Abstract
Background
Borderline personality disorder (BPD) is a chronic condition with a strong impact on patients‘ affective,cognitive and social functioning. Neuroimaging techniques offer invaluable tools to understand the biological substrate of the disease. We aimed to investigate gray matter alterations over the whole cortex in a group of Borderline Personality Disorder (BPD) patients compared to healthy controls (HC).
Methods
Magnetic resonance-based cortical pattern matching was used to assess cortical gray matter density (GMD) in 26 BPD patients and in their age- and sex-matched HC (age: 38±11; females: 16, 61%).
Results
BPD patients showed widespread lower cortical GMD compared to HC (4% difference) with peaks of lower density located in the dorsal frontal cortex, in the orbitofrontal cortex, the anterior and posterior cingulate, the right parietal lobe, the temporal lobe (medial temporal cortex and fusiform gyrus) and in the visual cortex (p<0.005). Our BPD subjects displayed a symmetric distribution of anomalies in the dorsal aspect of the cortical mantle, but a wider involvement of the left hemisphere in the mesial aspect in terms of lower density. A few restricted regions of higher density were detected in the right hemisphere. All regions remained significant after correction for multiple comparisons via permutation testing.
Conclusions
BPD patients feature specific morphology of the cerebral structures involved in cognitive and emotional processing and social cognition/mentalization, consistent with clinical and functional data.
Keywords: borderline personality disorder, neuroimaging, MRI
1. Introduction
Borderline personality disorder (BPD) is a chronic condition affecting 1 to 2%of the general population and up to 20% of psychiatric inpatients [31, 42]. Patients with BPD showed several areas of deficits. Among them affect regulation [36], in particular impulsivity and emotional instability, and social functioning [50-29], including deficit in the ability to infer their own and others‘ mental states, that is also referred to as Mentalizing, theory of Mind (ToM) or social cognition [48], represent a distinguishing features of the disease.
Neuroimaging techniques offer invaluable tools to understand the biological substrate of these deficits. Neurobiological studies link symptoms of BPD (impulsivity and emotional instability) to failures of frontolimbic functions [53] and suggest that emotional dysregulation is caused by prefrontal deficits, hyperactivity of the limbic system, or a combination of both [27]. Previous neuroimaging studies found that the most consistent structural difference in BPD patients is the reduced volume of the hippocampus and the amygdala [47, 61]. These structures are involved in emotion regulation and have been describe as key structures in the pathophysiology underpinning the disease.
Furthermore, a well-supported evidence underline the role of frontal cortex. In particular, some evidence suggest that BPD patients present abnormal areas in the prefrontal cortex (PFC),; in particular, the orbito-frontal cortex (OFC) [14, 53] and the adjacent ventro-medial cortex (including the anterior cingulate gyrus – ACG) [26] – the brain regions that underlie the modulation of emotional responses are the most affected areas. Reduced GM volumes in the dorsolateral cortex and in the left OFC were also reported in female adolescents with BPD [11]. Furthermore, BPD patients present a reduced gray matter (GM) volume in the posterior cingulate gyrus (PCG) [26, 34] and in the right parietal cortex [30].
These results were obtained mainly using region-of-interest or voxel-based morphometry approaches. In our knowledge, very few studies investigated cortical morphology in BPD. A recent study assessed cortical morphology using Freesurfer, an automated cortical surface reconstruction method, on a group of 25 BPD females [15]. The study showed morphologic abnormalities involving fronto-limbic and paralimbic regions of both hemisphere [15]. However, as the authors underlined in the discussion, their findings cannot be generalized since the sample included only females with BPD. Furthermore, Freesurfer analysis of cortical morphology, being automated, could have a series of limitations such as errors regarding processes of segmentation, normalization and skull extraction [15]. Another study on female BPD using Freesurfer package showed reduced cortical thickness in prefrontal cortex, left tempoparietal junction, bilateral temporal poles and bilateral paracentral lobules [7]. The authors concludes that the regions that exhibit cortical thinning in BPD patients resembles a network of cortical structures involved in social cognition [7].
Cortical Pattern Matching (CPM) [55] allows the accurate mapping of cortical gray matter density (GMD), by carefully aligning cortical structures, thereby increasing the accuracy and power of comparisons between samples. CPM was successfully used in other psychiatric patient groups [6, 19, 4] but it has not been used in BPD. Objective of the study was to investigate GM alterations over the whole cortex in a group of BPD patients compared to age- and sex-matched healthy controls (HC) to explore the biological characteristics of the disorder.
2. Materials and Methods
2.1 Subjects
Twenty-six patients meeting the DSM-IV criteria for BPD were enrolled in the study over a period of about 24 months. We included inpatients admitted to the Psychiatric Rehabilitation Unit of the IRCCS San Giovanni di Dio-Fatebenefratelli, a residential facility of the National Mental Health System for non-acute patients [16]. Patients were enrolled as a part of a wider study aiming at describing brain morphological features psychiatric disorder [43,44]. Exclusion criteria were: acute or lifetime schizophrenia, schizoaffective disorder, major depressive disorder with psychotic features, anorexia, drug or alcohol abuse in the last 3 months, or cognitive impairment. All patients underwent a multidimensional assessment including: Brief Psychiatric Rating Scale (BPRS) [40] to measure general psychopathology, Hamilton Depression Rating Scale (HAM-D) [25] to assess depressive symptoms, Personal and Social Performance scale [23] assessing global functioning, Toronto Alexithymia Scale (TAS) [3] to assess alexithymia (inability to express feelings with words), Barratt Impulsiveness Scale (BIS-11) [35] to measure impulsivity and State and Trait Anxiety Inventory (STAI) [52] for the assessment of anxiety.
Clinical diagnosis of the BPD group was asssessed by the Structured Clinical Interview Axis I Disorders (SCID I) and Axis II Personality Disorders (SCID II) for DSM-IV [17,18]. For each inpatient we collected all the relevant clinical information: demographics (age, sex, education), history of psychiatric disease (years), history of alcohol and drug abuse, presence of suicidal attempts, self-injurious behaviours or aggression, and drug therapy. Lifetime psychiatric comorbidities were as follows: 5 patients reported a lifetime history of depression, 3 eating disorder, 1 eating disorder and obsessive compulsive disorder (OCD), and 2 OCD; 3 satisfied criteria for antisocial personality disorder, 2 for dependent personality disorder. All inpatients received psychotropic medication.
A group of 26 HC was matched (1:1) to BPD patients by age and sex. Control subjects were part of a large sample of cognitive intact individuals enrolled in a previous MRI study on normal brain structure with MRI (ArchNor, Normative Archive of Structural Brain Magnetic Resonance Imaging), as described in detail elsewhere [21, 38]. Briefly, subjects who underwent a brain magnetic resonance (MR) scan at the Neuroradiology Units of the Poliambulanza Hospital in Brescia for reasons other than memory disturbance, cognitive impairment, degenerative diseases, or head trauma and whose MRI scan was negative were asked to participate to the study. Each subject underwent multidimensional assessment including clinical, neurological, and neuropsychological assessment.
The study was approved by the local ethics committee and written informed consent was obtained by all subjects. No compensation was provided for study participation.
2.2 Magnetic resonance imaging
Patients and controls underwent MRI performed on a 1.5 Tesla GE scanner. MR images were acquired with a gradient echo 3D protocol with the following parameters: TR = 20 ms, TE = 5 ms, flip angle = 30°, field of view = 220 mm, acquisition matrix 256×256, slice thickness 1.3 mm.
Cortical GM was studied using the CPM technique developed at the Laboratory of Neuro Omaging (LONI) of the University of California at Los Angeles [55].
2.3 Image pre-processing
The 3D images were reoriented along the AC-PC line and voxels below the cerebellum were removed with the MRIcro software (http://www.cabiatl.com/mricro/mricro/mricro.html). The anterior commissure was manually set as the origin of the spatial coordinates for an anatomical normalization algorithm implemented as part of the Statistical Parametric Mapping (SPM) software package ( available on http://www.fil.ion.ucl.ac.uk/spm/software/spm2/). A 12-parameter affine transformation was used to normalize each image to a customized template in stereotaxic space, created from the MRI scans of all the subjects included in the study.
2.4 Cortical gray matter mapping
Individual brain masks for each hemisphere were extracted from normalized images with the automatic method, EMS (expectation maximization segmentation; www.medicalimagecomputing.com/EMS) [58, 59], visually inspected, and manually corrected using DISPLAY, a three-dimensional visualization program that simultaneously displays the sagittal, coronal and axial slices of the brain (available on http://www.bic.mni.mcgill.ca/software/Display/Display.html), and allows the manual correction of errors in classifying brain and non-brain tissue. The resulting masks were applied to normalized images in order to obtain ‗skull-stripped‘ images of each hemisphere. A 3D model of hemispherical cortical surfaces was automatically extracted using intensity information [32]. Normalized images were segmented into GM, white matter and cerebrospinal fluid using an algorithm that employs partial volume correction and bias field correction [49]. Sulcal lines were traced by a single tracer (ML) on the cortical surfaces according to a previously validated anatomical delineation protocol [56].
For each subject, we manually traced 17 sulci on the lateral surface and 12 sulci were manually traced on the medial surface of each brain hemisphere; additional 3D lines were drawn to outline interhemispheric gyral limits. The reliability of manual outlining was assessed prior to experimental subject tracing with a standard protocol, requiring the same rater to trace all lateral and medial sulci of 6 test brains [51]. At the end of the reliability phase, the mean 3D deviation of the tracer from the gold standard was 3 mm everywhere for the medial sulci and 4.5 mm everywhere for the lateral sulci. Sulcal curves and cortical surfaces were flattened and averaged across subjects to create a population specific template based on all subjects in the study [55]. Averaged sulci were then used as landmarks to warp each subject's anatomy into the template. The same deformation was applied to the segmented images, thus allowing measurement of GM at thousands of homologous cortical locations. GM density (GMD) was computed at each cortical point as the proportion of tissue classified as GM in a sphere centered at that point, with a radius of 15 mm, and then averaged within each group to obtain the GMD mean.
We adopted a region-of-interest-based approach to quantify the average GMD within each Brodmann Area (BA). We applied a deformable BA atlas [37] to the left and right hemisphere average cortical surface models using CPM, enabling the tabulation of each subject's average GMD for each BA. We merge some BA in functional/morphological groups of BAs following a previously proposed combination [6].
2.5 Statistical analysis
To assess the homogeneity of variance and the data distribution, we carried out the Levene`s test and the Shapiro-Wilk test, respectively. When normally distributed and homogeneous, the demographic, clinical and brain features were compared using the appropriate parametric test (e.g., Student`s t-tests). When the data were not homogeneous and not normally distributed the non-parametric Mann-Whitney test was used, such as in the case of 5% of the GMD measures, in Brodmann areas measured by CPM technique.
P values were computed for the maps at the surface level by setting a significance threshold of p<0.05. Maps of systematic differences and percent differences were visualized using color codes on 3D models of the cortex, where the individual BAs were marked. A permutation test was applied to all maps to provide an overall p value for the effect, corrected for multiple comparisons [40]. This test computes the chance of the observed pattern occurring by accident by running n=10,000 permutations of the variables of interest, at a threshold of p=0.01 [54].
Throughout this paper, reference is made to different kinds of p values. The p values reported in the figures refer to t-test comparisons between cases and controls. These p values are those related to the specific effect size and power of our experiment, and are expressed using a color code. We then provide additional p values denoting the survival of such results in the permutation test to correct for multiple comparisons. When this p value at permutation testing is significant, the result of the permutation test may be regarded as the ―corrected ‖p value, i.e. the chance of observing such an extreme pattern of effects, or still greater effects, by accident. In both cases, the threshold for significance was 0.05.
3. Results
As shown in Table 1, patients with BPD and HC were well matched for age and sex. The BPD group differed from the HC group in years of education and global cognition (p<0.0005). BPD patients generally showed a long history of illness, and a moderate/marked degree of psychopathology, as assessed with the BPRS (Table 2). Their HAM-D scores were compatible with a moderate/severe degree of depression. A moderate level of anxiety resulted from the STAI trait and state evaluations, and alexithymia was significantly higher in BPD than in HC group (BPD: 53±11, HC:44±13, p<0.05 at Mann-Whitney test). The BPD group showed a high level of impulsivity, whereas global functioning mean score was compatible with a severe impairment in this measure. All patients received psychotropic medication: half of the patients received antipsychotic treatments, mostly benzodiazepines and 17 patients used mood stabilizers (65%, valproic acid was used by all but one), and one third of patients received antidepressant treatment, mainly the selective serotonin reuptake inhibitors (N=8) [Table 2].
Table 1.
Demographic features of 26 patients with Borderline Personality Disorder and their age- and sex-matched healthy controls
| BPD N=26 | HC N= 26 | p | |
|---|---|---|---|
| Sex (f) | 16 (61%) | 16 (61%) | 1.00 |
| Age, years | 38±11 | 38±11 | 0.5 |
| Education, years | 10±2 | 14±3 | <0.0005 |
BPD=Borderline Personality disorder; HC=healthy controls. P denotes significance on chi-squared test for dichotomous variables or Mann-Whitney tests for continuous variables.
Table 2.
Clinical and psychological features of 26 patients with Borderline Personality Disorder.
| BPD N=26 | |
|---|---|
| Years of Illness | 14 ±11 |
| Alcohol abuse | 20 (77%) |
| Substance abuse | 21 (81%) |
| Brief Psychiatric Rating Scale | 45 ±8 |
| Mild (24-48) | 17 (65%) |
| Moderate (49-72) | 9 (35%) |
| Moderate to severe (> 72) | 0 |
| Personal and Social Performance scale | 34±9 |
| Mild (>60) | 1 (4%) |
| Moderate (41 -60) | 3 (12%) |
| severe (< 40) | 22 (84%) |
| Hamilton Depression Rating Scale | 24±9 |
| normal (0-7) | 3 (12%) |
| Mild (8-13) | 2 (8%) |
| Moderate (14-18) | 4 (15%) |
| Severe (19-22) | 4 (15%) |
| Very severe (> 23) | 13 (50%) |
| State and Trait Anxiety Inventory – state | 49±13 |
| High anxiety | 18 (70%) |
| - trait | 56±11 |
| High anxiety | 22 (84%) |
| Toronto Alexithymia Scale | 53 ±11 |
| Non alex (<51) | 8 (31%) |
| Borderline (52-60) | 12 (46%) |
| alex (< 61) | 6 (23%) |
| Barratt Impulsiveness Scale 1 | 71 ±7 |
| Low (<68) | 5 (31%)1 |
| Moderate (68-89) | 11 (69%)1 |
| High (>90 | 0 |
| Pharmacotherapy | |
| Typical Antipsychotics | 7 (27%) |
| Atypical Antipsychotics | 14 (54%) |
| Antidepressants | 12 (31%) |
| Benzodiazepines | 24 (93%) |
| Mood stabilizers | 17 (65%) |
Barratt Impulsiveness Scale-11 was administered to 16 patients with BPD.
Cortical morphometry
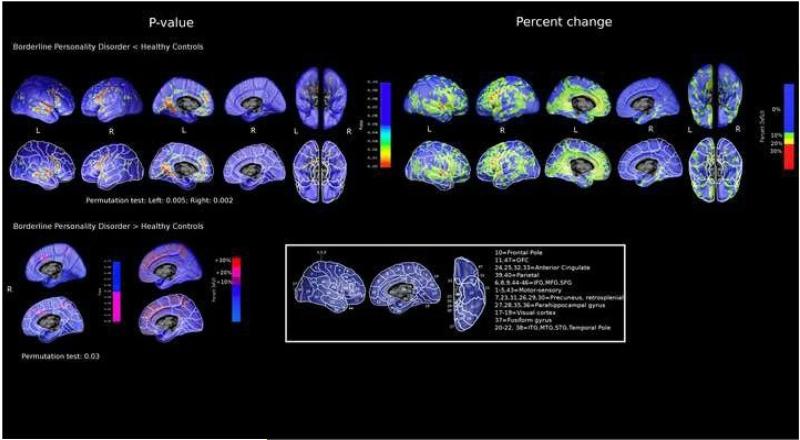
The BPD group showed widespread regions of cortical differences compared to the HC group. The most prominent feature we observed in patients was a lower GMD, with ‗peaks‘ of 20-30% lower tissue density in the bilateral superior temporal gyrus (STG) and in the inferior and middle frontal gyrus (IFG, MFG, Figure). In details, decreased GMD was detected bilaterally in the dorsal frontal cortex, in particular IFG, MFG, and superior frontal gyri (SFG) (Figure; average % GMD loss in the combined BAs 6, 8-10, 44-46: 6-7% to the left and right, p<0.005 on t-test; Table 3).
Figure.
Cortical morphology in 26 Borderline Personality Disorder (BPD) patients compared to 26 healthy controls. Pattern of percent gray matter local difference (right panel) and statistical significance (left panel) in BPD<HC and BPD>HC , with (lower row) and without (upper row) Brodmann areas' (BAs) boundaries highlighted
Table 3.
Average gray matter density (GMD), percentage difference between groups, and t-test p-values for individual Brodmann Areas (Bas), or functional/morphological groups of BAs, between the borderline personality disorder (BPD) and healthy control (HC) groups.
| BA | BPD N=26 | HC N=26 | % difference | p | ||
|---|---|---|---|---|---|---|
| Average GMD | 0.260±0,015 | 0.271±0,013 | −4% 1 | 0.005 | ||
| 11, 47 | Orbitofrontal Cortex | L | 0,248 ±0.018 | 0.264 ±0.021 | −6% 2 | 0.024 |
| R | 0.247±0.016 | 0,259±0,2074 | −5% 1 | 0.007 | ||
| 6,8-10, 44-46 | Dorsal frontal cortex | L | 0.252 ± 0,018 | 0,268 ± 0,129 | −6% 1 | 0.001 |
| R | 0.250 ± 0.017 | 0,264 ± 0.016 | −7% 1 | 0.004 | ||
| 24,25, 32,33 | Anterior cingulate cortex | L | 0.230 ± 0.025 | 0,250 ± 0.026 | −8% 1 | 0.007 |
| R | 0,213 ± 0.025 | 0.207 ± 0.024 | +3% 1 | 0.388 | ||
| 1-5, 43 | Sensorimotor cortex | L | 0.218 ± 0.016 | 0.226 ± 0.011 | −4% 1 | 0.062 |
| R | 0.220 ± 0.015 | 0,224 ± 0.014 | −2% 1 | 0.333 | ||
| 20-22, 38 | Temporal cortex | L | 0.271 ± 0.024 | 0.286 ± 0.017 | −5% 1 | 0.005 |
| R | 0.276 ± 0.023 | 0.286 ± 0.018 | −3% 1 | 0.107 | ||
| 27,28,35,36 | Medial temporal cortex | L | 0,369 ± 0.032 | 0,392 ± 0,028 | −6% 1 | 0.011 |
| R | 0,391 ± 0.026 | 0,400 ± 0.036 | −2% 1 | 0.086 | ||
| 37 | Fusiform gyrus | L | 0.299 ± 0.024 | 0.312 ± 0.019 | −4% 1 | 0.044 |
| R | 0.298 ± 0.020 | 0.308 ± 0.020 | −3% 1 | 0.081 | ||
| 39,40 | Parietal | L | 0.254 ± 0.024 | 0.259 ± 0.021 | −2% 1 | 0.453 |
| R | 0.262 ± 0.029 | 0,278 ± 0.026 | −6% 1 | 0.043 | ||
| 7, 23, 31, 26,29,30 | Precuneus, PC, Retrosplenial (+ some parietal) | L | 0,234 ± 0.025 | 0,253 ± 0.024 | −8% 1 | 0.009 |
| R | 0,227 ± 0.024 | 0,230 ± 0.024 | −1% 2 | 0.564 | ||
| 17-19 | Visual cortex | L | 0.262 ± 0.021 | 0.280 ± 0.016 | −7% 1 | 0.002 |
| R | 0.269 ± 0.022 | 0,269 ± 0.015 | 0% 1 | 0.564 |
Numbers denote means±S.D, % difference=mean difference in gray matter density in BPD compared to HC
p-values from t-test comparisons
from the non-parametric Mann-Whitney test .
See Figure for the topographic localization of each BA on the cortical 3D model.
BA=Brodmann Area
The anterior (ACC, BAs 24,25,32,33) and posterior (PCC, BAs 7,23,31,26,29,30) cingulate cortices showed lower density mainly in the left hemisphere (Figure; 8% average GMD loss; p=0.007 and p=0.009 for the ACC and PCC; Table 3). The parietal cortex (supramarginal and angular gyrus, BAs 39-40) was involved in the right (6% GMD loss in the combined BAs, p=0.04, Table 3).
The temporal lobe showed decreased density on the lateral (BAs 20-22, 38) and medial (BAs 27-28, 35-36) left cortex (5-6% GMD loss for the lateral and medial aspects, p<0.01; Table 3), including the parahippocampal gyrus. Finally, a large region of lower density mapped to the visual cortex (BAs 17-19; 7% GMD loss; Table 3) and the fusiform gyrus (BA 37; 4% GDM loss; p<0.05; Table 3).
Circumscribed regions of higher tissue density were detected in BPD compared to HC in the right hemisphere. These involved the superior frontal gyrus (BA 6, 8, 9), ACG (BA 32, 24; Figure) and the sensory-motor areas (BA 1-5, 43, Figure). These regions also survived the permutation test (P=0.03). The map of cortical gray matter differences was significant overall, after permutation testing (Left: 0.005; Right: 0.002), meaning that maps with a similar or greater proportion of significant voxels would only be achieved extremely rarely by random attribution of patients and controls to test groups.
4. Discussion
In this study we assessed the cortical mantle using cortical pattern matching in a group of BPD patients. Differences from controls consist mainly of GM density reductions, although we also observed regions with higher density in restricted areas. Abnormalities in the frontal (mainly prefrontal cortex) [53], cingulate [26] and right parietal cortex [30] are in line with previous structural MRI findings. Our results are also in line with the only two previous studies on cortical morphology [7,15]. In particular, de Araujo and colleagues using a different neuroimaging approach showed areas of alterations widely in frontolimbic regions and in parietal and posterior regions both in sense of a reduction and of enlargement compared with healthy controls [15].
Although differences in the analysis methods and study goals make it hard to compare findings across studies, the topographic involvement observed in our study is more extended than previously reported. This might be due to the older age of our patients, a longer duration of disease, or perhaps to the greater sensitivity of our mapping technique. It is known that BPD patients show some early alterations, such as those in the anterior cingulate cortex and in the OFC [13], while other features might develop later [13].
The abnormalities observed in our BPD sample involves cortical areas that subserve psychological functions that are compromised in BPD patients. In particular, frontolimbic areas are implicated in affective regulations and abnormalities in posterior/parietal areas have been linked to the presence of dissociative symptoms. Interestingly, most of the cerebral regions altered in our BPD patients are implicated in mentalizing/social cognition abilities that represent one of the main deficit in BPD patients. In particular, the medial prefrontal gyrus and the temporo-parietal junction and precuneus – whose GM density was significantly lower in BPD in agreement with a previus study [7] - are recognized as the neural basis of belief attribution [33, 1]. The GM reduction in these regions may explain the deficits in spontaneous and automatic belief attribution to others found in BPD patients [33]. Furthermore, patients with BPD showed lower GMD compared to HC in the ACG, fusiform gyrus, superior temporal sulcus and primary visual areas that contribute to process visual stimuli and to attribute motivation and social intension together with the amygdala that attribute the emotional salience to the stimuli[1, 2, 8]. Lastly, lower GMD in BPD was also found in areas identified as part of the mirror neuron system (inferior frontal gyrus/BA 44, superior temporal sulcus; premotor cortex), a network that seems to be especially relevant for the recognition of intentions [39].
In addition, consistently with previous findings in BPD, we found a lower GMD in the OFC [14]. The OFC is a key region involved in the modulation of emotional responses, and is connected with subcortical nuclei (i.e., the amygdale, hypothalamus and ventral striatum) that are strictly engaged in the emotional and motivational appraisal of social stimuli [5]. Typically, OFC is involved in emotion regulation and impulse controls. Functional studies have consistently reported BPD abnormalities in OFC responses while structural MRI results are more controversial with some showing reductions in OFC volumes in BPD [14, 53, 13, 11] while other studies did not find differences from healthy controls [45, 24].
Our data are generally consistent with the structural and functional anomalies typically reported for subjects with BPD. However, we also observed in BPD patients regions with greater cortical density. To date, regional volumetric enlargement in BPD subjects has been described only in subcortical regions such as the amygdala [63] and the putamen [9] although these results were contrasting. The fact that some regions (IFG, MFG, SFG, ACG, and motor-sensory areas) showed both an increase and a reduction of tissue density may be interpreted as a sign of a complex and abnormal developmental process, like in other psychiatric disorders [6, 43].
Our BPD subjects displayed a somewhat symmetric distribution of anomalies in the dorsal aspect of the cortical mantle, but a wider involvement of the left hemisphere in the mesial aspect in terms of lower density. These findings are in line with the ―emotional type ‖ hypothesis that assumes a different modulation of the right hemisphere for basic emotions and of the left hemisphere for more evolved social emotions [41]. This hypothesis is compatible with clinical and research observations describing BPD patients as able to recognize basic emotions, but unable to use them for adaptive aims. The emotional type hypothesis distinguishes between basic emotion, associated with innate displays and complex emotions, developed later during childhood and modulating facial emotional displays for social purposes [41]. In this model, the right hemisphere modulates schematic/primary emotions, whereas the left one modulates social emotions and associated display rules [41,20]. In line with this interpretation, in a PET study examining the neural substrates of ToM, the authors found that most of these activations were limited to the left medial hemisphere [12] supporting the idea that ToM funcitions mainly involved left hemisphere.
This study has both strengths and limitations. A major strength involves the spatial accuracy of our mapping technique leading to the comparisons, between subjects, of anatomical features over the whole cortical mantle. Commonly used methods such as manually tracing regions of interest, suffer the disadvantage of being spatially less detailed and typically strongly dependent on an a priori hypothesis. On the other hand, with the CPM technique is not possible to investigate the GMD of subcortical structures, and this makes it difficult to map some very deeply folded cortical regions, such as the insula, that might be relevant for this kind of disorder. A full characterization of the cortex in BPD requires further investigation with complementary techniques. Lastly, the presence in our sample of both females and males overcome the limitation the two previous two studies including only females. As underlined by the authors [15] the inclusion of only female patients prevents to extend the results to all BPD patients.
Some caveats should also be taken into account when interpreting our data. Our sample study is unselected, as a hospital-based cohort cannot be considered fully representative of the BPD population, and a replication is needed. Moreover, all patients were taking psychotropic medications. The impact of drugs on brain structures is a matter of debate, some psychotropic agents affecting neurons, such as fluoxetine [46] and atypical antipsychotics [10], while others, conversely, showing a ―positive ‖ (apparently trophic) effect of brain structure, such as lithium treatment [22] or atypical antipsychotics [10]. However, it is not clear whether the potential effect of drugs on brain morphology is stable over time or reversible after switching.
In neuroimaging studies, the potential confounding effect of medications is a major issue, and this is especially true when studying complex pathology such as BPD, where the majority of individuals may be receiving psychotropic medication. It might be possible to avoid the potential confounding effect of medication on neuroimaging measures by restricting the studies to medication-naive individuals. This strategy, however, would largely limit recruitment to a small group of participants who might not be representative of BPD populations typically managed in most clinical settings. Another approach, which may be possible in the future, is to attempt a meta-analysis of neuroimaging data coming from many cohorts, an approach which has recently been successfully applied to schizophrenia and bipolar disorder [28, 57, 58].
Another caveat regards the presence of substance and alcohol abuse in most BPD patients. There is a high degree of comorbidity ranging between 14% and 56%, with a median value of 52% [42] between BPD and alcohol and substance use disorders. Cross-sectional studies have found that 23–84% of BPD patients (median =65.1%) report meeting criteria for any substance use disorder [62]. Given the high prevalence of this comorbidity in our sample, it was impossible to stratify the group, so as to examine this possible confounding factor. A larger sample size could give us the opportunity to stratify for these variables and check their impact.
5. Conclusion
The present findings confirm cortical abnormalities in BPD patients. Interestingly, these abnormalities involves cortical areas that subserve psychological functions that are compromised in BPD patients. Besides the well supported frontolimbic and posterior/parietal alterations, linked with affective regulations and dissociative symptoms, respectively, most of the cerebral regions altered in our BPD patients are implicated in mentalizing/social cognition abilities that represent one of the main deficit in BPD patients. Neuroimaging studies offer invaluable tools to understand the biological substrate of these deficits.
Acknowledgements
The authors thanks Chiara Barattieri for revising in English and Ivan Villa for the MRI acquisitions.
Funding support. This work was financially supported by the Italian Ministry of Health, and AFaR (Associazione Fatebenefratelli per la Ricerca) — Rome, Italy. PT is also supported by NIH grants from the NIA, NIBIB, the National Library of Medicine, and the National Center for Research Resources (AG016570, EB01651, LM05639, RR019771).
References
- 1.Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Progress in Brain Research. 2006;156:363–78. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- 2.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 3.Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 4.Bearden CE, Thompson PM, Dutton RA, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–38. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–9. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 6.Boccardi M, Frisoni GB, Hare RD, Cavedo E, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Res. 2011;193:85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Bøen E, Westlye LT, Elvsåshagen T, et al. Regional cortical thinning may be a biological marker for borderline personality disorder. Acta Psychiatr Scand. 2013 Dec 6; doi: 10.1111/acps.12234. doi: 10.1111/acps.12234. [DOI] [PubMed] [Google Scholar]
- 8.Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, et al. Functional connectivity of the fusiform gyrus during a facematching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–24. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla P, Soloff PH, Sala M, Nicoletti MA, et al. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131:125–33. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Braus DF, Ende G, Weber-Fahr W, Demirakca T, Henn FA. Favorable effect on neuronal viability in the anterior cingulate gyrus due to long-term treatment with atypical antipsychotics: an MRSI study. Pharmacopsychiatry. 2001;34:251–3. doi: 10.1055/s-2001-18037. [DOI] [PubMed] [Google Scholar]
- 11.Brunner R, Henze R, Parzer P, Kramer J, et al. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: is it disorder specific? Neuroimage. 2010;49:114–20. doi: 10.1016/j.neuroimage.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 12.Calarge C1, Andreasen NC, O'Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160:1954–64. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- 13.Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, et al. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 2008;163:116–25. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.de Araujo Filho GM, Abdallah C, Sato JR, et al. Morphometric hemispheric asymmetry of orbitofrontal cortex in women with borderline personality disorder: A multi-parameter approach. Psychiatry Res. 2014;223:61–6. doi: 10.1016/j.pscychresns.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Araujo TB, de Araujo Filho GM, Sato JR, de Araújo CM, et al. Cortical morphology changes in women with borderline personality disorder: a multimodal approach. Rev Bras Psiquiatr. 2014;36:32–8. doi: 10.1590/1516-4446-2013-1120. [DOI] [PubMed] [Google Scholar]
- 16.de Girolamo G, Picardi A, Micciolo R, Falloon I, et al. Residential care in Italy. National survey of non-hospital facilities. Br J Psychiat. 2002;181:220–5. doi: 10.1192/bjp.181.3.220. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon JBW, Williams M, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) New York State Psychiatric Institute, Biometric Research Department; New York: 1994. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometric Research Department; New York: 1994. [Google Scholar]
- 19.Frisoni GB, Prestia A, Adorni A, et al. In vivo neuropathology of cortical changes in elderly persons with schizophrenia. Biol Psychiatry. 2009;66:578–85. doi: 10.1016/j.biopsych.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Gainotti G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia. 2012;50:205–18. doi: 10.1016/j.neuropsychologia.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Galluzzi S, Testa C, Boccardi M, Bresciani L, et al. The Italian Brain Normative Archive of structural MR scans: norms for medial temporal atrophy and white matter lesions. Aging Clin Exp Res. 2009;21:266–76. doi: 10.1007/BF03324915. [DOI] [PubMed] [Google Scholar]
- 22.Germanà C, Kempton MJ, Sarnicola A, et al. The effects of lithium and anticonvulsants on brain structure in bipolar disorder. Acta Psychiat Scand. 2010;122:481–7. doi: 10.1111/j.1600-0447.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 23.Gigantesco A, Vittorielli M, Pioli R, Falloon IR, Rossi G, Morosini P. The VADO approach in psychiatric rehabilitation: a randomized controlled trial. Psychiat Ser. 2006;57:1778–83. doi: 10.1176/ps.2006.57.12.1778. [DOI] [PubMed] [Google Scholar]
- 24.Goodman M, Hazlett EA, Avedon JB, Siever DR, et al. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J Psychiatr Res. 2011;45:803–7. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazlett EA, New AS, Newmark R, Haznedar MM, et al. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol Psychiatry. 2005;58:614–23. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 28.Hibar DP, van Erp TGM, Rasmussen J, et al. the ENIGMA-Bipolar Disorder Working Group . Meta-analysis of structural brain differences in bipolar disorder: the ENIGMA-Bipolar Disorder Project. OHBM; Seattle, WA: Jun, 2013. [Google Scholar]
- 29.Hill J, Pilkonis P, Morse J, Feske U, et al. Social domain dysfunction and disorganization in borderline personality disorder. Psychol Med. 2008;38:135–46. doi: 10.1017/S0033291707001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005;57:173–82. doi: 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Lenzenweger MF. Current status of the scientific study of the personality disorders: an overview of epidemiological, longitudinal, experimental psychopathology, and neurobehavioral perspectives. J Am Psychoanal Assoc. 2010;58:741–78. doi: 10.1177/0003065110386111. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald D, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance imaging. Proceedings of SPIE. 1994;2359:160–9. [Google Scholar]
- 33.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–8. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 34.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic structural changes in borderline personality disorder. J Psychiatr Res. 2008;42:727–733. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Putnam KM, Silk KR. Emotion dysregulation and the development of borderline personality disorder. Dev and Psychopathol. 2005;17:899–925. doi: 10.1017/s0954579405050431. [DOI] [PubMed] [Google Scholar]
- 37.Rasser PE, Johnston P, Lagopoulos J, Ward PB, et al. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. Neuroimage. 2005;26:941–51. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 38.Riello R, Sabattoli F, Beltramello A, Bonetti M, et al. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin Exp Res. 2005;17:329–36. doi: 10.1007/BF03324618. [DOI] [PubMed] [Google Scholar]
- 39.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev of Neuroscie. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 40.Roncone R, Ventura J, Impallomeni M, Falloon IR, et al. Reliability of an Italian standardized and expanded Brief Psychiatric Rating Scale (BPRS 4.0) in raters with high vs. low clinical experience. Acta Psychiat Scand. 1999;100:229–36. doi: 10.1111/j.1600-0447.1999.tb10850.x. [DOI] [PubMed] [Google Scholar]
- 41.Ross ED, Homan RW, Buck R. Differential hemispheric lateralization of primary and social emotions. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:1–19. [Google Scholar]
- 42.Rossi R, Ge Girolamo G. Epidemiologia del disturbo borderline di personalità e delle comorbilità in Asse I. In: Sanza M, Asioli F, Ferrannini L, editors. Disturbo Borderline di Personalità: continuità e discontinuità nel trattamento. Centro Scientifico Editore; Milan: 2010. [Google Scholar]
- 43.Rossi R, Lanfredi M, Pievani M, Boccardi M, Beneduce R, Rillosi L, et al. Volumetric and topographic differences in hippocampal subdivisions in borderline personality and bipolar disorders. Psychiatry Res. 2012;203:132–138. doi: 10.1016/j.pscychresns.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Rossi R, Pievani M, Lorenzi M, Boccardi M, et al. Structural brain features of borderline personality and bipolar disorders. Psychiatry Res. 2013;213:83–91. doi: 10.1016/j.pscychresns.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Rüsch N, van Elst LT, Ludaescher P, Wilke M, et al. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20:385–92. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- 46.Santarelli L, Saxe M, Gross C, Surget A, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 47.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 48.Sharp C, Fonagy P. Social cognition and attachment-related disorders. In: Sharp C, Fonagy P, Goodyer I, editors. Social Cognition and Developmental Psychopathology. Oxford University Press; Oxford: 2008. pp. 269–302. [Google Scholar]
- 49.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–76. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 50.Skodol AE, Gunderson JG, Shea MT, McGlashan TH, et al. The Collaborative Longitudinal Personality Disorders Study (CLPS): overview and implications. J Pers Disord. 2005;19:487–504. doi: 10.1521/pedi.2005.19.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sowell ER, Thompson PM, Rex D, Kornsand D, et al. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists Press, Inc.; Palo Alto: 1983. [Google Scholar]
- 53.Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 54.Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 56.Thompson PM, Woods RP, Mega MS, Toga AW. Mathematical/computational challenges in creating deformable and probabilistic atlases of the human brain. Hum Brain Map. 2000;9:81–92. doi: 10.1002/(SICI)1097-0193(200002)9:2<81::AID-HBM3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner JA, Hibar DP, Rasmussen J, et al. the ENIGMA-Schizophrenia Working Group . A Prospective Meta-Analysis of Subcortical Brain Volumes in Schizophrenia via the ENIGMA Consortium. OHBM; Seattle, WA: Jun, 2013. [Google Scholar]
- 58.van Erp TGM, Hibar DP, Rasmussen J, et al. the ENIGMA-Schizophrenia Working Group A Large-Scale Meta-Analysis of Subcortical Brain Volume Abnormalities in Schizophrenia via the ENIGMA Consortium, Society for Biological Psychiatry (SOBP) 2013 [Google Scholar]
- 59.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE T Med Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 60.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based bias field correction of MR images of the brain. IEEE T Med Imaging. 1999;18:885–896. doi: 10.1109/42.811268. [DOI] [PubMed] [Google Scholar]
- 61.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34:383–388. [PMC free article] [PubMed] [Google Scholar]
- 62.Whittle S, Yap MB, Yucel M, Sheeber L, et al. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc Cogni Affect Neurosci. 2009;4:247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanarini MC, Frankenbur FR, Weingeroff JL, Reich DB, et al. The course of substance use disorders in patients with borderline personality disorder and Axis II comparison subjects: a 10-year follow-up study. Addiction. 2011;106:342–8. doi: 10.1111/j.1360-0443.2010.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zetzsche T, Frodl T, Preuss UW, Schmitt G, et al. Amygdala volume and depressive symptomsin patients with borderline personality disorder. Biol Psychiat. 2006;60:302–310. doi: 10.1016/j.biopsych.2005.11.020. [DOI] [PubMed] [Google Scholar]