Abstract
To accomplish regenerative medicine, several critical issues in stem cell biology have to be solved, including the identification of sources, expanding populations, building them into organs, and assimilating them to the host. While many stem cells can now differentiate along certain lineages, knowledge on how to use them to build organs lags behind. Here we focus on topobiological events that bridge this gap, i.e., the regulation of number, size, axis, shape, arrangement, and architecture during organogenesis. Rather than reviewing detailed molecular pathways known to disrupt organogenesis when perturbed, we highlight conceptual questions at the topobiological level, and ask how cellular and molecular mechanisms can work to explain these phenomena. The avian integument is used as the Rosetta stone because the molecular activities are linked to organ forms which are visually apparent and have functional consequences during evolution as shown by the fossil record and extant diversity. For example, we show that feather pattern formation is the equilibrium of stochastic interactions among multiple activators and inhibitors. While morphogens and receptors are coded by the genome, the result is based on the summed physical-chemical properties on the whole cell surface and is self-organizing. For another example, we show developing chicken and duck beaks contain differently configured localized growth zones (LoGZ) and can modulate chicken beaks to phenocopy diverse avian beaks in Nature by altering the position, number, size, and duration of LoGZs. Different organs have their unique topology and we also discuss shaping mechanisms of the liver and different ways of branching morphogenesis. Multi-primordia organs (e.g., feathers, hairs, teeth) have additional topographic specificities across the body surface, an appendage field, or within an appendage. Promises and problems in reconstituted feather / hair follicles and other organs are discussed. Finally, simple modifications at the topobiological level may lead to novel morphologies for natural selection at the evolution level.
Introduction
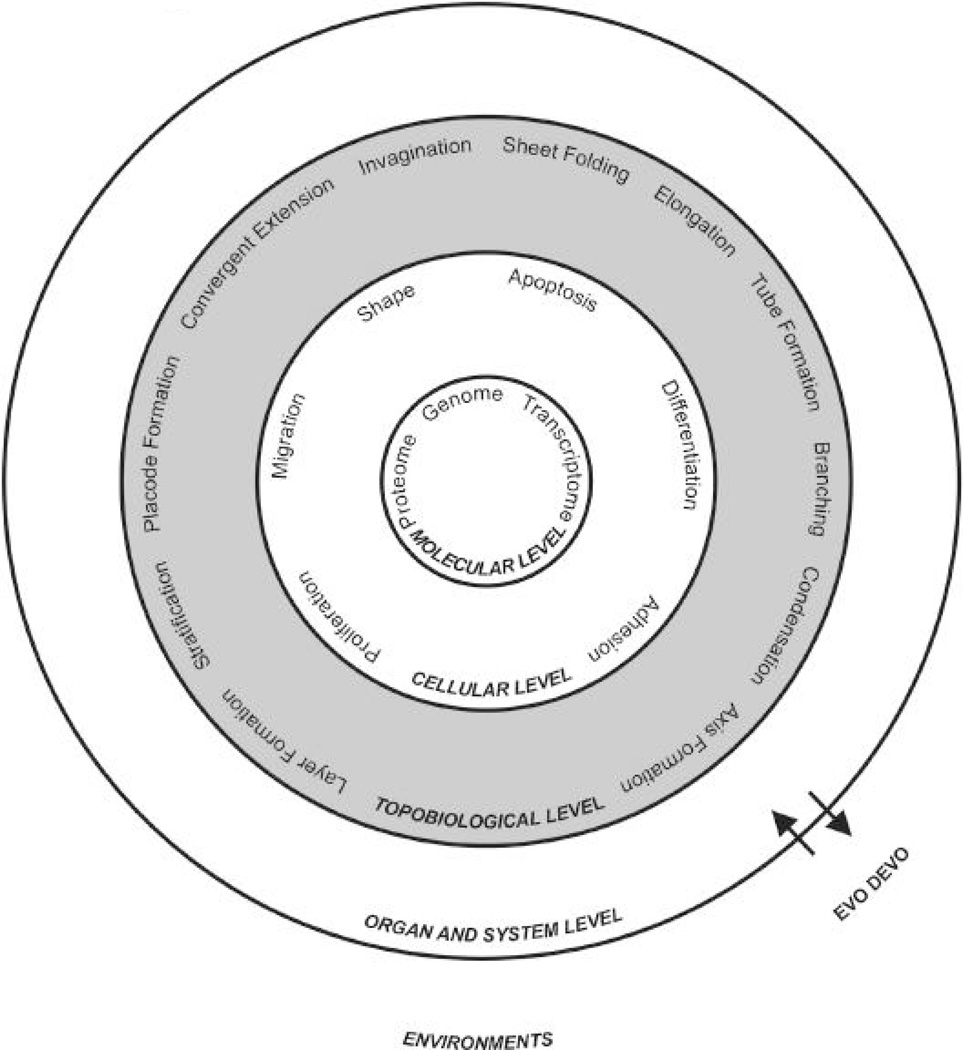
One of the most fundamental questions in biology is how the single dimension genomic codes are transformed into three dimensional forms which are even able to morph temporally. As the genomics of different organisms are gradually completed, in the post-genomic age, we need to learn more about how the molecular events are translated to biological structures and how cells are arranged in time and space to build an organ. In the last decade, many secreted regulatory pathways (e.g., SHH, BMP, WNT) were identified and developmental biologists gained a lot of new understanding and insight into the morphogenetic processes in development and diseases (Hogan and Kolodziej, 2002; Scott, 2000; Tickle, 2003; Moon et al., 2004). However, as we analyzed molecular pathways more, we gradually grew less satisfied that we could disrupt organ formation by mis-expressing certain molecular pathways, but did not know how the molecular pathways work together to build an organ. We have the ability to dissect molecular pathways and we know certain molecular pathways are essential, yet we do not know enough to assemble them into organs (Fig. 1).
Fig. 1. Levels of organ formation.
From molecules to the organism, there are different levels of interaction. Each level is important and inter-dependent, but also operates with different principles.
Maybe we should also look at a more global level in order to strive for integration of multiple molecular and cellular pathways. Maybe it is time to revisit the topobiology concept. As Dr. Gerald M. Edelman (1988a) muses "While the triumph of molecular biology answers the question on the chemical nature of genes and how hereditary traits are transmitted, it does not fully answer the question on how genes determine traits." He felt that "It is very difficult to account for the forms, patterns or shapes of complex animals simply by extrapolating from the rules governing the shape of proteins." and therefore turned to "the other side of biology", hence the birth of "Topobiology". He defined topobiology as "place dependent molecular interactions at the cell surface" (Edelman, 1988a). He emphasized the fundamental importance of cell proliferation, adhesion, migration, death and differentiation, and particularly the links of cell collectives by cell adhesion molecules, and the regulation of these links. A single cell is capable of proliferation, migration, shape changes, apoptosis, and differentiation, but cell adhesion, epithelial sheet morphogenesis, and tissue interactions require cell collectives. The topobiology concept focuses on multi-cellular activities to examine how multi-potential stem cells are organized into tissues and organs, with particular architectures, sizes and shapes.
The advent of genomics provides a "dictionary" of molecules, but we still lack the syntax of how this information is used. New understanding has been gained for studying molecular interactions, enhancer regulations, and pathway activities. These molecular events are integrated at the cellular level (Fig. 1). The basic information is genetically determined because the numbers of adhesion molecules or morphogen receptors on the cell membrane are pre-determined by the genome; however, the interaction among these cells is a physico-chemical phenomenon. Tissue and organ organization and structure reflect equilibrium of thousands of chemical reactions within a particular physical constraint. The importance of physico-chemical phenomena at this level has been pointed out previously (Newman and Frisch, 1979; Oster et al., 1985; Kiskowski et al., 2004). However, major research efforts and hence progress has been at the molecular and cellular level. The concept of topobiology did not get the attention it deserves and the parameters for topobiology remain mostly elusive. This knowledge is even more urgent now as we start to work on stem cells and hope to build an organ for regenerative medicine.
To understand how an organ is built, our laboratory has been using the avian integument as the Rosetta stone. Avian feathers and beaks are good models because the end points show distinct morphologies with functional consequences. Their evolution occurs through a series of novel topobiological events, which add evolutionary novelties which can be selected out by the environment. The accessibility of avian embryos and regenerating feather follicles provides excellent opportunities for tackling cellular and molecular events experimentally (Brown et al., 2003). Thus, they are excellent models to further develop the concept of topobiology. In this review we will first identify gaps that need to be bridged in stem cell biology and introduce progress that has been made in the topobiology of epithelial organs. The work on feather organogenesis has recently been of intense interest because of the many newly excavated feather related fossils from Northern China, and our effort to link molecular findings with these intermediate "proto-feather" morphologies (reviewed in Prum and Brush, 2002; Chuong et al., 2003; Sawyer and Knapp, 2003). The beak is used because the diverse beak shapes in Galapagos finches inspired Darwin's Evolution theory. The recent breakthrough by Tabin’s and our group (Abzhanov et al., 2004; Wu et al., 2004a) was praised in the accompanying Science commentary which said "Darwin will be pleased." (Pennisi, 2004). These works are examples demonstrating how Nature engineers organ forms on a grand scale of hundreds of millions of years in the context of Evo-Devo. We then briefly apply the topobiology concept to mammalian ectodermal organogenesis, liver shaping, lung branching, etc. We also discuss the regional specificity issue that we must face in engineering organs. At the end of the review we reflect on how understanding these principles may contribute to the engineering of stem cells.
Between stem cells and organs
Recently, stem cell biology has emerged as an important new discipline of translational research in the context of regenerative medicine. Several issues are important in stem cell biology research. They are A) identifying sources of stem cells, B) expanding stem cell populations while maintaining their properties, C) engineering stem cells to form the tissue / organ desired and D) having the engineered tissues / organs assimilate into the host. For the first issue, the research at this stage has been on embryonic stem cells and identifying possible sources of adult stem cells (Li and Xie, 2005; Toma et al., 2005; Lako et al., 2002; Fuchs and Segre, 2000). Somatic nuclei transfer technology has allowed the progress of therapeutic cloning (Hwang et al., 2005). For the second issue, scientists have worked on culture conditions and found some promising clues. For instance, Wnt has been found to help expand hematopoietic stem cells (Reya et al., 2003).
The third issue is how to engineer these cells to organ like structures and be useful for the host. This has proven to be of different difficulty levels for different types of organs. For hematopoietic cells, multiple blood cell types float in the blood stream without being organized into a particular form, and can function in response to cytokines. This lack of structural organization makes blood a relatively easy organ to work with, and as a result, hematopoietic stem cells have already been used successfully in clinical practice. The next level is to have engineered tissues that secrete needed extracellular factors required to alleviate disease conditions, such as insulin from pancreatic beta cells for diabetes (Lumelsky et al., 2001; Efrat, 2004), or dopamine secreted neurons for Parkinson’s disease (Snydeer and Olanow, 2005). The next challenging level is to be able to produce certain shapes suitable for functional morphology. For example, it is now possible to induce chondro-differentiation from mesenchymal cells in culture, but it is still very difficult to have these cells form the right contours on a cartilage or bone element. The use of a biodegradable polymer scaffold to generate auricular shaped cartilage (Shieh et al., 2004) can facilitate the process when a better solution is not available. It would be best to find out how Nature performs morphogenesis in development, but even Nature "forgets" how to do it during regeneration in the adult: during the body's effort to regenerate in response to osteoarthritis, bone spurs form which cause more damage. Even if we can have a functional tissue / organ entity, we still have to learn how to make them connect with the host. For example, a group of beating cardio-myocytes has to coordinate the motion of the whole myocardium and a group of transplanted neurons has to be connected with other parts of the brain. Finally, stem cell derived organs have to survive without being rejected by the host immune system or competed out by the native cells. Therefore, while stem cell engineering holds promise, there are many challenges before the knowledge is translated to clinical applications.
The focus of this review is on the third issue; how to engineer stem cells to form the tissue / organ desired. Suppose current stem cell research reaches a stage where we have enough stem cells that can be induced to form different differentiated phenotypes. How do we direct them to form organs? We need to position ourselves to answer these questions. Developmental biology used to be considered as a basic science operating in an ivory tower. Now scientists appreciate that tissue engineering and developmental biology are two sides of the same coin: when Nature does it, it is developmental biology; when humans do it, it is stem cell engineering. The best way to engineer stem cells is to learn how to guide them in Nature's way.
Topobiological transformation events in epithelial organ formation
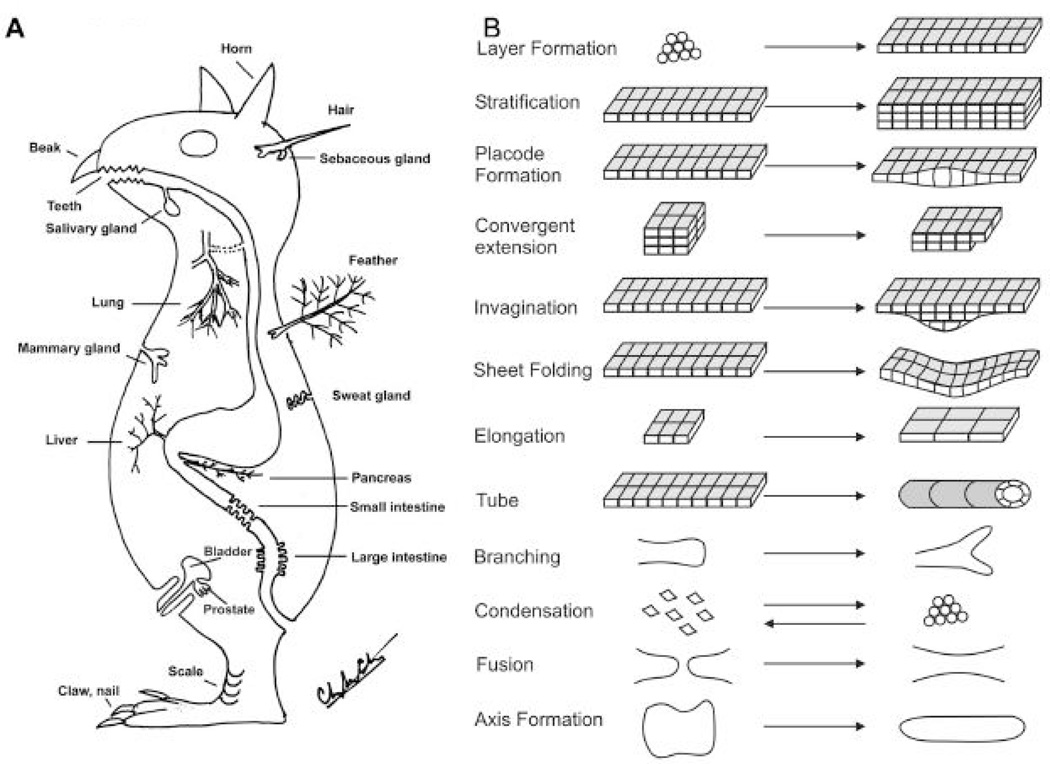
Here we use topological transformation to mean the conversion from one cell collective configuration to the other. It does not entirely fit the definition in mathematics (Columbia Encyclopedia, 2005), but we use the term to emphasize the geometry aspect of tissue morphogenesis: the forming and dissolution of cell groups, the shifting arrangements, the making and elimination of boundaries, the orientations, etc. In fact, the creation or removal of boundaries or breaking of epithelial sheets makes them topologically non-equivalent. The formation of epithelial organs involves topological transformations of a two dimension epithelial sheet into different structures (Fig. 2A). In ectodermal organ formation, they can evaginate to form bump like configurations (e.g., scale), some with elaborate surface (e.g., molar), protrusions (e.g., canine, claw), elongated filaments (e.g., hair), some with hierarchical branches (e.g., feathers), etc. They can also invaginate to form tubes (e.g., sweat glands), some with branching (salivary glands, mammary glands), follicles (e.g., hair, feather), etc. (Chuong, 1998). In the endoderm, similar topological transformations occur in the gut. Regional specialization of epithelia leads to the formation of the stomach, intestines, lungs, liver, and pancreas which form by budding from the gastro-intestinal tract during embryonic development. These apparently different epithelial organs actually share similar topobiological transformation events, i.e., an event that changes the topological configuration of cells before and after it happens. The involved molecular mechanisms have begun to be understood. Some examples are given (Fig. 2B).
Fig. 2. Topobiological transformation events during epithelial organ formation.
A). A prototype animal with ectodermal and endodermal organs. While these epithelial organs appear diverse, they share similar morphogenesis related signaling pathways and topobiological principles. Modified from Chuong edit 1998. The Molecular Basis of Epithelial Appendage Morphogenesis. B). Types of topobiological transformation events. These events are meaningful only at the level of cell groups (epithelial sheets, mesenchymal condensations), not at the single cell level. We need to learn more about how molecular mechanisms contribute to these events.
Layer formation
In this event, randomly arranged epithelial cells start to join with each other. The progeny of cell proliferation remain in the same sheet as the axial orientation of mitosis within the two dimension plane. Epithelial cell adhesion molecules such as E-cadherin were first shown to have this function (Nagafuchi et al., 1987).
Stratification
Some mitosis becomes asymmetric with a mitotic axis becoming perpendicular to the epithelial sheet. The daughter cells remaining in the basal layer can still proliferate (the beginning of stem cells), while the other daughter cells, now post-mitotic, start to pile up, forming multiple layers. Stratification enables the epithelia to form a multilayered barrier, protecting the organism from its environment and allows functional diversification. Recently, activation of the p63 pathway was shown to be involved in the stratification process (Koster et al., 2004; Koster and Roop, 2004). p63 is expressed early in the epidermal lineage when cells are still forming a single layer (Green et al., 2003; Koster et al., 2004). p63 null mice fail to form stratified epithelial derivatives (Mills et al., 1999).
Convergent extension
Convergent extension allows a change of shape of epithelial sheets by cell rearrangements. Lateral and medial cells become polarized and then the lateral cells intercalate between the medial cells, causing an extension along the anterior-posterior axis (Keller, 2002). This process was originally shown to be responsible for gastrulation in Xenopus and zebrafish (Keller, 1986), gut elongation in sea urchins (Ettensohn, 1985; Hardin and Cheng, 1986), the formation of the avian primitive streak (Wei and Mikawa, 2000) and shaping of the avian neural plate (Schoenwolf, 1991; Schoenwolf and Alvarez, 1989). It is likely to be a fundamental topological transformation process involved in other organ formation. Signaling along the noncanonical Wnt pathway is likely to be involved.
Invagination
Invagination of epithelial tissues is seen in the organization of the neuroepithelium in Xenopus (Schoenwolf and Alvarez, 1989). It also plays a critical role in tooth formation (Jernvall and Thesleff, 2000). The activation of wnt / β-catenin and the suppression of BMP by noggin leads to an invagination of the epithelial placode to initiate hair follicle formation (Jamora et al., 2003).
Tube formation
Tube formation can occur through re-arrangements of epithelial cells to form a lumen within an elongated cell cord. Tubular structures can form in many different ways. An epithelial sheet can curl and seal itself to form a tube. This occurs during neural tube formation (Colas and Schoenwolf, 2001). This involves cell shape changes forming a narrow apical region and a broad basal region. Tubes can also form by budding out from an epithelial surface. The lung is thought to branch out in this manner (Metzger and Krasnow, 1999; Hogan and Kolodziej, 2002). A mass of cells can invaginate to form a central cavity as occurs during salivary gland formation (Melnick and Jaskoll, 2000). Apoptosis may a play a role in this mechanism (Coucouvanis and Martin, 1995). In angiogenesis, hemangioblasts form an aggregate, called blood islands. The inner cells become hematopoietic stem cells while the outer cells become angioblasts which go on to multiply and differentiate into endothelial cells forming the blood vessels. So cords of hemangioblasts hollow out to form a tube (reviewed in Baron, 2003).
Branching
Branching is a used to increase the surface area for interactions with the environment, be it internal or external. Branching involves the splitting of the long axis into two. While the end results can be quite similar, they can be generated from very different mechanisms. It can be generated by differential growth or death. The process is seen in lung and mammary gland morphogenesis (see later, topobiology of other organs), as well as in feather barb branching.
Condensations and de-condensations
This involves increased cell adhesion that brings out a group of highly compacted cells, or the reverse of this process. Not only physically a cell collective forms or dissolves, there are also change of cell properties due to signaling initiated by cell contacts. The formation of dermal condensations is a very early step in feather formation (Chuong and Edelman, 1985a; Jiang and Chuong, 1992). The regulation of this process leads to periodic pattern formation (Please see the section on multi-primordium organs). The migration of neural crest cells is a good physiological examples of epithelial - mesenchymal transformation (Kang and Svoboda, 2005).
Fusion
When two cell collectives meet, the epithelial can remain as two entity with a surface boundary in between, or they boundary can disappears and two cell collectives fuse into one. This may occur through epithelial - mesenchymal transformation (Kang and Svoboda, 2005) or involves apoptosis.
Feather Morphogenesis
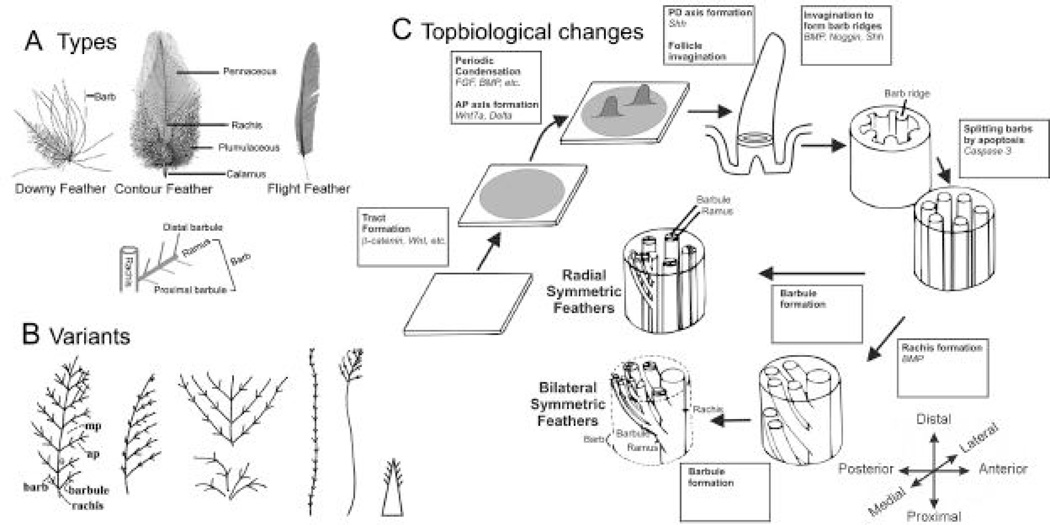
Feathers on the bird body show hierarchical branch patterns (Prum and Dyck, 2003). The major types of avian feathers include contour feathers, remiges, rectrices, downy feathers, etc. (Lucas and Stettenheim, 1972). A typical avian feather consists of a shaft (rachis) and barbs. The barbs are composed of a shaft (ramus) and many smaller branches (barbules) (Fig. 3A). Different feathers show variations in symmetry. Down feathers are radially-symmetric. Their rachis is absent or very short. Contour feathers have a weak bilateral symmetry. Flight feathers are bilaterally symmetric and some become bilaterally asymmetric (see below, Fig. 5). A contour feather can have a distal pennaceous region and a proximal plumulaceous region, so the feather can help the integument function for contour / communication display with the distal portion, but maintain warmth with its proximal plumulaceous portion (Fig. 9C). The plumulaceous regions are made of similarly shaped barbules both proximal and distal to the ramus. They are loose and fluffy. The pennaceous regions are made of groove-shaped proximal barbules and hook-shaped distal barbules. Therefore the distal barbules of a barb interlock with the proximal barbules of the barb above, forming a feather vane using a Velcro like mechanism.
Fig. 3. Feather types (A), variants (B), and topobiological events in development (C).
Panel A is adopted from Lucas and Stettenheim, 1972. Panel B is modified from Chuong, edit, 1998, “Molecular Basis of Epithelial Appendage Morphogenesis.” Landes Bioscience, Austin, TX. Panel C is modified from Chuong, C.-M. and Edelman, G.M. 1985. Expression of cell Adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J Cell Biol 101:1027–1043.
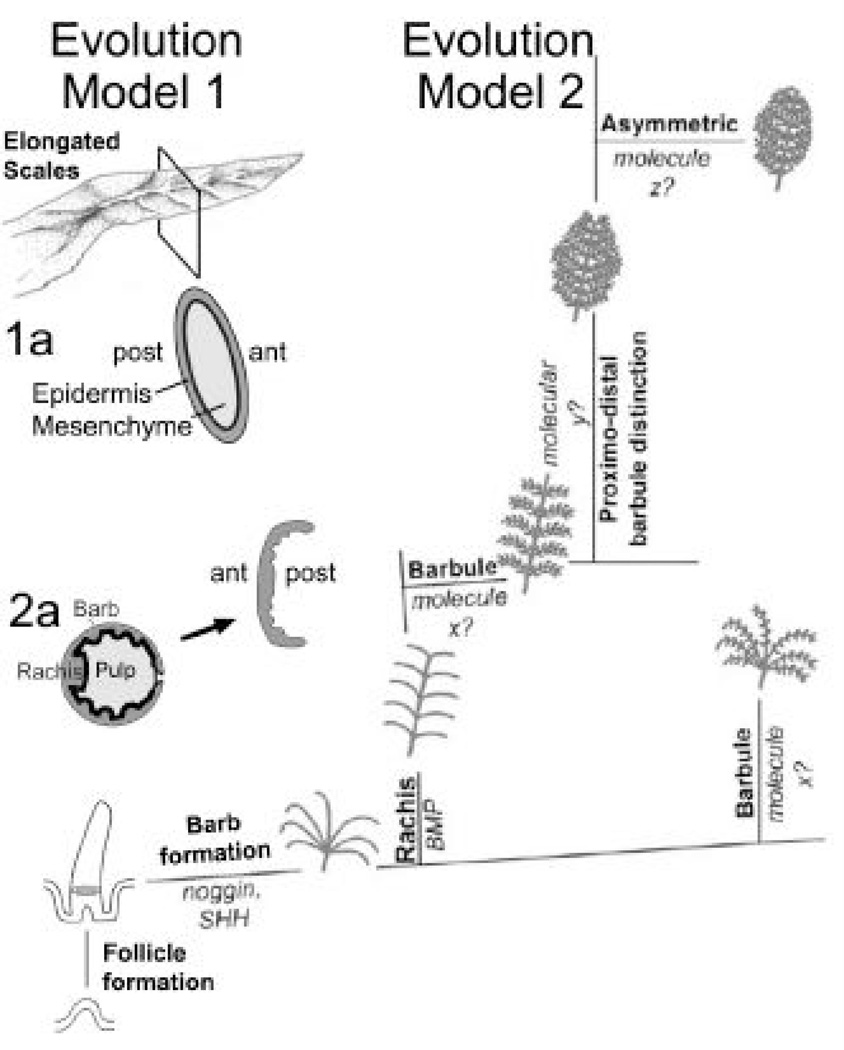
Fig. 5. Models on feather evolution.
Model 1 proposes elongated scales as the origin of the feather. Modified from Regal., 1975. Model 2 proposes that a series of novel topobiological transformation events, as evolutionary novelties, transform epidermal buds into complex feathers. Panel 1a and 2a are cross sections.
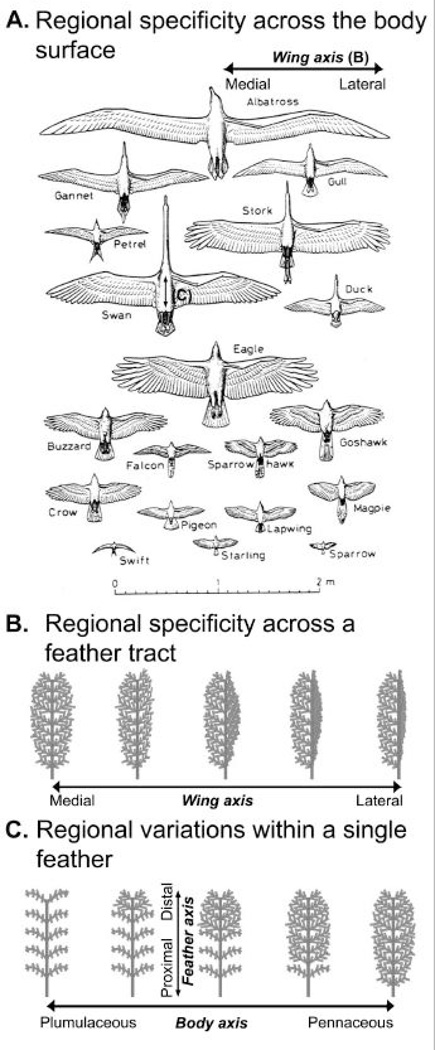
Fig. 9. Topographic regional specificities.
A) Regional specificity across the body surface is illustrated in different species of birds. They also fly using different modes with different wing shapes. B) Regional specificity across an appendage field is best demonstrated by the array of primary remiges on the wing. C) Intra-appendage regional specificity is best demonstrated by contour feathers on the trunk. Panel A is from Feduccia, (1999). “The Origin and Evolution of Birds.” Yale Univ. Press, New Haven, ed. 2.
Development
During avian embryonic development, feather formation starts with a placode, which is composed of elongated epithelia accompanied with dermal condensations (Sengel, 1976; Wu et al., 2004b). These feather primordia elongate and protrude out to form feather buds, topologically transforming a two dimensional flat epidermis into a three dimensional structure (Fig. 3C; Chuong and Edelman, 1985b). Feather buds are originally radially symmetric, but soon acquire anterior-posterior polarity through interactions with the epithelium. Feathers then start to elongate and develop a proximal-distal axis. Feathers form follicles which offer advantages over skin appendages that do not, such as scales. The follicular structure protects the epithelial stem cells and dermal papillae. Localization of the stem cells within a protected environment enables regeneration through natural feather molting cycles and induction by plucking. New cell proliferation at the follicle base pushes the more differentiated portions of the feather filament to the distal end. Feather filaments go through epithelial invaginations and evaginations to form the barb ridges, which precede the formation of the barbs and barbules. The barb ridges further differentiate into the barbule plates, axial plates and marginal plates. Barbule plate cells later keratinize to become the feather structure, while marginal plate and axial plate cells undergo apoptosis, die and become spaces (Fig. 4; Chang et al., 2004). The central pulp undergoes apoptosis allowing the feathers to unfold and assume their characteristic flat shapes, transforming a three dimension cylinder back to a two dimensional plane. Topobiological transformation events are listed in the boxes in Fig. 3C. In each process, signaling molecules are used in different ways (reviewed in Widelitz et al., 2003; Jiang et al., 2004; Wu et al., 2004b and references within), and some (e.g., BMP, SHH pathway members) are used repetitively in different contexts in the so called co-optive use of signaling modules (Harris et al., 2002).
Fig. 4. Pattern forming processes which regulates the number and size of multiple primordia within a field.
Part of A is adopted from Jiang et al., 1994, 2004. B is from Jiang et al., 1999.
With so many topological parameters involved, tuning of some of these parameters can lead to different feather shapes (Prum and Williamson, 2001), generating the diverse feather shapes in Nature. The range of feather variants can be appreciated in Bartels et al., (2003) and the interesting photos in Extraordinary Chickens (Green-Armytage, 2000). Schematic examples of these variants can be seen in Fig. 3B. To obtain different feather shapes, one can simply change the relative length of the rachis, barbs and barbules. For example, the left one represents the fluffy contour feathers of an ostrich, the second from the left is a strong flight feather of an eagle, the third from the left represents the contour feathers on the trunk of pheasants and the natal down. The one on the right represents the scale-like feathers of a penguin in which the rachis is enlarged while barbs and barbules are miniaturized. There are also the spectacular peacock tail contour feathers, and the many unusual decorative feathers found on birds of paradise.
An interesting point is that they are all keratinocytes built into different architectures. The variations do not just exist among different avian species, but can exist of the same individual. Furthermore, the epidermal stem cells can be guided by the dermal papilla to form different feather types in different skin regions (Cohen and Espinasse, 1961; our unpublished data).
Topobiology of multi-primordium organs
Some organs are made of multiple primordia. Each primordium can be considered as one organ, but they work together as a functional unit. This can be seen often in integument organs such as teeth, hairs, feathers, etc. All teeth have to work together to serve the function of breaking up food. Feathers in a tract also have to work together. A single feather does not permit flight, but together multiple pennaceous feathers can connect to form a feather vane as discussed earlier. While cells differentiate, the topology, i.e., the number, shape, size and arrangement of individual primordium are crucial for the way that particular organs work, and also provide a new level of functional integration and variation.
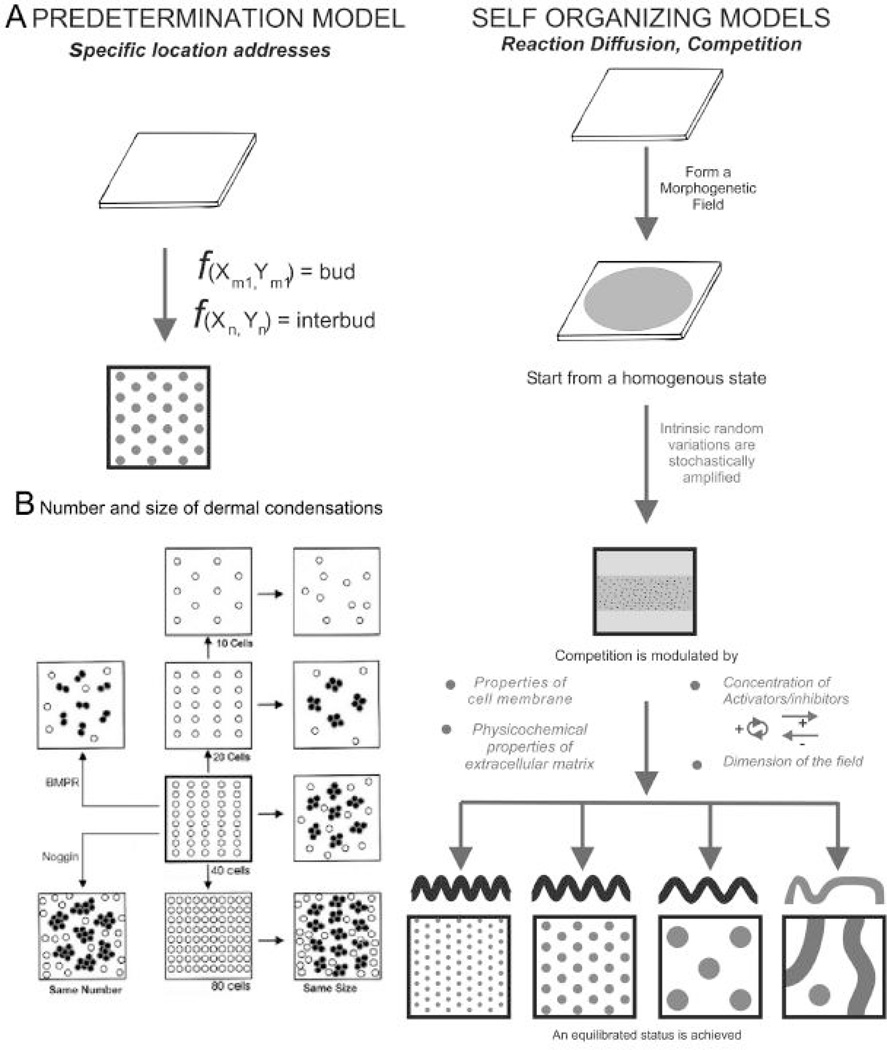
Feathers are laid out in exquisite patterns on the surface of the chicken embryo. These regular patterns have inspired scientist to think about how such regular patterns arise (Held, 1992). In general, one category of model considers that the fates of cells are predetermined by their position, whether the molecular coordinates exist in the form of specific enhancer sequences or as a morphogen gradient (Fig. 4). The other category considers the major driving force is based on physico-chemical phenomena. The reaction – diffusion mechanism has been used to describe periodic patterning in inanimate objects as well as in living systems (Turing, 1952; Gierer and Meinhardt, 1972; Nagorcka and Mooney, 1985; Moore et al., 1998; Jung et al., 1998). In reaction – diffusion, random fluctuations in molecular expression become amplified to form peaks and valleys. These however are unstable. The peaks and valleys were later postulated to be maintained and propagated through chemical interactions or mechanical forces. Meinhardt and Gierer (1974, 2000) proposed that some molecules distributed by a reaction – diffusion mechanism might stimulate the production of the periodic structures (activators) while some suppress their synthesis (inhibitors) through auto - and cross -catalysis. Activators also have the ability to further stimulate the production of activators as well as induce the production of inhibitors. Based on these models and our experimental results (Jiang et al., 1999, 2004; Jung et al., 1998), we propose a model for feather pattern formation. It consists of the following events. 1) Competent cells without specific identity are distributed in the field and move randomly. 2) Extra-cellular activators and inhibitors governed by a reaction-diffusion mechanism diffuse in the field. 3) Cells respond to activators and inhibitors stochastically and the results are manifested in changes of cell adhesion. 4) Cell cluster formations (dermal condensations) are reversible initially, then become committed once a threshold is reached. 5) The pattern reached is the result of competitive equilibrium. If the system is reset without changing any parameter, the pattern with similar topology will re-appear, but it will not be identical to the original pattern.
If feather patterns are pre-determined, scrambling the cells should not change their fates. The feather reconstitution model (Jiang et al., 1999) offered an opportunity to test this, because it allowed us to recombine a fixed sized epithelium with different numbers of mesenchymal cells. When increasing numbers of mesenchymal cells were used, we could expect either the same number of primordia with increased size, or the same size of primordia with increased numbers of primordia (Fig. 4B). Experimental results showed that for mesenchymal cells derived from the same region, the feather primordia were always the same size. When mesenchymal cell density is below the threshold, no primordia formed. At lower mesenchymal cell density, primordia appeared in random positions, not as aborted rows of a hexagonal lattice. As more cells were added, the number of primordia increased until they reached a maximal packing density, and feathers appeared to be arranged in a hexagonal pattern. However, this hexagonal pattern is a result of maximal packaging, not a consequence of preset molecular codes or positional values.
Thus the feather precursor cells at this stage are truly stem cells: they can become either bud or interbud cells. The size, number, and spacing of feather primordia can be regulated by altering the properties of cells or the micro-environment (Jiang et al., 1999; Shen et al., 2004). To help patients, dermatologists currently can implant hair follicles one by one into the alopecic scalp. We can foresee if all these parameters can be set right, the delivered stem cells should be able to self-organize into multiple hair follicles as they do during embryonic morphogenesis.
Evolution
During the morphological transformation from reptiles to birds, new challenges were imposed on early birds to re-engineer themselves from a tetrapod form mainly living on the land to a smaller bipedal animal with wings to live in the sky. The Jehol Biota spreading in Northern China is unique because it contains unique features and many plants and animals are preserved in outstanding condition (Zhou et al., 2003). It is particularly valuable for the analysis of the evolution of birds because birds evolved from reptiles during this period (Chatterjee, 1997; Chiapple, 1995; Feduccia, 1999). Early research suggested that feathers evolved from an elongation of scales enlisted for protection. It was then subdivided over time to form pennaceous and then plumulaceous feather types (Regal, 1975; Fig. 5, Model 1). Thus the order of formation is scales → elongated scales → the vane-like scale plates → partial pennaceous vanes with an rachis like central axis → bilaterally symmetric feathers → plumulaceous barbs → radially symmetric downy feathers (also see Wu et al., 2004b). From the developmental and molecular studies, Prum (1999; Prum and Brush, 2002) and us (Chuong et al., 2000; Yu et al., 2002) propose that the order of formation is: buds → follicle → cylindrical feather filaments → splitting to form radially symmetrically arranged barbs → radially symmetric downy feathers with plumulaceous barbules. By topologically changing the slanting angles of barb ridge organization, a rachis is created and the other lineage can lead to bilaterally symmetric plumulaceous feathers → bilaterally symmetric pennaceous vanes → bilaterally asymmetric vanes (Fig. 5, Model 2). This is also the order observed in development. In a broad sense of ontogeny repeating phylogeny, this probably occurred in evolution too. Indeed, a series of fossils were discovered representing intermediate forms of feathers or feather-like appendages from the Jehol Biota of China.
Furthermore, considering the topology of epithelium and mesenchyme, the scale is different from feathers (Fig. 5; Prum, 1999; Chuong et al., 2003). The scale dermis remains in the adult, and both anterior and posterior sides of scales are equivalent to the suprabasal side of the epidermis (Fig. 5, model 1a). In contrast, in the developing feather follicles, the cylindrical feather filament surrounds the mesenchymal pulp with the basement membrane facing inside. Upon maturation, apoptosis of the pulp epithelium and shedding of the feather sheath allows the feathers to open up. Thus the anterior and posterior side of the feather vane originally faces the suprabasal and basal layer, respectively (Fig. 5, model 2a; Chang et al., 2003). An elongated scale may show branches, and may be called a "non-avian feather" (Jones et al., 2000), but is not an avian feather.
From these results, a set of criteria is developed to define the true avian feathers (Chuong et al., 2003). It includes 1) possessing actively proliferating cells in the proximal follicle for a proximo – distal growth mode; 2) forming hierarchical branches of rachis, barbs and barbules, with barbs that can be bilaterally or radially symmetric, formed by differential cell death; 3) having a follicle structure, with a mesenchyme core during development; 4) when this matures, it consists of epithelia without a mesenchyme core with two sides of the vane facing the previous basal and supra-basal layers, respectively; and 5) having epithelial stem cells and the dermal papilla in the follicle which maintains the ability to molt and regenerate.
Work in molecular biology laboratories has allowed us to start to identify molecular pathways involved in each of these processes (Fig. 5; Yu et al., 2002; Harris et al., 2002). We have developed a novel feather plucking / regeneration model to mis-express genes in the regenerating feather stem cells (Yu et al., 2002). This allows us to gauge the contribution of each molecular pathway. We showed that BMPs promote rachis formation while noggin promotes barb branch formation. SHH is important to set up the spacing between barbs (Chang et al., 2004). Harris et al (2002) also showed that BMP2 and SHH mediate barb ridge formation, and have developed an activator / inhibition model to explain the branch patterning (Harris et al., 2005).
To summarize feather morphogenesis, we can see that the development and evolution of the feather does not require new "barb specific genes", or "barbule specific" genes. Instead, it co-opts the use of a morphogenesis signaling network to form a new topological arrangement (Harris et al., 2002) that becomes an evolutionary novelty. First, the formation of feather follicles shifted stem cells to reside in protected follicles in the dermis and proliferative cells in the growth zone were shifted to the proximal end of the follicles. This re-configuration allows continuous growth, unlimited length and molting cycles. To become more effective in thermo-regulation, the elongated and cylindrical appendages branched out to form more complex structures. The appendages branch to form barbs that provide a fluffier down feather coat, highly efficient in thermal regulation. Third, the rachis, the major branch of the bilateral feather, formed to define feathers of different shapes enabling the feather to become more effective in communication. Fourth, asymmetric barbules formed that interweave barbs into a vane, and enabled the birds to launch into the sky and fly. This series of novel topobiological transformation events opened the whole sky for the Aves class. In a way the sky niche is the best “patent award” given to birds for their successful evolutionary novelties.
Beak Morphogenesis
The recruitment of forelimbs as wings allowed a newly found mobility resulting from flight and opened vast eco-morphological possibilities. However, this came at a cost since animals now needed to develop a new feeding mechanism without the use of arms. This exerted selection pressures on the evolving structure of the face; a strong, lightweight, and effective feeding apparatus had to evolve. Furthermore, the beak had to show an ability to evolve through adaptive radiation to different eco-environments. The results are the amazing transformation of the snout into a large range of beak topologies adapted to different ecological niches (Zweers et al., 1997). At the global scale it involves a reptile snout - bird beak transformation. At the finer scale, it involves the fine tuning of Galapagos finches that inspired Darwin's evolution theory (Grant, 1986). At the developmental level, how are the different shapes of beaks produced (Fig. 6A)?
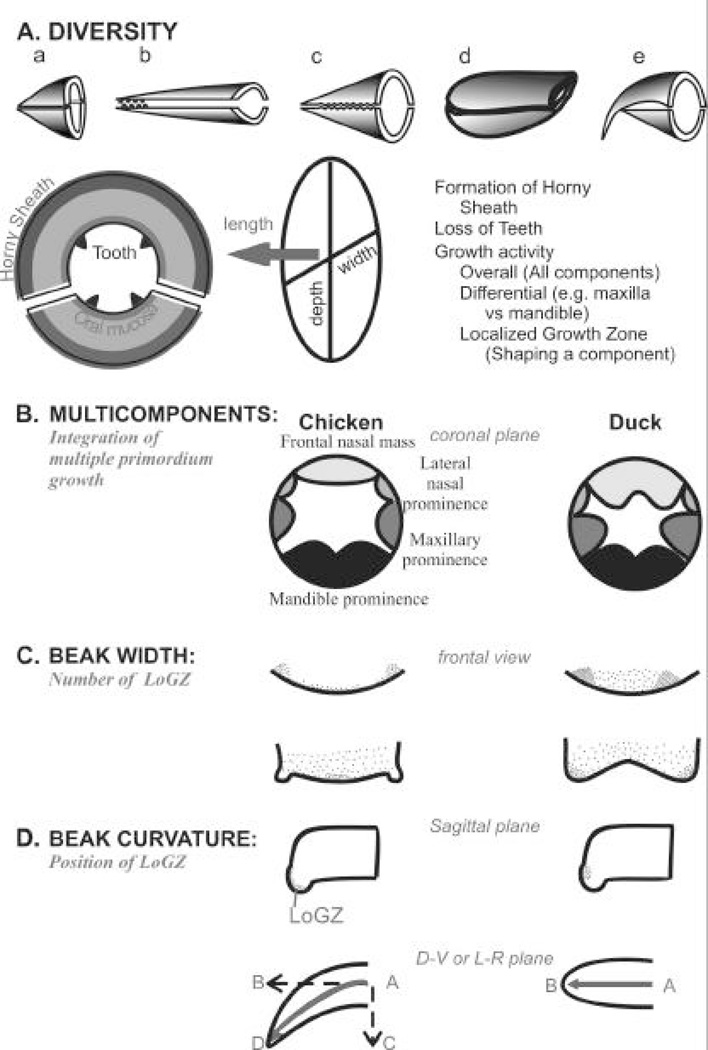
Fig. 6. Molecular shaping of the beak.
A. Diverse beak shapes, and the basic design of beaks. By positioning localized growth zone in different numbers and positions, the beak can become different shapes.
Development
The embryonic chick face is composed of multiple facial prominences (reviewed by Francis-West, et al., 1998, 2003; Helms and Schneider, 2003; Fig. 6B). Mesenchymal processes covered by epithelium surround the developing mouth. These prominences grow out together to form the face. The upper beak is formed from the FNM and MXP on the side. Lateral nasal masses have only smaller contributions and will not be emphasized here. The lower beak is derived from the paired mandibular prominences (MDP) which contain the two Meckel's cartilages. Cellular fate tracing with DiI labelling illustrates that cell populations centered around the nasal pits, the midline of the paired mandibular prominences, and at sites of fusion contribute most to the overall expansion (McGonnell et al., 1998). These data suggest that there are specific localized growth zones in these originally nearly round prominences. When the beak forms, the frontal nasal mass (FNM) and mandibular prominences assume an elongated shape, while MXPs remain short and ball-like. These developing facial prominences change shape substantially in developing stages leading to the formation of primary and secondary palates. Therefore the final shape and size of each prominence is the combination of the diffuse random growth and the directed localized growth in that prominence. Growth and morphogenesis of the prominences must be tightly coordinated to obtain the final distinct configuration of the face.
Experiments showed that the identity of facial prominences are specified early in the neural crest stage (Noden, 1983; Couly et al., 2002) and are coordinated by signaling molecules (Francis-West et al., 2003). An elegant experiment by transplanting duck crest into quail embryos (forming duail) and quail crest into duck embryo (quack) shows the beak morphology is in accord to the origin of the cephalic neural crest (Schneider and Helms, 2003). The identity of a MXP can be re-specified to a FNM by a combination of noggin and retinoic acid (Lee et al., 2001). BMP2, 7, FGF8, SHH, and HOX (MSXs) are involved in the formation of these prominences (Ashique et al., 2002a, b; Helms and Schneider, 2003; Hu and Helms, 1999; Hu et al., 2003; Richman et al., 1997; Barlow and Francis-West, 1997; Wilke et al., 1997; Creuzet et al., 2002). Recently an epithelial region in the FNM with juxtaposed FGF8 / SHH was shown to induce beak outgrowth (Hu et al., 2003). Indeed FGF 8 / SHH were shown to induce cranial chondrogenesis in vitro and in vivo (Abzhanov and Tabin, 2004).
While the facial morphology is determined by the crest cells (Schneider and Helms, 2003), we are interested in how chicken and duck faces develop differently in the late stages of morphogenesis. Recently, we showed that there are localized mesenchymal cell proliferative zones (LoGZ) in the FNM. In both chickens and ducks, there were two LoGZ at lateral FNM at (chicken H&H) stage 26. They converged into one in the chicken but remained as two in the duck. We showed that this region is enriched with BMP4, and further showed that BMP4 is involved in mediating LoGZ activity (Wu et al., 2004a; Fig. 6C). Independently, Dr. Tabin’s group pursued Galapagos island finch beaks directly. Using cDNA library subtraction, they also found the main candidate for beak diversity is BMP4. They went on to use chickens to show that BMP4 is functionally involved (Abzhanov et al., 2004). The concept is that a special activity may not be based on the presence or absence of a signaling molecule. Rather, the configuration of signaling molecule expressing cell clusters is important. This is further demonstrated in the cleft primary palate chicken mutant in which the abnormality is due to the failure of FGF8 to become restrictively expressed, not the absence or mutation of FGF8 (MacDonald et al., 2004). Therefore, BMPs are likely to be the major mediators of beak growth, while other morpho-regulatory molecules can act on the BMP pathway and in this way adjust its activity, and therefore the shape of the beak. How the messages in the chicken or duck neural crest cells are translated into the topological differences of localized growth zones in the FNM remain to be investigated.
Topology of multi-component organs
One unique aspect of the beak is that it represents a paradigm of "complex morphogenesis" in which an organ is made from multiple components, in contrast to "simple morphogenesis" in which the whole organ is sculpted from one primordium. Comparing the limb bud with facial morphogenesis, the limb bud is a paradigm of “simple morphogenesis". Developmental biologists have learned a lot of the molecular mechanisms of limb morphogenesis in the last decade (Dudley and Tabin, 2000; Niswander, 2003; Tickle, 2003; Capdevila and Izpisua-Belmonte, 2001). Through careful analyses of many laboratories, we now learned how molecular pathways (FGF, SHH, HOX, WNT, etc.) are involved in apical ectodermal ridge (AER), zone of polarizing activity (ZPA), and dorsal-ventral patterning that work together to shape the limb from a single primordium.
In contrast, the beak is made from the coordinated growth of multiple facial prominences. We try to define the following three categories of growth activities during beak morphogenesis. 1) Concerted "overall growth activities" are responsible for the global expansion of the face. 2) "Diffuse growth zone", the dispersed mesenchymal growth in each prominence contributes to different dimensions of the face. 3) The "localized growth zone" (LoGZ), which focuses on the temporal - spatial growth activities within individual prominence, molding specific shapes out of one prominence (Fig. 6). There appears to be a global overall growth activity in all facial prominences, and yet each facial prominence has its distinct localized growth zone. Some facial prominences have multiple localized growth zones. Thus, for the beak of each bird, a unique facial configuration emerges from the undulating landscape of global growth activities with peaks and valleys fine tuned by localized growth zones and localized apoptotic zones.
Complex morphogenesis offers more opportunities to generate morphological diversity (Fig. 6A), but the complex process is also prone to errors as seen in the high incidences of cleft palate / lips due to lack of coordination of cellular events (MacDonald et al., 2004). We can speculate a giant beak as seen in the Toucan may be produced when the "overall growth activity" is high. By increasing the "diffuse growth activity" in the maxilla or mandible alone, asymmetrically bigger upper / lower beaks may be generated as seen in parrots and pelicans. By adjusting the configurations of "localized growth zones", flat beaks like those in ducks, or vertical beaks like those seen in the sea gulls may be produced. By positioning the localized growth zone in a horizontal or oblique angle, beaks may grow straight as in the duck or curved as in the eagle. By sustaining the activity of a focused LoGZ, a long sharp beak as seen in the crane can be produced. The molecular bases of these interesting beak designs remain to be investigated.
Evolution
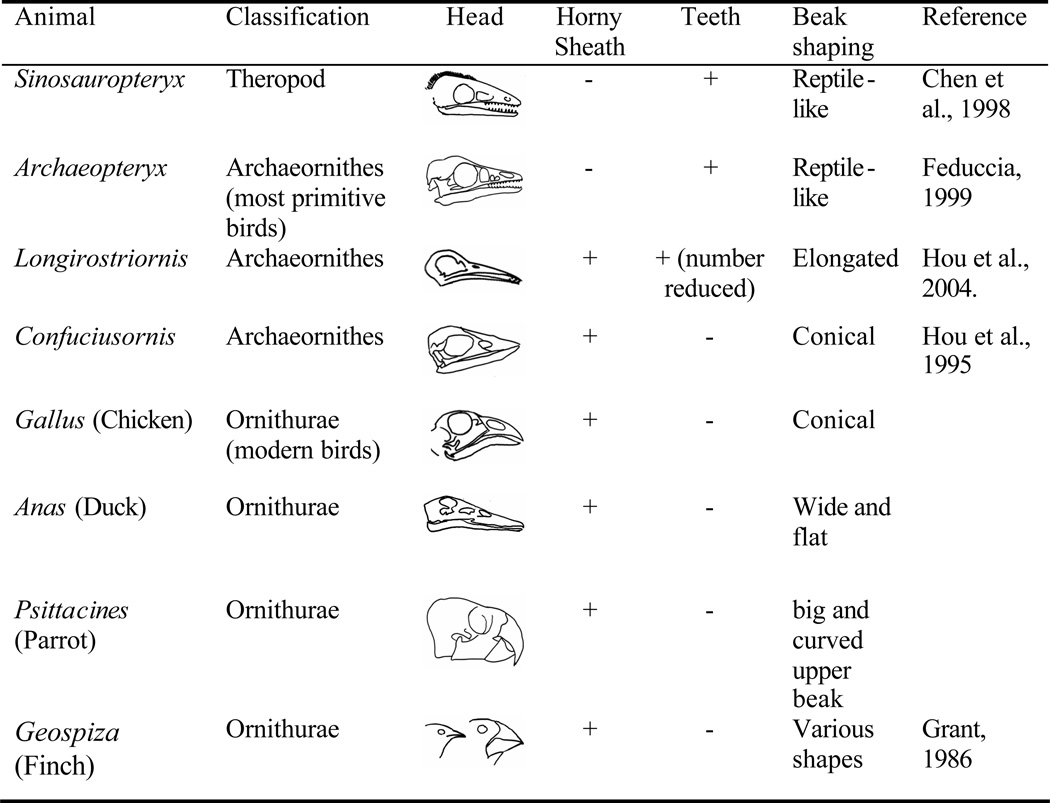
How do we define an "avian beak"? An avian beak requires the formation of a horny sheath, loss of teeth and the modification of the maxilla and mandibles into unique shapes. From the reptile to bird, the toothed jaws were gradually transformed into beaks. Indeed, in reptiles, beaks were seen in Psittacosaurus (a beaked dinosaur) and even in today's turtles. During the evolution of the beak, the trend is the gradual reduction and eventual loss of teeth, coupled with the formation of the horny sheath by thickened epidermal differentiation (Feduccia, 1999). Some Mesozoic birds existed representing intermediate stages (Fig. 7).
Fig. 7. Evolution of the beak.
Different shapes of snouts from reptiles, Mesozoic birds, and today's birds are represented.
Archaeopteryx had uniform reptilian teeth in both its upper and lower jaws. Longirostravis (125 million years ago) had a very long and slender rostrum and signs of the presence of a horny sheath (Hou et al. 2004). Ten small and conical shaped teeth are arranged in pairs and preserved in the distal snout. As this is the earliest wading bird, the preservation of teeth in the anterior snout may have facilitated securing its prey. The arboreal Confuciusornis is likely to be among the early birds that have formed a real beak with a complete loss of teeth in both of the upper and lower beak (Hou et al., 1996). The diversity of beaks is shaped by diet and reflects adaptive radiation (Lucas and Stettenheim, 1972; Feduccia, 1999). Darwin's finches in the Galapagos Islands are derived from a common ancestor and have evolved different sizes and shapes of beaks. The variation is subject to natural selection and environmental changes (Grant, 1986). In other birds, seed eaters such as chickens, quails and pigeons have conical beaks. Ducks have soft, leathery and flattened beaks for filtering food from the mud and water (Lucas and Stettenheim, 1972). Hawks have curved upper beaks for raptorial tearing.
To summarize beak morphogenesis, we have learned that beaks are made of the same differentiation materials (bone, horny sheath), but they form diverse shapes in different species. The different shapes are based on different topobiologically arranged cellular activities. By varying the proportion of the width, depth and length, different dimensions and their angles, the architecture of the beak is laid down. By modulating the number, size and positions of LoGZ, the beak can be further shaped (Fig. 6). We have learned that BMP pathway members, agonists and antagonists, may work as molecular candidates mediating the formation of a spectrum of morphologies for selection. Our experimental study with chickens showed that we can indeed produce beaks phenocopying those in Nature by modulating different developmental steps (Wu et al., 2004a). It is likely that the diversification of beak shapes was achieved by modulating prototypical molecular modules during the evolution of the beak. We now know that the BMP4 pathway is involved and can start by studying molecules related to this pathway.
Topobiology of other organs
Similar topobiological events take place in other organs as well. To continue the discussion of the integument, we have applied this concept to analyze the effect of tilting the balance of Bmp activity on the formation of various integument organs. We used KRT14 (human keratin 14 promoter) to drive the expression of chicken noggin in the basal layer of the integument. Ectodermal organ formation shares induction, morphogenesis, differentiation and regenerative phases. Since KRT14 driven expression of noggin suppressed Bmp activity at different stages of integument organ formation, the consequences are different (Plikus et al., 2004). When Bmps are suppressed at the induction stage, the number of hair follicles increases. When Bmps are suppressed at the morphogenesis stage, the size of genitals are increased. Suppressing Bmps also causes conversion of sweat glands and meimobian glands into hairs. Moderate reduction of BMP activity in claw morphogenesis causes splitting of the claw growth zone into multiple small growth zones and hence multiple nail plates. Complete suppression converts claw regions into epidermis. In addition, molar teeth change cusp shapes and sizes (Plikus et al., 2005). Thus, the change of phenotypes can be appreciated in the context of morpho-regulation (Edelman, 1988b). Since the changes of number, size and shape here are relatively minor, we also asked whether these should be considered as true pathology (pathology only if it is nonfunctional) or rather if they may be phenotypic variations that may be useful someday if the environment changes (Plikus et al., 2004). Topobiological analyses also have been used to analyze the change of cell adhesion during hair follicle morphogenesis (Muller-Rover et al., 1999). Invagination of hair placodes also has been successfully explained by increased expression of noggin and beta catenin (Jamora et al., 2003).
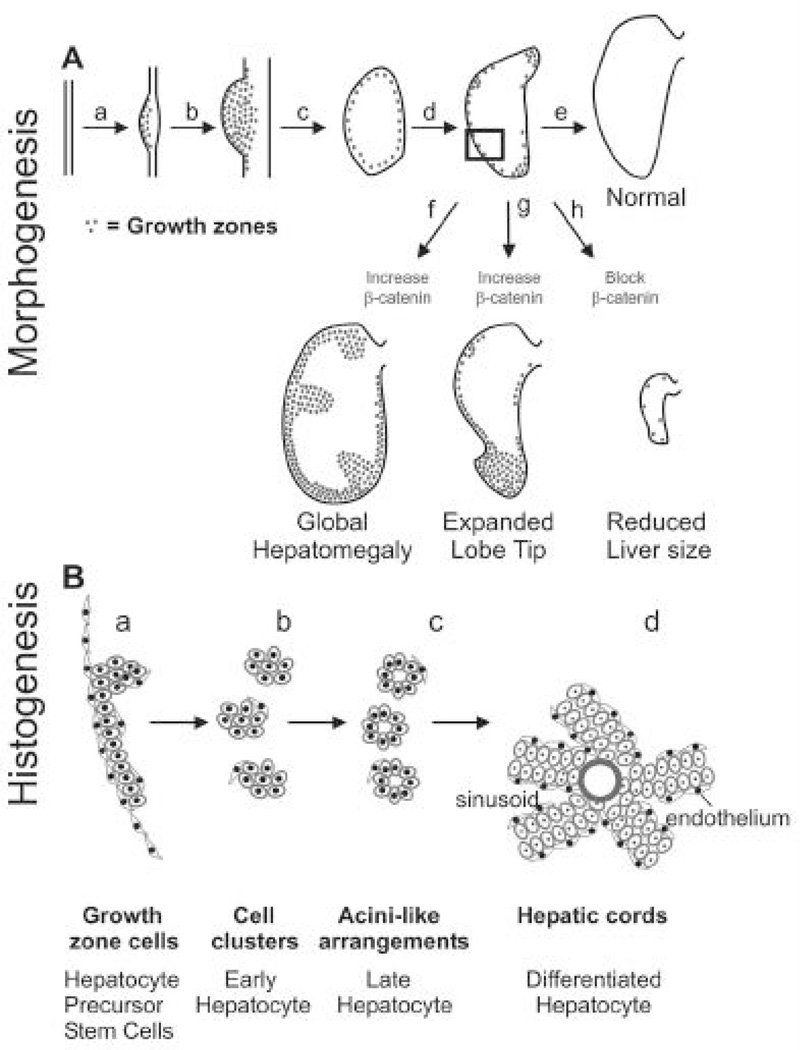
Among the visceral organs, the liver has a unique morphology with an asymmetric apex growing out from the liver lobes. We showed that initially there are diffuse growth activities and BrdU labeled cells are distributed all over the developing liver primordia in embryonic day 4 (E4) chicken embryos. At E7, proliferating cells become limited to the outermost layer of the developing liver primordia (Fig. 8). The duration of this stage determines the overall size of the liver. At E8, the proliferative zones become localized to the apex and a few regions in the outer margin to allow expansion in those specific regions, producing unique liver shapes (Suksaweang et al., 2004). Beta catenin mediates growth zone activity and different liver morphologies are produced when beta catenin is over expressed or suppressed (Suksaweang et al., 2004). As the liver primordia become mature toward the center, the hepatoblasts start to organize into a unique hepatic architecture, from layers to clusters, acini configuration and hepatic cords.
Fig. 8. Topobiological events in liver development.
Stippled region: growth zone. The distribution of the growth zone is changed from diffuse, to the outer layer of developing primordia, to selected regions of growing livers. From Suksaweang, et al., 2004, Morphogenesis of chicken liver: identification of localized growth. Developmental Biology. 266, 109–122.
In the lung, formation of branches increases the surface area for air sac / endothelial contact and is essential for its function. Branching occurs at the growing tips. Retinoic acid induces the expression of Fgf10 (Desai et al., 2004). Epithelial Shh helps to restrict the expression of mesenchymal Fgf10. Fgf10 defects lead to tracheobronchial truncations. Bmp4 further restricts Fgf10 expression along the proximal-distal axis (Bellusci et al., 1996; Affolter et al., 2003). Through a feedback loop, Fgf10 increases Bmp4 expression levels. It is thought that Shh present at the growing tip down regulates Fgf10 in the center, effectively splitting the field and inducing lung branching. Tgfβ1 is also expressed at branch sites and proximal regions of the branches. It promotes the deposition of extracellular matrix molecules and is believed to inhibit branching.
In the mammary gland, branching is largely dependent on Mmps (matrix metallo-proteinases). Branching occurs at the terminal end buds but also can occur along the side of the ducts by budding. As in the lung, branching of mouse mammary glands 1, 2, 3 and 5 appears to be dependent upon Fgf10 expression (Mailleux et al., 2002). The epithelial ducts are surrounded by myoepithelial cells and a dense stroma containing connective tissues and fibroblasts. Hormonal stimulation during estrous cycles leads to expanded growth and branching followed by regression during involution. Levels of Msx1 and possibly Msx2 drop during lactation and return during involution (Phippard et al., 1996) showing their possible regulation by hormones.
In contrast, branching of feather barbs occurs via a different mechanism. The feather filament cylinder forms first, and then cells between barb ridges go through apoptosis to sculpt out the spaces (Chang et al., 2004; Fig. 3). This is similar to digit separation in the limb. Thus, similar organ morphologies may be achieved through totally different topobiological mechanisms.
It should also be pointed out that in some organs, the end points of organogenesis can be chemical reactions (e.g., liver) or electric activities (e.g., brain). The topobiology concept was originally applied to brain function (Edelman, 1988a). For these, the topological arrangements are also important as they provide the essential anatomical constraints for cell groups to interact and connect. We chose integument organs because the consequence is obvious and helpful for us to decipher the topobiological principles.
Topographic specificity of multi-primordia organs
The multiplicity of certain ectodermal organs allows regional specification for diverse functions. The regional specificity can be considered at different hierarchical levels: i) across the whole body surface, ii) across an appendage field, and iii) within one appendage organ.
The regional specificity across the body surface can be appreciated clearly in humans. In our facial skin, eye brows, lips, palms, soles, nails, etc., different skin regions have fundamentally similar skin and skin appendage structures, but with topological variations for specialized functions (Chuong, 1998; Chuong et al., 2002). The mouse appears furry and the regional differences do not appear to be as apparent. We can see clear differences in vibrissae, tail skin, footpads, claws (Sundberg, 1994; Plikus et al., 2004; in press). Although not very obvious, there are also dorsal - ventral differences (Candille et al., 2004) and primary / secondary hair differences (Botchkarev et al., 2002). In other mammals, these differences can be exaggerated and different hair follicles respond differently to seasonal changes. The regional specificity is very clear in birds. There are downy feathers, contour feathers, flight feathers, tail feathers, scales, claws, beaks, combs, etc. (Lucas and Stettenheim, 1972; Fig. 9A) . Every small region is specialized to make the best use of the skin. Yet these regional diversifications are the results of evolutionary novelty and natural selection. The "proto-feathered" dinosaurs, Sinornithosaurus, about 120 million years ago had similar "protofeathers" all over the body without much appreciable regional specificity (Chen et al., 1998; Xu et al., 2001).
What are the molecular bases of these regional specificities? Classical tissue recombination experiments implied that the determinants are in the mesenchyme, if the epidermial cells maintain "stem cell" properties, competent in its multi-potentiality and not irreversibly committed (Fig. 10, the bidirectional arrows in the epidermal cell column). Differences in dorsal and ventral dermal progenitors have been defined (Fliniaux et al., 2004a), yet the molecular basis remains elusive. We have earlier observed Hox proteins expressed differently in different body regions of the developing feather buds, and have suggested the Hox code hypothesis for the regional specificity of the skin. The different Hox expression patterns observed in human dermal fibroblasts derived from different body regions are consistent with this hypothesis. The involvement of Tbx15 in the dorsal / ventral mouse coat is another exciting advance. With genome availability and microarray technology, a topographic mapping of skin regions over the body surface will provide insight to help zoom in on the molecular basis of regional specificity. This control of the specificity is also critical to regulating the type of ectodermal organs one may obtain from stem cells (Fig. 10).
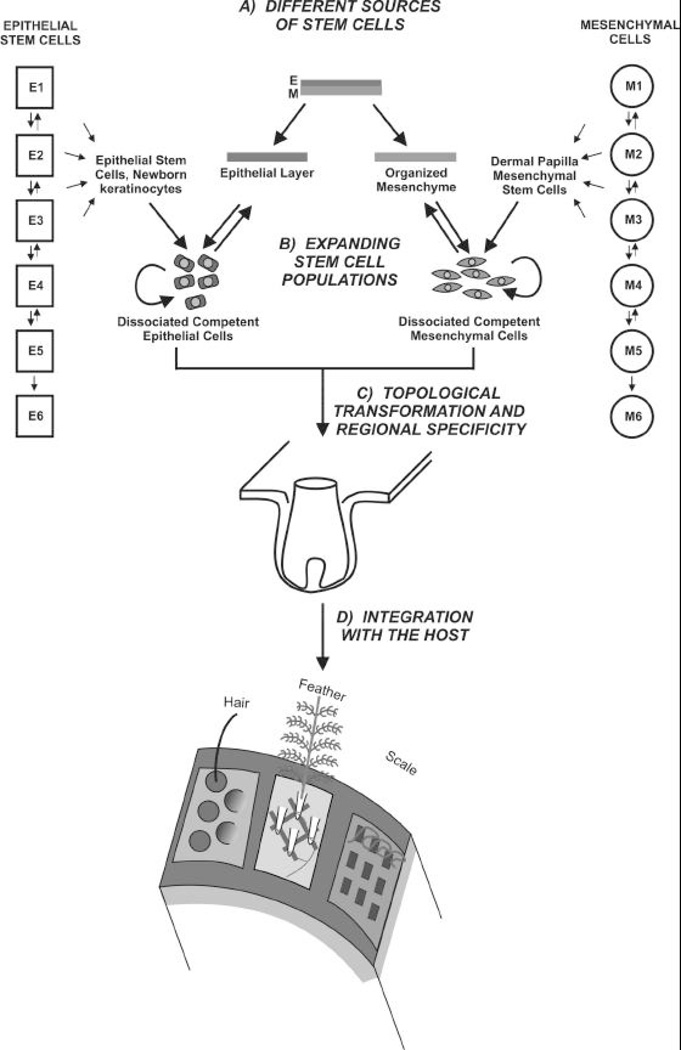
Fig. 10. Epithelial and mesenchymal cell recombination to generate new organs.
The four issues in stem cell biology (A-D) are highlighted, and ectodermal organ formation is used for illustration. A) Sources of stem cells can be from embryonic stem cells, adult stem cells, or somatic nucleus transplantation. Cells on the lateral columns indicate different stages during progression of stem cells. The downward arrows mean differentiation. The reverse arrows mean de-differentiation which eventually disappears, meaning cells are fully committed and their fates cannot be reversed anymore. B) Cell populations are expanded with the idea that the stem cell properties, self renewal and pluripotentiality, will not be lost or deregulated to become tumors. C) Competent epithelial stem cells and regional specific mesenchymal cells are combined in a proper environment to generate organs. If everything is set right, they can self-organize in normal morphogenesis. In tissue engineering, we need to learn these principles and the regulation of specificity. D) A single feather follicle would not be too useful if it is not connected to other parts of the body and coordinated as part of the system (Fig. 1, 10D). Ectodermal organs have to be connected with other systems via angiogenesis, myogenesis, neurogenesis to be fully integrated with the organism.
In the bird, the body regions are established by dividing the body surface into different fields or tracts during development (Sengel, 1976; Dhouailly et al., 2004; Jiang et al., 2004). By having multiple feathers in one feather tract, another level of topobiological specificity is possible across the feather tract. There are different modes of flight based on different wing shapes (Feduccia, 1999, Fig. 9A). The shape of the wing is made by the combination of the 20–30 flight feathers (remiges). Their relative lengths form the contour of the wing. Since the length of feather shaft is a function of the duration of the growth phase (like the anagen phase of the hair cycle), the shape of the wing becomes the spatial layout of multiple flight feathers from the medial to the lateral regions of the wing (in which the midline of the body is the medial. One can also consider this as the proximal - distal axis of the limb bud), each with its own temporal cycle regulation, but together add up to form a distinct shape of the wing. Another level of complexity is imposed on top of this array of flight feathers: the medial / lateral bilateral asymmetry (again, here we use the body axis, not the feather rachis as the reference point). According to aerodynamic engineering, the feather in the most lateral wing is most bilateral asymmetric, with the lateral vane much narrower than the medial vane (Fig. 9B). This feature was used to judge whether a fossil bird is a good flyer (Feduccia, 1999). Birds that give up flight (e.g., on isolated islands) soon lose this level of asymmetry over several generations. Two aspects of interest pertain to the molecular basis of this process: one is by what topobiological mechanism lateral / medial asymmetry is produced from the bilaterally symmetric flight feathers; the other is how this molecular activity can be displayed in a graduated medial - lateral fashion . In mammals, tooth fields have similar types of topological modulations to generate different sizes and shapes of incisors, canines, and molars (Jernvall and Thesleff, 2000; Plikus et al., 2005). These specializations do not exist in most reptiles or Mesozoic birds (Hou et al., 2003, 2004).
There are further regional variations within a single appendage organ. For example, the graded topological modulation of feathers can be seen in contour feathers. In the trunk, the functions of each feather are further divided along the proximal – distal axis. The distal region is made of pennaceous barbs (for contouring or communication), and the proximal domain is made of plumulaceous barbs (for thermal insulation) (Fig. 9C). Furthermore, the ratio of plumulaceous versus pennaceous regions changes gradually among adjacent feathers in the same feather tract, reflecting the need of different body parts to make the best balance between preserving body temperatures and streamlining body shapes. Such regional specific modulation of organ morphology makes the most effective use of every keratinocyte. In other organs, this type of sophisticated modification among cell groups may also exist (e.g, different brain regions, cortex laminations, neuronal circuits, Edelman, 1988a). Yet the feather is a good model because it lays out all topological arrangements clearly - the barbule represents a row of 10–20 keratinocytes connected in a head to tail fashion.
Integration of stem cells and organs to reach the level of system biology
We now come back to the stem cell issue. In the beginning, we emphasized that there are four types of issues that stem cell biology has to solve to achieve the goal of regenerative medicine (Fig. 10, A-D). Using the skin as an example, recent progress has led to new understanding in the inter-follicular epidermal stem cells (Watt, 2002) and hair bulge stem cells (Morris et al., 2004; Tumbar et al., 2004; Fig. 10A). We have learned the importance of the niche in regulating stem cell homeostasis (Fig. 10B). We also have learned that, to a limit, these epidermal progenitors can de-differentiate and trans-differentiate. Indeed it is most interesting to observe the conversion of part of the scales into feathers, amniotic membranes into feathers and hairs (Fliniaux et al., 2004b), sweat glands / Meibomian glands into hairs (Plikus et al., 2004), and even adult cornea epithelium into hairs (Pearton et al., 2005). Research in genetic and epigenetic regulation should shed more light on the control of cellular phenotypes.
Suppose this research bears fruit and we are able to form an organ; how then, do we direct it become part of the host and function in a useful manner? One idea situation is to have competent epidermal stem cells and inducing mesenchymal cells incubated in a micro-environment with proper chemical signaling and topological setting, then let them self-organize (Fig. 10). This type of approach was pioneered in Moscona's cell aggregate approaches to form feathers, retina, lentoid, livers, etc. (e.g., Garber et al., 1968; Vardimon et al., 1988). In these aggregates, a quite remarkable degree of histogenesis and chemical differentiation was achieved in the three dimension aggregates, yet their topological relationships are random. We constrained dissociated feather mesenchymal cells into a two dimension configuration, and overlayed this with a competent epithelial sheet. With this topological arrangement, we were able to obtain a reconstituted skin with an array of evenly spaced and oriented feather follicles (Jiang et al., 1999 and unpublished data). In the mouse, Lichti et al., (1995) mixed a population of competent epidermal and dermal cells in a chamber that was transplanted on a nude mouse. The cells sort out to form hair follicles. This procedure was simplified and improved to generate exogenous hair organs that are supported by the host and could cycle (Zheng et al., 2005). This is very good progress, albeit the hair filaments point to the center of the aggregates, forming a cyst. We still have to make the topobiological events right before stem cell engineering can be applied to humans.
Stem cell biology is just at its dawn. There are many critical issues to be solved and knowledge from multiple disciplines to be integrated. Assuming we have access to sources of stem cells and know, to a certain level, how to induce their differentiation, here we focus on the issue of guiding stem cells into organs. We identify the fundamental and practical importance of topobiological events in building the architecture of an organ. We turn to Nature to learn how she solves the simple to complex designs of ectodermal organs. Using feather and beak morphogenesis to decipher the principles, we observed a succession of topobiological transformation events, taking the epithelia from a flat sheet to more and more complex structures. Some of these topobiological principles are likely to be in operation in other organogenesis as well. These processes are important in development and morphological evolution, and have to be taken into consideration in tissue engineering. There may be a long way to go, but the process is exciting and the best is yet to come.
Acknowledgment
This work is supported by grants from NIH (CMC, AR42177, 47364, 47364; RW, CA83716). CMC thanks Dr. G. M. Edelman for his inspirations.
References
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev. Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int. J. Dev. Biol. 2002a;46:243–253. [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002b;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Baron MH. Embryonic origins of mammalian hematopoiesis. Exp. Hematol. 2003;31:1160–1169. doi: 10.1016/j.exphem.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J. Exp. Zoolog. B Mol. Dev. Evol. 2003;298:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J. Invest. Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Brown WR, Hubbard SJ, Tickle C, Wilson SA. The chicken as a model for large-scale analysis of vertebrate gene function. Nat. Rev. Genet. 2003;4:87–98. doi: 10.1038/nrg998. [DOI] [PubMed] [Google Scholar]
- Candille SI, Van Raamsdonk CD, Chen C, Kuijper S, Chen-Tsai Y, Russ A, Meijlink F, Barsh GS. Dorsoventral patterning of the mouse coat by Tbx15. PLoS Biol. 2004;2:30–42. doi: 10.1371/journal.pbio.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Yu M, Wu P, Jiang T-X, Yu H-S, Widelitz RB, Chuong C-M. Sculpting Skin Appendages Out of Epidermal Layers Via Temporally and Spatially Regulated Apoptotic Events. J. Invest. Dermatol. 2004;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. The Rise of Birds. Baltimore: John Hopkins University Press; 1997. [Google Scholar]
- Chen PJ, Dong ZM, Shen SN. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–152. [Google Scholar]
- Chiappe LM. The First 85 million years of Avian Evolution. Nature. 1995;378:349–355. [Google Scholar]
- Chuong C-M. Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience; 1998. [Google Scholar]
- Chuong C-M, Edelman GM. Expression of cell adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J. Cell. Biol. 1985a;101:1009–1026. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Edelman GM. Expression of cell adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J. Cell. Biol. 1985b;101:1027–1043. doi: 10.1083/jcb.101.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Chodankar R, Widelitz RB, Jiang TX. Evo-Devo of Feathers and Scales: Building complex epithelial appendages. Curr. Opin. Dev. Gen. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Nickoloff BJ, Elias PM, et al. What is the 'true' function of skin. Exp. Dermatol. 2002;11:159–187. doi: 10.1034/j.1600-0625.2002.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Oliver G, Ting S, Jegalian B, Chen HM, De Robertis EM. Gradient of homeoproteins in developing feather buds. Development. 1990;110:1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Chuong C-M, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J. Exp. Zoolog. B Mol. Dev. Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Espinasse PG. On the normal and abnormal development of the feather. J. Embryol. Exp. Morphol. 1961;9:223–251. [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev. Dyn. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- Columbia Encyclopedia. 2005 [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Vincent C, Le Douarin NM. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129:4301–4313. doi: 10.1242/dev.129.18.4301. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev. Biol. 2004;273:402–15. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Olivera-Martinez I, Fliniaux I, Missier S, Viallet JP, Thelu J. Skin field formation: morphogenetic events. Int. J. Dev. Biol. 2004;48:85–91. [PubMed] [Google Scholar]
- Dudley AT, Tabin CJ. Constructive antagonism in limb development. Curr. Opin. Genet. Dev. 2000;10:387–392. doi: 10.1016/s0959-437x(00)00101-5. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Topobiology: An introduction to molecular biology. Basic Books. 1988a:1–56. [Google Scholar]
- Edelman GM. Morphoregulatory molecules. Biochemistry. 1988b;27:3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Efrat S. Regulation of insulin secretion: insights from engineered beta-cell lines. Ann. N. Y. Acad. Sci. 2004;1014:88–96. doi: 10.1196/annals.1294.009. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA. Gastrulation in the sea urchin embryo is accompanied by the rearrangement of invaginating epithelial cells. Dev. Biol. 1985;112:383–390. doi: 10.1016/0012-1606(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Feduccia A. In: The Origin and Evolution of Birds. ed. 2. Feducia A, editor. New Haven: Yale Univ. Press; 1999. pp. 93–137. [Google Scholar]
- Fliniaux I, Viallet JP, Dhouailly D. Ventral vs. dorsal chick dermal progenitor specification. Int. J. Dev. Biol. 2004a;48:103–106. [PubMed] [Google Scholar]
- Fliniaux I, Viallet JP, Dhouailly D, Jahoda CA. Transformation of amnion epithelium into skin and hair follicles. Differentiation. 2004b;72:558–565. doi: 10.1111/j.1432-0436.2004.07209009.x. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mech. Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Robson L, Evans DJ. Craniofacial development: the tissue and molecular interactions that control development of the head. Adv. Anat. Embryol. Cell. Biol. 2003;169(III-VI):1–138. doi: 10.1007/978-3-642-55570-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA. Stem cells: a new lease on life. Cell. 2000;100:143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- Garber B, Kollar EJ, Moscona AA. Aggregation in vivo of dissociated cells. 3. Effect of state of differentiation of cells on feather development in hybrid aggregates of embryonic mouse and chick skin cells. J. Exp. Zool. 1968;168:455–472. doi: 10.1002/jez.1401680406. [DOI] [PubMed] [Google Scholar]
- Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Grant PR. In: Ecology and evolution of Darwin’s finches. Grant PR, editor. Princeton: Princeton Univ. Press; 1986. pp. 1–492. [Google Scholar]
- Green H, Easley K, Iuchi S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:15625–15630. doi: 10.1073/pnas.0307226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Armytage S. Extraordinary Chickens. New York: Harry N. Abrams, Inc; 2000. [Google Scholar]
- Held LI. Models for Embryonic Periodicity. New York: Karger; 1992. [PubMed] [Google Scholar]
- Hardin JD, Cheng LY. The mechanism and mechanics of archenteron elongation during sea urchin gastrulation. Dev. Biol. 1986;115:490–501. [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J. Exp. Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc. Natl. Acad. Sci. USA. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat. Rev. Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Hou LH, Chiappe L, Zhang FC, Chuong C-M. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91:22–25. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LH, Chuong CM, Yang A, Zeng XL, Hou JF. Fossil Birds of China. China: Yunnan Science and Technology; 2003. [Google Scholar]
- Hou LH, Martin LD, Zhou Z, Feduccia A. Early Adaptive Radiation of Birds: Evidence from Fossils from Northeastern China. Science. 1996;274:1164–1167. doi: 10.1126/science.274.5290.1164. [DOI] [PubMed] [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Hwang WS, Roh SI, Lee B, et al. Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science. 2005;308:1777–1783. doi: 10.1126/science.1112286. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Jiang T-X, Chuong C-M. Mechanism of feather morphogenesis: I. Analyses with Antibodies to adhesion molecules tenascin, N-CAM and integrin. Dev. Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jiang T-X, Jung HS, Widelitz RB, Chuong C-M. Self organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- Jiang T-X, Wideltz RB, Shen WM, Will P, Wu DY, Lin CM, Jung JS, Chuong C-M. Integument pattern formation involves genetic and epigenetic controls operated at different levels: Feather arrays simulated by a digital hormone model. Int. J. Dev. Biol. 2004;48:117–136. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TD, Ruben JA, Martin LD, Kurochkin EN, Feduccia A, Maderson PF, Hillenius WJ, Geist NR, Alifanov V. Nonavian feathers in a late Triassic archosaur. Science. 2000;288:2202–2205. doi: 10.1126/science.288.5474.2202. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev. Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Kang P, Svoboda KK. Epithelial-Mesenchymal Transformation during Craniofacial Development. J. Dent. Res. 2005;84:678–690. doi: 10.1177/154405910508400801. [DOI] [PubMed] [Google Scholar]
- Keller R. The cellular basis of amphibian gastrulation. In: Browder L, editor. The cellular basis of morphogenesis. Developmental Biology: A comprehensive Synthesis. New York: Plenum Press; 1986. pp. 241–327. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kiskowski MA, Alber MS, Thomas GL, Glazier JA, Bronstein NB, Pu J, Newman SA. Interplay between activator-inhibitor coupling and cell-matrix adhesion in a cellular automaton model for chondrogenic patterning. Dev. Biol. 2004;271:372–387. doi: 10.1016/j.ydbio.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes. Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. p63 and epithelial appendage development. Differentiation. 2004;72:364–370. doi: 10.1111/j.1432-0436.2004.07208002.x. [DOI] [PubMed] [Google Scholar]
- Lako M, Armstrong L, Cairns PM, Harris S, Hole N, Jahoda CA. Hair follicle dermal cells repopulate the mouse haematopoietic system. J. Cell. Sci. 2002;115:3967–3974. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- Lee SH, Fu KK, Hui JN, Richman JM. Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001;414:909–912. doi: 10.1038/414909a. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem Cell Niche: Structure and Function. Annu. Rev. Cell. Dev. Biol. 2005 doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lichti U, Scandurro AB, Kartasova T, Rubin JS, LaRochelle W, Yuspa SH. Hair follicle development and hair growth from defined cell populations grafted onto nude mice. J. Invest. Dermatol. 1995;104:43S–44S. doi: 10.1038/jid.1995.60. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR, editors. Avian Anatomy: Integument. Agriculture Handbook 362: Agricultural Research Services. Washington, D. C: US Department of Agriculture; 1972. [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Abbott UK, Richman JM. Upper beak truncation in chicken embryos with the cleft primary palate mutation is due to an epithelial defect in the frontonasal mass. Dev. Dyn. 2004;230:335–349. doi: 10.1002/dvdy.20041. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Clarke JD, Tickle C. Fate map of the developing chick face: analysis of expansion of facial primordia and establishment of the primary palate. Dev. Dyn. 1998;212:102–118. doi: 10.1002/(SICI)1097-0177(199805)212:1<102::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. Bioessays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Applications of a theory of biological pattern formation based on lateral inhibition. J. Cell. Sci. 1974;15:321–346. doi: 10.1242/jcs.15.2.321. [DOI] [PubMed] [Google Scholar]
- Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit. Rev. Oral. Biol. Med. 2000;11:199–215. doi: 10.1177/10454411000110020401. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Moore GP, Jackson N, Isaacs K, Brown G. Pattern and morphogenesis in skin. J. Theor. Biol. 1998;191:87–94. doi: 10.1006/jtbi.1997.0567. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Tokura Y, Welker P, Furukawa F, Wakita H, Takigawa M, Paus R. E- and P-cadherin expression during murine hair follicle morphogenesis and cycling. Exp. Dermatol. 1999;8:237–246. doi: 10.1111/j.1600-0625.1999.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagorcka BN, Mooney JR. The role of a reaction-diffusion system in the initiation of primary hair follicles. Theor. Biol. 1985;114:243–272. doi: 10.1016/s0022-5193(85)80106-5. [DOI] [PubMed] [Google Scholar]
- Newman SA, Frisch HL. Dynamics of skeletal pattern formation in developing chick limb. Science. 1979;205:662–668. doi: 10.1126/science.462174. [DOI] [PubMed] [Google Scholar]
- Niswander L. Pattern formation: old models out on a limb. Nat. Rev. Genet. 2003;4:133–143. doi: 10.1038/nrg1001. [DOI] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev. Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Oster GF, Murray JD, Maini PK. A model for chondrogenic condensations in the developing limb: the role of extracellular matrix and cell tractions. J. Embryol. Exp. Morphol. 1985;89:93–112. [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl. Acad. Sci. USA. 2005;102:3714–3719. doi: 10.1073/pnas.0500344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. Bonemaking protein shapes beaks of Darwin's finches. Science. 2004;305:1383. doi: 10.1126/science.305.5689.1383. [DOI] [PubMed] [Google Scholar]
- Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu YH, Maxson R, Hill RE, Dale TC. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong C-M. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am. J. Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JGM, Snead ML, Chai Y, Chuong C-M. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evolution and Development. 2005 doi: 10.1111/j.1525-142X.2005.05048.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M, Sundberg JP, Chuong C-M. The Mouse in Biomedical Research. Academic Press. NY; Morphogenesis of Skin and its Appendages. In press. [Google Scholar]
- Prum RO. Development and evolutionary origin of feathers. J. Exp. Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Prum RO, Dyck J. A hierarchical model of plumage: morphology, development, and evolution. J. Exp. Zoolog. B Mol. Dev. Evol. 2003;298:73–90. doi: 10.1002/jez.b.27. [DOI] [PubMed] [Google Scholar]
- Prum RO, Williamson S. Theory of the growth and evolution of feather shape. J. Exp. Zool. 2001;291:30–57. doi: 10.1002/jez.4. [DOI] [PubMed] [Google Scholar]
- Regal PJ. The evolutionary origin of feathers. Q. Rev. Biol. 1975;50:35–66. doi: 10.1086/408299. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Richman JM, Herbert M, Matovinovic E, Walin J. Effect of fibroblast growth factors on growth of facial mesenchyme. Dev. Biol. 1997;189:135–147. doi: 10.1006/dbio.1997.8656. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J. Exp. Zoolog. B. Mol. Dev. Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Alvarez IS. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development. 1989;106:427–439. doi: 10.1242/dev.106.3.427. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC. Cell movements driving neurulation in avian embryos. Development. 1991;(Suppl. 2):157–168. [PubMed] [Google Scholar]
- Scott MP. Development: the natural history of genes. Cell. 2000;100:27–40. doi: 10.1016/s0092-8674(00)81681-5. [DOI] [PubMed] [Google Scholar]
- Sengel P. Morphogenesis of Skin. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- Shen W-M, Will P, Galstyan A, Chuong C-M. Hormone-inspired self-organization and distributed control of robotic swarms. Autonomous Robots. 2004;17:93–105. [Google Scholar]
- Shieh SJ, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials. 2004;25:1545–1557. doi: 10.1016/s0142-9612(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Snyder BJ, Olanow CW. Stem cell treatment for Parkinson's disease: an update for 2005. Curr. Opin. Neurol. 2005;18:376–385. doi: 10.1097/01.wco.0000174298.27765.91. [DOI] [PubMed] [Google Scholar]
- Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong C-M. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev. Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP, editor. Handbook of Mouse Mutations with Skin and Hair Abnormalities: Animal Models and Biomedical Tools. CRC Press; 1994. [Google Scholar]
- Tickle C. Patterning systems--from one end of the limb to the other. Dev. Cell. 2003;4:449–458. doi: 10.1016/s1534-5807(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Phil. Trans. R. Soc. B. 1952;237:37–72. [Google Scholar]
- Vardimon L, Fox LL, Degenstein L, Moscona AA. Cell contacts are required for induction by cortisol of glutamine synthetase gene transcription in the retina. Proc. Natl. Acad. Sci. USA. 1988;85:5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. The stem cell compartment in human interfollicular epidermis. J. Dermatol. Sci. 2002;28:173–180. doi: 10.1016/s0923-1811(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mikawa T. Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development. 2000;127:87–96. doi: 10.1242/dev.127.1.87. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen J-Y, Wu P, Yu Z, Chuong C-M. Molecular Biology of feather morphogenesis: a testable model for evo-devo research. J. Exp. Zool. 2003;298B:109–122. doi: 10.1002/jez.b.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Dev. Dyn. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong C-M. Molecular shaping of the beak. Science. 2004a;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong C-M. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004b;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong C-M. The Morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong C-M. The biology of feather follicles. Int. J. Dev. Biol. 2004;48:181–191. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421:807–814. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- Zweers GA, Vanden Berge JC, Berkhoudt H. Evolutionary pattern of avian trophic diversification. Zoology. 1997;100:25–57. [Google Scholar]