Abstract
Allophanate/oxalurate-induced gene expression in Saccharomyces cerevisiae requires at least five transcription factors, four of which act positively (Gln3p, Gat1p, Dal81p, and Dal82p) and one negatively (Dal80p). Gln3p binds to and Gat1p is proposed to bind to single GATA sequences; Dal80p binds to pairs of specifically oriented and spaced GATA sequences, and Dal82p binds to a pathway-specific element, UISALL. Dal82p consists of at least three domains as follows: (i) UISALL DNA-binding, (ii) transcriptional activation, and (iii) coiled-coilDAL82. Here we show that the coiled-coilDAL82 domain possesses two demonstrable functions. (i) It prevents Dal82p-mediated transcription when inducer is absent. (ii) It is a major, although not exclusive, domain through which the inducer signal is received. Supporting the latter conclusion, a 38-amino acid fragment, containing little more than the coiled-coilDAL82 domain, supports oxalurate-inducible, Dal81p-dependent, reporter gene transcription. Dal81p is required for inducer responsiveness of LexAp-Dal82p and LexAp coiled-coilDAL82-mediated transcription but isn’t needed for inducer-dependent activation mediated by a Dal82p containing deletions in both the coiled-coilDAL82, UISALL-binding domains. There may be an interaction between Dal81p and the coiled-coilDAL82 domain since (i) Dal81p is required for transcription mediated by LexA-coiled-coilDAL82p and (ii) a Dal81p-Dal82p complex is detected by two-hybrid assay.
The transmission of environmental signals to the transcription apparatus has been extensively studied. The molecular communication networks vary widely, involving one or more of the following: protein-protein complex formation, post-translational protein modification, and subcellular localization/exclusion of regulatory proteins (1). The Saccharomyces cerevisiae allantoin pathway genes (DAL1-5 and DUR1-3) encode the permeases and enzymes required to degrade allantoin or its metabolic products to ammonia and carbon dioxide (2). Expression of these genes is subject to several major types of control. First and foremost is nitrogen catabolite repression (NCR)1 in which the GATA family transcription activators Gln3p and Gat1p are excluded from the nucleus and hence cannot reach their GATA-binding sites (3–11). In this way, DAL and DUR gene expression is drastically down-regulated when a good nitrogen source is available (2). When a poor nitrogen source is present, the GATA family transcription activators are nuclear, occupy their GATA-binding sites, and mediate DAL and DUR gene expression (4–8). In contrast to NCR, which is a global control mechanism shared by other nitrogen catabolic gene systems, induced DAL and DUR gene expression represents a second type of control (2). It is allantoin pathway-specific with the inducer being the last intermediate in the pathway, allophanate, or its non-metabolized analogue oxalurate (OXLU) (2).
The broad outlines of DAL gene induction have most recently been derived from analyses of the DAL7 promoter and transcription associated with it (12, 13). Three types of cis-acting sites are located upstream of DAL7 as follows: (i) UASNTR, the Gln3p-, Gat1p-binding sites, consisting of single GATA sequences, (ii) Dal80p-binding sites, consisting of pairs of GATA sequences in specific orientation and spacing, and (iii) UISALL sites that are specifically associated with induction (2, 12, 13). Increasing evidence supports the hypothesis that the repressor, Dal80p, competes with Gln3p and/or Gat1p for binding to the GATA sequences (3, 13, 14). In the absence of inducer, Dal80p-mediated repression prevails, and allantoin pathway genes are expressed at low basal levels (13). In the presence of inducer, Gln3p- Gat1p-mediated transcription prevails and the expression of these genes occurs at high levels. Induction requires the dodecanucleotide, UISALL, and two trans-acting factors, Dal81p and Dal82p (2); Dal82p binds UISALL (15), whereas the function of Dal81p is unknown. Although only 29 kDa, Dal82p possesses at least three demonstrable domains as follows: a UISALL-DNA binding domain (a.a. 1–85), a transcriptional activation domain (a.a. 66–99); and a putative (based on predicted sequence and secondary structure homology) C-terminal coiled-coil domain, designated coiled-coilDAL82 (a.a. 217–255) (16). Deletions within these domains result in loss of only one function. However, deletions within the overlapping region of the UISALL binding and transcriptional activation domains (a.a. 66–85) eliminate both functions. A LexA-Dal82p fusion protein devoid of the coiled-coilDAL82 supports 5-fold greater induced reporter gene expression than full-length wild type (16). The objective of this work is to further understand the roles played by the three Dal82p domains in the induction process.
MATERIALS AND METHODS
Plasmid Constructions
Plasmids, containing deletions of Dal82p binding and activation regions, were derived from pRD41 (15, 16), using the single-stranded template method (pSelect™ mutagenesis kit, Pro-mega Corp.). For each deletion, annealed complementary strand oligonucleotides, respectively, covering 25 bp 5′ and 25 bp 3′ of the desired deletion and maintaining the native reading frame were prepared. All constructs were sequenced to verify that no changes were made other than the designated deletion and to confirm maintenance of the correct reading frame.
Two types of LexAp-Dal82p plasmids were used as follows: 1) a “bait” plasmid for two-hybrid studies was a pEG202 derivative (contains HIS3 marker), and 2) LexAp fusion plasmids for activation studies were BTM116 derivatives (a close derivative of pEG202 containing a TRP1 marker) (both vectors were from Roger Brent’s laboratory (17)). The LexAp DNA binding domain in both plasmids was under control of the yeast ADH1 promoter. The pEG202 derivative, containing DAL82, was constructed using an EcoRI-BamHI adapter (5′-GATCCACCGAT-TCATCCATG-3′ and 5′-AATTCATGGATGAATCGGTG-3′) cloned into pEG202 digested with EcoRI and BamHI. This insert fragment generated the correct reading frame for fusing the LexAp DNA-binding domain to the first 8 Dal82p a.a. The remainder of DAL82 (BamHI-BglII fragment from pM08 (18) was cloned into the BamHI site yielding pSS8202. All of the above plasmids contain 2 μ sequences.
The pBTM116-based vector was constructed by cloning the 500-bp EcoRI-EcoRI fragment from pSS8202 into EcoRI-digested pBTM116. The remainder of DAL82 (a SalI-PstI fragment from pSS20) was then cloned into the SalI-PstI sites. pSS20 was constructed by subcloning the 3.9-kilobase pair EagI-ClaI fragment from pMO8 (18) into YCp50 yielding pSS82BTM. Various deletion derivatives of pSS82BTM were constructed by replacing wild type sequences between the BamHI and XhoI sites with sequences containing the desired deletion from the pRDΔ and pSSΔ plasmids (16).
To construct “prey” pVS8115, full-length DAL81 was cloned, in-frame with B42, into the XhoI site of pJG4-5 using SalI-BsmFI and PstI-SalI polymerase chain reaction adaptors. Full-length DAL81, including its promoter, from pPB14 (19) and pVS8115, was cloned into the SalI and NotI sites of 2-μm pRS423 to yield pSS42381. pSS42381 was used to test the effect of Dal81p overproduction in the two-hybrid assay (20). Construction of LexA-Gln3 pVS3BTM and pVS32 have been described (21).
β-Galactosidase Assay
Transformants, generally appearing 2–3 days after incubation (30 °C), were patched onto plates containing selective medium, incubated 2 days at 30 °C, and then inoculated into 50 ml of liquid YNB media (0.17% YNB without casamino acids or ammonium sulfate, 2% glucose, and 0.1% nitrogen source). Where indicated, inducer (OXLU) was added to a final concentration of 66 mg/liter. These cultures were grown overnight to a density of A600 = 0.45–1.00 and assayed as described earlier (22). Units of activity were calculated as Miller (23) (units = 1000 × A420/time (min) × volume (ml) × A600) except that 10 ml instead of 1 ml of culture were used. Each transformant was assayed in duplicate, and the reported β-galactosidase activities are the average of at least two separate, randomly selected transformants from the same transformation event and in many cases from two independent transformation events.
RESULTS
Dal82p-dependent Transcriptional Activation Requires Dal81p
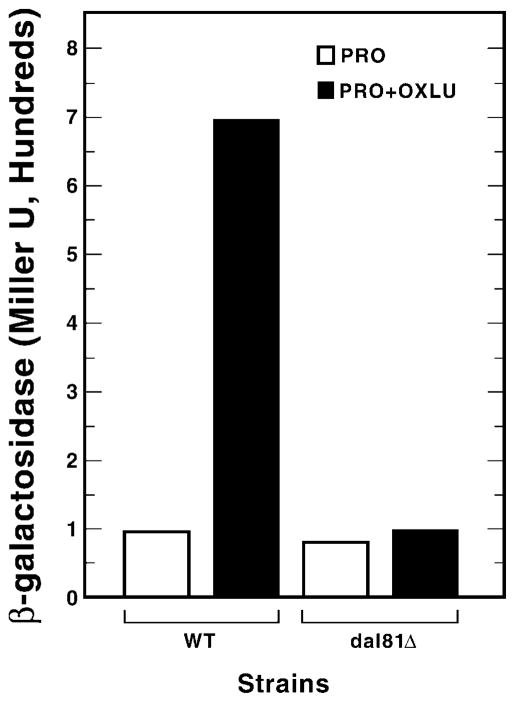
Dal82p supports transcriptional activation when tethered to a UAS-less promoter via LexAp (16). To establish the requirements for this activity, we assayed it in wild type (M1682-19b) and dal81Δ (PB200) strains growing in glucose/proline medium. LexA-Dal82p-mediated transcription requires both Dal81p and inducer, oxalurate (OXLU) (Fig. 1, pSS82BTM). It is important to note that OXLU uptake does not depend upon a permease whose production is Dal81p-dependent.2 Therefore, the Dal81p dependence of lacZ expression doesn’t derive indirectly from a Dal81p requirement for OXLU transport. A small deletion in the Dal82p activation domain (residues 89 to 95) destroys LexA-Dal82p-mediated transcription, demonstrating its absolute requirement for the activity we measured (Fig. 1, pSS480). pSS480 contains the same dal82 deletion as pRD480 which was demonstrated by Western analysis to produce a stable truncated protein. Furthermore, Dal82p produced from pSS480, although transcriptionally in-active, is able to bind DNA fragments containing UISALL (16).
Fig. 1. Requirement of Dal81p for transcriptional activation mediated by LexA-Dal82p (pSS82BTM) and its deletion derivatives.
M1682-19b (WT, wild type) PB200 (dal81Δ) were transformed with reporter pSH18-34 and the indicated pSS82BTM derivatives as follows: pSS480 lacks activation a.a. 89–95; pSS43 lacks coiled-coilDAL82 a.a. 220–255; pSS890 lacks a.a. 89–95 and 220–255; pSS489 lacks UISALL-DNA-binding a.a. 8–16; pSS820 lacks a.a. 8–16 and 220–255; and pSS889 lacks a.a. 8–16 and 89–95. Cultures were assayed after growth in glucose/proline medium with or without OXLU.
Since protein-protein interactions can occur through coiled-coil motifs such as the one in Dal82p, we reasoned that inducer-dependent transcription might derive from Dal81p being recruited to the reporter gene promoter via Dal82p, i.e. Dal82p acts as a bridge between DNA and Dal81p. To test this idea, we removed the putative C-terminal coiled-coilDAL82 (a.a. 217–255) (16). This mutation drastically altered the transcription profile (Fig. 2, pSS43). (i) Induced lacZ expression increased 5-fold with respect to wild type (pSS82BTM) and became largely, but not completely, inducer-independent (Fig. 1, WT, pSS43). (ii) High level expression, similar to that seen in the uninduced wild type, occurred in a dal81Δ, but addition of OXLU no longer increased expression (Fig. 1, pSS43). Again, an additional small activation domain deletion destroyed lacZ expression, demonstrating its epistasis to the coiled-coilDAL82 deletion (plasmid pSS890).
Fig. 2. Requirement of Dal81p for LexA-coiled-coilDAL82-mediated transcriptional activation.
The strains and conditions were as described in Fig. 1. pSSCCBTM, used in this experiment, contains the LexA DNA-binding domain fused to coiled-coilDAL82 a.a. 210–255. WT, wild type.
These experiments revealed several additional characteristics of Dal82p-dependent transcriptional activation. (i) The coiled-coilDAL82 domain mediates strong negative regulation of Dal82p-dependent activation when inducer is absent and continues this role, albeit to a lesser extent, when inducer is present. (ii) Relief of this down-regulation requires inducer and Dal81p whether or not the coiled-coilDAL82 domain is present. (iii) Dal82p-mediated transcription remains 2-fold inducer-responsive even when the coiled-coilDAL82 domain is absent; this response is Dal81p-dependent. (iv) Dal81p is not required for high level transcription if Dal82p lacks its coiled-coilDAL82 domain.
Since the Dal82p transcriptional activation and UISALL-binding domains partially overlap, we next investigated whether alteration of the UISALL-binding domain influenced the LexA-Dal82p-mediated transcriptional activation profile. Note that for this experiment we chose a UISALL-binding domain deletion that did not demonstrably affect transcriptional activation (16). Deleting the Dal82p UISALL-binding domain (amino acids 8–16) did not affect basal level expression but dramatically increased induced transcription to the same high level observed with the coiled-coilDAL82 deletion (Fig. 1, pSS489). Induced transcription, like that supported by wild type pSS82BTM, was completely Dal81p-dependent. In other words, deleting the coiled-coilDAL82 or a portion of the UISALL-binding domain yields the same phenotype in the presence of inducer. As with the coiled-coilDAL82 deletion, a Dal82p activation domain deletion was epistatic to one in the Dal82p UISALL-binding domain (Fig. 1, pSS889).
When deletions were introduced into both the Dal82p coiled-coilDAL82 and UISALL-binding domains, several changes occurred in the activation profile. (i) Reporter gene expression became fully Dal81p- and partially inducer-independent; wild type and dal81Δ strains behaved identically (Fig. 1, pSS820). The induced level with the double mutant, although 2–3 times that seen with the induced wild type LexA-Dal82p (pSS82BTM), was only half the induced level supported by pSS489 and pSS43, or about the same as with pSS43 in a dal81Δ (Fig. 2). (ii) pSS820 retained a clear inducer response, arguing that inducer played a role beyond Dal81p and the coiled-coilDAL82 domain. (iii) Maximum induced Dal82-mediated activation could be achieved by deleting either the coiled-coilDAL82 or UISALL-binding domains, but not both.
The important regulatory role demonstrated above for the coiled-coilDAL82 domain prompted us to determine whether it could function on its own; LexA was fused ahead of sequences encoding the coiled-coilDAL82 (a.a. 210–255, pSSCCBTM, Fig. 2). Remarkably, LexA-coiled-coilDAL82p supports OXLU-induced transcription, although more weakly (6–7-fold) than with full-length LexA-Dal82p (Fig. 2). Transcription is both inducer- and Dal81p-dependent, arguing that the coiled-coilDAL82p is structurally sufficient to mediate basic inducer responsiveness and Dal81p participation in it. On the other hand, inducer and Dal81p function through more than one domain of Dal82p. We base this conclusion on the observation that LexA-Dal82p lacking the coiled-coilDAL82 (pSS43) retains some Dal81p-dependent inducer responsiveness (Fig. 1).
Dal82p-dependent Transcriptional Activation Requires Gln3p
During characterization of cis-acting mutations in the Gln3p-binding sites of DAL7, we observed that single base substitutions in these GATA sequences destroyed their ability to support heterologous reporter gene expression (24). This phenotype could be effectively suppressed if we cloned a UISALL (Dal82p-binding site) adjacent to the mutated GATA sequence. Suppression required both Dal82p and its ability to bind to the UISALL element but did not require inducer or Dal81p (24). These observations pointed to a functional relationship between Gln3p and Dal82p. Therefore, we investigated whether LexAp-Dal82p-mediated transcription was affected by mutations in GLN3. Basal level (uninduced) reporter gene expression supported by full-length LexA-Dal82p and LexA-Dal82p lacking the UISALL-binding domain was largely Gln3p-dependent (Fig. 3A, pSS82BTM and pSS489). Only un-induced levels of β-galactosidase production were assayed because Gln3p is required for OXLU uptake. Although there was demonstrable Gln3p dependence of transcriptional activation supported by a coiled-coilDAL82 deletion, the requirement was modest (~2-fold) (Fig. 3B, pSS43). β-Galactosidase production supported by the coiled-coilDAL82, UISALL-binding domain double mutant (pSS820) was 25% lower than seen with the single coiled-coilDAL82 deletion (pSS43) in gln3Δ strain RR911 and was Gln3p-independent (Fig. 3B, pSS43 open bar and pSS820 open and filled bars). pSS820 supported greater lacZ expression than either pSS82BTM or pSS489 in a gln3Δ. Note that both Dal81p and Gln3p dependence are lost together in the coiled-coilDAL82, UISALL-binding domain double deletion pSS820, although transcription supported by pSS820 in a Gln3+ strain is still inducer-responsive.
Fig. 3. Requirement of Gln3p for transcriptional activation mediated by LexA-Dal82p (pSS82BTM) and its deletion derivatives.

Wild type TCY5 and gln3Δ RR911 were transformed with the indicated plasmids as described in Fig. 1. A and B show β-galactosidase production in glucose/proline grown cultures. C, compares β-galactosidase production in glucose/glutamine and glucose/proline media. WT, wild type.
In most cases, Gln3p dependence of the expression of a gene strongly correlates with it being NCR-sensitive (3), thereby providing an independent indication of Gln3p participation in the expression of a gene. In wild type strain TCY5, β-galactosidase production supported by pSS43 was 2-fold NCR-sensitive; a similar sized effect was observed relative to wild type in proline medium with a gln3Δ (Fig. 3C). Little if any NCR sensitivity occurred when the experiment was performed in gln3Δ (strain RR911) (Fig. 4C). That NCR sensitivity parallels Gln3p dependence of transcription supported by LexA-Dal82p lacking the coiled-coilDAL82 argues in favor of the Gln3p dependence.
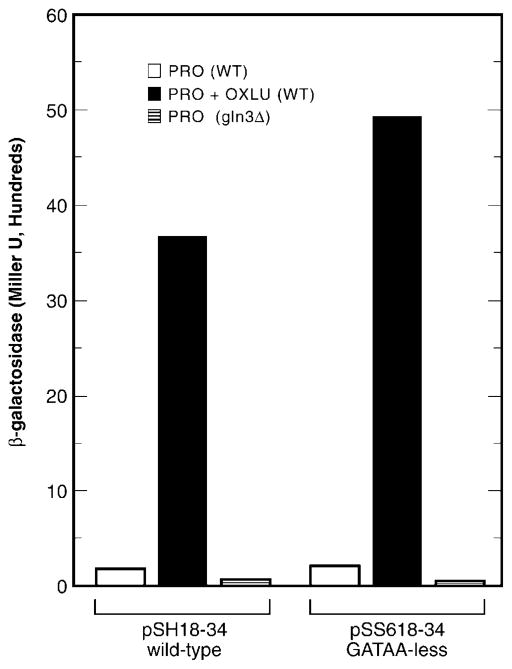
Fig. 4. LexA-Dal82p-mediated transcription supported by wild type reporter pSH18-34 or a derivative of this plasmid containing mutated GATA sequences (pSS618-34).
pSH18-34 or mutant pSS618-34 was used to transform either wild type TCY5 or gln3Δ. Transformants were grown on glucose/proline medium with or without OXLU prior to assaying reporter gene expression. WT, wild type.
A possible, trivial explanation for the Gln3p dependence of Dal82p-mediated activation might derive from the existence of Gln3p-binding sites near the LexAp-binding sites. If such sites exist, it is conceivable that we are reconstituting a more or less ordinary allantoin pathway gene promoter with binding of the Dal82p to the promoter occurring through LexAp-binding sites rather than a UISALL element. A computer search revealed two vector GATAA sequences 5 and 50 bp from the LexAp-binding sites. To determine whether these gratuitous sites accounted for the observed Gln3p dependence, we mutated them. β-Galactosidase production with wild type pSH18-34 was the same as with pSS618-34 in which the GATA sequences were mutated, arguing against the possibility of such an artifact (Fig. 4).
Dal81p and Dal82p Interact in a Two-hybrid Assay
One way Dal81p dependence of LexA-Dal82p-mediated transcription might occur is via formation of a Dal81p-Dal82p complex; data obtained with pSSCCBTM (Fig. 2), containing the coiled-coilDAL82 domain, are consistent with such a hypothesis. Because we were reluctant to tamper with the Dal82p activation domain, we used LexA-DAL82 pSS8202 as bait (Fig. 5). Without inducer, there was little transcription with negative control B42 activation domain pJG4-5 whether glucose or galactose was used as carbon source (Fig. 5B, prey, pJG4-5 is galactose-inducible). In proline + OXLU medium, irrespective of the carbon source provided, β-galactosidase production is high because of the ability of LexA-Dal82p (the bait) to support inducer-dependent activation. A similar result was observed when Dal81-B42 pVS8115 was used as prey (Fig. 5C). If overproducing full-length Dal81p influenced the transcriptional activation assay, we would have detected it here; there was no effect, and the profile was the same as with the B42 vector alone (Fig. 5D). It is also important to note the lack of β-galactosidase production with ammonia or glutamine as nitrogen source in the control experiments (Fig. 5, A–C). In contrast, when Dal81-B42 pVS8115 was the prey and galactose was the carbon source, high level β-galactosidase production occurred in minimal glutamine, ammonia, or proline medium, all devoid of inducer (Fig. 5D). Addition of inducer had no demonstrable effect upon the assay results except in glucose/proline medium where it was expected. In other words, the DAL81 prey yielded positive results in the absence of inducer only when the B42 activation domain was fused to it (Fig. 5, compare C and D).
Fig. 5. Two-hybrid assay with LexA-Dal82p as bait and B42-Dal81p as prey.
A, results obtained when control vectors were used as bait (pEG202) and prey (pJG4-5). B, β-galactosidase production in wild type EGY48 transformed with reporter pSH18-34, bait pSS8202 (LexA-Dal82p), and galactose-inducible B42 activation pJG4-5. Transformants were grown with either glucose or galactose. C is the same with the exception that the B42 activation plasmid was fused to Dal81p (pVS8115). The cultures were grown with the designated nitrogen sources as follows: GLN, glutamine; NH4, ammonia; PRO, proline. D, full-length Dal81p (but lacking the fused B42 activation domain) expressed from a 2-μm plasmid.
Although we would have preferred using a bait plasmid that was transcriptionally inactive whether or not inducer was present, the most straightforward interpretation of the data obtained in inducer-free medium is that the transcription we observed derived from the B42p activation domain of the Dal81p-B42 prey plasmid. The result argues in favor of a protein-protein interaction between Dal81p and Dal82p. High level reporter gene expression with repressive nitrogen sources supports this interpretation. If the activity derived solely from Dal82p-mediated activation, it would be NCR-sensitive (see Fig. 5).
Another observation supports the contention of a functional relationship between Dal81p and Dal82p. Overproduction of Dal81p or Gln3p, but not Dal82p, is toxic, resulting in slow growth (25).3 Simultaneous overproduction of Dal82p along with either Dal81p or Gln3p, however, suppresses this toxicity (data not shown).
SAGA Complex Requirements for LexAp-Gln3p and LexAp-Dal82p-mediated Activation
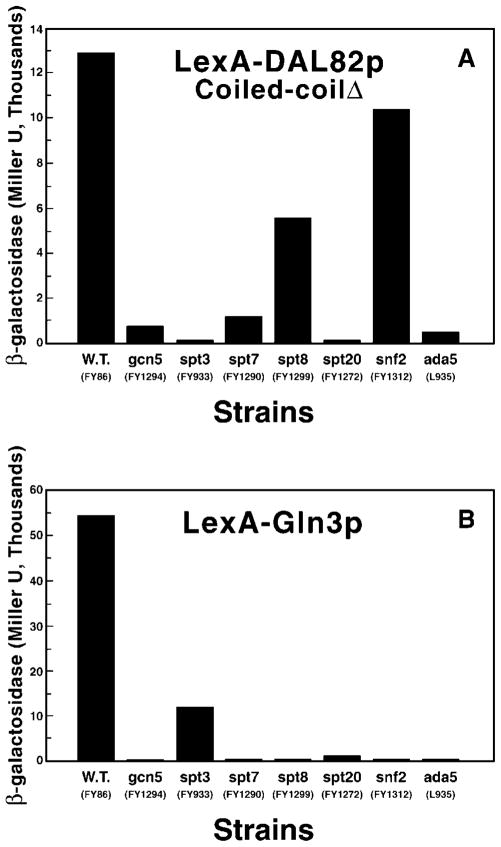
The partial requirement of Gln3p for Dal82p-mediated transcriptional activation (Fig. 3) and the growth phenotype suppression data just discussed raise the possibility that Gln3p might complex to Dal82p and thereby account for its activation capability. If such an interaction occurred, the SAGA components required for Dal82p-and Gln3p-mediated transcription would probably be similar since transcriptional activation would be achieved by Gln3p in both cases (26, 27). To evaluate this possibility, we compared the SAGA requirement profiles of transcription mediated by LexA-Dal82p lacking the coiled-coilDAL82 and LexA-Gln3p (Fig. 6). Coiled-coilDAL82 minus Dal82p was used for this experiment in preference to the inducer-dependent full-length protein, because Gln3p is required for uptake of inducer thereby compromising results obtained in the presence of inducer. The SAGA requirements of LexA-Gln3p and LexA-Dal82p differ for Spt3p, Spt8p, Spt20p, and Snf2p and maybe Spt7p and Gcn5p. LexAp-Dal82p-mediated activation possesses a greater requirement for Spt3p and smaller requirements for Spt8p, Snf2p, and perhaps Spt7p than that mediated by LexAp-Gln3p. Both proteins possess strong requirements for Spt20p/Ada5p.
Fig. 6. Requirement profiles of SAGA components for transcriptional activation mediated by LexAp-Dal82p (coiled-coilDAL82Δ).
(A) or LexAp-Gln3p (B). Strains with deletions in GCN5, SPT3, -7, -8, -20, SPT2, or ADA5 (strains listed in Table I) were transformed with reporter pSH18-34 and either pSS43 or pVS3BTM. In some cases pVS32, a plasmid similar to VS3BTM but with different selectable markers was used in place of pVS3BTM. Cultures were grown in glucose/proline medium and assayed as described in Fig. 1.
DISCUSSION
The above data provide further insight into the mechanism of allophanate-induced gene expression. Fig. 7 depicts a very preliminary working hypothesis whose function is to provide a way of organizing and visualizing the large number of observations and correlations we have made; it is not meant to propose/summarize a definitive explanation of the induction process. Multiple domains of Dal82p, the UISALL-binding protein, play significant roles in this process. The Dal82p C-terminal coiled-coilDAL82 domain appears to be central to down-regulating Dal82p-mediated transcription in the absence of inducer. Moreover, coiled-coilDAL82 is one of the domains through which Dal81p participates in the induction process as evidenced by the following observations: (i) a LexA-coiled-coilDAL82 fusion protein alone can mediate inducer- and Dal81p-dependent transcriptional activation, and (ii) transcription mediated by dal82 constructs lacking coiled-coilDAL82 is largely Dal81p-independent. Dal81p appears necessary to neutralize negative control of OXLU-induced transcription that occurs through coiled-coilDAL82. Similar results would be expected, however, if Dal81p was potentiating the elimination of negative control that occurs through another mechanism.
Fig. 7.

Working hypothesis used as a means to organize, visualize, and correlate data presented in this work.
Data in Fig. 5 lead us to conclude that a complex forms between Dal81p and Dal82p, possibly through the coiled-coilDAL82 domain. However, we were unable to demonstrate direct participation of coiled-coilDAL82 in complex formation experimentally because (i) a LexA-coiled-coilDAL82p bait does not appear to function in the two-hybrid assay with Dal81p as prey, and (ii) a coiled-coilDAL82 minus DAL82 construct (pSS43) cannot be used as a bait because of the high level transcription it supports on its own. The alternative approach to this question is to employ Dal81p as the bait. Unfortunately, characterization of the very large Dal81p and its potential domains are not available because so far the cloning experiments have, for unknown reasons, not been possible.
Although the coiled-coilDAL82 is a major domain associated with inducer and Dal81p function, it is not unique in this regard. Inducer responsiveness of the coiled-coilDAL82, UISALL-binding domain single deletion mutants (pSS43 and pSS489) and the double deletion mutant (pSS820) argues that the inducer signal, and the participation of Dal81p in responding to it, must occur through a second part of Dal82p as well. In addition, the induction characteristics of a coiled-coilDAL82 deletion alone and in combination with a binding site deletion as well as those of the LexA-coiled-coilDAL82p argues against the inducer simply binding to either Dal81p or Dal82p and seems to implicate an as yet unknown protein that is responsible for sensing the presence of the inducer. This reasoning is based on the following facts: (i) transcription mediated by the LexA-coiled-coilDAL82p (pSSCCBTM, Fig. 2) responds to inducer even though there is little more than the coiled-coilDAL82 present in the construct, (ii) LexA-Dal82p lacking the coiled-coilDAL82 (pSS43) remains inducer-responsive, and (iii) the dal82 UISALL DNA binding, coiled-coilDAL82 double deletion (pSS820) supports inducer-responsive reporter gene expression that also occurs in a dal81Δ recipient.
Another novel characteristic of the Dal81-Dal82 interaction is the negative regulation of the ability of Dal82p to mediate transcription in the absence of inducer. Here, although there is some loss of this negative regulation in a DNA-binding site deletion mutant, this domain does not seem to play as important a role as the coiled-coilDAL82. The centrality of the coiled-coilDAL82 in this function is further supported by the fact that one observes negative regulation of the ability of the isolated coiled-coilDAL82 to mediate reporter gene transcription in the absence of inducer. Dal82p has previously been characterized as a strictly positive regulatory protein. However, from this vantage point its coiled-coilDAL82 can also be considered a negative regulator of Dal82p-mediated transcription in the absence of inducer and that a potential function of Dal81p is to participate in the removal of this negative regulation or potentiation of its removal. There is another participant in the removal of negative regulation when inducer is present because limited induction remains in dal81 mutants (Fig. 1, pSS820).
A possible explanation for these results is that the coiled-coilDAL82, or some other part of the protein which is influenced by the coiled-coilDAL82, can mask the Dal82p transcriptional activation domain. By this reasoning, Dal81p, in the presence of inducer, interacts with the coiled-coilDAL82, and this complex unmasks the activation domain. The binding site deletion argues that a second portion of Dal82p participates in masking/unmasking the activation domain as well.
Separate from the Dal81p, Gln3p also participates in Dal82p-mediated transcription. Two lines of evidence support this proposal. (i) UISALL/Dal82p suppression of Gln3p-binding site mutation (mutations in the GATA sequence) phenotypes occurs in the absence of Dal81p and inducer (24). (ii) Dal82p participates in transcription mediated by CAR2 promoter fragments in the absence of inducer and Dal81p (28). Although there are no Gln3p-binding sites in CAR2, a functional UAS (RAP1-binding site) is required for UISALL to mediate transcription (28). Another possibility is that Dal81p forms a bridge between Dal82p and Gln3p which in turn interacts with the core transcription apparatus. This is less likely since (i) different subsets of SAGA components are required for LexA-Dal82p and LexA-Gln3p-mediated transcriptional activation, and (ii) Dal82p possesses a transcriptional activation domain and is able to support transcriptional activation in the absence of Gln3p or Dal81p. Objectively, however, we must also concede the existence of an alternative explanation for the SAGA results. It is possible that the Dal82p-Gln3p complex formation alters the conformation of Gln3p, and it is this alteration that is reflected in the Dal82p-specific SAGA component requirements. We have not distinguished between the two possibilities.
These arguments lead to our current hypothesis that the functional interaction that appears to exist between Gln3p and Dal82p is not a direct Gln3p-Dal82p protein-protein complex but involves components of the core transcription complex. The bridge could be the SAGA complex itself with Gln3p and Dal82p contacting it at different points. By this view, one would expect the existing indications of a Gln3p-Dal82p interaction (24) but without a demonstrable direct interaction. Although some components of the allophanate-mediated induction of transcription quite likely remain to be found (the hypothetical inducer binding protein), and the detailed protein-protein interactions are only now coming into focus, progress is being made toward filling in the missing pieces of the mechanism. This information will no doubt be prerequisite to understanding how UISALL-Dal82p-Dal81p and inducer are able to tip the equilibrium of Dal80p competing with Gln3p for binding to the GATA sequences upstream of the allantoin pathway genes in favor of Gln3p.
Table I.
Strains used in this work
| M1628–19b | MATa ura3-52 trp1-289 |
| PB200 | MATa trp1-289 dal81::hisG |
| TCY5 | MATα lys2 ura3 trp1 |
| RR911 | MATα lys2 ura3 gln3::hisG trp1::hisG |
| EGY48 | MATα 3lexAop::leu2 ura3 trp1 his3 |
| FY86a | MATα his3Δ200 ura3-52 leu2Δ1 |
| FY933 | MATα spt3Δ202 lys2-173R2 his3Δ200 ura3-52 leu2Δ1 |
| FY1272 | MATα spt20Δ200::ARG4 arg4-12 leu2Δ1 lys2-173R2 trp1Δ63 ura3-52 his3Δ200 |
| FY1290 | MATα trp1Δ63 leu2Δ1 spt7Δ402::LEU2 lys2-173R2 his3Δ200 ura 3-52 |
| FY1294 | MATα his3Δ200 gcn5Δ::HIS3 leu2Δ1 ura3-52 trp1Δ63 |
| FY1299 | MATα his3Δ200 trp1Δ63 lys 2-173R2 ura3-52 leu2Δ1 spt8Δ302::LEU2 |
| FY1312 | MATα leu2Δ1 snf2Δ::LEU2 trp1Δ63 ura3-52 lys2-173-R2 |
| L935 | MATα ura3 leu2 trp1Δ63 ada5Δ/spt20Δ his4 |
The last eight strains were generously provided by Fred Winston and colleagues (26).
Acknowledgments
We thank Dr. Fred Winston and colleagues for the SAGA mutant strains, Dr. Vladimir Svetlov for LexA-Gln3 pVS8115 and pVS32 as well as DAL81-B42 pVS8115, the University of Tennessee Yeast Group for suggested improvements to the manuscript, and Tim Higgins for preparing the figures.
Footnotes
This work was supported by National Institutes of Health Grant GM-35642.
The abbreviations used are: NCR, nitrogen catabolite repression; a.a., amino acid; bp, base pair; OXLU, oxalurate.
J. McKelvey and T. G. Cooper, unpublished observations.
S. Scott and T. G. Cooper, unpublished observations.
References
- 1.Thevelein JM, De Winde JH. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper TG. In: Mycota III. Marzluf G, Bambrl R, editors. Springer-Verlag; Berlin: 1994. pp. 139–169. [Google Scholar]
- 3.ter Schure EG, van Riel NA, Verrips CT. FEMS Microbiol Rev. 2000;24:67–83. doi: 10.1111/j.1574-6976.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck T, Hall MN. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas ME, Cutler NS, Lorenz M, Di Como CJ, Heitman J. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber S. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox K, Rai R, Distler M, Daugherty JR, Coffman JA, Cooper TG. J Biol Chem. 2000;275:17611–17618. doi: 10.1074/jbc.M001648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham TS, Andhare R, Cooper TG. J Biol Chem. 2000;275:14408–14414. doi: 10.1074/jbc.275.19.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edskes HK, Hanover JA, Wickner RB. Genetics. 1999;153:585–594. doi: 10.1093/genetics/153.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber Edskes HK, Gray VT, Wickner RB. Proc Natl Acad Sci U S A. 1999;96:1498–1503. doi: 10.1073/pnas.96.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edskes HK, Wickner RB. Proc Natl Acad Sci U S A. 2000;97:6625–6629. doi: 10.1073/pnas.120168697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo HS, Cooper TG. Mol Cell Biol. 1989;9:3231–3243. doi: 10.1128/mcb.9.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai R, Daugherty JR, Cunningham TS, Cooper TG. J Biol Chem. 1999;274:28026–28034. doi: 10.1074/jbc.274.39.28026. [DOI] [PubMed] [Google Scholar]
- 14.Daugherty JR, Rai R, ElBerry HM, Cooper TG. J Bacteriol. 1993;175:64–73. doi: 10.1128/jb.175.1.64-73.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorrington RA, Cooper TG. Nucleic Acids Res. 1993;21:3777–3784. doi: 10.1093/nar/21.16.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott S, Dorrington R, Svetlov V, Beeser AE, Distler M, Cooper TG. J Biol Chem. 2000;275:7198–7204. doi: 10.1074/jbc.275.10.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golemis EA, Gyuris J, Brent R. In: Current Protocols in Molecular Biology. Ausabel FM, Brent R, Kingston R, Moore D, Seidman J, Struhl K, editors. John Wiley & Sons, Inc; New York: 1996. pp. 1.1–1.28. [Google Scholar]
- 18.Olive MJ, Daugherty JR, Cooper TG. J Bacteriol. 1991;173:255–261. doi: 10.1128/jb.173.1.255-261.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bricmont PA, Daugherty JR, Cooper TG. Mol Cell Biol. 1991;11:1161–1166. doi: 10.1128/mcb.11.2.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christianson TW, Sikorski RS, Dante M, Shero JH, Heiter P. Gene (Amst) 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 21.Svetlov V. PhD thesis. University of Tennessee; Memphis, TN: 1997. Transcriptional Activator and Yeast Nitrogen Metabolism. [Google Scholar]
- 22.Guarente L, Mason T. Cell. 1983;32:1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- 23.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 24.Van Vuuren HJJ, Daugherty JR, Rai R, Cooper TG. J Bacteriol. 1991;173:7186–7195. doi: 10.1128/jb.173.22.7186-7195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minehart PL, Magasanik B. Mol Cell Biol. 1991;11:6216–6228. doi: 10.1128/mcb.11.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts SM, Winston F. Genetics. 1997;147:451–465. doi: 10.1093/genetics/147.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winston F, Sudarsanam P. Cold Spring Harbor Symp Quant Biol. 1998;63:553–561. doi: 10.1101/sqb.1998.63.553. [DOI] [PubMed] [Google Scholar]
- 28.Park HD, Scott S, Rai R, Dorrington R, Cooper TG. J Bacteriol. 1999;181:7052–7064. doi: 10.1128/jb.181.22.7052-7064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]