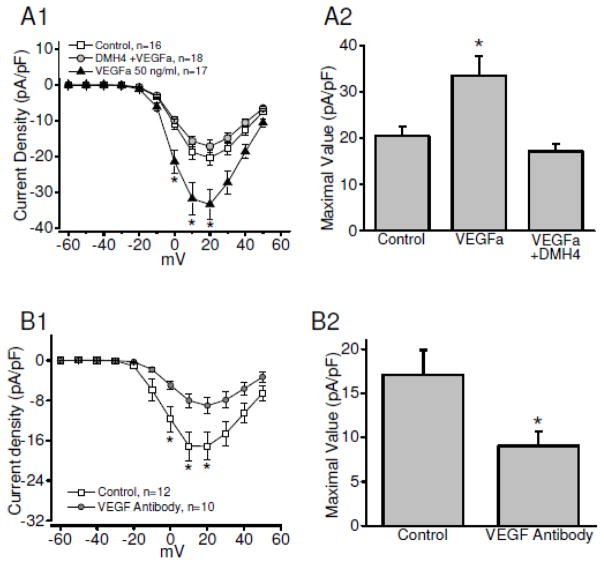
As demonstrated above, peptide Lv augmented L-VGCC activities through the VEGFR2 signaling pathway in chick cardiomyoytes (Figure 3A). While both VEGFR2 and VEGFa (an endogenous VEGFR2 agonist) are present in cultured embryonic or neonatal cardiomyocytes [32], thus far, there is no report on the activation of VEGFR directly eliciting an increase of L-VGCCs in cardiomyocytes. We found that treatment with VEGFa (50 ng/ml) for four hours significantly increased L-VGCC currents (Figure 5A). Treatment with the VEGFR2 antagonist (DMH4, 5μM) blocked the augmentation of L-VGCCs by VEGFa (Figure 5A). Treatment with a specific antibody against VEGFa (VEGF antibody) significantly decreased the L-VGCCs in cultured embryonic cardiomyocytes (Figure 5B), indicating that endogenous VEGF signaling was involved in the regulation of L-VGCCs in embryonic cardiomyocytes. These data support the notion that activation of VEGFR2 can augment the L-VGCCs in cardiomyocytes, which further confirms that peptide Lv mimics endogenous VEGF and increases L-VGCC currents in cardiomyocytes through activation of VEGFR2 signaling.
Figure 5. The endogenous agonist VEGFa mimics the effect of peptide Lv and enhances L-VGCC activity in chicken embryonic cardiomyocytes.
Cultured chicken cardiomyocytes were treated with control, VEGFa (50 ng/ml), or DMH4 (VEGFR2 specific inhibitor, 5 μM) in the presence of VEGFa (DMH4+VEGFa) for 4 hours prior to the electrophysiological recordings for L-VGCCs (A). The average current-voltage (I–V) relationships of L-VGCCs recorded from the control, VEGFa treated, VEGFa+DMH4 are shown in the left panel. VEGFa significantly increases the L-VGCC current density. Treatment with DMH4 (VEGFa+DMH4) significantly blocked the effect of VEGFa on L-VGCCs. The maximal values of the L-VGCC current densities (in pA/pF) are: control, 20.44±2.14; peptide Lv treated, 33.47±4.26; peptide Lv+DMH4, 17.17±1.64. * p < 0.05. (B) Treatment with an antibody specifically against VEGFa (VEGF antibody) for 24 h prior to the electrophysiological recordings decreased the L-VGCC currents in cardiomyocytes. The maximal values of the L-VGCC current densities (in pA/pF) are: control, 17.12±2.76; anti-VEGFa treatment, 9.01±1.62; * p < 0.05.
3.6. Peptide Lv stimulated the VEGFR2 activation in the human umbilical vein endothelial (HUVE) cells
We also tested the interaction between peptide Lv and VEGFR2 in cultured human umbilical vein endothelial cells (HUVECs) to verify that peptide Lv’s activity to activate VEGFR2 was not solely in chicken cardiomyocytes. HUVECs were chosen due to the high expression of VEGFR2 in vascular endothelial cells and the role of VEGFR2 signaling in angiogenesis [11]. VEGFR2 was co-immunoprecipitated with the anti-peptide Lv antibody (α-Peptide Lv) but not with the rabbit IgG from cultured HUVECs (Rabbit Ig; Figure 6A), which was similar to the co-IP result in embryonic chick hearts (Figure 2). To further validate if peptide Lv could also activate VEGFR2 (measured as pVEGFR2) in HUVECs as in chicken cardiomyocytes (Figures 3 and 4), cultured HUVECs were treated with 500 ng/ml peptide Lv for 0, 30, or 60 min before cells were harvested for Western immunoblotting. Antibodies against pVEGFR2 (tyr1054/1059), total VEGFR2, total ERK1/2, and pERK1/2 were used in the Western blots. Treatments with peptide Lv in cultured HUVECs increased both pVEGFR2 and pERK1/2 levels (Figure 6B). Therefore, peptide Lv was able to activate VEGFR2 and its downstream signaling pathway in cultured human endothelial cells, as well as in chicken cardiomyocytes.
Figure 6. Peptide Lv activates VEGFR2 signaling in HUVECs.
(A) Peptide Lv interacts with VEGFR2 in cultured HUVECs. Anti-peptide Lv antibody was able to co-immunoprecipiate VEGFR2 from the HUVEC lysate. (B) Peptide Lv stimulates autophosphorylation of VEGFR2 and ERK1/2 in cultured HUVECs. The HUVECs were treated with 500 ng/ml peptide Lv for 0, 30, and 60 min and harvested for Western blot analyses for pVEGFR2 (tyr1054/1059), VEGFR2, total ERK1/2, and pERK1/2. Both pVEGFR2 and pERK1/2 showed time-dependent increases after peptide Lv treatments. (C) Peptide Lv promotes cell proliferation of HUVECs. The HUVECs were cultured and treated with 0, 200, and 500 ng/ml peptide Lv for 48 hours. The MTT assays were carried out, and light absorbance was measured at 540 nm (OD 540). An increase of OD 540 reflects an increase in DNA synthesis and cell proliferation. Treatment of peptide Lv at 200 and 500 ng/ml significantly increased the OD 540 value compared to the control (n=6). * p < 0.05.
VEGF signaling regulates the proliferation, migration, and survival of endothelial cells and thus promotes angiogenesis [11]. Mutations of VEGFR2 result in deficits in blood-island formation and angiogenesis, which is lethal during embryonic development [33]. In response to angiogenic stimuli, endothelial cells proliferate, migrate, and coalesce to form a primitive vascular system and further recruit smooth muscle cells to give rise to mature blood vessels [34, 35]. Since peptide Lv activated VEGFR2 and its downstream signaling in both cardiomyocytes and vascular endothelial cells, we postulated that peptide Lv might enhance the transformation or production of embryonic endothelial cells thus promoting angiogenesis. The HUVECs were treated with peptide Lv (200 or 500 ng/ml) or vehicle for 48h, and then subjected to the tetrazolium dye (MTT) colorimetric assay to assess cell proliferation (Figure 6C). The cells treated with peptide Lv (both at 200 and 500 ng/ml) had a significant increase in the color absorbance at 540 nm indicating that there were more endothelial cells in the phase of proliferation after treatments with peptide Lv.
4. Discussion
We previously identified peptide Lv, a novel putative peptide that enhances L-VGCC activities in cone photoreceptors through increased expressions of both mRNA and protein levels [1]. In this report, we identified that VEGFR2 (KDR/FLK-1) was a potential receptor for peptide Lv, which was an underlying mechanism for the augmentation of L-VGCCs by peptide Lv in the cardiomyocytes. Peptide Lv was able to activate the phosphorylation of VEGFR2 (KDR/FLK-1) and its downstream signaling, while inhibition of VEGFR2(KDR/FLK1) signaling blocked the actions of peptide Lv. Our results suggest that peptide Lv can serve as a novel activator of VEGFR2 through its augmentation of L-VGCCs in cardiomyocytes and its action on promoting proliferation of endothelial cells. Furthermore, peptide Lv might have angiogenic properties and play a regulatory role in the cardiovascular system.
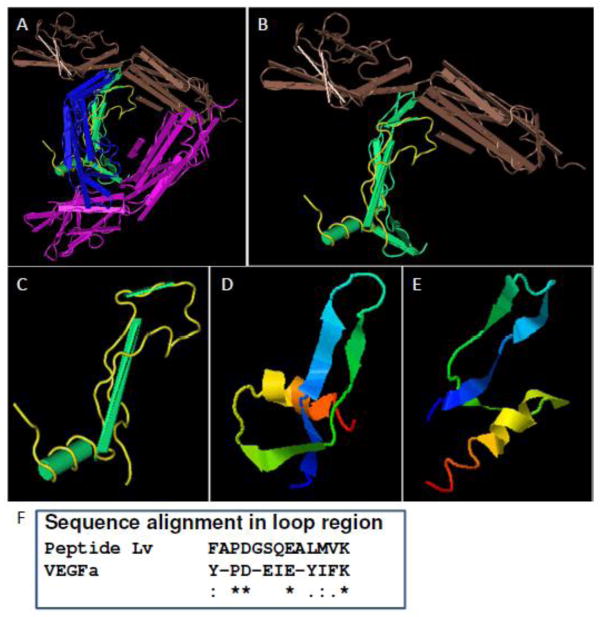
In the cardiovascular system, peptide hormones, such as somatostatin, angiotensin II, and natriuretic peptides (BNP and CNP), are known to be involved in the regulation of heart rate, cardiac contraction, and development [36–38]. Several VEGF family members, including VEGFa, VEGFb, VEGFc, VEGFd, platelet-derived growth factor (PDGF), and placental growth factor (PLGF), can activate VEGFRs to regulate angiogenesis and lymphangiogenesis [11]. Even though we compared the amino acid sequences of peptide Lv and the VEGF family, there is not much homology. Through I-TASSER [39], we obtained a predicted secondary structure for peptide Lv and found some similarities with VEGF members (Figure 7) [40]. Therefore, peptide Lv’s action as a novel activator for VEGFR2 may differ from other VEGF family members interacting with VEGFR2. However, whether peptide Lv could have a synergistic or competitive action with VEGF family members in the cardiovascular system needs future investigations. The mechanism by which peptide Lv interacts with VEGFR2 and promotes VEGFR2 dimerization [11] remains an interesting question to be addressed through further studies. Activation of VEGFR2 signaling elicits a rise of intracellular calcium concentration [30, 31] through an increase of the intracellular calcium store-related proteins or the conductance of transient receptor potential (TRP) channels in various cell types [41–43]. In cardiomyocytes, calcium influx through L-VGCCs triggers calcium release from intracellular calcium stores, activates Ca2+-dependent kinases, and leads to excitation-contraction coupling [13]. The VEGF-PLCγ pathway is known to control cardiac contractility in the embryonic heart [44]. Even though we showed that peptide Lv increased L-VGCC currents and the expression of L-VGCCα1 subunits through the VEGFR2 signaling pathway in cardiomyocytes, whether L-VGCCs are involved in the VEGF-mediated calcium-induced intracellular calcium release needs future investigation. We postulate that the peptide Lv augmentation of L-VGCC protein expression and currents might lead to increased cardiomyocyte contractions, since treatments with other neuropeptides, such as endothelin-1 and angiotensin-II, have shown such enhancement of Ca2+-dependent contraction in cardiomyocytes [45–47].
Figure 7. Predicted peptide Lv secondary structure shows potential similarities to the VEGFa and VEGFR2 interaction domains.
(A) The schematic structure of the VEGFa dimer (blue and green) and VEGFR2 (brown and purple) complex (MMDB ID: 96568, PDB ID: 3V2A). (B and C) The interaction domain located in VEGFa is in yellow. The loop region (yellow) in conjunction with two beta sheets is important for the interaction between VEGFa and VEGFR2. (D and E) The predicted secondary structures of peptide Lv were obtained from the I-TASSER program. The C-scores of the predicted structures are -2.30 (D) and -5 (E), respectively. Both predicted structures contain a helix domain and two beta sheets with a loop formed in between. There are partial similarities among the predicted peptide Lv secondary structure and the VEGFa domain where VEGFa interacts with VEGFR2. (F) The alignment of amino acid sequences in the loop region shows partial homology between peptide Lv and VEGFa. “*” represents identical amino acids. “:” represents highly conserved substitutions.
Our previous result from cone photoreceptors implies that the pertussis toxin (PTX) sensitive G-protein coupled receptors (GPCRs) might be potential candidates as the receptor for peptide Lv [1]. However, our proteomics analysis and co-IP data (Figure 2 and 6) suggested that VEGFR2, a tyrosine kinase family member [11], was a candidate receptor for peptide Lv in cardiomyocytes and vascular endothelial cells. Some members of the GPCR family are known to form a signaling complex with VEGFR2 and evoke both G-protein coupled and receptor-tyrosine kinase (RTK) activities [48]. The crosstalk between GPCRs, G-proteins, and RTKs are critical for endothelial cell proliferation, migration, and angiogenesis [49, 50]. The sphingosine1-phosphate receptor, a PTX sensitive GPCR, is able to form a complex with VEGFR2 and evoke G-protein coupled signaling and tyrosine kinase activity [48]. We observed that PTX blocked peptide Lv-induced augmentation on L-VGCCs and activation of pERK in cardiomyocytes (Supplemental data) and photoreceptors [1]. However, another GPCR inhibitor SCH202676, an allosteric modulator with the ability to inhibit ligand binding to GPCRs [51], did not block the action of peptide Lv in cardiomyocytes (Supplemental data). Pertussis toxin catalyzes ADP-ribosylation of G-proteins, thus impairing the interaction between the receptor and its associated G proteins and inhibits GPCRs [52, 53], while SCH202676 directly blocks the ligand binding sites on GPCRs [51]. Since SCH202676 did not reverse the action of peptide Lv in cardiomyocytes (Supplemental data), peptide Lv might not be directly mediated through GPCRs. Nevertheless, we cannot completely exclude the possibility that peptide Lv might elicit a crosstalk between G-protein mediated signaling and RTK.
In summary, we demonstrated that peptide Lv interacted with VEGFR2 and triggered receptor tyrosine kinase activity, as well as the downstream signaling pathway in cardiomyocytes. Peptide Lv enhanced Ca2+ entry by increasing L-VGCC currents through increasing the expression of L-VGCCα1 subunits. The interaction between peptide Lv and VEGFR2 represents a novel pathway regulating angiogenesis and cardiac physiology. Further investigation on the dynamic complex of peptide Lv, VEGFs, VEGFR2, and additional binding partners will be critical for understanding the physiological function of peptide Lv in the cardiovascular system.
Supplementary Material
Highlights.
Peptide Lv, a novel bioactive peptide, is able to augment the L-VGCCs in cardiomyocytes.
Peptide Lv interacts with VEGFR2 and activates its downstream signaling.
Inhibition of VEGFR2 blocks the action of peptide Lv on L-VGCCs in cardiomyocytes.
Peptide Lv promotes proliferation of endothelial cells.
Acknowledgments
This work was supported by R01EY017452 and R21 EY023339 from the National Eye Institute of the National Institutes of Health to GK, and a postdoctoral research award from the College of Veterinary Medicine and Biomedical Sciences at Texas A&M University to LS. We thank Dr. William Russell, Laboratory for Biological Mass Spectrometry at Texas A&M University for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shi L, Ko ML, Abbott LC, Ko GY. Identification of Peptide lv, a novel putative neuropeptide that regulates the expression of L-type voltage-gated calcium channels in photoreceptors. PLoS One. 2012;7:e43091. doi: 10.1371/journal.pone.0043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupe A, Li Y, Rowland C, Nowotny P, Hinrichs AL, Smemo S, Kauwe JS, Maxwell TJ, Cherny S, Doil L, Tacey K, van Luchene R, Myers A, Wavrant-De Vrieze F, Kaleem M, Hollingworth P, Jehu L, Foy C, Archer N, Hamilton G, Holmans P, Morris CM, Catanese J, Sninsky J, White TJ, Powell J, Hardy J, O’Donovan M, Lovestone S, Jones L, Morris JC, Thal L, Owen M, Williams J, Goate A. A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am J Hum Genet. 2006;78:78–88. doi: 10.1086/498851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Human molecular genetics. 1993;2:851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 4.Doucette L, Merner ND, Cooke S, Ives E, Galutira D, Walsh V, Walsh T, MacLaren L, Cater T, Fernandez B, Green JS, Wilcox ER, Shotland LI, Li XC, Lee M, King MC, Young TL. Profound, prelingual nonsyndromic deafness maps to chromosome 10q21 and is caused by a novel missense mutation in the Usher syndrome type IF gene PCDH15. European journal of human genetics: EJHG. 2009;17:554–564. doi: 10.1038/ejhg.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Lewanda AF, Eluma F, Jerald H, Choi H, Alozie I, Proukakis C, Talbot CC, Jr, Vander Kolk C, Bird LM, et al. Two craniosynostotic syndrome loci, Crouzon and Jackson-Weiss, map to chromosome 10q23–q26. Genomics. 1994;22:418–424. doi: 10.1006/geno.1994.1403. [DOI] [PubMed] [Google Scholar]
- 6.Nelen MR, Padberg GW, Peeters EA, Lin AY, van den Helm B, Frants RR, Coulon V, Goldstein AM, van Reen MM, Easton DF, Eeles RA, Hodgsen S, Mulvihill JJ, Murday VA, Tucker MA, Mariman EC, Starink TM, Ponder BA, Ropers HH, Kremer H, Longy M, Eng C. Localization of the gene for Cowden disease to chromosome 10q22–23. Nat Genet. 1996;13:114–116. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- 7.Nordmann Y, de Verneuil H, Deybach JC, Delfau MH, Grandchamp B. Molecular genetics of porphyrias. Annals of medicine. 1990;22:387–391. doi: 10.3109/07853899009147275. [DOI] [PubMed] [Google Scholar]
- 8.Pagani F, Pariyarath R, Garcia R, Stuani C, Burlina AB, Ruotolo G, Rabusin M, Baralle FE. New lysosomal acid lipase gene mutants explain the phenotype of Wolman disease and cholesteryl ester storage disease. Journal of lipid research. 1998;39:1382–1388. [PubMed] [Google Scholar]
- 9.Wayne S, Der Kaloustian VM, Schloss M, Polomeno R, Scott DA, Hejtmancik JF, Sheffield VC, Smith RJ. Localization of the Usher syndrome type ID gene (Ush1D) to chromosome 10. Human molecular genetics. 1996;5:1689–1692. doi: 10.1093/hmg/5.10.1689. [DOI] [PubMed] [Google Scholar]
- 10.Mendes-Pereira AM, Sims D, Dexter T, Fenwick K, Assiotis I, Kozarewa I, Mitsopoulos C, Hakas J, Zvelebil M, Lord CJ, Ashworth A. Genome-wide functional screen identifies a compendium of genes affecting sensitivity to tamoxifen. Proc Natl Acad Sci U S A. 2012;109:2730–2735. doi: 10.1073/pnas.1018872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 12.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther. 2002;300:724–728. doi: 10.1124/jpet.300.3.724. [DOI] [PubMed] [Google Scholar]
- 15.Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Advances in experimental medicine and biology. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- 16.Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O’Donnell P, Knowles JA, Weissman MM, Coryell W, Scheftner WA, Lawson WB, Levinson DF, Thompson SM, Potash JB, Gould TD. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarotskyy V, Gao G, Peterson BZ, Elmslie KS. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J Physiol. 2009;587:551–565. doi: 10.1113/jphysiol.2008.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko ML, Shi L, Grushin K, Nigussie F, Ko GY. Circadian profiles in the embryonic chick heart: L-type voltage-gated calcium channels and signaling pathways. Chronobiol Int. 2010;27:1673–1696. doi: 10.3109/07420528.2010.514631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 20.Sanada K, Hayashi Y, Harada Y, Okano T, Fukada Y. Role of circadian activation of mitogen-activated protein kinase in chick pineal clock oscillation. J Neurosci. 2000;20:986–991. doi: 10.1523/JNEUROSCI.20-03-00986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko GY, Shi L, Ko ML. Circadian regulation of ion channels and their functions. J Neurochem. 2009;110:1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez I, Greer CA, Mok MY, Mombaerts P. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet. 2000;26:18–19. doi: 10.1038/79124. [DOI] [PubMed] [Google Scholar]
- 23.Chikaev NA, Bykova EA, Najakshin AM, Mechetina LV, Volkova OY, Peklo MM, Shevelev AY, Vlasik TN, Roesch A, Vogt T, Taranin AV. Cloning and characterization of the human FCRL2 gene. Genomics. 2005;85:264–272. doi: 10.1016/j.ygeno.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dayanir V, Meyer RD, Lashkari K, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276:17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 28.Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 29.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 30.Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol. 1997;273:H687–694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham SA, Tran TM, Arrate MP, Bjercke R, Brock TA. KDR activation is crucial for VEGF165-mediated Ca2+ mobilization in human umbilical vein endothelial cells. Am J Physiol. 1999;276:C176–181. doi: 10.1152/ajpcell.1999.276.1.C176. [DOI] [PubMed] [Google Scholar]
- 32.Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, Ruozi G, Camporesi S, Sinagra G, Pepe M, Recchia FA, Giacca M. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]
- 33.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 34.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 35.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 36.Murray F, Bell D, Kelso EJ, Millar BC, McDermott BJ. Positive and negative contractile effects of somatostatin-14 on rat ventricular cardiomyocytes. J Cardiovasc Pharmacol. 2001;37:324–332. doi: 10.1097/00005344-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer J, Azer J, Hua R, Robbins C, Adamczyk A, McBoyle S, Bissell MB, Rose RA. The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J Mol Cell Cardiol. 2012;52:1122–1134. doi: 10.1016/j.yjmcc.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppanen VM, Alitalo K, Winkler FK, Ballmer-Hofer K. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood. 2012;119:1781–1788. doi: 10.1182/blood-2011-11-390922. [DOI] [PubMed] [Google Scholar]
- 41.Cheng HW, James AF, Foster RR, Hancox JC, Bates DO. VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1768–1776. doi: 10.1161/01.ATV.0000231518.86795.0f. [DOI] [PubMed] [Google Scholar]
- 42.Poteser M, Graziani A, Eder P, Yates A, Machler H, Romanin C, Groschner K. Identification of a rare subset of adipose tissue-resident progenitor cells, which express CD133 and TRPC3 as a VEGF-regulated Ca2+ entry channel. FEBS Lett. 2008;582:2696–2702. doi: 10.1016/j.febslet.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Thilo F, Liu Y, Loddenkemper C, Schuelein R, Schmidt A, Yan Z, Zhu Z, Zakrzewicz A, Gollasch M, Tepel M. VEGF regulates TRPC6 channels in podocytes. Nephrol Dial Transplant. 2012;27:921–929. doi: 10.1093/ndt/gfr457. [DOI] [PubMed] [Google Scholar]
- 44.Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, Fishman MC. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–1634. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiello EA, Villa-Abrille MC, Cingolani HE. Autocrine stimulation of cardiac Na(+)-Ca(2+) exchanger currents by endogenous endothelin released by angiotensin II. Circ Res. 2002;90:374–376. doi: 10.1161/hh0402.105373. [DOI] [PubMed] [Google Scholar]
- 46.Chu L, Takahashi R, Norota I, Miyamoto T, Takeishi Y, Ishii K, Kubota I, Endoh M. Signal transduction and Ca2+ signaling in contractile regulation induced by crosstalk between endothelin-1 and norepinephrine in dog ventricular myocardium. Circ Res. 2003;92:1024–1032. doi: 10.1161/01.RES.0000070595.10196.CF. [DOI] [PubMed] [Google Scholar]
- 47.Kaibara M, Mitarai S, Yano K, Kameyama M. Involvement of Na(+)-H+ antiporter in regulation of L-type Ca2+ channel current by angiotensin II in rabbit ventricular myocytes. Circ Res. 1994;75:1121–1125. doi: 10.1161/01.res.75.6.1121. [DOI] [PubMed] [Google Scholar]
- 48.Bergelin N, Lof C, Balthasar S, Kalhori V, Tornquist K. S1P1 and VEGFR-2 form a signaling complex with extracellularly regulated kinase 1/2 and protein kinase C-alpha regulating ML-1 thyroid carcinoma cell migration. Endocrinology. 2010;151:2994–3005. doi: 10.1210/en.2009-1387. [DOI] [PubMed] [Google Scholar]
- 49.Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY. The G protein G alpha(13) is required for growth factor-induced cell migration. Dev Cell. 2006;10:707–718. doi: 10.1016/j.devcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Fawzi AB, Macdonald D, Benbow LL, Smith-Torhan A, Zhang H, Weig BC, Ho G, Tulshian D, Linder ME, Graziano MP. SCH-202676: An allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- 52.Xiao RP, Ji X, Lakatta EG. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- 53.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.