Abstract
Current viewpoints concerning the bactericidal mechanisms of neutrophils are reviewed from a perspective that emphasizes challenges presented by the inability to duplicate ex vivo the intracellular milieu. Among the challenges considered are the influences of confinement upon substrate availability and reaction dynamics, direct and indirect synergistic interactions between individual toxins, and bacterial responses to stressors. Approaches to gauging relative contributions of various oxidative and nonoxidative toxins within neutrophils using bacteria and bacterial mimics as intrinsic probes are also discussed.
Keywords: Bactericidal mechanisms, Cationic antimicrobial peptides, Intraphagosomal probes, Myeloperoxidase, NADPH oxidase, Neutrophil-generated oxidants, Reactive oxygen species, Synergistic reactions
Overview of phagocytic killing by neutrophils
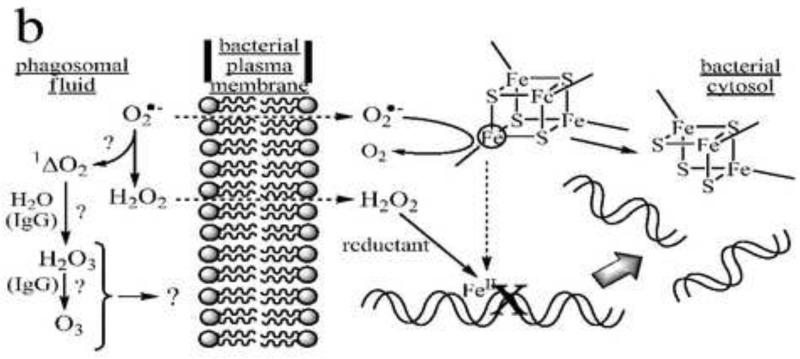
As part of the innate immune system, unicellular organisms are entrapped and destroyed within the vacuoles of phagocytic cells by processes that can involve both oxidative and nonoxidative reactions. Of the various classes of phagocytes, the most extensively studied from this perspective has been the neutrophil. This cell contains a particularly active inducible NADPH oxidase (NOX-2), that is assembled and activated upon agonist binding and generates superoxide anion (O2.−) in a respiratory “burst” that consumes O2 at a rate that is ~10-fold greater than basal levels [1,2]. When measured in large populations of cells, this stimulated respiration generally lasts for 20-30 min (e.g., [3]), although recent NADPH imaging studies suggests that individual cells undergo a shorter burst over 2-3 min that subsequently subsides to slightly elevated levels [4].1 Histological evidence [5,6] indicates that the developing phagosomal membrane is a primary site of this activity, where the NOX-2 respiratory chain is vectorially organized to generate O2.− within the phagosomal compartment from reductants present in the cytosol. Lysosomal degranulation is an essential part of the assembly of this respiratory chain, and also leads to encapsulation of myeloperoxidase (MPO) and other microbicidal proteins within the phagosome. Of these proteins, only MPO and lactoferrin are recognized as having potential for carrying out oxidative microbicidal reactions; the latter, however, is primarily in its apo (or metal-free) form, so that the prevailing opinion is that, rather than acting as a catalyst for oxidations, one of its funtions to sequester adventitious iron, thereby denying microbes access to this essential growth factor. On the other hand, MPO is a versatile enzyme which can catalyze one- or two-electron oxidations of several anions found in biological fluids (I−, Br−, Cl−, SCN−, NO −2) to generate bactericidal or bacteriostatic products [7-12]; the enzyme is unique among mammalian peroxidases, however, in possessing sufficient oxidizing potential to rapidly oxidize chloride ion to the potently microbicidal HOCl [13]. Indeed, the long-standing paradigm of oxidative microbial killing within neutrophil phagosomes is just these reactions, where NOX-2 generated O2.− or H2O2 derived from its dismutation react to form MPO compound I, followed by two-electron oxidation of Cl− to HOCl, regenerating the resting form of the enzyme in the process. The simultaneous expression of nonoxidative microbicidal mechanisms is evident from observations that MPO-deficient neutrophils (for leading references, see [14]) and normal neutrophils in anaerobic media [15,16] retain the capacity to kill microbes, albeit with efficiencies that are highly organism-specific [17,18]. Numerous neutrophil granule-derived microbicidal polypeptides and proteins have been identified [19]. Lysozyme [20], protegrins [21], defensins [22], bactericidal/permeability-increasing protein (BPI) [23], serum-derived group IIA phospholipase A2 (gIIA-PLA2) [19,24] and the neutral serine proteases, elastase [20] and cathepsin G [25], are particularly well-described components of nonoxidative microbicidal pathways in neutrophils. It is also worth noting that several nonvirulent strains of bacteria are killed within the time frame of phagocytosis; in one study from the Winterbourn laboratory, a sequential first-order kinetic model was used to estimate half-times for phagocytosis and killing, which gave values of t1/2 ~9 min and ~6 min, respectively, for the Gram-positive Staphylococcus aureus ATCC 27217 and t1/2 ~ 10 min and ~2 min for the Gram-negative Escherichia coli ATCC 25922 [26]. Several specific models for bactericidal action that have been proposed are illustrated in Fig. 1.
Fig. 1.
Four models of bactericidal action in neutrophils. Panel a: MPO-dependent oxidative killing. Granule-derived MPO catalyzes formation of HOCl from endogenous chloride and NOX-2-derived superoxide or hydrogen peroxide. HOCl and/or secondary chloramines formed by reaction with endogenous amines inactivate plasma membrane-localized proteins involved with energy transduction and biosynthesis (represented here as the F0F1-ATP synthase and a generic proton-symporting metabolite transport protein). Additional reactions of HOCl may involve formation of nitryl chloride (NO2Cl) if sufficient nitrite ion is present in the phagosomal fluid, and generation of hydroxyl and carbonate radicals following one-electron reduction by superoxide. Panel b: MPO-independent oxidative killing. Superoxide ion perfuses the bacterial membrane to react with iron-sulfur clusters in cytosolic dehydratases, releasing ferrous ion, which relocates to vulnerable target sites (here shown as genomic DNA). Subsequent site-specific oxidation by H2O2 in Fenton reactions leads to irreversible loss of function (here double-stranded cleavage of the DNA). (After Imlay and coworkers [53].) Additional (but presently controversial) pathways may involve singlet oxygen-initiated formation of hydrogen sesquioxide or ozone in antibody- or amino acid-catalyzed reactions. Panel c: Nonoxidative killing. Granule-derived cationic antimicrobial peptides (CAMP) aggregate in the bacterial plasma membranes to form membrane-spanning pores that dissipate ion gradients essential to homeostasis and energy transduction. In Gram-negative bacteria, granule-derived bactericidal permeability-increasing protein (BPI) binds to lipopolysaccharide (LPS), initiating a set of transformations that promote phospholipase (PLase) activation and access to and degradation of the bacterial membranes by these and other lytic proteins, including serum-derived complement (C7-C9) and group IIA-phospholipase A2 (PLA2). The latter gain access to the phagosome by binding to extracellular bacteria prior to phagocytosis. PG = peptidoglycan. (After Elsbach and coworkers [23,155].) Panel d: Redox-driven nonoxidative killing. Electrogenic NOX-2 respiration acts to polarize the phagosomal membrane, driving influx of electrolyte cations (here, K+). Hydrogen peroxide, formed as the respiratory end-product, is destroyed in catalatic reactions (here via MPO catalysis). The increased ionic strength causes release of granule-derived cationic seprocidins (e.g., cathepsin G, elastase) from anionic biopolymers, which then attack the bacterium. (After Segal and coworkers [41].)
Relative contributions of oxidative and nonoxidative mechanisms
The recognition that multiple mechanisms of killing exist has often been rationalized in terms of essential redundancies, where one type of mechanism (nonoxidative or, more narrowly, MPO-independent) serves as a back-up to the other (oxidative or MPO-dependent)—or vice-versa—in instances where the primary mechanism fails. Questions concerning the primacy of one or the other type are inevitably raised, but experimentation designed to gauge their relative contributions have not generally been very revealing if only because these results also depend upon the selected microbe. Consider, for example, the elegant studies by Winterbourn and associates in which rates of killing of Staphylococcus aureus ATCC 27217 by neutrophils were compared to rates of killing under conditions for which either normal neutrophil function was impaired or the bacterium was surface-modified by attachment of IgG-linked superoxide dismutase [27]. Making the assumptions that the bactericidal mechanisms acted independently and that the measured rates of killing paralleled their cumulative contributions, these researchers interpreted their data to indicate that about three-fourths of the killing is attributable to oxidative toxins arising from the respiratory burst, the bulk of whose formation is MPO-derived. In marked contrast to this result, Rosen and collaborators have reported that killing of Escherichia coli ATCC 11775 is equally as effective by MPO- or NOX-2-deficient neutrophils as by normal neutrophils [28], implying that nonoxidative bactericidal mechanisms were of paramount importance toward these cells. They also found that a putative marker for MPO-dependent reactions, inhibition of DNA synthesis [29], occurred in bacteria phagocytosed by neutrophils capable of expressing MPO microbicidal activity, but not in bacteria from cells deficient in MPO antimicrobial activity. Since this dysfunction undoubtedly constitutes a lethal event [29], the inference drawn from these observations was that both oxidative and nonoxidative mechanisms are operative in the normal neutrophils, but that because the MPO-dependent reactions are inconsequential to the outcome, they can be viewed as nonessential (or redundant). In subsequent work using a ΔoxyRS mutant of the same organism (which possesses impaired defenses against H2O2-derived oxidants), it was found that the mutant was more effectively cleared by the fully functional neutrophil than the NOX-2-deficient cell [30], so that in E. coli hypersensitized to phagocyte-generated oxidative toxins the oxidative mechanism is unmasked and becomes an important component of the now-enhanced bactericidal process.
Synergism in microbicidal action
Historically, the tendency to view these reactions in an “either-or” sense may have been fostered by the difficulties inherent in studying the underlying biochemical events directly within the phagosome, where few experimental techniques have been available to monitor particular reactions of interest against the large background of other overlapping and potentially interacting reactions (see, e.g., [30,31] for additional discussion of this point). As a consequence, most studies have involved in vitro examination of isolated neutrophil components, the collective properties of which are then used to construct models of phagosomal function. This approach may miss critical synergistic features of the microbicidal mechanisms, however, in which cooperation between various oxidative and nonoxidative processes work to promote killing, as well as constraints upon reactivity and medium conditions imposed by phagosomal compartmentation that are difficult or impossible to reproduce ex vivo. The latter arise in part because the intraphagosomal volume is very small and presumably contains a reaction environment that is quite different from the neutrophil cytosol, i.e., because the neutrophil plasma membrane everts upon forming the phagosome, the intraphagosomal medium is composed primarily of serum and the contents of fused granules. As a consequence, potential substrates for enzymatic reactions that generate toxic products that may be readily available in the external environment can be virtually absent from the phagosomal compartment [32]. Additionally, diffusion lengths of reactive intermediates to microbial targets are substantially shorter within the phagosome than are encountered ex vivo, promoting their reactions with the entrapped microbes at the expense of host-derived antioxidants [33]. Specific examples of these effects will be encountered in the subsequent discussion.
Oxidative and nonoxidative processes
Synergism between nominally independent microbicidal mechanisms is widespread, as has been repeatedly demonstrated by studies made at molecular, cellular, and organismal levels alike. A few pertinent examples taken from a much broader literature might serve to illustrate the point. Like MPO, the antimicrobial proteinase elastase is found in the primary granules of resting neutrophils and is translocated to the phagosome during particle ingestion. As early as 1976, Odeberg and Olsson reported that inclusion of native or denatured granulocyte elastase in MPO-based in vitro bactericidal media potentiated killing of both E. coli and S. aureus under conditions where elastase alone was not bactericidal [34]. Among the many observations that oxidation of proteins increases their susceptibility to proteolysis is the report by Vissers and Winterbourn that pretreatment of soluble plasma fibronectin by HOCl or exposure to the MPO/H2O2/Cl− chlorinating system greatly promoted proteolytic attack by elastase [35]. Similarly, in examining protein degradation within phagocytosed bacteria, Elsbach, Weiss and coworkers [36] found that the extent of degradation was highly dependent upon expression of MPO-mediated O2 metabolism, i.e., degradation was markedly diminished when either NOX-2 mediated respiration or MPO activity was suppressed. These experiments utilized a strain of E. coli that was killed equally effectively by normal and NOX-2 deficient human neutrophils, implying that, in this case, MPO enhancement of bacterial protein degradation was not essential to bactericidal action. Promotion of biopolymer degradation by exposure to oxidants is not limited to proteins. For example, van Rensburg and coworkers have reported that H2O2-mediated DNA strand breaks appearing in monocytes in the presence of activated neutrophils were not observed when MPO was inhibited; moreover, although HOCl alone did not elicit any DNA cleavage, when combined with H2O2 it increased the number of H2O2-mediated breaks and slowed the subsequent internal repair process [37]. (In these experiments, neutrophils were activated with a soluble agonist, phorbol myristate acetate so that H2O2 and the granule contents were externally discharged into the suspending medium.) The promotional effects of HOCl almost certainly do not involve direct attack upon DNA; as discussed below, the bactericidal reactions of HOCl appear to be associated with loss of function at the plasma envelope of the cells,rather than within the cytosol (Fig. 1a). Oxidant damage may also contribute to degradation of this membrane. An antimicrobial secretory phospholipase identified as gIIA-PLA2 accumulates in plasma at sites of inflammation as part of the acute phase response, where it is involved in digestion of bacterial phosphatidylglycerols to their corresponding lysophosphatides. In a recent study, Weiss and coworkers have demonstrated that phosphatidylglycerol degradation of cell suspensions of S. aureus in media containing gIIA-PLA2 is dramatically enhanced by phagocytosis of the bacteria by human neutrophils [38]. This promotional effect required both an active phospholipase and neutrophils containing a functioning NOX-2, but not expression of MPO activity. As discussed by the authors, these results may represent a newly discovered form of synergism involving cooperation between cellular O2-dependent and extracellular O2-independent antimicrobial systems.
An extreme example of synergism between MPO and elastase activities can be found in the response of genetically altered mice to challenge by potentially lethal levels of Klebsiella pneumonia [39]. Under conditions where mortality in normal mice was negligible, animals deficient in either of these enzymes rapidly succumbed following exposure to this pathogen. This implied synergism is not apparent in humans, however, where much has been made of the observation that clinical manifestations of MPO deficiency are almost nonexistent [40]. One suggested explanation for this difference is that murine neutrophils lack important granule-derived nonoxidative antimicrobial polypeptides such as α-defensins [20] and bactericidal permeability-increasing proteins [21] and are therefore more reliant on alternative microbicidal reactions, in this case involving MPO-generated oxidants. A dramatically different view of synergism between nonoxidative and nominally oxidative components leading to microbial killing has been proposed by Segal and coworkers [41] (Fig. 1d). According to their model, the central role of NOX-2 is not to generate reactive oxygen species, but to polarize the phagosomal membrane through electrogenic transmembrane electron transfer [42], thereby driving the influx of potassium ions. In this scenario, the attendant increase in osmolarity causes granule-derived microbicidal proteinases to be activated by release from proteoglycan complexes, which is presumably their normal inactive state when packaged within lysosomal granules. MPO is proposed to function solely as a catalase [43] to protect the neutrophil from H2O2, an inadvertent byproduct of the repiratory burst that presumably requires removal to prevent oxidative inactivation of the proteinases. In a following publication [44], the same group presented data supporting the existence of a high-conductance Ca2+-activated K+ channel within the phagosomal membrane that opened during respiratory activation of the neutrophil. This inventive model has attracted widespread attention and has stimulated much additional research, virtually none of which has been supportive. Particularly telling has been: (1) the inability of two groups [45,46] to confirm the existence of the high-flux K+ channel, a situation that has led to the formal retraction of the original report [44]; (2) the measurement of relatively high concentrations of stable chlorinated aromatic products generated in MPO-dependent reactions following phagocytic ingestion of bacteria [47-49] and bacterial mimics [3], clearly establishing the intracellular role of MPO as a chloroperoxidase; and (3) demonstrations that, at least in vitro, the MPO chlorinating system, but not H2O2 alone, rapidly inactivates neutrophil-derived elastase [39], i.e., just the opposite of the assumptions of the model. Furthermore, the measured permeability of H2O2 across neutrophil membranes is relatively high [50] so that when generated within the phagosomal compartment (where the surface area/volume ratio is large), it would not be expected to accumulate. Indeed, a kinetic modeling study based upon elementary rate constants measured for MPO reactions indicates that H2O2 concentrations would remain in the low micromolar concentrations during the respiratory burst of normal neutrophils, and not exceed ~30 μM even when active MPO was absent [50]. A further quantitiative prediction of this kinetic model is that almost all of the O2 consumed during the respiratory burst would be converted to HOCl by the intracellular MPO. Thus, the osmotic model proposed by Segal and coworkers (Fig. 1d) fails on both conceptual grounds and experimental observations to account for intraphagosomal killing. The notion that NOX-2 driven K+ influx might serve to maintain a pH-neutral phagosomal environment to activate seprocidins remains an interesting possibility. Nonetheless, in a recent comprehensive review, Murphy and DeCoursey have concluded that the charge compensation within the phagosome necessary for maintenance of NOX-2 respiration is provided primarily by H+ influx through a voltage-gated proton channel [51].
Superoxide and hydrogen peroxide
Synergism can also exist between different host- and bacterially-derived oxidants. The most well-recognized of these is the cooperative bactericidal effects of NOX-2 generated O2•− and H2O2. Acting alone, neither of these reactive oxygen species (ROS) is microbicidal at levels thought to exist during the neutrophil respiratory burst. However, as Imlay and coworkers have demonstrated in a series of studies, prior exposure of bacteria to O2•− renders them sensitive to H2O2-mediated killing. These researchers suggest a plausible experimentally supported scenario (Fig. 1b) in which superoxide damage to active site iron-sulfur clusters in cytosolic dehydratases leads to internal release of iron, which redistributes throughout the cell, including at binding sites on the bacterial DNA; subsequent site-specific H2O2-driven Fenton reactions may then lead to lethal events such as double-strand cleavage [52-55]. When present in the external medium of bacterial suspensions, iron-based Fenton systems are generally not very bactericidal [56] (for an exception, see [57]). However, the presence of even trace amounts of copper ion can render the media containing micromolar amounts of H2O2 highly toxic; moreover, cuprous ion alone in anaerobic media has exhibited toxicities somewhat greater than a Cu2+/H2O2/ascorbate Fenton system [56]. A useful point of comparison is the relative in vitro toxicities of copper with that of HOCl, generally considered to be highly microbicidal; whereas the toxicity of Cu2+/H2O2/ascorbate is roughly comparable to that of HOCl toward E. coli (LD90 ~ 108-109 molecules/bacterium), the toxicity of CuCl alone in anaerobic media is two orders of magnitude greater (LD90 ~ 3×106 molecules/bacterium) [33]. Recent mechanistic studies by the Imlay group have again implicated dehydratases as important targets of copper ion-mediated damage [58,59]. The data support a specific bactericidal mechanism involving cuprous ion inactivation of isopropylmalate dehydratase, an enzyme essential to the synthesis of branched chain amino acids. Interestingly, this inactivation could also occur in anaerobic solutions, consistent with our earlier toxicity studies. Additional studies demonstrated that, unlike Fe, intracellular Cu did not promote DNA damage, which is surprising because copper is generally more effective than iron at catalyzing H2O2 strand cleavage [60,61]. In any event, the dramatic enhancement of toxicity that is attained when even trace levels of copper are present in in vitro bactericidal assay media suggests the possibility of synergism wherein host derived metal ions are introduced from serum or granule proteins into the developing phagosome. Copper and iron are present in serum as metalloproteins and metal-protein complexes, and granule lactoferrin also contains some iron. However, despite considerable effort, a metal-releasing mechanism has not yet been found. Ceruloplasmin and transferrin, which are the principal copper- and iron-binding serum proteins, respectively, as well as lactoferrin have been shown to retain their metal-binding capacity when extensively oxidized by HOCl or MPO-derived halogenating agents [62-65]. Digestion of transferrin by Pseudomonas aeruginosa-derived elastase has been reported to generate iron-containing fragments capable of catalyzing hydroxyl radical formation in O2•−/H2O2-driven reactions; the extent of damage was enhanced by inclusion of neutrophil elastase, but neither this proteinase alone or in combination with other representative proteinases was able to generate a Fenton catalyst, despite extensive digestion of the enzymes [65]. These particular studies involved lengthy prior digestion of the transferrin (24-48 h), so that the results appear more relevant to considerations of extracellular tissue damage at sites of infection than to intraphagosomal killing.
Superoxide and hypochlorous acid
An interesting analog of the Haber-Weiss reaction: O2•− +H2O2 → O2 + OH• + OH−, is the corresponding reaction with HOCl, i.e., O2•− + HOCl → O2 + OH•+ Cl− [66,67] (Fig.1a). Unlike the reaction with H2O2, which must be metal-catalyzed to proceed at an appreciable rate, metal ion-independent one-electron reduction of HOCl proceeds at a rate that is sufficiently rapid to be important within the phagosome during stimulated respiration [68], conditions where both O2•− and HOCl are in relatively high concentration [3,50]. Indeed, evidence for MPO-dependent OH• formation under comparable conditions has been obtained in in vitro environments utilizing both neutrophils stimulated with soluble agonists and cell-free enzymatic systems [66]. This reaction is unlikely to be important to intraphagosomal microbicidal action, however. Killing of bacterial suspensions by radiolytically-generated OH• is very inefficient (typically, < 0.1% of the toxicity of an equivalent amount of HOCl) [33]; however, this direct comparison may be highly misleading in the sense that studies utilizing added hydroxyl radical scavengers suggest that only the subfraction of OH• generated within the bacterium is lethal [69,70]. When corrected for the relative external and cellular internal volumes of the suspensions, the apparent toxicity of – radiolytically generated OH• within the bacterial cytosol is an impressive 103 to 104-fold larger than that of HOCl. Nonetheless, within the phagosome, the formation and reactions of O2•− and HOCl undoubtedly occur external to the entrapped bacterium. Because the phagosomal milieu presents numerous alternative targets for OH• in the form of serum- and granule-derived antioxidants and biomolecules [33] and because it is a very reactive and therefore nondiscriminant oxidant, any OH• formed by this reaction probably is either efficiently scavenged or reacts with nonlethal sites on the bacterial envelope. This viewpoint is supported by kinetic modeling studies that probe the fate of intraphagosomally-generated OH• [33]. The prospect exists that oxidative damage to the cell could be mediated by carbonate radical (CO3•−), however. This oxidant is considerably less reactive, and therefore more long-lived and selective in its target sites than OH• [71,72]. Modeling studies suggest that carbonate radical would be an effective toxin if generated within the phagosome, even in the presence of natural scavengers [33], and radiolytically-generated CO3•− has been shown to be microbicidal toward both bacteria (E. coli) and yeast (Saccharomyces cerevisiae) in suspension [69,70]. At issue here is whether or not the reaction, OH• + HCO3− H2O + CO3•−, will compete within the phagosome with other reactions of OH•; the rate constant for this reaction (8.5×106 M−1 s−1 [73]) is several orders of magnitude below the near-diffusion limited rate constants usually measured for reactions of OH•, so that the relative extent of this reaction depends critically upon the levels of bicarbonate achieved in neutrophils during glucose-driven stimulated respiration.
Neutrophil-derived oxidants and nitrogen oxides
Synergistic effects between phagocyte-generated oxidants and reactants produced by other cell types that might contribute to cellular defense mechanisms have been reported. Two prominent examples are the reaction between HOCl and nitrite ion to generate nitryl chloride (NO2Cl), i.e., HOCl + NO2− → NO2Cl + OH− , and related species [74,75], and the H2O2 toxicity-enhancing effects of nitric oxide (NO•) [76,77]. Nitrite and NO levels at sites of infection could be elevated through induction of the highly active form of nitric oxide synthetase (iNOS) in macrophages. This activity is easily demonstrated in isolated murine macrophages and murine-based macrophage-like hybridomas (e.g.,[78-80]), but whether or not human macrophages express this enzyme is a matter of considerable debate (e.g., [81-83]). Although both HOCl and NO2Cl are capable of chlorinating phenolic rings [32,75], the product yields in an in vitro model reaction was found to be considerably greater for HOCl, calling to question potential advantages of conversion to nitryl chlorides for in vivo modification of tyrosyl groups; moreover, under physiological conditions, the rate constant for conversion of HOCl to NO2Cl is relatively low so that at reasonable physiological levels of NO −2, this reaction is not expected to compete with other available intraphagosomal partners for HOCl [32]. Although NO• alone is virtually nontoxic toward E. coli, it can dramatically potentiate killing by H2O2 [76,77]. This potentiation does not appear to involve direct reactions between H2O2 and NO• or its catabolic products (N2O3, NO2−), but could very well arise from inhibition of normal bacterial defenses against H2O2, including catalase and glutathione peroxidase [76,84-86]. The physiological relevance of this phenomenon is also in doubt because the effect drops off significantly with NO• concentration; for example, in a representative in vitro bactericidal assay system, at 1.0 atm (~2 mM) NO•, killing by 1 mM H2O2 was essentially complete within 30 min, whereas at 0.05 atm (0.1 mM) NO•, killing under these conditions was marginal (~ 20%) [77]. Based on this scaling, promotion of H2O2-mediated killing would be negligible at lower, physiologically relevant, levels of NO•.
Reactions involving singlet oxygen (1 ΔO2)
Hypochlorous acid reacts with the hydroperoxide anion (HO −2) to generate electronically excited singlet oxygen in quantitative yield according to the reaction: HOCl + HO2− → O2(1Δg) + H2O + Cl− [87]. Singlet oxygen is far more reactive than is O2 in its 3Σu electronic ground state, and is a major contributor to the “photodynamic effect” involved with light-sensitized cell damage [88]. Consequently, the reaction between HOCl and HO2− has attracted attention as a potential mechanism for intraphagosomal killing in neutrophils. However, because the reactant species are not the predominant forms in solution, the apparent rate constant is relatively low; in the physiological range (pH 5-8), k ~ (0.5-2)×103 M−1 s−1 [87]. Since the intraphagosomal milieu contains far more reactive partners for HOCl (see, e.g., [89-94]), this reaction is unlikely to compete favorably for MPO-generated HOCl. Indeed, MPO/H2O2/Cl− reactions in cell-free in vitro systems under optimal conditions produce only barely detectable amounts of 1Δg O2, as measured by the fluorescence emission accompanying its decay to the electronic ground state [95]. Singlet oxygen formation by stimulated neutrophils is apparently not detected by this very sensitive method, although analogous reactions involving eosinophil peroxidase-catalyzed generation of the less reactive hypobromous acid (HOBr) readily form 1Δg O2, which is also visible when eosinophils are stimulated with soluble agonists [89]. However, singlet oxygen has been trapped from stimulated neutrophils and the cell-free MPO/H2O2/Cl− system alike as the stable endoperoxide of 2,9-diphenylanthracene (DPA); with neutrophils, 1ΔgO2 yields were stated to be as high as ~20% of the O2 consumed in the respiratory burst [96]. Even higher yields were attained with activated peritoneal macrophages [97], which are virtually devoid of MPO [92]. These latter results imply the existence of an alternative mechanism for singlet oxygen generation, quite possibly the dismutation reaction of superoxide ion, i.e., 2O2•− + 2H+ → H2O2 + O2(1Δg) [96,97]. One cannot help but notice, however, that the data upon which these conclusions are based involve very small changes in optical spectra of the DPA, and results from earlier trapping studies utilizing 14C-labeled cholesterol dispersed on polyacrylamide beads led to the opposite conclusions, namely, that at most 0.2% of the O2 product formed during superoxide dismutation was in its 1Δg electronic state [98] and, more generally, that activated neutrophils formed only trace amounts of 1Δg O2 during stimulated respiration accompanying phagocytosis of the beads [99]. Singlet oxygen generation by neutrophils has resurfaced recently as an issue because it has been proposed as a precursor to more potent microbicidal oxidants, specifically ozone (O3) and, possibly, dihydrogen trioxide (H2O3); these species are thought to be formed in reactions catalyzed by antibodies [100,101] and selected amino acids [102] (Fig. 1b). The evidence supporting O3 formation in neutrophils, which constitutes primarily identification of products obtained with trapping agents, is equivocal, however, because the probe molecules have been shown to be considerably less selective than originally thought [103,104], and in fact react directly with O2•− [105] or are potential substrates in MPO-catalyzed oxidations. The presumed catalytic mechanisms have not been satisfactorially rationalized and remain ill-defined. In any event, the amount of singlet oxygen formed during stimulated respiration in neutrophils by most accounts appears to be minor, which in turn restricts the upper limits for intraphagosomal yields of O3 and related oxidants by these mechanisms to very low values.
Issues impacting upon experimental design
Bacterial responses to stress
Several additional factors exist that tend to compromise attempts to extrapolate ex vivo results to the physiological milieu. In the first instance, the microbe is not a passive witness to its own demise and can mount defenses against both phagocyte-generated oxidative and nonoxidative toxins. Among these are protective responses to oxidative stressors that may include, in addition to those involving the well-recognized O2•−-responsive SoxR(S) and H2O2-responsive OxyR and PerR regulators [106], a distinct pattern of response to exposure to HOCl [107,108]. Additional recognized defense mechanisms include secretion of virulence factors that inhibit trafficking and proper assembly of phagocyte NOX-2 respiratory chains; this type of response is exemplified by Salmonella typhimurium [109-111]. Reaction to exposure to an inimical oxidizing environment may occur in bacteria within timescales associated with phagocytosis. Using gene arrays, Rosen and collaborators have demonstrated large changes in the mRNA content of E. coli 11775 attending phagocytosis by neutrophils [30]. Specifically, their analysis detected 28 genes that were significantly (defined as greater than 60%) upregulated and another 49 genes that were significantly downregulated following phagocytosis by normal neutrophils. These changes were evident within 7 minutes (approximately the time scale required for phagocytosis and activation of NOX-2 [3,26]) and underwent only minor changes in relative amounts of mRNA thereafter over a span of 30 min. Prominent among the transcripts in increased abundance were those regulated by the OxyR transcription factor. A distinct pattern of response was observed for bacteria ingested by NOX-2-deficient neutrophils including, notably, no significant change in the levels of H2O2-responsive mRNA. Although the consequences of these changes in mRNA levels to the bacterial proteome were not directly examined, the resistance of these bacteria to phagocyte-mediated oxidative killing was apparent from comparisons of susceptibilities of NOX-2 replete and deficient neutrophils [30]. These differences presumably arose from expression of the OxyR gene products in the fully competent bacteria.
This type of global remodeling of the bacterial genome in response to microbicidal agents appears to be quite general. For example, DeLeo and coworkers have shown that exposure of a virulent strain of S. aureus to sublethal amounts of HOCl, H2O2, or a mixture of primary (azurophilic) granule proteins caused changes in expression of as many as 1200 genes, representing changes in almost one-half of the bacterial gene pool; distinct patterns of up- and down-regulation were observed for the various toxins [108]. Moreover, the collective changes observed for each of the toxins were also observed for bacteria that had been phagocytosed by neutrophils [112]. Cationic antimicrobial proteins have also been shown to upregulate two-component histidine kinase-response regulator systems in several pathogenic bacteria, including S. typhimurium [113], P. aeruginosa [114], and Streptococcus pyogenes [115]. Inasmuch as these regulons control expression of numerous genes, their upregulation resulted in extensive changes to the bacterial proteome. This apparent capacity for rapid remodeling of the bacterial proteome within the developing phagosome or, more generally, at sites of infection, may confound attempts to extrapolate in vitro results to the cellular milieu. Specifically, much of what is known about microbicidal mechanisms, particularly of oxidative toxins, has been obtained by exposing cultures to bolus additions of the oxidants and examining the metabolic consequences. Under these conditions, the bacteria are killed virtually instantaneously upon exposure to lethal levels of the oxidants [116] and do not have sufficient time to develop endogenous defenses or enzymatic repair systems. Consequently, the mechanisms that have been deduced from studies with isolated bacteria could be less important in the phagosome.
Kinetic effects
Physical constraints arising from intraphagosomal compartmentation also have the capacity to affect the outcome of biological oxidations, albeit in the opposite direction. Target distributions of very fast reactions are often skewed in environments where the reactants are asymmetrically generated because significant reactant concentration gradients can develop when diffusion becomes a limiting kinetic factor. This effect is perhaps most evident in considerations of the hydroxyl radical, whose chemical reactivity is so great that it is confined to react with target molecules in the immediate vicinity of its site of generation. This recognition has led to widespread adoption of the “site-specific” mechanism in biological Fenton reactions [53,60] wherein the distribution of the catalyst over its binding sites dictates the loci of oxidative damage within the cell. Although HOCl shows a far greater intrinsic selectivity in its reactions with biological components than OH•, highly electrophilic centers can react with rate constants that exceed 107 M−1 s−1 [89]. Furthermore, the very high bacterial toxicity exhibited by HOCl and chloramines [33,117,118], coupled to the rapid rates of killing noted earlier, support the notion that the lethal target sites on the bacterium contain essential functional groups that are among the most reactive toward these chlorine oxidants. As a cationic protein, MPO is often found to bind avidly to microbial envelopes [119-121]. The corresponding asymmetric topographical distribution of catalyst presents an opportunity for directing the initially generated HOCl toward the most reactive, and presumably vulnerable, sites on the bacterial envelope, much as envisioned for the Fenton “site-specific” mechanism. However, the consequences of this asymmetric distribution of catalyst quickly disappear as the most reactive sites are exhausted and diffusional processes become more rapid than the remaining chemical reactions, allowing a more uniform distribution of oxidant to be achieved within the compartment. These considerations become an issue in experiments where specific reactions, for example, tyrosine chlorination [47-49], have been used to estimate the relative extent of reactions of neutrophil-generated chlorinating agents toward host versus bacterial proteins. These reactions are inherently quite slow and, as such, cannot capture the site-directing effects that MPO binding would have favoring reaction of very reactive, and potentially lethal, sites on the bacterial envelope.
Reactions occurring outside the phagosome
Another potentially confounding factor to attempts to quantitatively map the intracellular distribution of enzymatic oxidation and chlorination reactions is the apparent generation of ROS and HOCl at sites other than the phagosomal and plasma membranes. In the resting neutrophil, the membrane-localized components of NOX-2 are stored primarily in non-azurophilic granule fractions [122]. During phagocytosis and respiratory activation, granule fusion leads to recruitment of some of this activity to the phagosome, along with MPO obtained from azurophilic granules. However, cytochemical, immunological and chemical probe studies [6,123-125] all suggest that the cytochrome b558-containing granules are also activated toward ROS production during stimulation, particularly when soluble agonists are used. Moreover, some of these reactions are MPO-dependent [123,125], further suggesting that fusion of azurophilic and secondary granules might be occurring apart from their incorporation into the phagosome. The biological function of such events is obscure, since most of the oxidative toxins generated at these sites should be effectively scavenged by antioxidant systems within the neutrophil cytosol before encountering remotely located (i.e., extracellular or intraphagosomally entrapped) bacteria. A yet more curious and extensive structural remodeling of the neutrophil is the formation of so-called NETs (neutrophil extracellular traps), which are extracellular fibrils formed by active elaboration of primarily neutrophil chromatin and granule proteins [14,126-128]. These NETs form on timescales associated with phagocytosis, bind and kill bacteria, and can degrade bacterially-derived virulence factors; their formation at sites of acute infection in tissues has been demonstrated by confocal microscopy with immunofluorescence staining. Although the relative importance of these extracellular microbicidal constructs to host defense remains to be established, they comprise an entirely different reaction environment in the sense that access to enzymatic substrates is not limited by intraphagosomal compartmentation [32]. One might, for example, expect to find greater participation of other halogenation and nitration reactions in the extracellular MPO-dependent microbicidal reactions where restrictions on the amount of available substrates are relaxed. Even this assumption contains an element of uncertainty, however, as discussed in the following paragraph.
Asynchronous stimulation of phagocytes
Under most laboratory conditions, stimulated neutrophils and activated human macrophages do not appear to express iNOS activity. However, low levels of activity have been reported for human neutrophils and macrophages by some investigators, most notably when the cells were isolated from diseased patients or following priming with proinflammatory cytokines (e.g., [81,129,130] and references cited therein). The potential importance of timing to cellular expression of a particular oxidant is nicely illustrated by the capacity of mouse macrophages to generate peroxynitrite anion (ONOO−) via the concerted action of its NOX-2 and iNOS enzymatic reactions. Although the iNOS gaseous product, NO•, is inherently nontoxic, it rapidly undergoes radical-radical combination with O2•− to form ONOO−. The latter is a powerful oxidant capable of reacting directly with many biological substrates or, upon addition of Lewis acids, of undergoing O-O bond homolysis to strongly oxidizing [131-133] and microbicidal [70] secondary radicals (OH•, CO3•−). Additionally, the bulk of the NO• formed is ultimately degraded, forming the MPO substrate, NO −2 as one of its catabolic end-products. In macrophages, which strongly express iNOS, induction of activity is not observed until several hours post-stimulation [79,80]. In contrast, the NOX-2 driven respiratory burst occurs immediately upon stimulation and, as in populations of neutrophils, subsides within the first half-hour post-stimulation [80]. Consequently, in synchronously stimulated populations of cells, ONOO− formation is not observed. However, copious formation of ONOO− has been demonstrated in a mouse macrophage-like cell line (J774) under conditions where the cells had been primed to synthesize iNOS several hours before respiratory stimulation, i.e., where the synthase activity was maximal [134]. A similar strategy has been used to demonstrate iNOS formation in human neutrophils, albeit at a considerably lower level of activity. Specifically, incubation of neutrophils with proinflammatory cytokines for 16 h was shown by immunostaining and autoradiographic methods to elicit both iNOS mRNA and protein, the latter co-localizing with MPO in the neutrophil azurophilic granules [130]. Moreover, immunohistochemical methods were used to demonstrate iNOS-dependent formation of 3-nitrotyrosine on bacteria ingested by the primed neutrophils. Under the conditions of these experiments, iNOS-dependent reactivity could be found in approximately one-fifth of the neutrophils. Although the investigators attributed this reaction to intermediary formation of ONOO− [135,136], alternative possibilities exist [137]. For one, iNOS could equally serve as an endogenous source of NO −2, which can also nitrate tyrosyl groups via the intermediacy of MPO-generated NO •2. In any event, the findings suggest that the capacity of neutrophils and macrophages to generate alternative oxidants in physiological environments may depend upon the immediate history of the cells, as well as the identity of activating cytokines found in the physiological milieu. To quote Nathan [81]: “In sum, it is difficult to recreate reproducibly in vitro the macrophage-priming or –activating environments that arise in infected or inflamed human hosts.”
Direct detection and characterization of intraphagosomal reactions
The use of extrinsic probes, which may include the bacteria themselves, to directly monitor reactions occurring within the neutrophil phagosome has provided a means to monitor in real time both the reaction environment and nature of oxidative toxins to which the engulfed microbes are exposed. Studies utilizing pH-senstive particulate probes [3,138-140] and dyeimpregnated bacteria [141] have consistently shown that the phagosome in normal human neutrophils initially either undergoes a slight alkanization to pH ~7.8 [3,138,141] or remains unchanged [139,140] over the duration of the respiratory burst, in contrast to NOX-2 deficient or NOX-2 inhibited cells, which undergo immediate acidification [139-141]. Factors that have been suggested to contribute to the retardation of intraphagosomal acidification in normal neutrophils include proton consumption during respiratory generation of H2O2 [141], and reduced fusogenic incorporation of granule-derived proton-pumping V-type ATPases into the phagosomal membrane [140], and activation of voltage-gated proton influx [9]. Slow subsequent intraphagosomal acidification over the ensuing 60 minutes to values that are reported to be as low as pH ~6 has also been reported [3,138]. It should be noted that these measurements utilize HOCl-reactive fluorescent dyes so that interference from MPO-catalyzed reactions can be problematic. Results from experiments that in which azide ion is added to inhibit MPO activity [3,140] are perhaps more relevant to the behavior of MPO-deficient neutrophils, so that further work addressing the effect of MPO-mediated chlorination reactions upon proton translocation pathways across the phagosomal membrane seems warranted. Killing of bacteria can occur essentially on the timescales of respiratory activation and phagocytosis [26]; as mentioned above, killing rates have been used to infer that MPO-catalyzed reactions are primary contributors to neutrophil-mediated bactericidal mechanisms of S. aureus [27]. The question of whether or not bactericidal amounts of HOCl can be generated within the phagosome during this period has been addressed experimentally through the use of micron-sized fluoresceinconjugated polyacrylamide particles that contained a cystamine linker which could be cleaved on demand to allow post-phagocytic recovery of the dye [3]. Approximately 10% of the O2 consumed in the respiratory burst was isolated as chlorofluoresceins derived from the ingested particles, a number that corresponded well with both kinetic modeling results suggesting nearly stoichiometric formation of HOCl from NOX-2 generated O −2 [50] and the measured distributions of chlorotyrosines between host and bacterial proteins (~10% bacterial) using 14C-labeling methods [47-49]. The actual amount of oxidant trapped was ~6×107 HOCl/particle, which is just sufficient to overtly kill laboratory strains of bacteria, as determined by cell-free viability assays [142]. An interesting comparison is presented by S. cerevisiae; ~10-fold greater amounts of HOCl are required to kill this yeast, suggesting that even optimal production of this oxidant within neutrophil phagosomes may be insufficient to account for its fungicidal activity [143].
Bactericidal mechanisms
Given the diverse chemistry exhibited by the various putative neutrophil-generated toxins, it may ultimately become possible to infer the identity of the intraphagosomal microbicidal mechanisms by the nature of the lethal lesions inflicted upon the microbes. For example, earlier research from several laboratories involving determination of metabolic dysfunctions caused by titrimetric exposure to HOCl, chloramines, or the cell-free MPO-H2O2-Cl− microbicidal system led to the conclusion that the plasma membrane was the primary locus of these chlorine-based bactericidal reactions. Typically, one observes sigmoidal titrimetric behavior for survival curves in which the initial additions of HOCl are involved in reactions that appear inconsequential to viability, following which killing becomes very sensitive to further additions of small amounts of HOCl. Bacterial death is paralleled by loss in inner membrane-localized functions, including active metabolite transport, F0F1-ATP synthase activity, electron transport, inhibition of genomic DNA binding capacity essential to DNA synthesis, and loss of protein translocation capacity [29, 142,144-150]. Many of these functions are linked to respiratory or substrate-level oxidative phosphorylation. Not surprisingly, then, cellular death is also paralleled by extensive phosphoanhydride bond hydrolysis within the intracellular adenine nucleotide pool, converting essentially all of the ATP to AMP [147]. Without available ATP reserves or the capacity to regenerate them, the lethal HOCl-induced lesions cannot be repaired and, at this point, the cell is functionally dead [142,150]. In contrast, target molecules within the cytosol that are highly susceptible to inactivation do not lose intracellular activity until an amount of HOCl that is several-fold greater than that required to kill the bacterium is added, consistent with an “outside-in” topographic progression of oxidation of reactive sites. This pattern is a direct consequence of the exceptionally wide range of reactivity exhibited by HOCl toward biomolecules [50,89,91-94,144,150], the rate constants for which span at least 11 orders of magnitude. These rates approach diffusion-control for the most reactive biomolecules, yet for others, they are so low that HOCl is virtually inert toward them. This “outside-in” reactivity pattern is elegantly demonstrated by recent studies on intraphagosomal oxidation of bacterial methionine groups to methionine sulfoxide [149]. In this study, Rosen and coworkers showed that this reaction in E. coli was MPO-dependent. Moreover, cellular death correlated with oxidation of methionine groups located on the inner membrane and/or within the cytosol, as opposed to the outer envelope and periplasm, despite the fact that the latter groups were nearly completely oxidized before significant damage was observed at the internal sites. Causative relationships between methionine oxidation and death were probed by using bacteria with altered capabilities for methionine sulfoxide repair. Whereas a doubly-deficient mutant strain lacking omethionine sulfoxide reductases (msrA− msrB−) was more susceptible to HOCl-mediated killing than the wild-type analog, a plasmid-bearing (msrA+)-overexpressing strain was relatively resistant to killing, implying that methionine oxidation reactions are involved in bacterial death. This study is unique in identifying a very reactive protein substituent (k (HOCl) = 4×107 M−1 s−1 [150]) as contributory to MPO-mediated bacterial killing.
In contrast, MPO-independent oxidative killing might well proceed according to the model advanced by Imlay and coworkers, as described earlier [52-55], in which site-directed Fenton chemistry targets vulnerable molecules in the bacterial cytosol and the bacterial DNA (Fig. 1b). Among potentially diagnostic reactions are the cleavage of bacterial genomic and plasmid DNA, which would be promoted by coordination of redox-active metal ions [53,60]. Several studies have indicated that neutrophil-inflicted damage to bacterial DNA is minimal, however (e.g., [152] and references cited therein). Somewhat surprisingly, we recently found that genomic DNA in E. coli was extensively cleaved following exposure to HOCl in modest (3-5 fold) excess of LD90 [153]. This reaction, which was not reflected in proportionate damage to plasmid DNA, was provisionally attributed to damage to the nuclear bases being amplified by loss of DNA repair processes such that the chain-cleaving enzymes of the base excision pathways were still functional, but the lack of available ATP precluded enzymatic rebuilding of the cleaved polymer chain. If confirmed, this scenario implies that HOCl-inactivated bacteria are hypersensitive to lysis of their chromosomal DNA, irrespective of the identity of the agents that modify the nuclear bases. Since most studies have been made with plasmid DNA as target molecules, it might prove instructive to revisit the question of neutrophil-inflicted chromosomal DNA damage.
The antimicrobial peptides and proteins that have been described as nonoxidative toxins share the common feature of being strongly cationic in neutral solutions; considerable evidence indicates that, individually or collectively, expression of toxicity requires strong electrostatic association with the negatively charged bacterial cell envelope. One might then anticipate that microbicidal action by this set of toxins would include reactions that compromise the integrity of the envelope (Fig. 1c). The cytosolic membrane, in particular, represents a point of vulnerability since loss of its ion and metabolite impermeabilities would almost certainly constitute a lethal event . Protegrins and related antimicrobial peptides aggregate in phospholipid bilayer membranes to form membrane-spanning pores that accelerate electrolyte leakage. As a consequence, the membrane is no longer able to maintain sufficient transmembrane potential to perform essential chemiosmotically-driven work. Recent studies indicate that as few as ~800 protegrin molecules are capable of killing a single E. coli in this fashion [21]. More generally, serum gII A-PLA2 phospholipase and the antimicrobial proteases found in neutrophil granules and serum cause lytic damage that degrades the bacterial envelope [19,21,23,31,38,153,154], and proteins involved with cell wall synthesis and repair are among those strongly upregulated by exposure to cationic antimicrobial peptides [112-115]. The nature of the lethal lesions inflicted by these nonoxidative toxins differ from what has previously been observed for HOCl and MPO-catalyzed bactericidal systems. In these cases, the cytosolic membranes of the dead cells appear to be intact, as judged by their retention of a normal protonmotive force [156], proton conductivities, and glycerol permeation rates [157]. In general, the structural components of the bacterial cell envelopes are among the biomolecules that are virtually inert toward HOCl. This dramatic difference in transmembrane permeation characteristics following exposure to the two classes of toxins could possibly form the basis for gauging their relative contributions to bactericidal action in various organisms.
Monitoring the fate of individual bacteria
Distinguishing among these possibilities might not require recovery and characterization of the phagocytosed microbe if suitable endogenous bacterial probes can be developed. A start in this direction has been made utilizing green fluorescent protein (GFP) as a reporter group [158-160]. This protein has been expressed in the cytosol of E. coli [159] and several strains of S. aureus [160], with qualitatively similar results being reported. Briefly, among various potential phagocyte-generated oxidants, fluorescence quenching occurred only upon exposure of the bacteria to HOCl. Loss of the characteristic GFP optical absorption band indicates that this reaction involves oxidative destruction of the chromophore [159]. The amount of HOCl required for this reaction was ~10 times greater than that required to kill the cells. Notably, chloramine (NH2Cl), which is an intrinsically less reactive chlorinating agent than HOCl [161], was unable to quench fluorescence even from homogeneous solutions containing purified GFP; similarly, exposure of GFP to the prominent granule-derived seprocidins, cathepsin G and elastase, did not affect its fluorescence properties [159]. Following phagocytosis, decreases in bacterial fluorescence were observed only under conditions where both NOX-2 and MPO activities were expressed, implicating MPO-dependent oxidative mechanisms in the reactions. With S. aureus, intraphagosomal killing was incomplete, and the extent of killing correlated closely with the magnitude of fluorescence loss, suggesting that fluorescence quenching is a marker for bacterial cell death [158]. One confounding anomaly exhibited by these reactions is that intraphagosomal fluorescence quenching exhibited a pronounced delay following phagocytosis, and was not evident until 45-60 min post-NOX-2 activation. This behavior contrasts markedly with that of externally localized fluorescence probes, whose reactions to form chlorinated products closely track HOCl generation during the respiratory burst [3]. This temporal delay, which exceeds by several-fold the duration of the respiratory burst, almost certainly excludes direct reaction of HOCl with GFP in the bacterial cytosol as the cause of loss in fluorescence. Chloramines formed as secondary chlorinating agents [117,118] might be involved, but these would have to be unusually reactive (e.g., [162,163]) since it is clear that simple chloramines are insufficiently reactive to quench GFP fluorescence. Alternatively, macromolecular aggregation of GFP might contribute indirectly to modification of the properties of the chromophore. Slow protein-protein and protein-DNA cross-linking within E. coli following treatment with HOCl has been demonstrated [153,164]; however, immunoblotting analysis of bleached GFP recovered from phagocytosed S. aureus suggests that the nonfluorescent GFP protein is intact and unmodified [160]. An alternative scenario (which we discounted in our original publication [159]) is that the fluorescence losses reflect changes in internal pH within the dead bacteria. Whereas viable GFP-expressing E. coli are capable of maintaining their basal fluorescence intensity for hours in weakly acidic (~pH 5) nutrient-depleted media, bacteria that have been HOCl- or heat-inactivated or otherwise treated with protonophores to reduce their intracellular ATP levels [151] rapidly lose fluorescence in proportion to the acidity of their environment (Margo Roemeling, unpublished experiments). At 60 min post-phagocytosis, the phagosome may have become sufficiently acidic to significantly quench GFP fluorescence within the dead bacteria [3]; if this is confirmed, one might infer that the viability of individual phagocytosed bacteria could be determined from the acid-base properties of their cytosolic GFP. One should emphasize, though, that the chemistry leading to intraphagosomal fluorescence losses remains to be established. Regardless of the outcome, it seems likely that use of these probes can provide unique information on the fate of phagocytosed microbes. For example, it is already evident from the MPO-dependence of the fluorescence losses that HOCl formed in the respiratory burst is a major neutrophil-generated bactericidal agent. However, these changes occur in a microenvironment that is relatively well protected from direct oxidative insult by HOCl, requiring postulation of either unidentified secondary oxidants derived from HOCl or as-yet unidentified physiological changes that are an indirect consequence of MPO-mediated oxidative damage as causative agents. Even at the current level of understanding, the use of GFP-expressing bacteria offers a convenient, perhaps unique, means of obtaining stochastic information that could find widespread application in systematically investigating the influence of the numerous extraneous factors (e.g., reaction environment, neutrophil history, microbial metabolic capabilities) affecting the outcome of phagocytosis—a point that is beautifully illustrated in the studies by Nauseef and collaborators [160].
Summary
In the 128 years since Metchnikoff's seminal discovery of phagocytes at Messina, a great deal has been learned concerning the workings of the innate immune system. Our understanding of the underlying microbicidal chemistry is still in its infancy, however. One focal point has been the role of MPO-generated HOCl which, after considerable recent scrutiny, remains as a major component of bactericidal action in normal neutrophils functioning in in vitro aerobic environments. How this activity translates to actual physiological environments, where O2 tensions may vary considerably and symbiosis may be critical to favorable outcomes, is uncertain. Corollary challenges include understanding microbicidal mechanisms in functional but MPO-deficient cells, where oxidative killing mechanisms are clearly suboptimal, and determining how neutrophils deal with more refractory organisms such as fungi, where lethal doses may exceed the HOCl-generating capacity of the cell. Although much is to be learned concerning these matters, many of the issues raised appear amenable to experimentation. One area where rapid progress can be anticipated is the use of individual microbes as probes for real-time monitoring of their own intraphagosomal reactions. Endeavors in this area would be considerably augmented by continued improvements in our currently rudimentary understanding of the microbicidal mechanisms of putative neutrophil-generated toxins.
Highlights.
Current viewpoints of the modes of neutrophil bactericidal action are summarized
Synergistic interactions between microbicidal components are outlined
Challenges in extrapolating ex vivo data to intracellular reactions are delineated
The use of bacteria as in vivo probes of intraphagosomal reactions is illustrated
Acknowledgments
I am very grateful to the Department of Biochemistry and Biophysics at Oregon State University for providing resources through a courtesy appointment as Visiting Professor that greatly facilitated my writing this review. Research in my laboratory has been supported by the National Institutes of Health through grant AI-15842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Even more complex behavior has been reported for adherent polarized neutrophils that, when stimulated, undergo dramatic changes in temporal oscillations of their NAD(P)H content and spaciotemporal waves that propagate the length of the cell to cause localized periodic release of superoxide [Kindzelskii, A. L.; Petty, H. R. Proc. Natl. Acad. Sci. USA 99:9207-9212; 2002]. The physiological significance of this behavior is not evident.
References
- 1.DeLeo FR, Allen L-AH, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 2.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Q, Griffin DA, Barofsky DA, Hurst JK. Intraphagosomal chlorination dynamics and yields determined using unique fluorescent bacterial mimics. Chem. Res. Toxicol. 1997;10:1080–1089. doi: 10.1021/tx9700984. [DOI] [PubMed] [Google Scholar]
- 4.Pan L, Zhang X, Song K, Tang B, Cai W, Wu X, Rupp RA, Xu J. Real-time imaging of autofluorescence NAD(P)H in single human neutrophils. Appl. Optics. 2009;48:1042–1046. doi: 10.1364/ao.48.001042. [DOI] [PubMed] [Google Scholar]
- 5.Ohno Y-I, Hirai K-I, Kanoh T, Uchino H, Ogawa K. Subcellular localization of H2O2 production in human neutrophils stimulated with particles and an effect of cytochalasin B on the cells. Blood. 1982;60:253–260. [PubMed] [Google Scholar]
- 6.Seguchi H, Kobayashi T. Study of NADPH oxidase-activated sites in human neutrophils. Electron Microscopy. 2002;51:87–91. doi: 10.1093/jmicro/51.2.87. [DOI] [PubMed] [Google Scholar]
- 7.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327:487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtmüller PG, Burner U, Obinger C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide and thiocyanante. Biochemistry. 1998;37:17923–17930. doi: 10.1021/bi9818772. [DOI] [PubMed] [Google Scholar]
- 9.van Dalen C, Winterbourn CC, Senthilmohan R, Kettle AJ. Nitrite as a substrate and inhibitor of myeloperoxidase. J. Biol. Chem. 2000;275:11638–11644. doi: 10.1074/jbc.275.16.11638. [DOI] [PubMed] [Google Scholar]
- 10.Burner U, Furtmüller PG, Kettle AJ, Koppenol WH, Obinger C. Mechanisms of reaction of myeloperoxidase with nitrite. J. Biol. Chem. 2000;275:20597–20601. doi: 10.1074/jbc.M000181200. [DOI] [PubMed] [Google Scholar]
- 11.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Furtmüller PG, Zederbauer M, Jantschko W, Helm J, Bogner M, Jakopitsch C, Obinger C. Active site structure and catalytic mechanisms of human peroxidases. Arch. Biochem. Biophys. 2006;445:199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Furtmüller PG, Arnhold J, Janschko W, Pichler H, Obinger C. Redox properties of the couples compound I/compound II and compound II/native enzyme of human myeloperoxidase. Biochem. Biophys. Res. Commun. 2003;301:551–557. doi: 10.1016/s0006-291x(02)03075-9. [DOI] [PubMed] [Google Scholar]
- 14.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Rosler J, Schulze I, Wahn V, Papayannopoulos V, Zychinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vel WAC, Namavar F, Verweig MJ, Pubben ANB, McLaren DM. Killing capacity of human polymorphonuclear leukocytes in aerobic and anaerobic conditions. J. Med. Microbiol. 1984;18:173–180. doi: 10.1099/00222615-18-2-173. [DOI] [PubMed] [Google Scholar]
- 16.Beaman L, Beaman BL. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu. Rev. Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- 17.Mandel GL. Catalase, superoxide dismutase and virulence of Staphylococcus aureus. J. Clin. Invest. 1975;55:561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klebanoff SJ. Phagocytic cells: products of oxidative metabolism. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates. Raven Press; New York: 1988. pp. 391–444. [Google Scholar]
- 19.Elsbach P, Weiss J, Levy O. Oxygen-independent antimicrobial systems of phagocytes. In: Gallin JI, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 801–817. [Google Scholar]
- 20.See e.g., Voet D, Voet JG. Biochemistry. 2nd ed. Wiley; New York: 1995. [Google Scholar]
- 21.Bolintineanu D, Hazrati E, Davis HT, Lehrer RI, Kaznessis YN. Antimicrobial mechanism of pore-forming protegrin peptides: 100 pores to kill E. coli. Peptides. 2010;31:1–8. doi: 10.1016/j.peptides.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer RI. Primate defensins. Nature Rev. Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 23.Elsbach P, Weiss J, Levy O. Integration of antimicrobial host defenses: role of the bactericidal/permeability-increasing protein. Trends Microbiol. 1994;2:324–328. doi: 10.1016/0966-842x(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 24.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 25.Hof P, Mayr I, Huber R, Korzus E, Potempa J, Travis J, Powers JC, Bode W. The 1.8 angstrom structure of human cathepsin G in complex with suc-val-pro-pheP-(OPh)2: a Janus-faced proteinase with two opposite specificities. EMBO J. 1996;15:5481–5491. [PMC free article] [PubMed] [Google Scholar]
- 26.Hampton MB, Vissers MCM, Winterbourn CC. A single assay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J. Leuk. Biol. 1994;55:147–152. doi: 10.1002/jlb.55.2.147. [DOI] [PubMed] [Google Scholar]
- 27.Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen H, Michel BR. Redundant contribution of myeloperoxidase-dependent systems to neutrophil-mediated killing of Escherichia coli. Infect. Immun. 1997;65:4173–4178. doi: 10.1128/iai.65.10.4173-4178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen H, Orman J, Rakita RM, Michel BR, VanDevanter DR. Loss of DNA-membrane interactions and cessation of DNA synthesis in myeloperoxidase-treated Escherichia coli. Proc. Natl. Acad. Sci. USA. 1990;87:10048–10052. doi: 10.1073/pnas.87.24.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudinger BJ, Oberdoerster MA, Lewis PJ, Rosen H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J. Clin. Invest. 2002;110:1151–163. doi: 10.1172/JCI15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Revs. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q, Hurst JK. Relative chlorinating, nitrating and oxidizing capabilities of neutrophils determined with phagocytosable probes. J. Biol. Chem. 1997;27:32767–32772. doi: 10.1074/jbc.272.52.32767. [DOI] [PubMed] [Google Scholar]
- 33.Lymar SV, Hurst JK. Role of compartmentation in promoting toxicity of leukocyte-generated strong oxidants. Chem. Res. Toxicol. 1995;8:833–840. doi: 10.1021/tx00048a003. [DOI] [PubMed] [Google Scholar]
- 34.Odeberg H, Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect. Immun. 1976;14:1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vissers MCM, Winterbourn CC. Oxidative damage to fibronectrin. 1. The effects of the neutrophil myeloperoxidase system and HOCl. Arch. Biochem. Biophys. 1991;285:53–59. doi: 10.1016/0003-9861(91)90327-f. [DOI] [PubMed] [Google Scholar]
- 36.Weiss J, Kao L, Victor M, Elsbach P. Respiratory burst facilitates the digestion of Escherichia coli killed by polymorphonuclear leukocytes. Infect. Immun. 1987;55:2142–2147. doi: 10.1128/iai.55.9.2142-2147.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rensburg CEJ, Van Staden AM, Anderson R, Van Rensburg EJ. Hypochlorous acid potentiates hydrogen peroxide-mediated DNA-strand breaks in human mononuclear leucocytes. Mutation Research. 1992;265:255–261. doi: 10.1016/0027-5107(92)90054-6. [DOI] [PubMed] [Google Scholar]
- 38.Femling JK, Nauseef WM, Weiss JP. Synergy between extracellular group IIA phospholipase A2 and phagocyte NADPH oxidase in diegestion of phospholipids of Staphylococcus aureus ingested by human neutrophils. J. Immunol. 2005;175:4653–4661. doi: 10.4049/jimmunol.175.7.4653. [DOI] [PubMed] [Google Scholar]
- 39.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumonia and inactivating neutrophil elastase: effects on host defense. J. Immunol. 2005;174:1557–1565. doi: 10.4049/jimmunol.174.3.1557. [DOI] [PubMed] [Google Scholar]
- 40.Nauseef WM, Root RK, Malech HL. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J. Clin. Invest. 7:1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 42.Henderson LM, Chappell JB, Jones OT. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kettle AJ, Winterbourn CC. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–10212. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- 44.Ahluwalla J, Tinker A, Clapp LH, Duchen MR, Abramov AY, Pope S, Nobles M, Segal AW. The large conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Femling JK, Cherny VV, Morgan D, Rada B, Davis AP, Czirják G, Enyedi P, England SK, Moreland JG, Ligeti E, Nauseef WM, DeCoursey TE. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J. Gen. Physiol. 2006;127:659–672. doi: 10.1085/jgp.200609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am. J. Physiol. Cell Physiol. 2007;293:C45–C54. doi: 10.1152/ajpcell.00450.2006. [DOI] [PubMed] [Google Scholar]
- 47.Chapman ALP, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 2002;277:9757–9762. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- 48.Rosen H, Crowley JR, Heinecke JW. Human neutrophils use the myeloperoxidase—hydrogen peroxide—chloride system to chlorinate, but not nitrate bacterial proteins during phagocytosis. J. Biol. Chem. 2002;277:30463–30468. doi: 10.1074/jbc.M202331200. [DOI] [PubMed] [Google Scholar]
- 49.Painter RG, Valentine VG, Lanson NA, Jr., Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. Implications for microbial killing. J. Biol. Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 51.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim. Biophys. Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keyer K, Gort AS, Imlay JA. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 1995;177:6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx− mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elzanowska H, Wolcott RG, Hannum DM, Hurst JK. Bactericidal properties of hydrogen peroxide and copper or iron-containing complex ions in relation to leukocyte function. Free Radic. Biol. Med. 1995;18:437–449. doi: 10.1016/0891-5849(94)00150-i. [DOI] [PubMed] [Google Scholar]
- 57.Rosen H, Klebanoff SJ. Role of iron and ethylenediaminetetraacetic acid in the bactericidal activity of a superoxide anion-generating system. Arch. Biochem. Biophys. 1981;208:512–519. doi: 10.1016/0003-9861(81)90539-7. [DOI] [PubMed] [Google Scholar]
- 58.Macomber L, Rensing C, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chevion M. A site-specific mechanism for free radical induced biological damage: The essential role of redox-active transition metals. Free Radic. Biol. Med. 1988;5:27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- 61.Tachon P. Ferric and cupric ions requirement for DNA single-strand breakage by hydrogen peroxide. Free Radical Res. Commun. 1989;7:1–10. doi: 10.3109/10715768909088155. [DOI] [PubMed] [Google Scholar]
- 62.Winterbourn CC, Molloy AL. Susceptibilities of lactoferrin and transferring to myeloperoxidase-dependent loss of iron-binding capacity. Biochem. J. 1988;250:613–616. doi: 10.1042/bj2500613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halliwell B, Aruoma OI, Wasil M, Gutteridge JMC. The resistance of transferrin, lactoferrin and caeruloplasmin to oxidative damage. Biochem. J. 1988;256:311–312. doi: 10.1042/bj2560311a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark RA, Pearson DW. Inactivation of transferrin iron binding capacity by the neutrophil myeloperoxidase system. J. Biol. Chem. 1989;264:9420–9427. [PubMed] [Google Scholar]
- 65.Britigan BE, Edeker BL. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J. Clin. Invest. 1991;88:1092–1102. doi: 10.1172/JCI115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramos CL, Pou S, Britigan BE, Cohen MS, Rosen GM. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J. Biol. Chem. 1992;267:8307–8312. [PubMed] [Google Scholar]
- 67.Candeias LP, Patel KB, Stratford MRL, Wardman P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett. 1993;333:151–153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- 68.Long CA, Bielski BHJ. Rate of reaction of superoxide radical with chloride-containing species. J. Phys. Chem. 1980;84:555–557. [Google Scholar]
- 69.Wolcott RG, Franks BS, Hannum DM, Hurst JK. Bactericidal potency of hydroxyl radical in physiological environments. J. Biol. Chem. 1994;269:9721–9728. [PubMed] [Google Scholar]
- 70.King DA, Sheafor MW, Hurst JK. Comparative toxicities of putative phagocyte-generated oxidizing radicals toward a bacterium (Escherichia coli) and a yeast (Saccharomyces cerevisiae). Free Radic. Biol. Med. 2006;41:765–774. doi: 10.1016/j.freeradbiomed.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 71.Adams GE, Aldrich JE, Bisby RH, Cundall RB, Redpath JL, Willson RL. Selective free radical reactions with proteins and enzymes: reactions of inorganic radical anions with amino acids. Radiat. Res. 1972;49:278–289. [PubMed] [Google Scholar]
- 72.Chen S-N, Hoffman MZ. Rate constants for the reactions of carbonate radical with compounds of interest in neutral aqueous solutions. Radiat. Res. 1973;56:40–47. [PubMed] [Google Scholar]
- 73.Czapski G, Lymar SV, Schwarz HA. Acidity of the carbonate radical. J. Phys. Chem. A. 1999;103:3447–3450. [Google Scholar]
- 74.Johnson DW, Margerum DW. Non-metal redox kinetics: a reexamination of the mechanism of the reaction between hypochlorite and nitrite ions. Inorg. Chem. 1991;30:4845–4851. [Google Scholar]
- 75.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J. Biol. Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 76.Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell JB. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hurst JK, Lymar SV. Toxicity of peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chem. Res. Toxicol. 1997;10:802–810. doi: 10.1021/tx970008v. [DOI] [PubMed] [Google Scholar]
- 78.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharides. Proc. Natl. Acad. Sci. USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. J. Biol. Chem. 2001;276:34051–34058. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- 80.Palazzolo-Ballance AM, Suquet C, Hurst JK. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry. 2007;46:7536–7548. doi: 10.1021/bi700123s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nathan C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nathan C. Role of iNOS in human host defense. Science. 2006;312:1874. doi: 10.1126/science.312.5782.1874b. [DOI] [PubMed] [Google Scholar]; Liu P, Adams JS, Modlin RL. Response. Science. 2006;312:1874–1875. [Google Scholar]
- 83.Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J. Clin. Invest. 2011;121:3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nunoshiba T, deRojas-Walker T, Wishnok JS, Tannenbaum SR, Demple B. Activation by nitric oxide of an oxidative stress response that defends Escherichia coli against activated macrophages. Proc. Natl. Acad. Sci. USA. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown GC. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995;232:188–191. doi: 10.1111/j.1432-1033.1995.tb20798.x. [DOI] [PubMed] [Google Scholar]
- 86.Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Tanaguchi N. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J. Biol. Chem. 1995;270:21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- 87.Held AM, Halko DJ, Hurst JK. Mechanisms of chlorine oxidation of hydrogen peroxide. J. Am. Chem. Soc. 1978;100:5732–5740. [Google Scholar]
- 88.Foote CS. Mechanisms of photosensitized oxidation. Science. 1968;162:963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- 89.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA. 1981;78:210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grisham MB, Jefferson MM, Melton DF, Thomas EL. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem. 1984;259:10404–10413. [PubMed] [Google Scholar]
- 91.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 92.Pattison DI, Davies M. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- 93.Pattison DI, Hawkins CL, Davies MJ. What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem. Res. Toxicol. 2009;22:807–817. doi: 10.1021/tx800372d. [DOI] [PubMed] [Google Scholar]
- 94.Nagy P, Winterbourn CC. Redox Chemistry of Biological Thiols. Adv. Mol. Toxicol. 2010;4:183–222. [Google Scholar]
- 95.Kanofsky JR, Hoogland H, Wever R, Weiss SJ. Singlet oxygen production by human eosinophils. J. Biol. Chem. 1988;263:9692–9696. [PubMed] [Google Scholar]
- 96.Steinbeck MJ, Khan AU, Karnovsky MJ. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J. Biol. Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 97.Steinbeck MJ, Khan AU, Karnovsky MJ. Extracellular production of singlet oxygen by stimulated macrophages quantified using 9,10-diphenylanthracene and perylene in a polystyrene film. J. Biol. Chem. 1993;268:15649–15654. [PubMed] [Google Scholar]
- 98.Foote CS, Shook FC, Abakerli RA. Chemistry of Superoxide Ion. 4. Singlet Oxygen is not a major product of dismutation. J. Am. Chem. Soc. 1980;102:2503–2504. [Google Scholar]
- 99.Foote CS, Abakerli RB, Clough RL, Lehrer RI. On the question of singlet oxygen production in polymorphonuclear leucocytes. In: DeLuca MA, McElroy WD, editors. Bioluminescence and Chemiluminescence. Academic Press; Orlando: 1983. pp. 81–89. [Google Scholar]
- 100.Wentworth P, Jr., McDunn JE, Wentworth AD, Takeuchi C, Nieva J, Jones T, Bautista C, Ruedi JM, Gutierrez A, Janda KD, Babior BM, Eschenmoser A, Lehrer RA. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 101.Babior BM, Takeuchi C, Ruedi J, Gutierrez A, Wentworth P., Jr. Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. USA. 2003;100:3031–3034. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamashita K, Miyoshi T, Arai T, Endo N, Itoh H, Makino K, Mizugishi K, Uchiyama T, Sasada M. Ozone production by amino acids contributes to killing of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:16912–16917. doi: 10.1073/pnas.0807952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kettle AJ, Winterbourn CC. Do neutrophils produce ozone? An appraisal of current evidence. BioFactors. 2005;24:41–45. doi: 10.1002/biof.5520240105. [DOI] [PubMed] [Google Scholar]
- 104.Smith LL. Oxygen oxysterols, ouabain, and ozone: a cautionary tale. Free Radic. Biol. Med. 2004;37:318–324. doi: 10.1016/j.freeradbiomed.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 105.Kettle AJ, Clark BM, Winterbourn CC. Superoxide converts indigo carmine to isatin sulfonic acid. Implications for the hypothesis that neutrophils produce ozone. J. Biol. Chem. 2004;279:18521–18525. doi: 10.1074/jbc.M400334200. [DOI] [PubMed] [Google Scholar]
- 106.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pallazolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, Kreiswirth BN, Skaar EP, DeLeo FR. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 2008;180:500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 109.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM, Dinauer MC, Mastroeni P, Fang FC. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 110.Vasquez-Torres A, Fantuzzi G, Edwards CK, III, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc. Natl. Acad. Sci. USA. 2001;98:2561–2565. doi: 10.1073/pnas.041618998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallois A, Klein JR, Allen L-AH, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 112.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM;, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 113.Bader MW, Navarre WW, Shiau W, Nikaido H, Frye JG, McClelland M, Fang FC, Miller SI. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 2003;50:219–230. doi: 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- 114.McPhee JB, Lewenza S, Hancock REW. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 115.Voyich JM, Braughton KR, Sturdevant DE, Vuong C, Kobayashi SD, Porcella SF, Otto M, Musser JM, DeLeo FR. Engagement of the pathogen survival response used by Group A Streptococcus to avert destruction by innate host defense. J. Immunol. 2004;173:1194–1201. doi: 10.4049/jimmunol.173.2.1194. [DOI] [PubMed] [Google Scholar]
- 116.Albrich JM, Hurst JK. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982;144:157–161. doi: 10.1016/0014-5793(82)80591-7. [DOI] [PubMed] [Google Scholar]
- 117.Thomas EL. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect. Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas EL. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: Effect of exogenous amines on antibacterial action against Escherichia coli. Infect. Immun. 1979;25:110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Selvaraj RJ, Zglinczynski JM, Paul BB, Sbarra AJ. Enhanced killing of myeloperoxidase-coated bacteria in the myeloperoxidase-hydrogen peroxide-chloride ion (−1) system. J. Infect. Dis. 1978;137:481–485. doi: 10.1093/infdis/137.4.481. [DOI] [PubMed] [Google Scholar]
- 120.Ramsey PG, Martin T, Chi E, Klebanoff SJ. Arming of mononuclear phagocytes by eosinophil peroxidase bound to Staphylococcus aureus. J. Immunol. 1982;128:415–420. [PubMed] [Google Scholar]
- 121.Miyasaki KT, Zambon JJ, Jones CA, Wilson ME. Role of high-avidity binding of human neutrophil peroxidase in the killing of Actionobacillus actinomycetemcomitans. Infect. Immun. 55:1029–1036. doi: 10.1128/iai.55.5.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocytes. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 123.Lundqvist H, Follin P, Khalfan L, Dahlgren C. Phorbol myristate acetate—induced NADPH oxidase activity in human neutrophils: only half the story has been told. J. Leuk. Biol. 1996;59:270–279. doi: 10.1002/jlb.59.2.270. [DOI] [PubMed] [Google Scholar]
- 124.Vaissiere C, Le Cabec V, Maridonneau-Parini I. NADPH oxidase is functionally assembled in specific granules during activation of human neutrophils. J. Leuk. Biol. 1999;65:629–634. doi: 10.1002/jlb.65.5.629. [DOI] [PubMed] [Google Scholar]
- 125.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antiox. Redox Signaling. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 126.Brinkman V, Reichard U, Goosman C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 127.Fuchs TA, Abed U, Goosman C. Novel cell death program leads to neutrophil extracellular traps. J. Cell. Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 129.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 130.Evans TJ, Buttery LDK, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl. Acad. Sci. USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Radi R, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 132.Coddington JW, Hurst JK, Lymar SV. Hydroxyl radical formation during peroxynitrous acid decomposition. J. Am. Chem. Soc. 1999;121:2438–2445. [Google Scholar]
- 133.Bonini MG, Radi R, Ferrer-Sueta G, Ferreira AM, Da C, Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem. 1999;274:10802–10806. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 134.Alvarez MN, Trujillo M, Radi R. Peroxynitrite formation from biochemical and cellular fluxes of nitric oxide and superoxide. Methods Enzymol. 2002;359:353–366. doi: 10.1016/s0076-6879(02)59198-9. [DOI] [PubMed] [Google Scholar]
- 135.Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- 136.Goldstein S, Czapski G, Lind J, Merényi G. Tyrosine nitration by simultaneous generation of •NO and O •−2 under physiological conditions. How the radicals do the job. J. Biol. Chem. 2000;275:3031–3036. doi: 10.1074/jbc.275.5.3031. [DOI] [PubMed] [Google Scholar]
- 137.Hurst JK. Whence nitrotyrosine? J. Clin. Invest. 2002;109:1287–1289. doi: 10.1172/JCI15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cech P, Lehrer RI. Phagolysosomal pH of human neutrophils. Blood. 1984;63:88–95. [PubMed] [Google Scholar]
- 139.Hurst JK, Albrich JM, Green TR, Rosen H, Klebanoff S. Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J. Biol. Chem. 259:4812–4821. [PubMed] [Google Scholar]
- 140.Jankowski A, Scott CC, Grinstein S. Determinants of the phagosomal pH in neutrophils. J. Biol. Chem. 2002;277:6059–6066. doi: 10.1074/jbc.M110059200. [DOI] [PubMed] [Google Scholar]
- 141.Segal AW, Geisow M, Garcia R, Harper A, Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 142.Barrette WC, Jr., Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolution of ATP production. Biochemistry. 1989;28:9172–9178. doi: 10.1021/bi00449a032. [DOI] [PubMed] [Google Scholar]
- 143.King DA, Hannum DM, Qi J-S, Hurst JK. HOCl-mediated cell death and metabolic dysfunction in the yeast Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2004;423:170–181. doi: 10.1016/j.abb.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 144.Hurst JK, Barrette WC., Jr. Leukocyte oxygen activation and microbicidal oxidative toxins. CRC Crit. Rev. Biochem. Mol. Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- 145.Rosen H, Rakita RM, Waltersdorph AM, Klebanoff SJ. Myeloperoxidase-mediated damage to the succinate oxidase system of Escherichia coli. Evidence for selective inactivation of the dehydrogenase component. J. Biol. Chem. 1987;242:15004–15010. [PubMed] [Google Scholar]
- 146.Rakita RM, Michel BR, Rosen H. Myeloperoxidase-mediated inhibition of microbial respiration: damage to Escherichia coli ubiquinol oxidase. Biochemistry. 1989;28:3031–3036. doi: 10.1021/bi00433a044. [DOI] [PubMed] [Google Scholar]
- 147.Hurst JK, Barrette WC, Jr., Michel BR, Rosen H. Hypochlorous acid and meloperoxidase-catalyzed oxidation of iron-sulfur clusters in bacterial respiratory dehydrogenases. Eur. J. Biochem. 1991;2002:1275–1282. doi: 10.1111/j.1432-1033.1991.tb16500.x. [DOI] [PubMed] [Google Scholar]
- 148.Hannum DM, Barrette WC, Jr., Hurst JK. Subunit sites of oxidative inactivation of Escherichia coli by HOCl. Biochem. Biophys. Res. Commun. 1995;212:868–874. doi: 10.1006/bbrc.1995.2049. [DOI] [PubMed] [Google Scholar]
- 149.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci, USA. 2009;106:18686–18691. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 151.Barrette WC, Jr., Hannum DM, Wheeler WD, Hurst JK. Viability and metabolic capability are maintained by Escherichia coli, Pseudomonas aeruginosa, and Streptococcus lactis at very low adenylate energy charge. J. Bacteriol. 1988;170:3655–3659. doi: 10.1128/jb.170.8.3655-3659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fox HB, De Togni P, McMahon G, Levy SB, Robinson JS, Karnovsky MJ, Babior BM. Fate of the DNA in plasmid-containing Escherichia coli minicells ingested by human neutrophils. Blood. 1987;69:1394–1400. [PubMed] [Google Scholar]
- 153.Suquet CC, Warren JJ, Seth N, Hurst JK. Comparative study of HOCl-inflicted damage to bacterial DNA ex vivo and within cells. Arch. Biochem. Biophys. 2010;493:135–142. doi: 10.1016/j.abb.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lichtenstein AK, Ganz T, Nguyen T-M, Selsted ME, Lehrer RI. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J. Immunol. 1988;140:2686–2694. [PubMed] [Google Scholar]
- 155.Elsbach P, Weiss J. Phagocytic cells: oxygen-independent antimicrobial systems of phagocytes. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates. Raven Press; New York: 1988. pp. 445–470. [Google Scholar]
- 156.Barrette WC, Jr., Albrich JM, Hurst JK. Hypochlorous acid-promoted loss of metabolic energy in Escherichia coli. Infect. Immun. 1987;55:2518–2525. doi: 10.1128/iai.55.10.2518-2525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Albrich JM, Gilbaugh JH, III, Callahan KB, Hurst JK. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane and transport systems of Escherichia coli. J. Clin. Invest. 1986;78:177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Espey MG, Xavier S, Thomas DD, Miranda KM, Wink DA. Direct real-time evaluation of nitration with green fluorescent protein in solution and within human cells reveals the impact of nitrogen dioxide vs. peroxynitrite mechanisms. Proc. Natl. Acad. Sci. USA. 2002;99:3481–3486. doi: 10.1073/pnas.062604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Palazzolo AM, Suquet CC, Konkel ME, Hurst JK. Green fluorescent protein-expressing Escherichia coli as a selective probe for HOCl generation within neutrophils. Biochemistry. 2005;44:6910–6919. doi: 10.1021/bi047342s. [DOI] [PubMed] [Google Scholar]
- 160.Schwartz J, Leidal KG, Femling JK, Weiss JP, Nauseef WM. Neutrophil bleaching of GFP-expressing Staphylococci: probing the intraphagosomal fate of individual bacteria. J. Immunol. 2009;183:2632–2641. doi: 10.4049/jimmunol.0804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hurst JK, Carr PAG, Hovis FE, Richardson RJ. Hydrogen peroxide oxidation by chlorine compounds. Reaction dynamics and singlet oxygen formation. Inorg. Chem. 1981;20:2435–2438. [Google Scholar]
- 162.Pattinson DI, Davies MJ. Kinetic analysis of the role of histidine chloramines in hypochlorous acid mediated protein oxidation. Biochemistry. 2005;44:7378–7387. doi: 10.1021/bi0474665. [DOI] [PubMed] [Google Scholar]
- 163.Peskin AV, Midwinter RG, Harwood DT, Winterbourn CC. Chlorine transfer between glycine, taurine, and histamine: reaction rates and impact upon cellular reactivity. Free Radic. Biol. Med. 2004;37:1622–1630. doi: 10.1016/j.freeradbiomed.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 164.Kulcharyk PA, Heinecke JW. Hypochlorous acid produced by the myeloperoxidase system of human phagocytes induces covalent cross-links between DNA and protein. Biochemistry. 2001;40:3648–3656. doi: 10.1021/bi001962l. [DOI] [PubMed] [Google Scholar]