Abstract
Objective
Caregivers of adolescents and young adults (AYA) with complex medical conditions, including brain tumor survivors, have protracted and often complex roles, yet a gap exists in understanding their perceived competence. The aim of this study is to test a hypothesized model based on the theoretical and empirical literature: better caregiver health, better survivor health, and better family functioning contribute directly to fewer caregiving demands, which in turn contribute to greater caregiver competence.
Method
Telephone interviews using structured self-report questionnaires were conducted in this cross-sectional study with a sample of 186 caregivers (mothers) of childhood brain tumor survivors aged 14–40 years old who live with at least one parent. Structural equation modeling (SEM) was used to test the hypothesized model.
Results
The final SEM model suggests that survivor health and family functioning directly predict caregiver competence. Caregiver health indirectly predicts caregiver competence through caregiver demands and then family functioning. Family income directly predicts family functioning. The model showed adequate fit (CFI = 0.905, TFI = 0.880, and RMSEA = 0.081). Overall, the model accounted for 45% of variance in caregiver competence.
Conclusions
For this sample of caregivers of AYA with medically complex conditions, family functioning and the health of survivors are both important to how they evaluate their skills as caregivers. The results of this study underscore the crucial role of care models that focus on optimizing the health of the survivor, caregiver, and family, along with supporting a family centered approach to their care.
Keywords: caregiving demands, caregiving competence, family functioning, medically complex adolescents and young adults, adolescent and young adult brain tumor survivors
Advances in treatment now enable the survival into young adulthood of up to 90% of children with once fatal conditions such as cancer, cystic fibrosis, and neurodegenerative diseases (Cohen et al., 2011; Perrin, Guyer, & Lawrence, 1992). Unlike their healthy peers, these adolescent and young adult (AYAs) survivors often have personal (e.g., delayed developmental milestones, employment, interpersonal relationships), medical (e.g., increasing health complications), and institutional (e.g., economics, education, insurance changes, transportation, transition to adult health care) challenges impeding their transition to independence. Their caregivers, usually their mothers, typically live dramatically altered lives and often continue to function in primary caregiving roles as these children enter adulthood (James et al., 2002; Kuo, Cohen, Agrawal, Berry, & Casey, 2011). Often unpredictable, these caregiving demands may remind them that their children are vulnerable and undermine how competent they feel as caregivers (Gravelle, 1997; Maltby, Kristjanson, & Coleman, 2003).
Caregivers of AYA childhood brain tumor survivors share similar experiences with caregivers of AYA with other serious health conditions because their children survived longer than expected, have multiple chronic conditions, and face challenges when transitioning to independence (Eiser, Eiser, & Greco, 2002). The tumors and their treatment (i.e., surgery, chemotherapy, and cranial and/or spinal irradiation) can result in late effects, including one of the most severe risk profiles for childhood cancer survivors (chronic morbidities and reduced health-related quality of life) and for their caregivers (ongoing care demands) (Deatrick, Mullaney, & Mooney-Doyle, 2009; Forinder & Norberg, 2010; Hutchinson, Willard, Hardy, & Bonner, 2009; Oeffinger et al., 2006; Radcliffe, Bennett, Kazak, Foley, & Phillips, 1996).
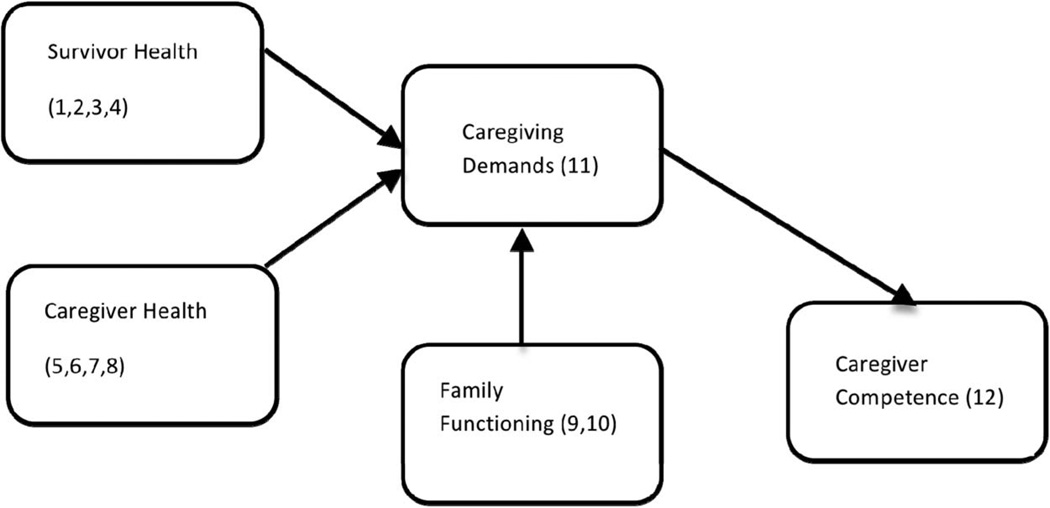
To better understand the functioning of these caregivers, particularly their demands and competency, a review of the empirical literature and a related theoretical model by Raina and colleagues (2004) were used to construct the hypothesized model for this study (see Figure 1). Raina’s stress process model is based on life span caregiving research to predict the psychological and physical health of caregivers according to the following domains: background and contextual factors, child characteristics, caregiver stress/strain, intrapsychic factors, and coping/supportive factors (Raina et al., 2004). In subsequent research, the domains in the model were empirically supported in studies of the psychological and physical health of caregivers for infants, children, and adolescents with serious critical and chronic conditions and cancer (Klassen et al., 2007; Klassen et al., 2011; Raina et al., 2005). These domains were used in the model for this study (see Table 1 and Figure 1).
Figure 1.
Perceived competence for caregivers of childhood brain tumor survivors. Depicted are the domains for the conceptual model and measures for the study. Measures are (1) Pediatric oncology Quality of Life Measure (POQOL); (2) Survivor Demographic Form; (3) Treatment intensity; (4) Medical Sequelae; (5) State–Trait Anxiety Inventory (STAI); (6) Brief Symptom Inventory (BSI) Global Severity Index; (7) (Post Traumatic Stress) Reaction Index (RI); (8) Medical Outcomes Study Short Form 36 Health Survey (SF 36); (9) parent Demographic Form; (10) Family Assessment Device (FAD); (11) Bakas Caregiver Outcomes Scale (BOCOS); (12) Family Management Measure (FaMM) Condition Management Ability Scale.
Table 1.
Comparison of Raina (2004) and Perceived Competence Models: Domains, Factors, Variables, and Measures
| Raina (2004) domains | Raina factors | Competence domains | Competence variables | Competence measures |
|---|---|---|---|---|
| Background/contextual | Socio-economic status | Demographic characteristics | Socio-economic status | Parent report of family Demographics |
| Child characteristics | Function & behavior | Survivor health | HRQOL-emotional | POQOL-emotional |
| HRQOL-physical | POQOL-physical | |||
| Treatment intensity | Treatment intensity | |||
| Late effects | Medical Sequelae | |||
| Post-treatment cognitive ability | Parent report of survivor Demographics | |||
| Outcomes | Psychological & physical health | Caregiver Health | Physical health | SF 36 |
| State and trait anxiety | STAI | |||
| Overall psychological distress | BSI | |||
| Post-traumatic Stress | RI | |||
| Caregiving Stress/Strain | Caregiving demands | Caregiving demands | Caregiving demands | BOCOS |
| Intrapsychic | Self-perception | Caregiver competence | Caregiver competence | FaMM condition management ability |
| Coping & support | Family functioning | Family functioning | General family functioning | FAD general functioning |
The health of the survivor, caregiver, and family are central to the hypothesized model in terms of their influence on caregiver demands. Survivor health includes their emotional health-related quality of life (HRQL), physical HRQL, cognitive late effects, age, time since treatment, treatment intensity, medical sequelae, and life threat because of their risk for complex morbidities and caregiver demands (Oeffinger et al., 2006). Caregiver health includes overall psychological functioning, state anxiety, trait anxiety, posttraumatic stress symptoms, marital status, and physical health, all of which have been identified as being critical to performing as caregivers and meeting the demands of childhood cancer survivors, including brain tumors (Klassen et al., 2007; Litzelman, Catrine, Gangnon, & Witt, 2011). Therefore, caregiver heath transitioned from being an outcome in Raina’s (2004) Model to influencing caregiving demand in this study. Because caregivers and survivors do not function in isolation, evaluation must also extend to the role of the family in determining how caregivers experience the demands of their role. Specifically, family functioning is important in explaining cognitive and behavioral outcomes in children and AYA with brain tumors (Carlson-Green, Morris, & Krawiecki, 1995; Foley, Barakat, Herman-Liu, Radcliffe, & Molloy, 2000; Hocking et al., 2011). Family functioning includes the ability of the family to work as a unit with the caregiver as well as their economic resources because better family functioning and financial resources are associated with the perception of fewer caregiver demands in general childhood cancer populations (Hutchinson et al., 2009; Klassen et al., 2011). In families of brain tumor survivors, members are often concerned about the following factors: the survivors’ abilities before and after treatment; implications of the brain tumor for the future of the child and family; balance between the needs of the family and needs of the child; amount of time and work taken for condition management; ability to meet the needs of the child over the long-term within the context of loss and recovery; and relationships, shared views, mutual support, and flexibility (Anclair, Hoven, Lannering, & Boman, 2009; Aukema, Last, Meeteren, & Grootenhuis, 2011; Deatrick et al., 2009). These concerns continue to impact the family well into adulthood (Hovén, Lannering, Gustafsson, & Boman, 2011; Hutchinson et al., 2009), and therefore family functioning is included in the model for this study as influencing caregiver demand along with survivor and caregiver health.
Caregiving demands are also potentially important to the perceived competence of the caregiver and are examined in most research conducted with caregivers of young children with complex conditions including brain tumors (Hutchinson et al., 2009). The demands or burdens experienced by caregivers are associated with reports of their health; caregivers who experience more demands report less optimal health (Klassen et al., 2007; Klassen et al., 2011; Raina et al., 2004; Vance, Eiser, & Horne, 2004). As Raina proposed, caregivers who perceive that their survivor has less than optimal health undergo more caregiver demands (Bandura, 1991; Hovén et al., 2011), a relationship which is also tested in this research. While used as an outcome measure in Raina’s model, caregiver health is tested in terms of its association with caregiver demands because of the long term impact of caregivers’ emotional and physical health on managing the demands of that role (Deatrick et al., 2009; Hardy et al., 2008; Raina et al., 2004).
Less attention has been directed toward the caregiver’s perceived mastery or competence for actually managing these demands. Caregiver competence is correlated with care recipient outcomes in both adult brain tumor (Sherwood et al., 2007) and childhood chronic illness (Knafl et al., 2011) samples. In terms of caregivers, those who are less healthy (physically and psychologically) experience more demands, and similarly, those who experience more demands feel less competent (Boydell, Stasiulis, Greenberg, Greenberg, & Spiegler, 2008; Klassen et al., 2011; Sherwood et al., 2007). Thus, based on the model proposed for this research (shown in Figure 1), the following hypothesis was tested: better caregiver health, better survivor health, and better family functioning contribute directly to fewer caregiving demands, which in turn contribute to greater caregiver competence.
Method
Participants and Procedures
Based on recommendations for AYA research by the National Cancer Institute, sample criteria (i.e., survivor age, types of tumors) were broad to detect similarities and differences within the sample (Department of Health and Human Services, National Institutes of Health, National Cancer Institute, & LIVESTRONG Young Adult Alliance, 2006). In addition, the broad age range was used as a proxy for survivor development in the hypothesized model to detect for the influence of age on the perceptions of caregiving competence within this cross sectional sample. Mothers whose survivors met the following inclusion criteria were eligible: (a) at least 5 years from diagnosis of a brain tumor, (b) at least 2 years from discontinuation of treatment, and (c) current age 14 through 40 years. In addition, mothers were eligible to participate if they (a) resided in the same household as the survivor and (b) viewed themselves as assuming a major responsibility for the survivor’s care. Mothers were excluded if (a) they were younger than 21, (b) their survivor was married or living in a partnered relationship, (c) their survivor was diagnosed with a genetically based brain tumor (e.g., neurofibromatosis), mental retardation, or developmental delay before cancer, or (d) they did not speak English.
The study protocol was approved by the relevant Institutional Review Boards. Possible subjects were identified using two strategies: (a) a database of a large mid-Atlantic children’s hospital and (b) consecutive entry of newly eligible cases as they were identified in neuro-oncology and survivorship outpatient clinics in the same institution. Unsolicited mailings were sent to caregiver addresses in of all children treated for brain tumors over the last 30 years (n = 1,077) that were identified using facility databases. In addition, caregivers were prescreened using medical records data and approached in the outpatient oncology clinic setting (n = 63) making a total of 1,140 potential participants. Next, a research assistant screened all subjects who had given permission to be contacted and then obtained consent by telephone. Thirty percent (n = 327) of those contacted by mail responded; of those, 37% were ineligible, 17% refused, and 46% provided consent. Ninety percent (n = 57) of caregivers contacted in clinic responded to the invitation to participate; of those, 5% were ineligible, 28% refused, and 67% provided consent. Caregivers (n = 190) were scheduled for telephone data collection. Data collection was completed by 186 (98%) and 4 (2%) were lost to follow-up after consenting and scheduling.
Measures
Using the following measures, data were gathered from caregivers (total of 259 items which took an average of 85 minutes to complete) and professionals and tested for their correlation with caregiver competence. Those indicators that were significantly correlated were used as indicators for model domains, including survivor health, caregiver health, family functioning, caregiving demands, family income, and competence of caregivers.
Mother Self-Report
Survivor health
The Pediatric Oncology Quality of Life scale measured the mother’s perception of the physical aspects of the survivor’s health related quality of life (HRQL physical) and emotional aspects (HRQL emotional) in which lower scores indicate better HRQL. Construct validity data supports the association of these scales of the Pediatric Oncology Quality of Life scale with other validated measures in the expected directions (Goodwin, Boggs, & Graham-Pole, 1994), including discriminating Treatment Status (on or off treatment) for physical HRQL (p < .0006; Bijttebier et al., 2001) and correlating moderately with maternal-reported child anxiety for emotional HRQL (0.64; Kazak & Barakat, 1997). Internal consistency reliability for this sample was 0.79 (emotional) and 0.87 (physical). The survivor’s cognitive late effects (Cognitive Rating) were rated by the mother using a single item 10-point rating of their child’s posttreatment cognitive ability. A higher score indicates poorer functioning.
Caregiver health
The Global Severity Index (GSI) of the Brief Symptom Inventory serves as a normed measure of the mother’s overall psychological distress. Higher scores indicate more distress (Derogatis, 1993; Derogatis & Melisaratos, 1983). The State/Trait Anxiety Inventory (STAI) is a normed measure and assesses current (State) and personality (Trait) anxiety for both clinical and nonclinical populations on a 0–4 point scale. Higher scores indicate higher levels of anxiety (Novy, Nelson, Goodwin, & Rowzee, 1993; Speilberger, 1983). The Reaction Index (RI) uses a 5-point scale to measure the mother’s posttraumatic stress symptoms of intrusion, avoidance, and arousal. Higher item totals reflect greater frequency of symptoms. Construct validation data supports a strong association of the RI with a clinical diagnosis of posttraumatic stress disorder (0.91) (Pynoos, Frederick, Nader, & Arroyo, 1987; Pynoos et al., 1993; Stuber, Christakis, Houskamp, & Kazak, 1996). Internal consistency reliability with this sample was 0.87. The Medical Outcomes Study: Short Form 36 Health Survey (Short Form 36 Health Survey [SF-36]) is a normed instrument that measures the mother’s general health.
Caregiving demands
Bakas Caregiver Outcomes Scale (BCOS) measures current subjective demands of caregiving, including changes in the caregiver’s own well-being, social functioning, and health as a result of providing care, on a −3 to + 3 scale. Higher scores represent positive changes. Construct validation data supports a moderate association (r = .32) of the BCOS with the Sf-36 (Bakas, Champion, Perkins, Farran, & Williams, 2006). Internal consistency reliability for this sample was 0.87.
Family functioning
The General Functioning Scale of the Family Assessment Device (FAD) was used in this study to measure mother’s perceptions of how well the family works. Item responses are averaged across items. Higher scores indicate poorer functioning with the cut-off score for poorer functioning being 2.0. Construct validity is well established with the General Functioning Scale discriminating between nonclinical and clinical samples (p < .001) (Epstein, Baldwin, & Bishop, 1983). Internal consistency reliability for this sample was 0.89.
Caregiver competence
Condition Management Ability (Caregiver Competence) of the Family Management Measure assesses the caregiver’s perceptions about the manageability of the survivor’s condition. Higher scores indicate that the caregiver views the survivor’s condition as more manageable. Construct validation data demonstrated moderate correlations in the expected directions with the Family Assessment Device (−0.35) and the (child) Functional Status II instrument (0.32) (Knafl et al., 2011). Test–retest reliability during instrument development was 0.79 and internal consistency reliability for this Study 0.74.
Demographic information
The following data were gathered from the caregivers about themselves and the survivor: age, education, employment, marital status, race and ethnicity, family income, time since treatment, and health insurance.
Health Professional Report
Treatment intensity and medical sequelae
Intensity of treatment and medical late effects ratings were based on chart review. The information about each patient was abstracted from the medical chart using the treatment flow sheets, letters, and treatment summaries (e.g., radiation summary). The chart was abstracted by graduate level students who had been trained to identify relevant data. The data were then rated by a pediatric oncologist and a pediatric oncology nurse practitioner after patient identifiers were removed.
The Treatment Intensity Rating and the Medical Sequelae Rating (Kazak et al., 2012; Werba et al., 2007) were modified and pilot tested for use with the brain tumor population. Two investigators rated each survivor (Intensity Treatment Rating-Interrater reliability κ = 0.97; Medical Sequelae- Interrater reliability κ = 0.94). The intensity of the child’s treatment regimen was rated on a 5-point ordinal scale from minimum through most. Codes included the following: (1) minimal, resection only; (2) average, focal radiation and/or nonintensive chemotherapy; (3) moderate, moderate chemotherapy (with or without focal radiation but no craniospinal radiation); and (4) intensive, craniospinal radiation (with or without moderate, nonintensive chemotherapy) or high dose chemotherapy with stem cell rescue; and (5) most intensive, craniospinal radiation and intensive chemotherapy with stem cell rescue. The medical sequelae or late effects were rated on a 4-point ordinal scale from minimum (no limitations) to severe restrictions of daily activities (life threatening condition).
Statistical Analysis
Participants’ data were summarized by descriptive statistics, including frequencies and percentages for categorical variables and mean and standard deviation for continuous variables. Demographic predictors and indicators of the five components of the theoretical model (survivor health, caregiver health, caregiving demands, family functioning, and caregiver competence) were then selected by calculating bivariate correlations among the study variables for the (SEM) analysis. The maximum likelihood estimation method of SEM analysis employing Mplus (Version 6.0) and robust standard errors was used to assess the hypothesized model (see Figure 1), which had two latent constructs (survivor health and caregiver health). Standardized parameter estimates were reported and statistical significance of all paths in the model was evaluated by t tests. Multiple fit indices were used to assess the model fit (McDonald & Ho, 2002); including comparative fit index (CFI), Tucker-Lewis index (TFI), and root mean square error of approximation (RMSEA; Hu & Bentler, 1999).
To find the most parsimonious model that was empirically and theoretically justified after the hypothesized model was tested, adjustments were made to improve fit by eliminating sources of error, adjusting paths that were not statistically significant, and consulting the literature. The readjusted models were then retested.
Results
Sample Characteristics
The 186 caregivers’ ranged in age from 30 to 69 years (M = 51.55; SD = 6.36). The survivors ranged in age from 14 to 39 years (M = 20.52 [5.28]). Table 2 and Table 3 describe other demographics for caregivers and survivors respectively. The sample of caregivers was primarily White (88.7%) and married or living with a partner (79%). Nearly half of the caregivers were employed full time (47.3%) and attended or graduated from high school (29%) or college (29%). More than half of survivors were male (56.5%), attended school or work (41%), and had moderate restrictions to daily living (47.9%). Half (50%) of the survivors’ brain tumors were located in the posterior fossa region and the histology of the tumors were primarily low grade glioma (51%) or primitive neuroectodermal tumor/medulloblastoma (27%). Sample characteristics are similar to the 33% (n = 157) of brain tumor survivors living at home with their parents who responded to the baseline mailed survey from the Childhood Cancer Survivor Study (CCSS) (Armstrong et al., 2009; St. Jude Children’s Research Hospital, 2013) in terms of their mean age, gender, inability to work or go to school, and diagnoses.
Table 2.
Caregiver and Family Demographic Data (n = 186)
| Characteristic | Frequency | Percent |
|---|---|---|
| Caregiver race | ||
| African American | 17 | 9.10 |
| Asian and Pacific Islander | 4 | 2.10 |
| White | 165 | 88.70 |
| Caregiver ethnicity | ||
| Hispanic caregiver | 6 | 3.20 |
| Non-Hispanic caregiver | 179 | 96.20 |
| Unknown caregiver | 1 | 0.50 |
| Caregiver employment | ||
| Employed full time | 88 | 47.30 |
| Employed part time | 47 | 25.30 |
| Not employed | 51 | 27.40 |
| Caregiver education | ||
| College | 54 | 29 |
| Graduate school | 33 | 17.70 |
| High school/voc or less than HS | 54 | 29 |
| Other | 5 | 2.70 |
| Some college | 40 | 21.50 |
| Family income (dollars) | ||
| <20k | 9 | 4.80 |
| 20 to 29K | 7 | 3.80 |
| 30 to 39K | 15 | 8.10 |
| 40 to 49K | 12 | 6.50 |
| 50 to 59K | 6 | 3.20 |
| 60 to 74K | 17 | 9.10 |
| 75 to 99K | 33 | 17.70 |
| 100 to 149K | 35 | 18.80 |
| Above 150K | 42 | 22.06 |
| Not reported | 10 | 5.40 |
| Caregiver marital status | ||
| Single/divorced/widow | 39 | 21.00 |
| Married/with partner | 147 | 79.00 |
Table 3.
Demographic and Medical Data for Survivors (n = 186)
| Characteristic | Frequency | Percent |
|---|---|---|
| Survivor education and work (n = 1 missing) | ||
| No school or work | 33 | 17.70 |
| School | 74 | 39.80 |
| Work with or without school | 78 | 41.90 |
| Survivor age in years | ||
| 14–19 | 82 | 44.1 |
| 20–24 | 73 | 39.2 |
| 25–29 | 19 | 10.2 |
| 30–39 | 12 | 6.5 |
| Treatment intensity | ||
| 1. Resection only | 62 | 33.30 |
| 2. Focal radiation ± non-intensive chemotherapy | 55 | 29.60 |
| 3. Moderate chemotherapy ± focal radiation, but no CSI | 19 | 10.20 |
| 4. Craniospinal radiation ± moderate/non-intensive chemotherapy or intensive chemotherapy and stem cell rescue | 45 | 24.20 |
| 5. Craniospinal radiation and intensive chemotherapy and stem cell rescue | 5 | 2.70 |
| Medical sequelae | ||
| 1. Minimal to no limitation in activity | 31 | 16.70 |
| 2. Mild restriction of daily activity | 48 | 25.80 |
| 3. Moderate restriction of daily activity | 89 | 47.90 |
| 4. Severe restriction of daily activity; life threatening | 18 | 9.70 |
| Tumor locations | ||
| Posterior fossa/cerebellar | 94 | 50.54 |
| Cortical/subcortical: all lobes | 33 | 17.74 |
| Suprasellar/hypothalamic (included pituitary) | 29 | 15.59 |
| Pineal | 7 | 3.76 |
| Thalamic/basal ganglia | 6 | 3.23 |
| Ventricle (supratentorial) | 6 | 3.23 |
| Brain stem (tectal) | 5 | 2.69 |
| Optic nerve/chiasm | 4 | 2.15 |
| Brain NOS | 2 | 1.08 |
| Diagnoses | ||
| Low grade glioma | 94 | 50.54 |
| Primitive neuroectodermal tumors (PNET)/medulloblastoma | 51 | 27.42 |
| Craniopharyngioma | 14 | 7.53 |
| High grade glioma | 7 | 3.76 |
| Ependymoma | 5 | 2.69 |
| Germinoma & nongerminoma germ cell | 5 | 2.69 |
| Choroid Plexus | 4 | 2.15 |
| Others | 6 | 3.23 |
Structural Model Testing
Table 4 presents the means, SDs, and ranges for all of the study measures and Table 5 presents the bivariate correlations among the study variables. The following variables were not related to outcome variables and were not included in the SEM analysis: professional assessment of the treatment intensity and medical sequelae, caregiver education, caregiver assessment of life threat and treatment intensity, time since treatment, marital or partner status, and age of survivors. The initial test of the Model (see Figure 1), hypothesizing that survivor health, caregiver health, and family functioning predict caregiver demands and that caregiver demands predict caregiver competence, yielded a poor fit (CFI = 0.770, TFI = 0.708, RMSEA = 0.125). In addition, the path from family functioning to the caregiving demands was not significant.
Table 4.
Descriptive Statistics for Measures
| n | Mean | Minimum | Maximum | SD | |
|---|---|---|---|---|---|
| BCOS | 184 | 63.73 | 27.00 | 105.00 | 14.08 |
| GSI (normed) | 184 | 51.31 | 0.00 | 80.00 | 13.62 |
| State (normed) | 185 | 47.46 | 21.00 | 76.00 | 15.74 |
| Trait (normed) | 185 | 48.91 | 25.00 | 75.00 | 10.65 |
| SF-36 | 186 | 72.69 | 5.00 | 100.00 | 19.86 |
| FAD | 185 | 1.75 | 1.00 | 3.25 | 0.45 |
| Caregiver competence | 183 | 47.81 | 31.00 | 60.00 | 6.81 |
| HRQL-emotional | 186 | 14.25 | 6.00 | 41.00 | 7.89 |
| HRQL-physical | 186 | 25.05 | 9.00 | 57.00 | 12.68 |
| Cognitive rating | 186 | 5.61 | 1.00 | 10.00 | 3.23 |
| RI | 182 | 21.99 | 0 | 65 | 12.33 |
| Time since treatment | 185 | 12.65 | 5.00 | 39.00 | 6.33 |
Table 5.
Bivariate Correlations Among Study Variables
| BCOS | GSI normed |
State norm |
Trait norm |
Cognitive rating |
RI | FAD | Caregiver competence |
HRQL physical |
HRQL emotional |
SF-36 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GSI norm | − .347** | ||||||||||
| State | − .460** | .597** | |||||||||
| Trait | .324** | .670** | .669** | ||||||||
| Cognitive rating | − .162* | .127 | .099 | .096 | |||||||
| RI | .336** | .538** | .476** | .565** | .156 | ||||||
| FAD | .288** | .313** | .387** | .425** | .228** | .213* | |||||
| Caregiver Competence | .298** | − .288** | − .362** | −.363** | − .399** | .355* | − .430** | ||||
| HRQL-Physical | .257** | .149* | .259** | .173* | .493** | .310* | .130 | − .537** | |||
| HRQL-Emotional | .192** | .281** | .381** | .245** | .270** | .291* | .257** | − .412** | .576** | ||
| SF-36 | .218** | − .433** | − .334** | −.397** | −.161* | .361* | − .191** | .277** | − .183* | −.154* | |
| Income | − .027 | .121 | − .080 | −.153* | − .158 | −.085 | −.152* | .227** | − .250** | − .274** | .247** |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
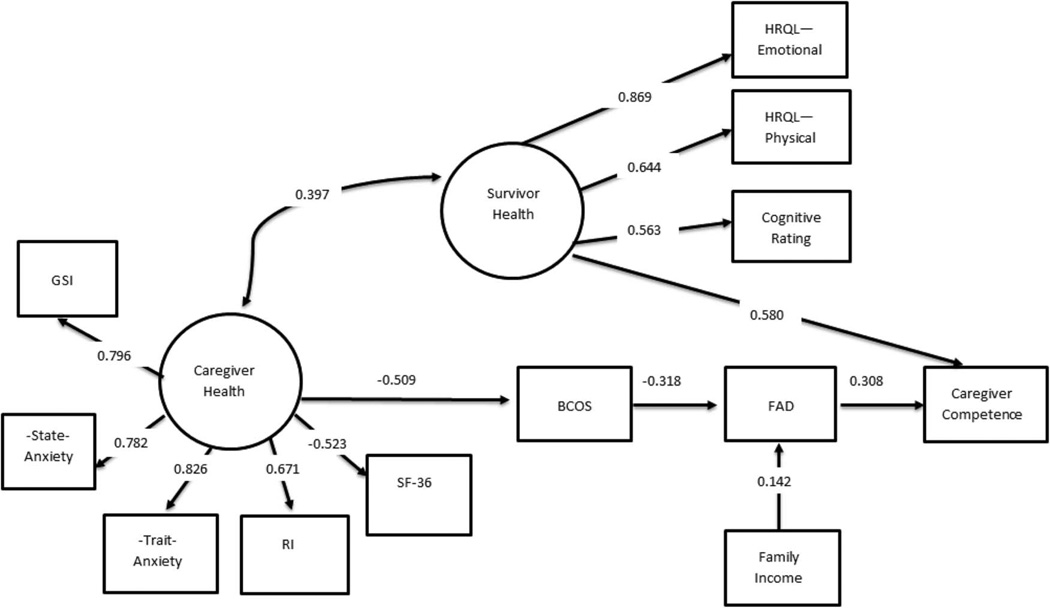
The model was adjusted twice to attempt to improve the fit while being consistent with empirical and theoretical literature. First, both family functioning and demand were tested as mediators of caregiver health and caregiver competence and survivor health was retained as an independent predictor of caregiver ability. The model fit indices were improved (CFI = 0.930, TFI = 0.909, RMSEA = 0.069) but the path from caregiving demands to caregiver competence was not significant. Second, the model was adjusted so that caregiving demands predicted family functioning, which predicted caregiver competence with survivor health remaining as an independent predictor of caregiver competence. This final model with standardized estimates of path coefficients is presented in Figure 2. The final SEM model suggested that survivor health and family functioning predicted caregiver competence, that is, better survivor health and family functioning were associated with better caregiver competence. Better caregiver health indirectly predicted better caregiver competence through decreased perceived caregiver demands and then better family functioning. Higher family income directly predicted better family functioning. Finally, caregiver health and survivor health were moderately correlated. The model showed adequate fit: CFI = 0.904, TFI = 0.878, RMSEA = 0.081. All the measures have significant loadings on the latent constructs (p < .01). All the paths are significant with p < .01 except the effect of income on family functioning is borderline significant with p = .052. Overall the model accounted for 45% of variance in the caregiver competence.
Figure 2.
Final model explaining caregiving competence: survivor, caregiver, and family factors. All the paths are significant at the p < .01 except the effect of income on family functioning is significant at p = .052.
Discussion
The results of the study confirm the importance of family, survivor, and caregiver factors for explaining caregivers’ perceived competence and verify the importance of a family centered approach to their care. Family functioning and the health of the survivor directly influenced the caregiver’s sense of competence or mastery.
The direct relationship of family functioning with caregiving competence emphasizes the central role of family in the adaption of the caregiver to his or her role. Instead of being predicted by caregiver demand as hypothesized, this study revealed that caregivers’ assessment of their role mastery is influenced most strongly by the functioning of their family. This study measured the influence of general family functioning, which includes communicating, problem solving, and decision making because of their consistency with the tasks faced by caregivers for AYA survivors (Alderfer et al., 2008; Epstein et al., 1983). As such, the study goes beyond what is presently known about family functioning and caregiving, as research has generally focused on the influence of family functioning on the psychosocial functioning of the caregiver (Given & Sherwood, 2006; Klassen et al., 2007). These insights provide support for family oriented models when considering the care of AYA with serious health conditions, including those with neurocognitive problems (Hocking et al., 2011; Peterson & Drotar, 2006). This study, however, does not include the important perspectives of these survivors who are dependent on their families for their everyday functioning (Hovén et al., 2011). Additionally, the inclusion of family income in predicting family functioning is certainly supported in both pediatric and adult caregiving research, highlighting the risks of sociodemographic disadvantage (Raina et al., 2004). This is important to consider in future research regarding the complex role that sociodemographic issues play for the survivor, caregiver, and family.
The health of the survivor directly influences the caregiver’s sense of competence, rather than through caregiving demands as hypothesized. Ratings were based solely on caregivers’ perceptions because professional ratings (treatment intensity and medical late effects) were dropped from the model because of statistical nonsignificance. This phenomenon has been noted in previous studies involving the HRQL of cancer survivors; that is, the perceived treatment intensity and medical late effects often derived from survivors or family members are more highly correlated with outcomes than the objective ratings of the treatment team often derived from chart reviews (Hobbie et al., 2000). In fact, other investigators have verified the importance of the caregivers’ perceptions about the patients’ HRQL to their role adaptation. For instance, caregivers’ perceptions about problem behaviors of adult survivors of adult brain tumors directly affected their depressive symptoms and also indirectly affected depressive symptoms by lowering levels of caregiving mastery (Sherwood et al., 2007). In addition, age and time since diagnosis were dropped from the model because of statistical nonsignificance. Therefore, these issues may be important to understanding other phenomenon such as the caregivers’ overall psychosocial adjustment; however, they may not be as important to explaining caregivers’ perceptions of competence. In fact, another study of long-term survivors of childhood brain tumors also found that other issues overshadowed age and time since diagnosis in explaining family related phenomenon (Hovén et al., 2011). The results of this study concerning caregivers for AYA survivors of childhood brain tumors confirm previous research as well as provide new insights into the role of the survivor’s health in caregivers’ appraisals about their caregiving.
As hypothesized, the caregivers’ own health directly predicted caregiving demands which in turn are related to family functioning and finally to caregiver competence. For these caregivers, their health not only becomes important to how they estimate the demands of caregiving, but it may also limit their ability to access resources, their energy for the effort for caregiving, and their motivation.
The primary limitations of this cross sectional study are participation rate and representativeness of the sample, including issues related to diversity. This study is most representative of white, married, non-Hispanic maternal caregivers who are educated and who have economic resources. The national incidence of brain tumors in Black children is lower than that of other racial groups, a phenomenon complicated by the following factors: poorer survival rates, tumor histology, and age at diagnosis (Gurney, Smith, & Bunin, 1999). Nonetheless, the sample for this study did not contain an adequate number of Black children and caregivers, and thus, it is not entirely representative of national incidence rates (Central Brain Tumor Registry of the United States, 2012). In addition, even though being married or partnered was not statistically related to the perception of caregiver competence, the implications for family functioning should be considered within the context of the characteristics of this sample because 79% of the sample was married or partnered. Future research can be designed to better understand the contribution of other family members such as fathers and secondary caregivers. Finally, this sample was compared with an AYA brain tumor sample from the CCSS who live at home with their parents in terms of their mean age, ability to work or attend school, and diagnoses (Armstrong et al., 2009; St. Jude Children’s Research Hospital, 2013). Although these factors are similar in both samples, the age distributions could be compared because the baseline sampling frame on the CCSS analysis included only 15–25 year olds. (The CCSS is now following survivors longitudinally to better understand change over time as well as older survivors.) As recommended by the scientific community (Department of Health and Human Services et al., 2006) the cross sectional study reported in this manuscript used a broad age range as a proxy for survivor development to detect influence of age on the perceptions of caregiving competence.
In addition, all of the measures were completed by one reporter, the caregiver, and single source data can potentially inflate the relationships among variables in the model. Those completed by the professionals were not included in this analysis because they were not statistically related to the outcome of the model. Those completed by survivors (n = 136) were not included in this analysis because of uneven caregiver and survivor sample sizes. Other potential survivor respondents were unable to complete the surveys because of neurocognitive delays. Future analyses will focus on questions regarding the health related quality of life and family functioning using data generated from the 136 survivors.
In summary, the results using data about 186 mothers and survivors who live at home with their parents represent novel information and the largest data set of its kind. This study is an important first step in testing a model that can be used in subsequent cross sectional and longitudinal studies with AYA survivors of brain tumors as well as with other AYA with serious health conditions. Future studies need to modify the model to include additional family members, especially fathers and other important caregivers. In fact, this study lays the foundation for future research studying longitudinal outcomes as well as identifying potentially actionable targets for future interventions focused on the individual or the family. Based on the results of this study, either family functioning or survivor’s health can be targeted to improve competence for caregivers of AYA brain tumor survivors. For instance, interventions targeted to survivor health could emphasize recovery expectations and reframe notions about the survivor’s functioning through family systems and cognitive–behavioral interventions (Thirlaway & Upton, 2009). Alternatively, interventions regarding the survivor’s health using a family ecological perspective could enable the caregiver and survivor to better access coordinated care (Aukema et al., 2011; Kazak, Segal-Andrews, & Johnson, 1995).
Whereas most caregiver interventions have targeted the demands or burden perceived by the individual caregiver, two comprehensive reviews of psychosocial caregiver/family interventions called for interventions designed to improve relationships within the family (Chesla, 2010; Martire, Lustig, Schulz, Miller, & Helgeson, 2004). Understanding how caregivers view their competence vis-à-vis functioning in their family may not only improve relationships within their families but also those with their providers (Knafl, Deatrick, & Havill, 2012).
Families are crucial to the ongoing experience of the caregiver and possibly to their ability to sustain their ongoing roles. Therefore, they need to be integrated into clinical practice standards and guidelines as well as policy initiatives. A recent clinical report from pediatric providers provides clinical guidelines and algorithms for patient-centered and family centered care when transitioning adolescents to adult care, including youth with special health care needs. In addition, Schwartz and colleagues (2011) offer a social-ecological model of AYA readiness for transition that includes individual, family, health care, community level issues. Finally, recent deliberations by the Institute for Family-Centered Care (2013), based on the Institute of Medicine’s work regarding patient quality and safety, highlight the role of family involvement in providing safe care across the life span. Additional professional and public policy is clearly needed to address issues for this population of AYA, their caregivers, and families.
Acknowledgments
We thank the members of the Writers Seminar of the CHOP/PENN Psychosocial Research Curriculum, supported by a K05 Award to Anne E. Kazak, Ph.D. (CA128805) for reading and reviewing prior drafts of this paper, and Emily Watts, RN, BSN for providing editorial assistance. This work was supported by Grant R01 NR009651 from the National Institutes of Nursing Research, National Institutes of Health and an Oncology Nursing Foundation/American Brain Tumor Foundation Grant.
Contributor Information
Janet A. Deatrick, University of Pennsylvania School of Nursing
Wendy Hobbie, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania and University of Pennsylvania School of Nursing.
Sue Ogle, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Michael J. Fisher, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania and University of Pennsylvania Perelman School of Medicine
Lamia Barakat, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania and University of Pennsylvania Perelman School of Medicine.
Thomas Hardie, Drexel University and University of Pennsylvania School of Nursing.
Maureen Reilly, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Yimei Li, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania and University of Pennsylvania Perelman School of Medicine.
Jill P. Ginsberg, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania and University of Pennsylvania Perelman School of Medicine
References
- Alderfer MA, Fiese B, Gold J, Cutuli J, Holmbeck G, Goldbeck L, Patterson J. Evidence-based assessment in pediatric psychology: Family measures. Journal of Pediatric Psychology. 2008;33:1046–1061. doi: 10.1093/jpepsy/jsm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anclair M, Hoven E, Lannering B, Boman K. Parental fears following their child’s brain tumor diagnosis and treatment. Journal of Pediatric Oncology Nursing. 2009;26:68–74. doi: 10.1177/1043454208323912. [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness K, Leisenring W, Packer R. Long-term outcomes among adult survivors of childhood nervous system malignancies in the childhood cancer survivor study. Journal of the National Cancer Institute. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukema EJ, Last B, Meeteren A, Grootenhuis M. Explorative study on the aftercare of pediatric brain tumor survivors: A parents’ perspective. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1637–1646. doi: 10.1007/s00520-010-0995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Champion V, Perkins S, Farran C, Williams L. Psychometric testing of the revised 15-item Bakas caregiving outcomes scale. Nursing Research. 2006;55:346–355. doi: 10.1097/00006199-200609000-00007. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social cognitive theory of self-regulation. Organizational Behavior and Human Decision Processes. 1991;50:248–287. [Google Scholar]
- Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I’ll show them: The social construction of (in) competence in survivors of childhood brain tumors. Journal of Pediatric Oncology Nursing. 2008;25:164–174. doi: 10.1177/1043454208315547. [DOI] [PubMed] [Google Scholar]
- Bijttebier P, Vercruysse T, Vertommen H, Gool S, Uyttebroeck A, Brock P. New evidence on the reliability and validity of the pediatric oncology quality of life scale. Psychology & Health. 2001;16:461–469. [Google Scholar]
- Carlson-Green B, Morris RD, Krawiecki N. Family and illness predictors of outcome in pediatric brain tumors. Journal of Pediatric Psychology. 1995;20:769–784. doi: 10.1093/jpepsy/20.6.769. [DOI] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States. 2012 CBTRUS statistical report tables. Bethesda, MD: National Institutes of Health; 2012. [Google Scholar]
- Chesla CA. Do family interventions improve health? Journal of Family Nursing. 2010;16:355–377. doi: 10.1177/1074840710383145. [DOI] [PubMed] [Google Scholar]
- Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SKM, Simon TD, Srivastava R. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatrick JA, Mullaney EK, Mooney-Doyle K. Exploring family management of childhood brain tumor survivors. Journal of Pediatric Oncology Nursing. 2009;26:303–311. doi: 10.1177/1043454209343210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, National Institutes of Health, National Cancer Institute, & LIVESTRONG Young Adult Alliance. Closing the gap: Research and cancer care imperatives for adolescents and young adults with cancer. Report of the adolescent and young adult oncology progress review group. Bethesda, MD: National Institutes of Health; 2006. (NIH Publication Number 06–6067) Retrieved from www.livestrong.org. [Google Scholar]
- Derogatis L. Brief symptom inventory: Administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Derogatis LR, Melisaratos N. The brief symptom inventory (BSI): An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Eiser C, Eiser R, Greco V. Parenting a child with cancer: Promotion and prevention-focused parenting. Pediatric Rehabilitation. 2002;5:215–221. doi: 10.1080/1363849031000078610. [DOI] [PubMed] [Google Scholar]
- Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. Journal of Marital and Family Therapy. 1983;9:171–180. [Google Scholar]
- Foley B, Barakat L, Herman-Liu A, Radcliffe J, Molloy P. The impact of childhood hypothalamic/chiasmatic brain tumors on child adjustment and family functioning. Children’s Health Care. 2000;29:209–222. [Google Scholar]
- Forinder U, Norberg AL. “Now we have to cope with the rest of our lives.” Existential issues related to parenting a child surviving a brain tumour. Supportive Care in Cancer. 2010;18:543–551. doi: 10.1007/s00520-009-0678-3. [DOI] [PubMed] [Google Scholar]
- Given B, Sherwood PR. Family care for the older person with cancer. Seminars in Oncology Nursing. 2006;22:43–50. doi: 10.1016/j.soncn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Goodwin DAJ, Boggs SR, Graham-Pole J. Development and validation of the pediatric oncology quality of life scale. Psychological Assessment. 1994;6:321–328. [Google Scholar]
- Gravelle AM. Caring for a child with a progressive illness during the complex chronic phase: Parents’ experience of facing adversity. Journal of Advanced Nursing. 1997;25:738–745. doi: 10.1046/j.1365-2648.1997.1997025738.x. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Smith MA, Bunin GR. CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LA, Smith M, Gurney JG, Linet M, Tamara T, Young J, Bunin G, editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1999, SEER program, National Cancer Institute. Bethesda, MD: National Cancer Institute; 1999. pp. 51–64. [Google Scholar]
- Hardy K, Bonner M, Masi R, Hutchinson K, Willard V, Rosoff P. Psychological functioning in parents of adult survivors of childhood cancer. Journal of Pediatric Hematology and Oncology. 2008;30:153–159. doi: 10.1097/MPH.0b013e31815814d9. [DOI] [PubMed] [Google Scholar]
- Hobbie WL, Stuber M, Meeske K, Wissler K, Rourke M, Ruccione K, Kazak A. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. Journal of Clinical Oncology. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- Hocking MC, Hobbie WL, Deatrick JA, Lucas MS, Szabo MM, Volpe EM, Barakat LP. Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. The Clinical Neuropsychologist. 2011;25:942–962. doi: 10.1080/13854046.2011.580284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovén EI, Lannering B, Gustafsson G, Boman KK. Persistent impact of illness on families of adult survivors of childhood central nervous system tumors: A population-based cohort study. Psycho-Oncology. 2011 doi: 10.1002/pon.2067. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hutchinson KC, Willard VW, Hardy KK, Bonner MJ. Adjustment of caregivers of pediatric patients with brain tumors: A cross-sectional analysis. Psycho-Oncology. 2009;18:515–523. doi: 10.1002/pon.1421. [DOI] [PubMed] [Google Scholar]
- Institute for Family Centered Care. Partnering with Patients and Families: A Mini Toolkit. 2013 Retrieved from http://www.ipfcc.org/tools/downloads.html.
- James K, Keegan-Wells D, Hinds PS, Kelly KP, Bond D, Hall B, Speckhart B. The care of my child with cancer: Parents’ perceptions of caregiving demands. Journal of Pediatric Oncology Nursing. 2002;19:218–228. doi: 10.1177/104345420201900606. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Barakat L. Parenting stress: Parenting stress and quality of life during treatment for childhood leukemia predicts child and parent adjustment after treatment ends. Journal of Pediatric Psychology. 1997;22:749–758. doi: 10.1093/jpepsy/22.5.749. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Hocking MC, Ittenbach RF, Meadows AT, Hobbie W, Derosa BW, Reilly A. A revision of the intensity of treatment rating scale: Classifying the intensity of pediatric cancer treatment. Pediatric Blood and Cancer. 2012;59:96–99. doi: 10.1002/pbc.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak A, Segal-Andrews A, Johnson K. Pediatric psychology research and practice: A family/systems approach. In: Roberts M, editor. Handbook of pediatric psychology. New York, NY: The Guilford Press; 1995. pp. 84–104. [Google Scholar]
- Klassen AF, Raina P, McIntosh C, Sung L, Klaassen RJ, O’Donnell M, Dix D. Parents of children with cancer: Which factors explain differences in health-related quality of life? International Journal of Cancer. 2011;129:1190–1198. doi: 10.1002/ijc.25737. [DOI] [PubMed] [Google Scholar]
- Klassen A, Raina P, Reineking S, Dix D, Pritchard S, O’Donnell M. Developing a literature base to understand the caregiving experience of parents of children with cancer: A systematic review of factors related to parental health and well-being. [See comment] Supportive Care in Cancer. 2007;15:807–818. doi: 10.1007/s00520-007-0243-x. [DOI] [PubMed] [Google Scholar]
- Knafl K, Deatrick JA, Gallo A, Dixon J, Grey M, Knafl G, O’Malley J. Assessment of the psychometric properties of the family management measure. Journal of Pediatric Psychology. 2011;36:494–505. doi: 10.1093/jpepsy/jsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafl K, Deatrick J, Havill N. Continued development of the family management style framework. Journal of Family Nursing. 2012;18:11–34. doi: 10.1177/1074840711427294. [DOI] [PubMed] [Google Scholar]
- Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Archives of Pediatrics and Adolescent Medicine. 2011;165:1020–1026. doi: 10.1001/archpediatrics.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzelman K, Catrine K, Gangnon R, Witt WP. Quality of life among parents of children with cancer or brain tumors: The impact of child characteristics and parental psychosocial factors. Quality of Life Research. 2011;20:1261–1269. doi: 10.1007/s11136-011-9854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby H, Kristjanson L, Coleman M. The parenting competency framework: Learning to be a parent of a child with asthma. International Journal of Nursing Practice. 2003;9:368–373. doi: 10.1046/j.1440-172x.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is it beneficial to involve a family member? A meta-analysis of psychosocial interventions for chronic illness. Health Psychology. 2004;23:599–611. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- McDonald RP, Ho MH. Principles and practice in reporting structural equation analyses. Psychological Methods. 2002;7:64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- Novy DM, Nelson DV, Goodwin J, Rowzee RD. Psychometric comparability of the state-trait anxiety inventory for different ethnic subpopulations. Psychological Assessment. 1993;5:343–349. [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT Childhood Cancer Survivor, S. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Perrin J, Guyer B, Lawrence JM. Health care services for children and adolescents. The Future of Children. 1992;2:58–76. [Google Scholar]
- Peterson CC, Drotar D. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: Review of research, clinical evidence, and future directions. Clinical Child Psychology and Psychiatry. 2006;11:349–366. doi: 10.1177/1359104506064980. [DOI] [PubMed] [Google Scholar]
- Pynoos R, Frederick L, Nader K, Arroyo W. Life threat and posttraumatic stress in school age children. Archives of General Psychiatry. 1987;44:1057–1063. doi: 10.1001/archpsyc.1987.01800240031005. [DOI] [PubMed] [Google Scholar]
- Pynoos R, Goenjian A, Tashjian M, Karakashian M, Manjikian R, Manoukian G, Fairbanks L. Posttraumatic stress reactions in children after the 1988 Armenian earthquake. British Journal of Psychiatry. 1993;163:239–247. doi: 10.1192/bjp.163.2.239. [DOI] [PubMed] [Google Scholar]
- Radcliffe J, Bennett D, Kazak A, Foley B, Phillips P. Adjustment in childhood brain tumor survival: Child, mother, and teacher report. Journal of Pediatric Psychology. 1996;21:529–539. doi: 10.1093/jpepsy/21.4.529. [DOI] [PubMed] [Google Scholar]
- Raina P, O’Donnell M, Rosenbaum P, Brehaut J, Walter SD, Russell D, Wood E. The health and well-being of caregivers of children with cerebral palsy. Pediatrics. 2005;115:626–636. doi: 10.1542/peds.2004-1689. [DOI] [PubMed] [Google Scholar]
- Raina P, O’Donnell M, Schwellnus H, Rosenbaum P, King G, Brehaut J, Wood E. Caregiving process and caregiver burden: Conceptual models to guide research and practice. BMC Pediatrics. 2004;4:1–13. doi: 10.1186/1471-2431-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LA, Tuchman L, Hobbie W, Ginsberg J. A social-ecological model of readiness for transition to adult-oriented care for adolescents and young adults with chronic health conditions. Child: Care, Health and Development. 2011;37:883–895. doi: 10.1111/j.1365-2214.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- Sherwood PR, Given BA, Given CW, Schiffman RF, Murman DL, von Eye A, Remer S. The influence of caregiver mastery on depressive symptoms. Journal of Nursing Scholarship. 2007;39:249–255. doi: 10.1111/j.1547-5069.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- Speilberger CD. Manual for the state-trait anxiety inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- St. Jude Children’s Research Hospital. Childhood Cancer Survivor Study. 2013 [Data File]. Retrieved from https://ltfu.stjude.org/childhood-cancer-survivor-study.
- Stuber ML, Christakis D, Houskamp B, Kazak A. Post trauma symptoms in childhood leukemia survivors and their parents. Psychosomatics: Journal of Consultation Liaison Psychiatry. 1996;37:254–261. doi: 10.1016/S0033-3182(96)71564-5. [DOI] [PubMed] [Google Scholar]
- Thirlaway K, Upton D. Evaluating lifestyle psychology. In: Thirlaway K, Upton D, editors. The psychology of lifestyle: Promoting healthy behavior. London, UK: Routledge; 2009. pp. 235–257. [Google Scholar]
- Vance YH, Eiser C, Horne B. Parents’ views of the impact of childhood brain tumours and treatment on young people’s social and family functioning. Clinical Child Psychology and Psychiatry. 2004;9:271–288. [Google Scholar]
- Werba BE, Hobbie W, Kazak AE, Ittenbach RF, Reilly AF, Meadows AT. Classifying the intensity of pediatric cancer treatment protocols: The intensity of treatment rating scale 2.0 (ITR-2) Pediatric Blood & Cancer. 2007;48:673–677. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]