Abstract
Objective
The authors performed meta-analyses of randomized controlled trials to examine the effects of cognitive training on attention-deficit/hyperactivity disorder (ADHD) symptoms, neuropsychological deficits, and academic skills in children/adolescents with ADHD.
Method
The authors searched Pubmed, Ovid, Web of Science, ERIC, and CINAHAL databases through May 18, 2014. Data were aggregated using random-effects models. Studies were evaluated with the Cochrane risk of bias tool.
Results
Sixteen of 695 nonduplicate records were analyzed (759 children with ADHD). When all types of training were considered together, there were significant effects on total ADHD (standardized mean difference [SMD] = 0.37, 95% CI = 0.09–0.66) and inattentive symptoms (SMD = 0.47, 95% CI = 0.14–0.80) for reports by raters most proximal to the treatment setting (i.e., typically unblinded). These figures decreased substantially when the outcomes were provided by probably blinded raters (ADHD total: SMD = 0.20, 95% CI = 0.01–0.40; inattention: SMD = 0.32, 95% CI = −0.01 to 0.66). Effects on hyperactivity/impulsivity symptoms were not significant. There were significant effects on laboratory tests of working memory (verbal: SMD = 0.52, 95% CI = 0.24–0.80; visual: SMD = 0.47, 95% CI = 0.23–0.70) and parent ratings of executive function (SMD = 0.35, 95% CI = 0.08–0.61). Effects on academic performance were not statistically significant. There were no effects of working memory training, specifically on ADHD symptoms. Interventions targeting multiple neuropsychological deficits had large effects on ADHD symptoms rated by most proximal assessors (SMD = 0.79, 95% CI = 0.46–1.12).
Conclusion
Despite improving working memory performance, cognitive training had limited effects on ADHD symptoms according to assessments based on blinded measures. Approaches targeting multiple neuropsychological processes may optimize the transfer of effects from cognitive deficits to clinical symptoms.
Key Words: ADHD, nonpharmacological, working memory, executive functions, evidence-based psychiatry
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset condition characterized by pervasive patterns of inattention and/or impulsivity-hyperactivity that often persist into later life.1 Combinations of pharmacological and psychological approaches are recommended for its treatment.2 Although medication is efficacious in randomized controlled trials (RCT) in the short/medium-term and is indicated as the first-line treatment (at least for severe cases2), it has a number of potential limitations—each affecting some patients. These include the following: partial response or nonresponse3; possible adverse effects4; uncertainty about long-term costs and benefits5; poor adherence6; and negative medication-related attitudes from patients, parents, or clinicians.7 Psychological treatments such as behavioral parent training are also widely used. However, a recent meta-analysis8 found no effects on ADHD symptoms when only ratings by assessors blind to treatment allocation were considered.
In recent years, cognitive training has been investigated as a potential ADHD treatment.9 Building on evidence of brain plasticity from rehabilitation science and contemporary developmental neuroscience, cognitive training is premised on the notion that key brain networks implicated in ADHD can be strengthened, and the cognitive processes they subserve improved, through controlled exposures to information processing tasks.10 Thus, it is argued that cognitive training can reduce ADHD symptoms and improve functioning by targeting neuropsychological deficits thought to mediate ADHD pathophysiology. In keeping with the complex nature of ADHD neuropsychology,11 cognitive training approaches have targeted a range of deficits (e.g., attentional control, working memory, inhibitory control). Currently, such training is typically delivered via computers using adaptive procedures, whereby training task difficulty is automatically increased across sessions to continually challenge the patient at the boundaries of his or her competence. This has been shown in neuroimaging studies to be necessary for sustaining neuronal changes.12,13
The efficacy of cognitive training for ADHD was addressed in a meta-analysis of nonpharmacological treatments for ADHD by Sonuga-Barke et al.14 on behalf of the European ADHD Guidelines Group (EAGG). This meta-analysis focused solely on RCTs. Importantly, it addressed the issue of blinding by comparing outcomes rated by individuals most proximal to the therapeutic setting (often unblinded and invested in the patient and/or intervention) and those provided by reporters judged to be probably blinded. Effects of cognitive training on ADHD symptoms calculated using unblinded ratings were highly significant (standardized mean difference [SMD] = 0.64, 95% CI = 0.33–0.95). These effects dropped substantially (SMD = 0.24) and became statistically nonsignificant (95% CI = −0.24 to 0.72) when probably blinded measures were used. However, these results should be considered as preliminary because only 6 RCTs were included. The authors concluded that more evidence was required, especially from trials in which assessments were effectively blinded, before cognitive training could be supported as an ADHD treatment. A second meta-analysis by Rapport et al.,9 published more recently and exploring a wider range of outcomes, found similar effects. However, compared to Sonuga-Barke et al.,14 this more recent meta-analysis included only 2 additional peer-reviewed RCTs with outcomes related to ADHD core symptoms. Moreover, to increase statistical power, Rapport et al.9 also included non-RCTs and pooled across design types, making effect size estimates of the effects of cognitive training on ADHD core symptoms and related neuropsychological impairment difficult to interpret.
A significant number of new RCTs of cognitive training for ADHD, not available for inclusion in these previous 2 meta-analyses,9,14 have been published in the past 2 years, reflecting the current interest in cognitive training in this field. The greater number of trials now available allows a much more definitive estimate of the effects of cognitive training to be made. In the present article, we update the first EAGG cognitive training meta-analysis to include these new trials, and we extend its focus to cover effects on neuropsychological processes and academic functioning, which were not addressed in the previous EAGG meta-analysis.14 The focus on neuropsychological processes is important for 2 reasons. First, neuropsychological deficits are postulated to mediate the pathways between originating causes and disorder onset: improvements in neuropsychological functioning may therefore be a prerequisite for ADHD symptom reduction.15 Second, they are associated with functional impairments in their own right, independent of their association with ADHD symptomatology, especially in social and academic contexts.16 A broad range of training approaches have been used with ADHD populations. In the meta-analysis by Sonuga-Barke et al.,14 given the small number of studies available, trials with different techniques had to be pooled to generate an effect size estimate. However, given the increased number of trials now available, our aim was to explore training-type specific effects through the use of subanalyses where sufficient numbers of trials existed.
Method
The EAGG protocol for nonpharmacological interventions for ADHD was registered on the International Prospective Register of Systematic Reviews PROSPERO (http://www.crd.york.ac.uk/PROSPERO, protocol number: CRD42011001393). The same protocol was followed here.
Inclusion and Exclusion Criteria
Only RCTs including interventions aimed to directly train a cognitive function were retained. As reported by the Cochrane group,17 to ensure high levels of methodological adequacy and to avoid the inevitable bias caused by dependence on investigators agreeing to provide data from unpublished studies, only published studies were included. Trials were included if participants had an ADHD diagnosis (any subtype) or met accepted cut-offs on validated ADHD rating scales and were between 3 and 18 years of age. Trials involving children with ADHD comorbid with rare disorders (e.g., fragile X syndrome) only were excluded. Control conditions allowed were “treatment as usual,” “wait list,” “active/placebo/sham” (i.e., involving other forms of computer-based activity or alternative training regimen). Trials were not excluded if patients received medication as part of normal treatment. In an extension of the EAGG protocol,14 trials could be included in this updated meta-analysis despite not reporting an ADHD outcome if they reported neuropsychological and/or academic outcomes.
Search Strategy
Sonuga-Barke et al.14 included studies up to April 3, 2012. Here, using the same search strategy, our final search date was May 18, 2014. Supplement 1, available online, reports details about the search strategy and syntax for each database. Parallel searches were conducted separately by the first 2 authors.
Outcome Measures
For consistency with previous EAGG meta-analyses8 and to provide a robust estimate of effects, outcome domains were analyzed only if 5 or more RCTs were available. The outcomes analyzed were: ADHD symptoms (total ADHD as well as inattention and hyperactivity/impulsivity symptoms), parent ratings of executive functioning (e.g., Behavior Rating Inventory of Executive Function [BRIEF]), standardized measures of reading and arithmetic ability, and laboratory-based measures of verbal and visual working memory, inhibition, and attention. For neuropsychological outcomes, only scores from tasks different from those used for training were included in the analysis.
Study Selection
Articles’ titles and abstracts were screened independently by the first 2 authors. Final inclusion was based on the full text. Trials were blindly double coded for eligibility by the first 2 authors (S.C., M.F). Disagreement was resolved by the senior author for 3 trials.
Risk of Bias Assessment
Two authors independently assessed trial risk of bias using 5 domains of the Cochrane Collaborations tool17: namely, selection bias, performance bias, detection bias, attrition bias, and other bias. If there was disagreement between the 2 raters, the final rating was established through consensus with the involvement of the senior author. This occurred for 4 trials.
Data Extraction and Statistical Analysis
Trial information was entered into RevMan 5.0.18 Data extraction was independently performed and cross-checked by the first 2 authors. SMD was calculated as mean pre- to posttreatment change in the intervention group minus the mean pre- to posttreatment change in the control group divided by the pooled pretest standard deviation with a bias adjustment.19 SMDs for each trial were combined using the inverse variance method. Given the inherent heterogeneity of studies, random effects models were used. The I2 statistic was calculated a posteriori to estimate between-trial SMD heterogeneity. For the most proximal analysis, parent ratings, if available, were used for home-based interventions, and teacher ratings were used for school-based interventions, except when it could be inferred from the manuscript’s text that teachers were less blinded than parents for home-based interventions and parents less blinded than teachers for school-based interventions (2 trials20,21). Probably blinded assessments were those made by an individual judged likely to be unaware of treatment allocation. In trials in which more than 1 such measure was available, the best-blinded measure was chosen. For home-delivered interventions, teachers’ ratings were usually judged to be blinded, whereas for school-based interventions, parents were judged to be blinded except where this could be inferred not to be the case from the text20,21 or from e-mail exchange with the authors. As per protocol, where direct observations were available, we selected these over rating-scale scores. This decision was based on the judgement that direct observations are likely, in general, to be better blinded than parent- or teacher-rated outcomes, even when the latter are made in a setting other than the therapeutic setting. Where multiple measures were available for a single outcome (as was sometimes the case for laboratory tasks), the measure most frequently reported across included trials and/or that which was judged to tap the core of the construct was selected. Sensitivity analyses were conducted including only trials meeting the following criteria: use of active/sham control; use of working memory training; use of training targeting more than 1 neuropsychological domain (termed here “multiple process training”); and use of no/low medication (i.e., with <30% of participants receiving medications). We also performed an additional sensitivity analysis excluding the study by Gray et al.,22 in which all participants had a diagnosis of ADHD plus coexisting intellectual disability. Publication bias was assessed with funnel plots and Egger’s tests. Finally, we also conducted a meta-regression analysis, using the metareg command in STATA,23 to assess the relationship between age and SMD for most proximal and probably blinded assessments of ADHD core symptoms. This analysis was conducted to establish whether the efficacy of cognitive training varied across age, a finding that could be of clinical significance.
Results
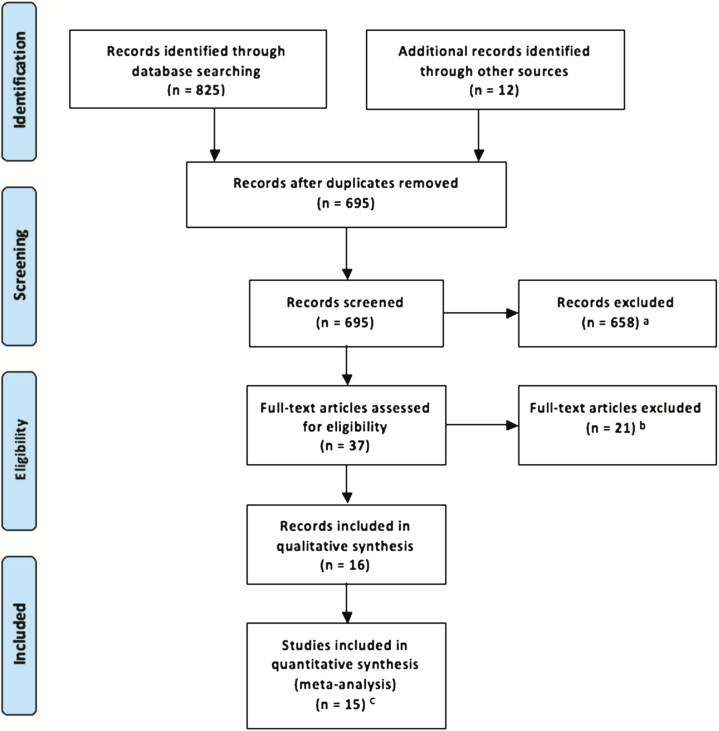
Fifteen trials (reported in 16 papers) met entry criteria (see Table S1, available online). Studies not included in the meta-analysis are listed (with reasons for their exclusion) in Table S2 (available online). Figure 1 reports the trial selection flowchart. Table 1 gives information about retained trials. Results of all analyses are summarized in Table 2. Six trials were on working memory training, 4 on attention training, 2 combined attention and working memory training, 2 inhibition and working memory training, and 1 trial provided a general executive function training covering working memory, inhibition, and cognitive flexibility. All training schedules had an “adaptive” component, that is, task difficulty was increased across sessions to track performance improvement. Eight trials had an active control condition. Six trials were implemented at home, 5 at school, 2 at either school or home, 1 trial in the clinic, and 1 at the welfare service/children center, home or laboratory. Five trials had no/low medication levels. Figure S1 (available online) depicts the graphic output for the risk of bias assessment. Risk of bias was generally low or unclear. No trials were scored as “high risk” with regard to “random sequence generation,” “allocation concealment,” and “incomplete outcome data,” and only 3 and 4 trials scored high for “blinding of participants/personnel” and “blinding of outcome assessment,” respectively (the rating of each study is available upon request).
Figure 1.
Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of selection of studies (last search updated May 18, 2014). Note: aA total of 259 studies were not on attention-deficit/hyperactivity disorder (ADHD); 342 were not on cognitive training; 7 were not randomized controlled trials (RCTs); 47 were reviews; 3 were studies in adults; and 1 was a study protocol. bReasons for exclusion of each study are reported in Table S2, available online. cEgeland et al.24 and Hovik et al.25 refer to the same study.
Table 1.
Characteristics of Studies Included in the Meta-Analysis
| Trial |
Design |
Training |
Sample |
Outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author (Year) | Type | Control | Length of Training (Days) and FU | Type | Setting | n T C |
Meds T (%) C (%) |
Age (mo) | ADHD M-Prox |
ADHD P-Blind |
Included Neuropsychology Outcomes | Academic Functioning |
| Klingberg (2005)26 | 2 groups | NA-WMT | 35 FU: 3 mo |
WMT RoboMemo43 |
School/home | 26 27 |
0a 0 |
116 (mean) | Parent | Teacher | Digit span (verbal WM); span board (visual WM); stroop accuracy (inhibition) | N/A |
| Shalev (2007)27 | 2 groups | Computer games | 56 No FU |
Attention training | Clinic | 20 16 |
0b 0b |
72–156 | Parent | Parent | N/A | In-house tests |
| Johnstone (2010)28 | 2 groups | NA-WMT | 35 No FU |
Inhibitory and WMT | Home | 20 20 |
47c 78c |
95–149 | Parent | Parent | No go errors % (inhibition) | N/A |
| Rabinerd (2010)29 | 3 groups | Waitlist | 98 FU: within 1 y |
Attention training Captain’s Log44 | School/home | 25 25e |
7 | NS | Teacher | N/A | N/A | Woodcock-Johnson test |
| Steinerf (2011)20 | 3 groups | Waitlist | 120 No FU |
Attention/WMT BrainTrain44 |
School | 13 15 |
60 | 148.8 ± 10.8 (mean) | Parent | Teacher | N/A | N/A |
| Tuchag (2011)30 | 3 groups | Visual perception training | 28 No FU |
Attention training AixTent | Welfare service, home or lab | 16 16 |
100 100 |
124–138 | N/A | N/A | Vigilance omissions (inattention) | N/A |
| Johnstoneh (2012)31 | 3 groups | Waitlist | 35 FU: 6 wk |
Adaptive inhibitory training and WMT | Home | 22 20 |
90 | 95–145 | Parent | NA | Counting span (verbal WM); Go NoGo, RT incongruent stimuli (inhibition); oddball task correct (attention) | N/A |
| Gray (2012)22 | 2 groups | Adaptive math training Academy of Math | 35 No FU |
Adaptive WMT RoboMemo43 |
School | 32 20 |
98 | 144–204 | Teacher | N/A | Digit span back (verbal WM); CANTAB spatial WM (visual WM); D2 test total (attention) | Wide-Range Achieve- ment |
| Green (2012)32 | 2 groups | NA-WMT | 25 No FU |
Adaptive WMT RoboMemo43 |
Home | 12 14 |
67 14 |
84–168 | Parent | Parent | WISC index (verbal WM) | N/A |
| Van der Oord (2012)33 | 2 groups | Waitlist | 35 FU: 9 wk |
Adaptive EF training (inhibition, WM, flexibility) | Home | 18 22 |
66 | 96–144 | Parent | Teacher | N/A | N/A |
| Tamm (2013)34 | 2 groups | Waitlist | 56 No FU |
Adaptive attention training Pay Attention! |
School | 45 46 |
65 73 |
84–180 | Parent | Clinician | Digit span (verbal WM); D-KEFS scaled score (inhibition), omissions (inhibition) | N/A |
| Chacko (2013)35 | 2 groups | NA-WMT | 35 No FU |
Adaptive WMT RoboMemo43 |
Home | 44 41 |
27 32 |
84–132 | Parent | Teacher | AWMA listening (verbal WM); dot matrix (visual WM); CPT commissions (inhibition); omissions (attention) | Wide-Range Achieve-ment |
| Egelandi (2013)24 | 2 groups | TAU | 25 FU: 8 mo |
Adaptive WMT RoboMemo43 |
School | 33 34 |
68 | 120–144 | Teacher | N/A | Stroop interference score (inhibition; CPT focus (attention) | Logos Test |
| Hovik (2013)25 | 2 groups | TAU | 25 FU: 8 mo |
Adaptive WMT RoboMemo43 |
School | 33 34 |
68 | 120–144 | Teacher | N/A | Digit span (verbal WM); Leiter visual span (visual WM) | N/A |
| Steinerf (2014)21 | 3 groups (neurofeed-back, cognitive training, control) | TAU | 91 No FU |
Adaptive attention and WMT | School | 34 36 |
41 55 |
100.8 ± 14.8 (mean) | Parent | Direct observa-tion (BOSS) | N/A | N/A |
| Van Dongen-Boomsma (2014)36 | 2 groups | NA-WMT | 35 No FU |
Adaptive WMT (Cogmed RoboMemo43) | Home, except for 1 participant | 26 21 |
0 0 |
71.5–87.6 | Investigator | Teacher | Digit span (verbal WM); Knox Cubes (visual WM); Stroop difference (inhibition); Sustained attention dots: SDRT (attention) | N/A |
Note: Studies are listed in chronological order of publication and are followed by study reference number, as in Table S1, available online; long-term follow-up is listed under “Length of Training” after first outcome measurement when available, and “n” is the number of individuals in the treatment (T) and control (C) conditions. ADHD = attention-deficit/hyperactivity disorder; AWMA = automated working memory assessment; BOSS = Behavioral Observation of Students in Schools; CANTAB = Cambridge Neuropsychological Test Automated Battery; D-KEFS = Delis-Kaplan Executive Function System; EF = executive functions; FU = follow-up; MProx = most proximal rater; N/A = not applicable; NA-WMT = non-adaptive working memory training; NS = not specified; PBlind = probably blinded rater; RT = reaction time; SDRT = Spatial Delayed Response Task; TAU = treatment as usual; WISC = Wechsler Intelligence Scale for Children; WMT = working memory training.
Two children discontinued stimulants more than 1 year before the study, 1 child discontinued stimulant medication 1 week before the study, and the other participants were stimulant naive.
Four participants in the treatment group and 3 in the control group received psychostimulants throughout the duration of the study. None were medicated either during the training sessions or during the pre- and posttesting sessions.
Participants were asked to refrain from taking ADHD medication in the 24 hours before testing.
This study also included an arm on computer-assisted instruction that was not considered for the present meta-analysis.
A total of 27 additional participants were allocated to computer-assisted instruction.
This trial also included an arm of neurofeedback (www.playattention.com).
Results of this study are also reported in Lange KW, Tucha L, Hauser J, Lange KM, Stasik D, Tucha O. Attention training in attention deficit hyperactivity disorder. Aula Abierta 2012;40:55-60.
This study also included a “software with attention monitoring” arm, which was not included in the present meta-analysis for consistency with interventions included in the other studies retained in the meta-analysis.
This paper and Hovik et al.14 refer to the same study and present analyses on different outcomes.
Table 2.
Summary of Results Showing Pooled Standardized Mean Differences (SMD) Between Treatment and Control Arms for Each Outcome
| Outcome | Trials Included | Measure | Study n | Effect |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|
| SMD | 95% CI | p | I2 | p | ||||
| ADHD total | All | MProx | 14 | 0.37 | 0.09 to 0.66 | .01 | 71 | <.001 |
| PBlind | 11 | 0.20 | 0.01 to 0.40 | .04 | 30 | .16 | ||
| Active control | MProx | 7 | 0.16 | 0.23 to 0.55 | .41 | 71 | <.001 | |
| PBlind | 6 | 0.22 | −0.09 to 0.53 | .17 | 42 | .13 | ||
| WMT | MProx | 6 | 0.00 | −0.31 to 0.31 | 1.00 | 56 | .05 | |
| MPT | MProx | 5 | 0.79 | 0.46 to 1.12 | <.001 | 36 | .18 | |
| MED | MProx | 5 | 0.19 | −0.16 to 0.54 | .30 | 56 | .06 | |
| PBlind | 5 | 0.11 | −0.10 to 0.32 | .31 | 0 | .74 | ||
| Inattention | All | MProx | 11 | 0.47 | 0.14 to 0.80 | <.01 | 76 | <.001 |
| PBlind | 9 | 0.32 | −0.01 to 0.66 | .06 | 69 | <.001 | ||
| Active control | MProx | 5 | 0.30 | −0.17 to 0.76 | .21 | 72 | <.001 | |
| WMT | MProx | 5 | 0.22 | −0.18 to 0.62 | .28 | 66 | <.001 | |
| MED | MProx | 5 | 0.35 | −0.09 to 0.79 | .29 | 71 | .02 | |
| Hyper/Imp | All | MProx | 9 | 0.14 | −0.07 to 0.35 | .18 | 28 | .28 |
| PBlind | 8 | 0.18 | −0.01 to 0.37 | .06 | 0 | .50 | ||
| Active control | MProx | 5 | 0.01 | −0.25 to 0.22 | .91 | 0 | .60 | |
| WMT | MProx | 5 | 0.02 | −0.24 to 0.21 | .89 | 0 | .68 | |
| Executive function rating | All | MProx | 6 | 0.35 | 0.08 to 0.61 | .01 | 22 | .22 |
| Working memory (visual) | All | Objective | 5 | 0.47 | 0.23 to 0.70 | <.01 | 69 | <.001 |
| Active control | Objective | Insufficient trials (n = 4) | ||||||
| WMT | Objective | 5 | 0.47 | 0.23 to 0.70 | <.01 | 69 | <.001 | |
| Working memory (verbal) | All | Objective | 8 | 0.52 | 0.24 to 0.80 | <.01 | 48 | .06 |
| Active control | Objective | 5 | 0.58 | 0.23 to 0.94 | .001 | 45 | .12 | |
| WMT | Objective | 6 | 0.57 | 0.29 to 0.82 | <.001 | 32 | .19 | |
| Inhibition | All | Objective | 6 | 0.07 | −0.15 to 0.28 | .53 | 2 | .4 |
| Attention | All | Objective | 7 | 0.14 | −0.19 to 0.48 | .41 | 58 | .03 |
| Reading | All | Standardized tests | 5 | 0.09 | −0.09 to 0.27 | .33 | 23 | .26 |
| Arithmetic | All | Standardized tests | 5 | 0.01 | −0.13 to 0.11 | .84 | 0 | .44 |
Note: Significant effects are expressed in boldface. Table reports only measures for which 5 or more trials were available. Where only most proximal rater (MProx) is reported, there were insufficient trials with probably blinded rater (PBlind) measures. Active controls = all trials with an active control arm such as easy or non-adaptive training; ADHD = attention-deficit/hyperactivity disorder; All = all trials meeting inclusion criteria with available measure; MED = trials in which <30% of participants were treated with ADHD medication; MPT = multiple process training; WMT = all trials using just working memory training.
ADHD Symptoms
ADHD symptoms (total score or inattention or hyperactivity/impulsivity separately) were an outcome in up to 14 trials. Probably blinded measures were available in up to 11 trials (Table 2).
When most proximal assessments were the outcome, there was a moderate but significant effect on total ADHD and inattention symptoms but no effect on hyperactivity/impulsivity (Figure 2; SMD and CI data for all outcomes are presented in Table 2). In sensitivity analyses (Figures S2 and S3, available online), considering only trials with an active control, the effects were no longer statistically significant for any ADHD core symptoms outcomes. There was no effect of working memory training when implemented on its own (Figures S2 and S3, available online). In contrast, multi-process training approaches (i.e., approaches targeting more than 1 neuropsychological domain) gave a large effect size for total ADHD symptoms (Figure S2, available online). Between-study heterogeneity of effect sizes was high and significant for total ADHD and inattention symptoms.
Figure 2.
Forest plots for meta-analysis of effects on attention-deficit/hyperactivity disorder (ADHD) core symptoms assessed by the most proximal and probably blinded raters. Note: Cogn = cognitive; std = standard.
When analyses were restricted to probably blinded measures (Figure 2), in general, effect sizes were reduced with small and statistically marginal effects for all ADHD outcomes. In a sensitivity analysis (Figure S4, available online), effect sizes dropped further to nonsignificant levels when only trials with an active control arm were included. There were insufficient studies (n < 5) for an analysis of probably blinded measures in multi-component training trials, as well as for a number of other sensitivity analyses.
When analysis was restricted to no/low medication trials, effects on total ADHD symptoms were not significant for either most proximal or, when available, probably blinded assessments in any ADHD core symptom–related outcome (Table 2).
Neuropsychological Outcomes
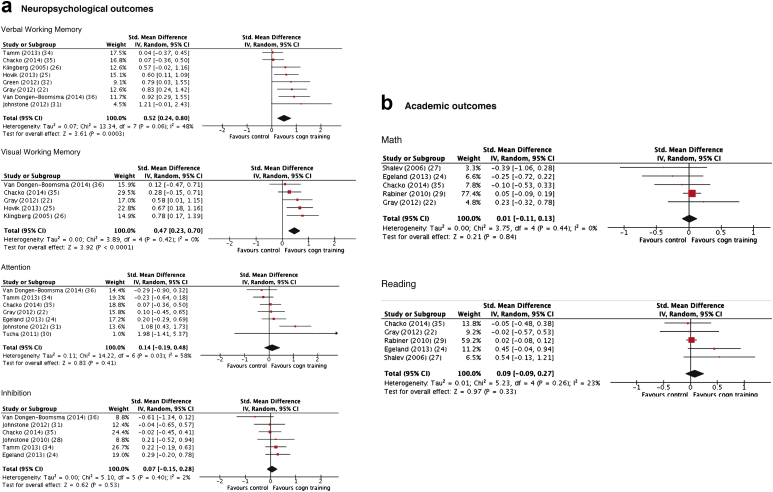
Eight trials included laboratory measures of verbal, and 5 trials visual working memory (Table 2). There was a large and significant effect of cognitive training on both components (Figure 3), which was maintained in sensitivity analyses considering trials with active controls only or working memory training trials only (Figure S5, available online; sensitivity analyses were not performed for visual working memory because of an insufficient number of trials). The number of trials using multi-component training and no/low medication trials was insufficient to perform sensitivity analyses. There were no significant effects of training on laboratory tests of inhibition (6 trials) or attention (7 trials) (Figure 3). Six trials included most proximal ratings of executive functioning using the BRIEF rating scale (Figure S6, available online). These demonstrated a small-to-moderate, significant SMD. There was an insufficient number of trials with ratings of executive functioning to perform planned sensitivity analyses.
Figure 3.
Forest plots for meta-analysis of effects on neuropsychological and academic outcomes. Note: Cogn = cognitive; std = standard.
Academic Ability
Five trials included standardized measures of reading and 5 of arithmetic. There were no significant effects in either domain (Figure 3). There was an insufficient number of trials to perform planned sensitivity analyses.
Publication Bias
Funnel plots and results of Egger’s test are reported in Supplement 2 (available online). For both meta-analyses of ADHD symptoms scored by most proximal and probably blinded raters, the test failed to reach the p < 0.05 level, suggesting no significant publication bias.
Meta-Regression Analysis
For most proximal or probably blinded assessments of ADHD core symptoms, there was no significant effect of age on SMD (see Supplement 3, available online).
Sensitivity Analysis Excluding the Study by Gray et al.22
The main results considering most proximal assessment of ADHD core symptoms were substantially unchanged, as reported in Figure S7 (available online). As this study was not included in “probably blinded” analyses, no sensitivity analysis was conducted considering probably blinded assessment.
Discussion
There are 2 perspectives on cognitive training in ADHD. From 1 perspective, cognitive training is a front-line ADHD treatment: this is based on the hypothesis that because causal pathways to disorder are mediated by neuropsychological deficits, strengthening deficient neuropsychological functions should reduce ADHD symptoms and associated impairment. From the second perspective, it is perceived as an adjunctive treatment that reduces impairment associated with neuropsychological deficits commonly seen in children with ADHD, independent of any effects on core ADHD symptoms itself. The current meta-analysis, including an additional 10 RCTs compared to the previous study by Sonuga-Barke et al.,14 provided little support for cognitive training as a front-line ADHD treatment. There were statistically significant effects on ADHD symptoms when considering raters most proximal to treatment delivery, especially for symptoms of inattention. However, these effects were reduced substantially when analyses were limited to trials with an active control arm or where assessors were probably blind to treatment allocation. The evidence was somewhat stronger for the benefits of cognitive training as an adjunctive treatment aimed at reducing neuropsychological impairment. There were large and highly significant improvements on objective tests of both visual and verbal working memory, although there were no effects on inhibition or inattention. Furthermore, the effects of cognitive training on working memory did not extend to the academic outcomes explored.
The substantial drop in SMDs between most proximal and probably blinded analyses for ADHD symptoms is similar to the pattern seen in previous meta-analyses of nonpharmacological treatments using the EAGG protocol (e.g., behavioral intervention;8 neurofeedback14). This is probably caused by the inflation of effect size estimates that inevitably occurs when one relies on raters who are both likely to be aware of treatment allocation and heavily invested in the delivery and outcome of treatment. It is also possible that probably blinded and most proximal assessments differed in some way that reduced the sensitivity of the former to treatment-related change. However, the same measurement approaches were used for each (some parent, some teacher, and some direct observation measures). Another possibility is that most proximal assessments accurately captured treatment effects established in the therapeutic setting but that these effects did not generalize to the settings in which probably blinded assessments were made. However, in a substantial minority of trials (Table 1), especially those with an active control arm, probably blinded measures were collected in the treatment setting, and the effects for these trials were no larger than those for trials in which they were collected in a different setting.
The trials included in the meta-analysis used a wide range of training approaches targeting different neuropsychological processes. There was a sufficient number of trials to look at 2 classes of intervention individually, which was not possible in the previous meta-analysis by Sonuga-Barke et al.14: namely, training of working memory only, and training focusing on multiple neuropsychological domains. The results for trials implementing working memory training only departed in a striking way from the most proximal/probably blinded pattern described above. Effects on ADHD were negligible even considering most proximal measures. This suggests that this form of training, which has been widely promoted for use with patients with ADHD (as discussed by Rapport et al.,9), has little or no efficacy for core ADHD symptoms. On the other hand, the SMD for most proximal assessment of ADHD symptoms was substantially larger for trials based on training targeting multiple domains than for all studies as a whole. Unfortunately, there was an insufficient number of trials (n = 4) with probably blinded measures to corroborate these effects using independent sources. The superiority of these approaches may be due to the typically greater number of training sessions in multi- compared to single-component approaches (in our analysis, an average of 9 weeks compared to 6 weeks, respectively). However, the finding opens up the interesting possibility that multi-component training models may be more successful for ADHD, given the complex and heterogeneous nature of the condition. Because children with ADHD differ from one another in their neuropsychological profile, and because children may be affected by more than 1 deficit,37,38 multi-component training may be used to target a series of neuropsychological domains that may be more important than working memory alone in the pathophysiology of ADHD symptoms. The development and evaluation of multi-component training models should be a future priority.
The effects on neuropsychological outcomes were restricted to working memory, which were substantial, with no effects on inhibitory or attentional control. There were significant effects on parents’ ratings of executive function, but these could not be corroborated by independent blinded evidence. All 6 trials that included a working memory outcome were working memory training trials. Therefore, although these trials produced “near transfer” of training effects to untrained working memory measures, there was no evidence of “far transfer” to other neuropsychological processes. Crucially, there was also no evidence that these effects generalized to important areas of everyday functioning, which themselves are influenced by working memory ability,16 such as reading and arithmetic. This finding may be relevant in clinical practice. Indeed, parents may currently favor cognitive training with the hope that they can improve academic performance. Our results show that this is not supported by empirical evidence.
The success of working memory training in improving working memory performance draws into even sharper relief its failure to improve ADHD symptoms, suggesting dissociation between neuropsychological functioning and disorder. There are 4 possible explanations for this: first, that working memory deficits do not, in fact, mediate ADHD pathophysiology39; second, that although they do mediate the development of ADHD, they have become entrenched and not susceptible to the type of training implemented in trials conducted to date; third, that training as currently implemented targets types of working memory not fundamental to the deficits in ADHD9; and fourth, that training produces only peripheral, practice-like effects on working memory, with no profound impact on the brain networks underpinning neuropsychological deficits responsible for ADHD. Whether or not working memory deficits are part of the causal mechanism underpinning ADHD, based on our results, strengthening working memory appears to be neither a necessary nor a sufficient condition for ADHD symptom reduction. In this regard, our findings suggest that choosing substrates that have emerged from experimental research as treatment targets may not necessarily translate into clinical benefits. This possible dissociation between candidate mechanisms of a disorder and clinical targets is important when adopting pathophysiology-based research approaches such as the Research Domain Criteria (RDoC).40 From a clinical standpoint, developing techniques to extend transfer from the effects on core working memory processes to broader neuropsychological processes and important domains of impairment and/or clinical presentation is the most pressing challenge for the future. The reasons for the lack of effect on inhibitory and attentional control are hard to determine on the basis of the current analysis, given the small number of trials that specifically targeted these domains. Although we might predict that training targeting multiple deficit domains would show effects on these neuropsychological processes, there were insufficient trials with multi-component training and measures of inhibition and/or attention to test this. Approaches focusing on motivational or energetic processes may also be valuable (i.e., training to increase delay of gratification).41
A number of limitations need to be taken into account when interpreting the current analysis. First, there was significant SMD heterogeneity for some analyses (most proximal total ADHD, symptoms of inattention, and visual working memory). This leaves open the possibility that cognitive training may be effective under specific circumstances in individual trials. Given the limited number of trials available, we were unable to identify specific features of positive trials (apart from therapeutic content, working memory training). Second, only a minority of trials (n = 5) reported using intention-to-treat analyses, a situation that may have inflated the effects for some outcomes, as participants who are more difficult to treat or who perceive the treatment as less beneficial may drop out of trials; however, drop-out was relatively low in most trials. Third, despite the recent substantial increase in the number of available cognitive training trials, there was an insufficient number of trials to evaluate training approaches targeting specific neuropsychological constructs other than working memory training. Fourth, there were insufficient trials to run analyses for some important outcomes (e.g., functional impairment, IQ), as well as for sensitivity analyses or analyses restricted to probably blinded measures for a number of outcomes. Fifth, too few trials included long-term outcomes (Table 1) to allow an evaluation of the extent to which effects on clinical symptoms grew over time or effects on neuropsychological processes persisted. Sixth, no trials were restricted to individuals with both ADHD and the specific neuropsychological deficit to be trained. As a consequence, effect sizes for both neuropsychological deficits and ADHD symptoms may have been truncated: in the former case because there would be little room for improvement where no deficit existed; in the latter case because targeting a neuropsychological deficit that was not causing the condition would be unlikely to reduce symptoms of the core condition. Seventh, in the neuropsychological domains, diverse measures from different tasks (still tapping the same domain, however) were combined across studies to allow the calculation of pooled SMD estimates. Eighth, it is important to understand whether initial symptom-related and neuropsychological treatment effects persist over time and generalize to other domains, if they do. There were insufficient trials that examined long-term outcomes to address this issue. Finally, the categorization of studies as “probably blinded,” although carried out according to previously agreed and clear decision rules set out in the protocol, is limited by an inevitable degree of uncertainty because of limitations in the information reported in some trials.
In summary, the current meta-analysis found limited evidence for the clinical value of cognitive training for children with ADHD outside of the narrow confines of specific targeted neuropsychological processes (i.e., working memory training improved working memory function). Given the evidence for neuropsychological heterogeneity in ADHD, future efforts should be directed at developing protocols to target a broader range of neuropsychological deficits. Furthermore, therapeutic innovation is required to enhance the “far transfer” of specific neuropsychological gains to everyday patterns of functional impairment through more ecologically valid training approaches.42 Future trials should more consistently include active control arms, a broader range of functional outcomes, and long-term follow-up.
Footnotes
Supplemental material cited in this article is available online.
This article can be used to obtain continuing medical education (CME) at www.jaacap.org.
Support for meetings and analyses was received from Brain Products GMBH, Janssen-Cilag, Eli Lilly and Co., Medice, Shire, and Vifor. No honoraria were received, and funders had no input for the review and meta-analysis process or the writing of this article.
The European ADHD Guidelines Group (EAGG) is a workgroup of the European Network for Hyperkinetic Disorder (EUNETHYDIS) and consists of the following members and associates co-opted to work on this review (listed in alphabetical order): T. Banaschewski, MD, PhD; D. Brandeis, PhD; J. Buitelaar, MD, PhD; D. Coghill, MD; S. Cortese, MD, PhD; D. Daley, PhD; M. Danckaerts, MD, PhD; R.W. Dittmann, MD, PhD; M. Döpfner, PhD; M. Ferrin, MD, PhD; C. Hollis, MD, PhD; M. Holtmann, MD, PhD; E. Konofal, MD, PhD; M. Lecendreux, MD; A. Rothenberger, MD; P. Santosh, MD; J.A. Sergeant, PhD; E. Simonoff, MD; E.J. Sonuga-Barke, PhD; C. Soutullo, MD, PhD; H-Ch. Steinhausen, MD, PhD; J. Stevenson, PhD; A. Stringaris, MD, PhD; E. Taylor, MD; S. van der Oord, PhD; I. Wong, PhD; and A. Zuddas, MD.
The authors thank the following individuals for providing additional information on studies and/or advice: Corrado Barbui, MD, PhD, Verona University, Italy; Andrea Cipriani, MD, PhD, Oxford University, UK; Jens Egeland, PhD, Oslo University, Norway; Stuart J. Johnstone, PhD, University of Wollongong, Australia; Marianna Purgato, PhD, Johns Hopkins University, USA; David Rabiner, PhD, Duke University, USA; Lilach Shalev-Mevorah, PhD, Tel-Aviv University, Israel; Leanne Tamm, PhD, Cincinnati Children’s Hospital, USA; Rosemary Tannock, PhD, Hospital for Sick Children, Toronto, Canada; Olivier Tucha, PhD, Groningen University, Netherlands; and Martine van Dongen-Boomsma, MD, Radboud University Nijmegen, Netherlands.
Disclosure: Dr. Cortese has received royalties from Argon Healthcare Italia. Dr. Ferrin has received economic support from the Instituto de Salud Carlos III, Consejeria de Salud Junta de Andalucia, Gobierno de Navarra (Beca Jeronimo Ayanz), and Fundación Alicia Koplowitz. Prof. Buitelaar has served as a consultant to/advisory board member of/ and/or speaker for Janssen Cilag BV, Eli Lilly and Co., and Servier. Prof. Daley has provided educational talks for Eli Lilly and Co. and Shire; has attended an advisory board for Eli Lilly and Co.; has received support for educational travel from Eli Lilly and Co., Shire, and HP Pharma; and has received funding from Shire. Prof. Dittmann has received compensation for serving as a consultant or speaker for, or he or the institution he works for have received research support or royalties from the companies or organizations indicated: the European Union (EU FP7 Programme), US National Institute of Mental Health (NIMH), German Federal Ministry of Health/Regulatory Agency (BMG/BfArM), German Federal Ministry of Education and Research (BMBF), German Research Foundation (DFG), Volkswagen Foundation, Ferring, Janssen-Cilag, Eli Lilly and Co., Otsuka, Shire, and Theravance. He owns Eli Lilly and Co. stock. Prof. Holtmann has served as an advisor or consultant for Eli Lilly and Co., Novartis, Shire, and Bristol-Myers Squibb and has received conference attendance support or was paid for public speaking by AstraZeneca, Bristol-Myers Squibb, Janssen-Cilag, Eli Lilly and Co., Medice, Neuroconn, Novartis, and Shire. Dr. Santosh has received research funding from the EU (FP7 Programme) and the National Institute for Health Research (NIHR). He is also a director and shareholder of HealthTracker, Ltd., UK, and HighStreet Medical Dental, Gillingham, UK. Dr. Stringaris has received grant or research support from the Wellcome Trust, the NIHR, and the Department of Health UK. He has received royalties from Cambridge University Press for his book The Maudsley Reader in Phenomenological Psychiatry. Prof. Zuddas has received research grants from the EU (FP7 Programme), Italian National Institute of Health, Sardinian Secretary of Public Health, Eli Lilly and Co., Shire, and Vifor and has served as speaker, adviser, or consultant for AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Co., Lundbeck, Schering-Plough, Shire, and Vifor. He has been a member of Data Safety Monitoring Boards for Otsuka and Lundbeck. Prof. Sonuga-Barke has served on the speakers’ board of Shire and Janssen-Cilag; has served as a consultant to Shire and NeuroTechnology Solutions, Ltd.; has received research support from Janssen-Cilag and Shire; has served on the advisory board of Shire and NeuroTechnology Solutions, Ltd.; and has received conference support from Shire. Profs. Brandeis and Stevenson report no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Ramos-Quiroga J.A., Montoya A., Kutzelnigg A., Deberdt W., Sobanski E. Attention deficit hyperactivity disorder in the European adult population: prevalence, disease awareness, and treatment guidelines. Curr Med Res Opin. 2013;29:1093–1104. doi: 10.1185/03007995.2013.812961. [DOI] [PubMed] [Google Scholar]
- 2.Taylor E., Dopfner M., Sergeant J. European Clinical Guidelines for Hyperkinetic Disorder—first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I7–I30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 3.Faraone S.V., Biederman J., Spencer T.J., Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese S., Holtmann M., Banaschewski T. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54:227–246. doi: 10.1111/jcpp.12036. [DOI] [PubMed] [Google Scholar]
- 5.Molina B.S., Hinshaw S.P., Swanson J.M. MTA at 8 Years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler L.D., Nierenberg A.A. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122:184–191. doi: 10.3810/pgm.2010.01.2112. [DOI] [PubMed] [Google Scholar]
- 7.Kovshoff H., Williams S., Vrijens M. The Decisions Regarding ADHD Management (DRAMa) study: uncertainties and complexities in assessment, diagnosis and treatment, from the clinician's point of view. Eur Child Adolesc Psychiatry. 2012;21:87–99. doi: 10.1007/s00787-011-0235-8. [DOI] [PubMed] [Google Scholar]
- 8.Daley D., Van der Oord S., Ferrin M. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry. 2014;53:835–847. doi: 10.1016/j.jaac.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Rapport M.D., Orban S.A., Kofler M.J., Friedman L.M. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. 2013;33:1237–1252. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Vinogradov S., Fisher M., de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonuga-Barke E.J., Coghill D. The foundations of next generation attention-deficit/hyperactivity disorder neuropsychology: building on progress during the last 30 years. J Child Psychol Psychiatry. 2014;55:e1–e5. doi: 10.1111/jcpp.12360. [DOI] [PubMed] [Google Scholar]
- 12.Lewis C.M., Baldassarre A., Committeri G., Romani G.L., Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poldrack R.A., Gabrieli J.D. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- 14.Sonuga-Barke E.J., Brandeis D., Cortese S. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- 15.Coghill D., Nigg J., Rothenberger A., Sonuga-Barke E., Tannock R. Whither causal models in the neuroscience of ADHD? Dev Sci. 2005;8:105–114. doi: 10.1111/j.1467-7687.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmes J., Gathercole S.E., Dunning D.L. Poor working memory: impact and interventions. Adv Child Dev Behav. 2010;39:1–43. doi: 10.1016/b978-0-12-374748-8.00001-9. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane Collaboration. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. Available at: www.cochrane-handbook.org
- 18.Cochrane Collaboration . Nordic Cochrane Centre; Copenhagen: 2011. RevMan, version 5.1. [Google Scholar]
- 19.Morris S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386. [Google Scholar]
- 20.Steiner N.J., Sheldrick R.C., Gotthelf D., Perrin E.C. Computer-based attention training in the schools for children with attention deficit/hyperactivity disorder: a preliminary trial. Clin Pediatr (Phila) 2011;50:615–622. doi: 10.1177/0009922810397887. [DOI] [PubMed] [Google Scholar]
- 21.Steiner N.J., Frenette E.C., Rene K.M., Brennan R.T., Perrin E.C. Neurofeedback and cognitive attention training for children with attention-deficit hyperactivity disorder in schools. J Dev Behav Pediatr. 2014;35:18–27. doi: 10.1097/DBP.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 22.Gray S.A., Chaban P., Martinussen R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. J Child Psychol Psychiatry. 2012;53:1277–1284. doi: 10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp . StataCorp; College Station, TX: 2013. Stata Statistical Software: release 13. [Google Scholar]
- 24.Egeland J., Aarlien A.K., Saunes B.K. Few effects of far transfer of working memory training in ADHD: a randomized controlled trial. PLoS One. 2013:e75660. doi: 10.1371/journal.pone.0075660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovik K.T., Saunes B.K., Aarlien A.K., Egeland J. RCT of working memory training in ADHD: long-term near-transfer effects. PLoS One. 2013;8:e80561. doi: 10.1371/journal.pone.0080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingberg T., Fernell E., Olesen P.J. Computerized training of working memory in children with ADHD-a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Shalev L., Tsal Y., Mevorach C. Computerized progressive Attentional (CPAT) Program: effective direct intervention for children with ADHD. Child Neuropsychology. 2006;13:382–388. doi: 10.1080/09297040600770787. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone S.J., Roodenrys S., Phillips E., Watt A.J., Mantz S. A pilot study of combined working memory and inhibition training for children with AD/HD. Atten Defic Hyperact Disord. 2010;2:31–42. doi: 10.1007/s12402-009-0017-z. [DOI] [PubMed] [Google Scholar]
- 29.Rabiner D.L., Murray D.W., Skinner A.T., Malone P.S. A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol. 2010;38:131–142. doi: 10.1007/s10802-009-9353-x. [DOI] [PubMed] [Google Scholar]
- 30.Tucha O., Tucha L., Kaumann G. Training of attention functions in children with attention deficit hyperactivity disorder. Atten Defic Hyperact Disord. 2011;3:271–278. doi: 10.1007/s12402-011-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnstone S.J., Roodenrys S., Blackman R. Neurocognitive training for children with and without AD/HD. Atten Defic Hyperact Disord. 2012;4:11–23. doi: 10.1007/s12402-011-0069-8. [DOI] [PubMed] [Google Scholar]
- 32.Green C.T., Long D.L., Green D. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics. 2012;9:639–648. doi: 10.1007/s13311-012-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Oord S., Ponsioen A.J., Geurts H.M., Brink E.L., Prins P.J. A Pilot Study of the Efficacy of a Computerized Executive Functioning Remediation Training With Game Elements for Children With ADHD in an Outpatient Setting: Outcome on Parent- and Teacher-Rated Executive Functioning and ADHD Behavior. J Atten Disord. 2012 doi: 10.1177/1087054712453167. in press, http://dx.doi.org/10.1177/1087054712453167. [DOI] [PubMed] [Google Scholar]
- 34.Tamm L., Epstein J.N., Peugh J.L., Nakonezny P.A., Hughes C.W. Preliminary data suggesting the efficacy of attention training for school-aged children with ADHD. Dev Cogn Neurosci. 2013;4:16–28. doi: 10.1016/j.dcn.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chacko A., Bedard A.C., Marks D.J. A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. J Child Psychol Psychiatry. 2014;55:247–255. doi: 10.1111/jcpp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dongen-Boomsma M., Vollebregt M.A., Buitelaar J.K., Slaats-Willemse D. Working memory training in young children with ADHD: a randomized placebo-controlled trial. J Child Psychol Psychiatry. 2014;55:886–896. doi: 10.1111/jcpp.12218. [DOI] [PubMed] [Google Scholar]
- 37.Pauli-Pott U., Becker K. Neuropsychological basic deficits in preschoolers at risk for ADHD: a meta-analysis. Clin Psychol Rev. 2011;31:626–637. doi: 10.1016/j.cpr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Frazier T.W., Demaree H.A., Youngstrom E.A. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 39.Coghill D.R., Hayward D., Rhodes S.M., Grimmer C., Matthews K. A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychol Med. 2014;44:1087–1099. doi: 10.1017/S0033291713001761. [DOI] [PubMed] [Google Scholar]
- 40.Sonuga-Barke E.J. Editorial: 'What's up, (R)DoC?'—can identifying core dimensions of early functioning help us understand, and then reduce, developmental risk for mental disorders? J Child Psychol Psychiatry. 2014;55:849–851. doi: 10.1111/jcpp.12293. [DOI] [PubMed] [Google Scholar]
- 41.Sonuga-Barke E.J. On the reorganization of incentive structure to promote delay tolerance: a therapeutic possibility for AD/HD? Neural Plast. 2004;11:23–28. doi: 10.1155/NP.2004.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreau D., Conway A.R. The case for an ecological approach to cognitive training. Trends Cogn Sci. 2014;18:334–336. doi: 10.1016/j.tics.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Cognitive Medical Systems AB. RoboMemo®. Stockholm: Cognitive Medical Systems AB, 2005.
- 44.BrainTrain. Captain’s Log. Richmond, VA: BrainTrain, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.