Abstract
Objective To determine whether the immunogenicity of a single dose infant priming schedule of serogroup C meningococcal (MenC) conjugate vaccine is non-inferior to a two dose priming schedule when followed by a booster dose at age 12 months.
Design Phase IV open label randomised controlled trial carried out from July 2010 until August 2013
Setting Four centres in the United Kingdom and one centre in Malta.
Participants Healthy infants aged 6-12 weeks followed up until age 24 months.
Interventions In the priming phase of the trial 509 infants were randomised in a 10:10:7:4 ratio into four groups to receive either a single MenC-cross reacting material 197 (CRM) dose at 3 months; two doses of MenC-CRM at 3 and 4 months; a single MenC-polysaccharide-tetanus toxoid (TT) dose at 3 months; or no MenC doses, respectively. Haemophilus influenzae type b (Hib)-MenC-TT vaccine was administered to all infants at 12 months of age. All infants also received the nationally routinely recommended vaccines. Blood samples were taken at age 5, 12, 13, and 24 months.
Main outcome measure MenC serum bactericidal antibody assay with rabbit complement (rSBA) one month after the Hib-MenC-TT vaccine. Non-inferiority was met if the lower 95% confidence limit of the difference in the mean log10 MenC rSBA between the single dose MenC-CRM and the two dose MenC-CRM groups was >−0.35.
Results The primary objective was met: after a Hib-MenC-TT booster dose at 12 months of age the MenC rSBA geometric mean titres induced in infants primed with a single MenC-CRM dose were not inferior to those induced in participants primed with two MenC-CRM doses in infancy (660 (95% confidence interval 498 to 876) v 295 (220 to 398)) with a corresponding difference in the mean log10 MenC rSBA of 0.35 (0.17 to 0.53) that showed superiority of the single over the two dose schedule). Exploration of differences between the priming schedules showed that one month after Hib-MenC-TT vaccination, MenC rSBA ≥1:8 was observed in >96% of participants previously primed with any of the MenC vaccine schedules in infancy and in 83% of those who were not vaccinated against MenC in infancy. The MenC rSBA geometric mean titres induced by the Hib-MenC-TT boost were significantly higher in children who were primed with one rather than two MenC-CRM doses in infancy. Only priming with MenC-TT, however, induced robust MenC bactericidal antibody after the Hib-MenC-TT booster that persisted until 24 months of age.
Conclusions MenC vaccination programmes with two MenC infant priming doses could be reduced to a single priming dose without reducing post-boost antibody titres. When followed by a Hib-MenC-TT booster dose, infant priming with a single MenC-TT vaccine dose induces a more robust antibody response than one or two infant doses of MenC-CRM. Bactericidal antibody induced by a single Hib-MenC-TT conjugate vaccine dose at 12 months of age (that is, a toddler only schedule), without infant priming, is not well sustained at 24 months. Because of rapid waning of MenC antibody, programmes using toddler only schedules will still need to rely on herd protection to protect infants and young children.
Trial registration Eudract No: 2009-016579-31; NCT01129518; study ID: 2008_06 (http://clinicaltrials.gov).
Introduction
Control of invasive meningococcal C (MenC) disease has been achieved in the United Kingdom, the Netherlands, Canada, and Australia, where MenC conjugate vaccines have been introduced in the routine national childhood immunisation programmes.1 Three licensed meningococcal conjugate vaccines are in use: two MenC polysaccharide protein conjugate preparations utilising the cross reacting material 197 (MenC-CRM), a non-toxic mutant of diphtheria toxoid (Menjugate, Novartis Vaccines and Diagnostics, Siena, Italy; and Meningitec, currently marketed by Nuron Biotech, Schaffhausen, Switzerland), and one MenC polysaccharide-tetanus toxoid (MenC-TT) formulation (NeisVac-C; currently marketed by Pfizer, New York). The impact of MenC vaccination was evident within a few years, despite differences between countries in MenC vaccination schedules, which include whether or not an infant “priming” dose is used, the number of these priming doses, and whether to accompany introduction with a mass catch up MenC immunisation campaign for older age groups.2
Starting in 1999 the UK was the first country to launch routine MenC conjugate vaccination for all infants concurrently with staggered catch-up vaccination of individuals aged 1-25 years up until 2002.3 Since then the MenC schedule has been changed twice. In 2006, the original three dose MenC infant priming schedule administered at 2, 3, and 4 months of age was reduced to a 3 and 4 month schedule with the addition of a Hib-MenC-TT boost at 12 months of age.3 This decision was based on results of clinical trials that showed that the serological threshold of MenC serum bactericidal antibody assay with rabbit complement (rSBA) of ≥1:8, accepted to be protective against invasive meningococcal disease,4 was observed in >98% of vaccinated children after two infant MenC priming doses5 6 and on estimates of rapid waning of MenC vaccine effectiveness after infant vaccination.7 Subsequently in 2013, MenC infant priming was further reduced to a single dose at 3 months, with retention of the 12 month old booster and the introduction of another booster dose at age 13-14 years to sustain MenC immunity through adolescence.8
The rationale for a single MenC conjugate vaccine dose in infancy is based on limited data from studies that looked at the immunogenicity after the first9 10 or a single priming MenC vaccine dose at 2 months of age,11 or the effect a single priming dose of different MenC glycoconjugate formulations had on the immunogenicity of a Hib-MenC-TT booster dose administered to children aged 12 months.12 No randomised controlled studies have directly compared the effect on the immunogenicity of a Hib-MenC-TT booster after different reduced MenC infant immunisation schedules. We investigated such differences and assessed the corresponding persistence of MenC bactericidal antibody at 24 months of age.
Methods
Participants and recruitment
We enrolled 509 healthy infants, born at 37-42 weeks’ gestation and aged between 6-12 weeks, in a phase IV open labelled randomised controlled trial carried out between 5 July 2010 and 1 August 2013 in four centres in the UK (Oxford, Bristol, London, and Southampton) and one centre in Malta. An invitation letter was sent to the parents of all children due for their routine immunisations, and parents who expressed an interest for their child to participate in the study were called to ensure eligibility. Eligible infants were then asked to visit Mater Dei Hospital in Malta or were seen in their homes in the UK. The study was divided into three phases: the primary vaccination phase (from 2-5 months of age); the booster phase (from 12-13 months of age), and the persistence phase (at 24 months of age).
Exclusion criteria included known immunosuppression, a family history of immunodeficiency, administration of blood products, previous vaccination (except with the BCG, hepatitis B, and rotavirus vaccines for the primary phase, and with the combined diphtheria, tetanus, acellular pertussis, inactivated polio, and Haemophilus influenzae type b (DTaP-IPV-Hib), and the 13 valent pneumococcal conjugate vaccines used in the primary phase as well as the hepatitis A, influenza, and varicella-zoster vaccines for the booster phase), previous infection with MenC, allergic reactions to any vaccine components, a history of seizures or any neurological disorder, and severe acute/chronic illness at the time of enrolment.
Visits and vaccines
Participants were randomised in a 10:10:7:4 ratio into four different groups: a single infant dose MenC-CRM group, a two infant dose MenC-CRM group, a single infant dose MenC-TT group, and a control group (no infant MenC vaccine), respectively (table 1). Infants in the MenC-CRM groups were immunised with a MenC-CRM conjugate vaccine (Menjugate) at age 3 months or at 3 and 4 months. Those in the single infant dose MenC-TT group received one dose of the MenC-TT vaccine (NeisVac-C) at 3 months of age, while infants in the control group did not receive any MenC vaccine priming doses. All infants were vaccinated with a combined DTaP-IPV-Hib vaccine (Pediacel, Sanofi Pasteur MSD, Lyon, France) at 2, 3, and 4 months of age and with the 13 valent pneumococcal conjugate vaccine (PCV13, Prevenar 13, Pfizer, New York) at 2 and 4 months of age. In the booster phase, infants in all groups were vaccinated with the Hib-MenC-TT vaccine (Menitorix, GlaxoSmithKline Biologicals, Rixensart, Belgium) as well as the routine PCV13 vaccine at 12 months of age. All infants received the combined measles, mumps, and rubella (MMR) vaccine at 13 months. Blood samples were obtained for serologic assays at 5, 12, 13, and 24 months of age (table 1).
Table 1.
Timelines of vaccination and blood sampling with different infant meningococcal C (MenC) vaccination schedules
| Study group | Randomisation ratio | Phase | Persistence at 24 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary | Booster | ||||||||
| 2 | 3 | 4 | 5 | 12 | 13 | ||||
| Single infant dose MenC-CRM group | 10 | — | MenC-CRM | — | — | Hib-MenC-TT | — | — | |
| Two infant dose MenC-CRM group | 10 | — | MenC-CRM | MenC-CRM | — | — | — | ||
| Control group | 4 | — | — | — | — | — | — | ||
| Single infant dose MenC-TT group | 7 | — | MenC-TT | — | — | — | — | ||
| Routine vaccines all groups | — | DTaP-IPV-Hib, PCV13 | DTaP-IPV-Hib | DTaP-IPV-Hib, PCV13 | — | PCV13 | MMR | — | |
| Blood sampling | — | — | — | — | ✓ | ✓ | ✓ | ✓ | |
MenC-CRM (Menjugate); MenC-TT (NeisVac-C); Hib-MenC-TT (Menitorix); DTaP-IPV-Hib (Pediacel); PCV13 (Prevenar 13); CRM=cross reacting material 197; TT=tetanus toxoid; Hib=Haemophilus influenzae type b.
Participants in each study site were randomised according to a computer generated list produced by the Oxford Vaccine Group in Oxford. Stata version 10.0 was used to generate the randomisation codes with permuted block size of 30 and stratification by centre. Allocation concealment until the point of enrolment was achieved through the use of opaque sealed envelopes. Study staff and parents of participants were not masked to group allocation after enrolment.
Serologic assays
Meningococcal serogroup C antibody was measured as described by Maslanka and colleagues13 using a MenC rSBA targeting the Neisseria meningitidis C11 (C:16:P1.7-1,1) strain and baby rabbit serum as the complement source (Pel-Freeze Incorporated, Rodgerson, AZ). MenC rSBA titres were expressed as the reciprocal of the final serum dilution giving ≥50% killing at 60 minutes. An rSBA threshold of ≥1:8 was taken as indicative of protection.4 A threshold of ≥1:128 was also included as a more conservative protective threshold.14 MenC rSBA assays were carried out at the Vaccine Evaluation Unit, Public Health England, Manchester Laboratory, Manchester Royal Infirmary, Manchester, UK.
Safety evaluation
After each immunisation, vaccinated infants were observed for 15 minutes for any immediate reactions. Parents completed a diary card for five days from the day of immunisation and recorded all local and systemic adverse events. Any solicited or unsolicited adverse events as well as serious adverse events occurring from the day of vaccination to the subsequent visit were noted.
Statistical analysis
Our primary objective was to show non-inferiority of the MenC geometric mean titres one month after the 12 month dose of the Hib-MenC-TT vaccine between the single infant dose MenC-CRM and the two infant dose MenC-CRM groups. The geometric mean titre expresses the mean (calculated on log transformed data) back in the original units and gives a meaningful expression of central tendency of the antibody response in a study population. Geometric mean titres (and their 95% confidence intervals) were calculated by taking the antilog of the mean (and 95% confidence interval) log10 transformed MenC rSBA titres. Titres <4 (the lower limit of detection of the assay) were given an arbitrary value of 2 to be able to log10 transform these values to conduct the analysis. Non-inferiority was met if the lower 95% confidence limit of the difference in the mean log10 MenC rSBA between the single infant dose MenC-CRM group minus the two infant dose MenC group was >−0.35 (equivalent to a non-inferiority margin of >−10%) at one month after Hib-MenC-TT vaccination. This margin was derived from published data measured one month after a MenAC polysaccharide vaccine challenge was administered to infants aged 12 months who had been primed with MenC-TT at 2 and 4 months of age.11 Based on this margin of −0.35, with at least 160 participants enrolled in each of the single infant dose MenC-CRM and two infant dose MenC-CRM groups, the power to show the primary objective at 2.5% one sided level of significance was 90% (allowing for a 12.5% dropout rate). Sample size calculations for the additional two arms of the study resulted in an unusual 10:10:7:4 allocation ratio. Details of all calculations are included in appendix table A. The study design was not intended to compare reactogenicity rates between the different schedules and so no sample size calculation was carried out for these secondary outcomes.
The analysis of the outcome variables was based on the intention to treat population. We also performed an analysis on the completers population to complement the intention to treat population analysis. Participants were included in the intention to treat population if they had at least one dose and at least one assessment after baseline and in the completers population if they received all vaccine doses and had all planned assessments. Models used in the analyses contained the terms dose group (four levels) and study centre (five levels).
We performed analysis of variance (ANOVA) of the log10 transformed rSBA titres at each blood sampling visit and present results as geometric mean titres with 95% confidence intervals. Binary variables were analysed with logistic regression with results of a comparison between two levels of a factor reported as odds ratios (95% confidence interval). P<0.05 was considered significant. We used STATA 13 and StatXact 9 for the immunogenicity analyses and SAS v9.3 for the safety analyses.
Results
Out of the 509 infants enrolled in the study, 497 completed the primary immunisation phase, 478 the booster phase, and 453 the persistence phase (fig 1).
Fig 1 MenC vaccination schedules and flow of infants in intention to treat population
Demography
The mean age of the participants at enrolment (n=509) was 8.5 weeks (range 6.9-10.6), 51.7% (263) were boys, and 90.2% were white. At the booster and persistence phases the mean age of the infants was 12.5 months (11.9-13.6) and 24.1 months (22.1-27.3), respectively.
Immunogenicity
Although we planned to adjust for study centre, adjustment made no difference to the primary analysis or to any of the secondary analyses. All results presented are unadjusted.
Primary objective
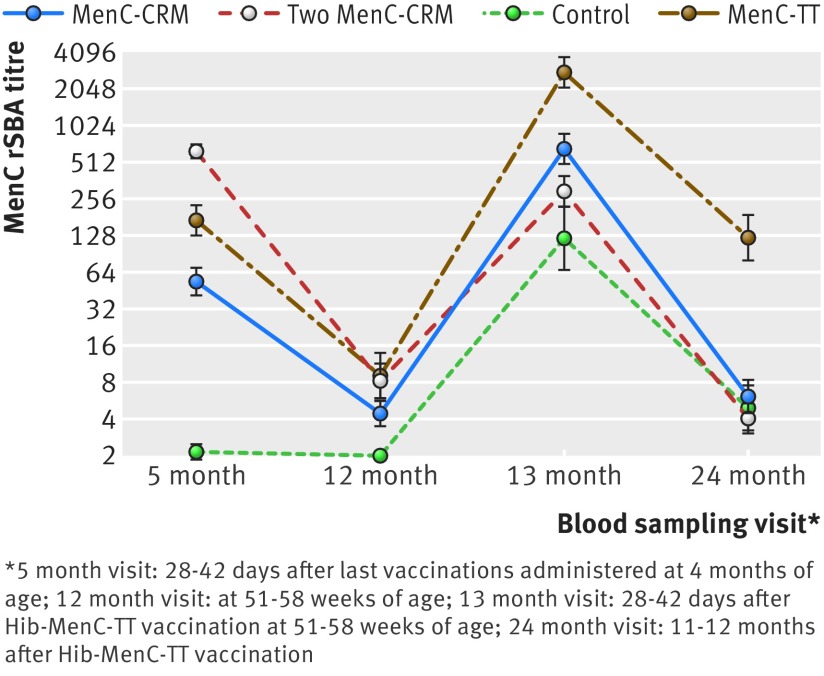
Our primary objective was met as the immunogenicity of one priming dose of MenC-CRM at 3 months was non-inferior (and in fact was superior) to two MenC-CRM doses given at 3 and 4 months of age, when assessed at 13 months of age after the Hib-MenC-TT booster. One month after administration of the Hib-MenC-TT vaccine at 12 months of age, participants in the single infant dose and two infant dose MenC-CRM groups had MenC rSBA geometric mean titres of 660 (95% confidence interval 498 to 876) and 295 (220 to 398), respectively (fig 2 and appendix table B). These corresponded to a difference of 0.35 (0.17 to 0.53) in the mean log10 MenC rSBA between the single infant dose and two infant dose MenC-CRM groups. Therefore, as the lower 95% confidence limit was >−0.35 (that is, greater than the equivalent non-inferiority margin of −10%), the primary objective of the study was met. The immunogenicity of the Hib-MenC-TT vaccine after the single infant dose MenC-CRM schedule was actually superior to the immunogenicity of the two dose MenC-CRM infant priming regimen as shown by the 95% confidence intervals of the difference, which did not cross 0.
Fig 2 Geometric mean titres (and 95% confidence interval) for meningococcal C (MenC) rabbit serum bactericidal antibody (rSBA) at visits performed at age 5, 12, 13, and 24 months according to type of MenC priming schedule (intention to treat population)
Primary immunisation phase
At 5 months of age two MenC-CRM doses resulted in a significantly higher proportion of infants with MenC rSBA ≥1:8 (100% v 84%, P<0.001) and ≥1:128 (99.3% v 48.6%; P<0.001) (table 2) as well as higher MenC rSBA geometric mean titres (620.5 v 53.6; P<0.001) compared with a single MenC-CRM dose (fig 2 and appendix table B). Similarly, the percentage of vaccinated infants with MenC rSBA above the thresholds of protection after a two dose MenC-CRM priming schedule was higher compared with one priming dose of MenC-TT (100% v 93.9% (P=0.004) with MenC rSBA ≥1:8 and 99.3% v 79.8% (P<0.001) with MenC rSBA ≥1:128, respectively); an observation that was also reflected in higher MenC rSBA geometric mean titres (620.5 v 169.4; P<0.001). A comparison of the immunogenicity of single doses of the different MenC glycoconjugates showed that priming with one dose of the MenC-TT vaccine resulted in a higher percentage of infants with MenC rSBA above the predefined cut off for protection and induced higher MenC rSBA geometric mean titres than a single MenC-CRM dose (93.9% v 84.0% (P=0.018) with MenC rSBA≥1:8 and MenC rSBA geometric mean titres of 169.4 v 53.6 (P<0.001), respectively).
Table 2.
Percentage of participants with meningococcal C (MenC) rabbit serum bactericidal antibody (rSBA) ≥1:8 and ≥1:128 thresholds of protection (ITT population) after vaccination with MenC-cross reacting material 197 (MenC-CRM) or MenC-tetanus toxoid (MenC-TT) conjugate formulations
| Study group | MenC rSBA ≥1:8 (95% CI)* by age (months) | MenC rSBA ≥1:128 (95% CI)* by age (months) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 12 | 13 | 24 | 5 | 12 | 13 | 24 | ||
| Single infant dose MenC-CRM group | 84 (77 to 90) | 26 (19 to 34) | 97 (93 to 99) | 31 (23 to 39) | 49 (40 to 57) | 7 (3 to 12) | 92 (86 to 96) | 11 (6 to 18) | |
| Two infant dose MenC-CRM group | 100 (97 to 100) | 41 (33 to 50) | 97 (92 to 99) | 20 (13 to 28) | 99 (96 to 100) | 13 (7 to 20) | 81 (73 to 87) | 6 (2 to 11) | |
| Control group | 2 (0 to 10)† | 0 | 83 (71 to 92) | 27 (16 to 41) | 2 (0 to 10)† | 0 | 69 (54 to 80) | 11 (4 to 22) | |
| Single infant dose MenC-TT group | 94 (87 to 98) | 40 (30 to 50) | 100 (96 to 100) | 82 (73 to 89) | 80 (71 to 87) | 23 (15 to 32) | 99 (94 to 100) | 69 (59 to 79) | |
*Presented numbers have been rounded up; accurate numbers are presented within text.
†One participant in control group was found to have MenC rSBA titre of 1:128 at 5 month blood sampling visit that was then undetectable at 12 month visit. This was likely from transplacentally acquired maternal antibody.
Booster phase
Before Hib-MenC-TT vaccination at 12 months of age, waning of the immune response after the different MenC vaccine priming schedules was evident, with only 26-41% of vaccinated infants still having MenC rSBA ≥1:8. MenC rSBA geometric mean titres decreased 77-fold, 12.8-fold, and 18.2-fold after infant vaccination with two MenC-CRM doses, a single MenC-CRM dose, or a single MenC-TT dose, respectively (fig 2 and appendix table B). Although the two infant dose MenC-CRM priming schedule resulted in a significantly higher percentage of infants with MenC rSBA ≥1:8 (but not with MenC rSBA ≥1:128) compared with a single infant dose MenC-CRM schedule, we saw no significant differences compared with the single infant dose MenC-TT schedule. For recipients of the MenC-TT vaccine in infancy, the proportion with MenC rSBA above the threshold for protection as well as the MenC rSBA geometric mean titres were higher than a single MenC-CRM infant priming dose (39.8% v 25.9% (P=0.023) with MenC rSBA ≥1:8, and MenC rSBA geometric mean titres of 9.3 v 4.4, P<0.002).
One month after Hib-MenC-TT vaccination at 12 months of age there was no difference in the percentage of infants with MenC rSBA ≥1:8 after a single MenC-CRM or MenC-TT infant priming dose compared with two MenC-CRM infant priming doses (97.1% and 100% v 92.1%, respectively), although all three schedules resulted in significantly higher rates of seroprotection against MenC compared with those who were not primed in infancy (table 2). The percentage of vaccinated infants with MenC rSBA ≥1:128, however, was significantly higher after single dose MenC-CRM/TT infant priming compared with two MenC-CRM infant doses or no priming at all (91.9% and 98.8% v 81.0% and 68.5%, respectively), which was also reflected in significantly higher MenC rSBA geometric mean titres between these groups (fig 2 and appendix table B). Although after the Hib-MenC-TT boost we saw no significant differences in the percentage of infants with MenC rSBA ≥1:8 between a single infant priming dose of MenC-CRM and MenC-TT, the percentage of infants with MenC rSBA ≥1:128 (table 2) as well as the MenC rSBA geometric mean titres were significantly higher in those who had been primed with MenC-TT (fig 2).
Persistence phase
Twelve months after Hib-MenC-TT vaccination, only 19.7%, 30.9%, and 27.3% of those who had been primed with two MenC-CRM doses, one MenC-CRM dose, or who had not been primed at all in infancy, respectively, still had MenC rSBA ≥1:8. We found no significant differences in the percentage of infants with MenC rSBA≥1:8 or in MenC rSBA geometric mean titres between the single or two infant dose MenC-CRM groups compared with the control group, though significantly more participants who were primed with one MenC-CRM dose in infancy had MenC rSBA ≥1:8 compared with those who received two MenC-CRM infant priming doses (table 2), a finding that was not reflected in significant differences between the MenC rSBA geometric mean titres persisting in the participants within the MenC-CRM primed groups (fig 2 and appendix table B). In contrast, 82.1% of children who were primed with one MenC-TT dose in infancy had MenC rSBA ≥1:8 and 69.5% had MenC rSBA ≥1:128; values that were significantly higher than those persisting after all other schedules (table 2). In addition, although the MenC rSBA geometric mean titres persisting after a single dose MenC-TT priming and Hib-MenC-TT boosting schedule had declined over 12 months, they were still significantly much higher than after all the other schedules (fig 2 and appendix table B).
Safety
Primary vaccination phase
There were no significant differences in the frequency of local adverse events at 3 months of age between any of the MenC priming schedules (all 95% confidence intervals for the odds ratios for group comparisons included 1.0), with local pain, erythema, swelling, and induration being reported in each of the MenC groups, excluding the control group, in up to 22%, 45%, 16%, and 25% of infants, respectively (appendix table C). Similarly we found no significant differences when we compared the frequency of systemic adverse events, with the most frequently reported symptoms being increased sleepiness in up to 50% and irritability in up to 66% of infants in each group (appendix table C). Fever ≥38°C was reported in ≤1% in each group. One participant in the two infant dose MenC-CRM group was admitted to hospital because of a vaccine related serious adverse event consisting of a haematoma at the site of the first MenC vaccine. This infant was subsequently diagnosed with factor VIII deficiency and made a complete recovery but was withdrawn from the study.
Booster phase
The priming regimen before Hib-MenC-TT vaccine administration at 12 months of age did not result in any observable significant differences in the frequency of local adverse events between any of the groups (all 95% confidence intervals for the odds ratios for group comparisons included 1.0), with up to 29% having pain, 76% erythema, 23% swelling, and 31% experiencing induration at the Hib-MenC-TT injection site in each group. Similarly, differences in the expected systemic adverse events between the groups were not significant, with drowsiness, irritability, and diminished appetite being the most commonly reported side effects in up to 39%, 61%, and 35%, respectively, in each group (appendix table C). Fever was the least frequent systemic adverse event, occurring in 10% of participants in each group. No vaccine related serious adverse events occurred in the booster phase.
Discussion
The number of MenC doses used in the current vaccination schedules in different countries within Europe, as well as internationally, varies from none to one or two infant priming doses followed by another dose in the second year of life, with or without a further boost in adolescence (table 3).15 16 17 18 19 This randomised controlled study provides data directly supporting a reduction in the routine MenC immunisation schedule from two infant doses to a single infant dose prime plus booster: a change that has been implemented in the UK since June 2013.8
Table 3.
Current international infant meningococcal C (MenC) vaccination schedules
| Country and region | No of doses (before age <2 years) | Recommended age of vaccination | Adolescent vaccination | |
|---|---|---|---|---|
| <12 months | 12-24 months | |||
| America (North) | ||||
| Canada15: | ||||
| Manitoba | 1 | — | 12 months | Yes |
| New Brunswick | 1 | — | 12 months | Yes* |
| Newfoundland and Labrador | 1 | — | 12 months | Yes* |
| Quebec | 1 | — | 12 months | Yes |
| Nova Scotia | 1 | — | 12 months | Yes |
| Nunavut | 1 | — | 12 months | Yes* (if no previous dose) |
| Ontario | 1 | — | 12 months | Yes* |
| Prince Edward Island | 1 | — | 12 months | Yes* |
| Saskatchewan | 1 | — | 12 months | Yes* |
| British Columbia | 2 | 2 months | 12 months | Yes |
| Northwest Territories | 2 | 2 months | 12 months | Yes† |
| Yukon | 2 | 2 months | 12 months | Yes |
| Alberta | 3 | 2, 4 months | 12 months | Yes* |
| USA16 | Nil‡ | — | — | Yes* |
| America (South) | ||||
| Brazil17 | 3 | 3, 5 months | 15 months | No |
| Australia18 | ||||
| Whole country | 1 | — | 12 months* | No |
| Europe19 | ||||
| Austria | 1 | — | 12-14 months | Yes* |
| Belgium | 1 | — | 15 months | No |
| Cyprus | 1 | — | 12-13 months | No |
| France | 1 | — | 12-23 months | No |
| Germany | 1 | — | 11-23 months | No |
| Italy | 1 | — | 13-15 months | No |
| Netherlands | 1 | — | 14 months | No |
| Portugal | 1 | — | 12 months | No |
| Iceland | 2 | 6, 8 months | — | No |
| Spain | 2 | 2 months | 12 months | Yes |
| UK | 2 | 3 months | 12-13 months§ | Yes |
| Greece | 3 | 2, 4 months | 6 months-5 years | Yes* |
| Ireland | 3 | 4, 6 months | 13 months | No |
*Given as conjugate MenACWY vaccine.
†In Northwest Territories MenACWY conjugate vaccine is administered to post-secondary school students attending schools outside territory.
‡Four dose series of Hib-MenCY-TT or MenACWY-CRM at 2, 4, 6, and 12-15 months only in children at increased risk of meningococcal disease.
§Hib-MenC-TT.
MenC vaccine efficacy data have shown that MenC rSBA titres ≥1:8, and more conservatively ≥1:128, are correlated with protection against MenC disease on a population level.14 Priming with two MenC-CRM doses at 3 and 4 months of age does not offer any advantage over priming with a single MenC-CRM or MenC-TT dose at 3 months of age because MenC antibodies wane below these thresholds for most infants in all three groups, and at least 97% of children had MenC rSBA ≥1:8 after a Hib-MenC-TT boost at 12 months of age, irrespective of the number of MenC doses used for infant priming. Our findings are similar to those reported in another study, which showed that 98% of infants had MenC rSBA ≥1:8 in response to a 12 month Hib-MenC-TT booster dose after one MenC infant priming dose.12 That study, however, made no comparison with the response to a two dose MenC infant priming schedule or a control group.
Intriguingly, priming with a single MenC-CRM dose induced higher post-Hib-MenC-TT rSBA geometric mean titres than two priming doses, suggesting that the administration of a greater amount of MenC antigen during priming reduces the subsequent immune response to the 12 month MenC conjugate vaccine booster dose. The underlying mechanism, which is not reflected in the frequencies of MenC-specific memory B cells in peripheral blood detected at 5, 12, or 13 months,20 could still be related to differences in numbers of memory B cells if the pool is considered to be resident in lymphoid tissues and therefore inaccessible with peripheral blood sampling. Furthermore, this phenomenon might be the result of dose dependent differences in carrier protein that manifest when different MenC glycoconjugate vaccine formulations are used for priming and boosting. A similar effect has also been observed in children challenged with a MenC pure polysaccharide formulation after infant priming with one dose of MenC-TT, which induced significantly higher post-boost MenC rSBA geometric mean titres than two dose MenC-TT infant priming.11 The relatively reduced post-booster response seen with an increase in the number of priming doses of MenC conjugate vaccine is not the same as the hyporesponsiveness that occurs in children repeatedly vaccinated with a pure polysaccharide MenC vaccine compared with others who are being vaccinated with the same MenC polysaccharide formulation for the first time.21 The latter is thought to result from the terminal differentiation of B cells into plasma cells without the formation of memory B cells, which is induced by repeated immunisation with a T cell independent antigen that, as a net result, depletes the MenC specific B cell pool.22
Two months after infant vaccination, one MenC-TT dose was significantly more immunogenic than one dose of MenC-CRM (fig 2 and table 2); and after a Hib-MenC-TT boost at 12 months of age, the MenC rSBA geometric mean titres were significantly higher in those primed with MenC-TT than all other study groups (fig 2 and appendix table B). Such differences in immunogenicity are known to persist after a MenC boost in the second year of life, irrespective of whether MenC-TT or MenC-CRM are used for boosting.23 Furthermore, at 2 years of age 82% of vaccinated children primed with MenC-TT, whose MenC rSBA geometric mean titres were significantly higher than the MenC rSBA geometric mean titres measured in those primed with a MenC-CRM schedule and in those who were not primed at all, still had MenC rSBA ≥1:8 in contrast with ≤30% of those primed with other MenC schedules. Despite evidence of immune memory after MenC disease and vaccination,24 the antibody response after MenC exposure is not rapid enough to prevent disease in those with a MenC rSBA titre <1:8,7 showing the importance of generating high rSBA geometric mean titres after booster, leading to a higher proportion of children maintaining rSBA titres above the 1:8 threshold through early childhood.
Such results are assumed to indicate differences in immunogenicity of the vaccines that relate to the T cell help induced by the different carrier proteins, though there are other manufacturing differences between MenC-TT and MenC-CRM that make it difficult to formally draw this conclusion. Differences in the persistence of post-boost MenC bactericidal antibody are consistent with observations from other studies of the persistence of MenC bactericidal antibody after priming with different MenC glycoconjugates in infancy.25 26 Our findings show that it would be more rational to prime infants with MenC-TT, rather than MenC-CRM, and boost with Hib-MenC-TT.
The proportions of infants with a MenC rSBA titre ≥1:8 after immunisation with a single dose of Hib-MenC-TT at 12 months of age, without infant priming, reached up to 83%; a proportion that might be acceptable in countries where MenC disease is currently under control. The introduction of just a single dose of MenC vaccine at 12 months of age might not be appropriate in other countries where herd immunity has not been established through the initiation of the programme with a “catch up campaign” and subsequent adolescent boosting (used to maintain herd immunity). A routine 12 month only MenC immunisation programme, in the absence of such herd immunity, would leave unvaccinated infants as well as vaccinated children, whose immunity has waned over time, at risk. The low titres of bactericidal antibodies in infancy and from 2 years of age onwards observed with just a single MenC vaccination at 12 months of age suggest prevention of breakthrough cases in infants and preschool children depend on herd immunity induced by a catch up vaccination campaign that could then be sustained through adolescent boosting. A single MenC toddler dose was successful in controlling MenC disease in the Netherlands,27 Australia,28 and Canada,29 where infants were protected through herd protection induced by an initial catch up campaign targeting older children and adolescents. An alternative, as in the US, is to provide the first MenC dose in adolescence.30 A MenC priming dose at 12 months of age, however, might still be important for a robust anamnestic response after a MenC boost in adolescence.31 Indeed if herd immunity in the UK is maintained through a robust adolescent MenC booster programme, the 3 month infant MenC vaccine might conceivably be dropped from the vaccination programme without any change in the current excellent population protection. Furthermore, the anticipated introduction of a routine MenB vaccination schedule in infancy, with a MenB vaccine that contains relatively well conserved meningococcal subcapsular proteins that might also be common among different meningococcal strains independent of the capsular polysaccharide type,32 is predicted to protect against other serogroups, including some clones of MenC, in the first 12 months of life, potentially supporting the removal of the infant MenC doses.
Strengths and limitations
The inclusion of a control group in this study made it possible to compare the post-boost immunogenicity of the different MenC priming schedules with that induced by administration of the first MenC conjugate vaccine dose in MenC vaccine naive participants at 12 months of age. Follow-up of bactericidal antibody up to 24 months of age helped us to investigate if differences in immunogenicity between the different infant MenC priming schedules are long lasting: an effect that impacts planning of MenC immunisation programmes. We did not look at the differences in antibody that could have been induced by boosting the different MenC-CRM priming schedules with a MenC-CRM booster dose because only Hib-MenC-TT is currently being used as a booster dose in the second year of life in the UK. Future studies looking at the persistence of bactericidal antibodies in children older than 2 years would show if the differences seen between the MenC-TT and MenC-CRM priming schedules or the control group are sustained. The response to a MenC boost in adolescents previously primed with a single MenC dose at 12 months of age compared with age matched MenC naive controls also merits further investigation.
The lack of significant differences in the local adverse events at the MenC injection site at 3 months as well as at the Hib-MenC-TT site at 12 months of age, and in the corresponding systemic adverse events, between the different schedules could possibly have resulted from our study being underpowered to detect differences in reactogenicity between the groups. Such differences could be investigated in a larger study.
Conclusions
In countries where the incidence of invasive MenC disease in infancy has been controlled or practically eliminated after a routine MenC vaccination programme, two MenC infant priming doses could be reduced to a single priming dose without loss of immediate post-booster immunogenicity and without any effect on reactogenicity. Unlike MenC-TT, priming with MenC-CRM or administering the first MenC dose at the age of 12 months does not result in bactericidal antibody that is sustained at 24 months of age above the accepted protective threshold for most young children. Implementation of MenC vaccine prime and boost schedules with MenC tetanus toxoid conjugates seems more likely to induce sustained protection against MenC disease in early childhood. In the absence of any infant MenC vaccine priming doses, the protection provided by just one MenC vaccine dose administered at 12 months of age would strongly rely on the persistence of herd protection, induced by a previous catch up MenC immunisation campaign, which could then be maintained by a booster in adolescence.
What is already known on this topic
Different prime and boost schedules for MenC glycoconjugate vaccines are effective in controlling MenC disease
Infant protection induced by a single MenC glycoconjugate vaccine dose at 2 or 3 months of age is similar to that induced by two or three doses
What this study adds
Increasing the number of MenC-CRM doses in infancy reduces the subsequent immune response to a MenC glycoconjugate booster
When boosting with Hib-MenC-TT vaccine, priming with a single MenC-TT dose in infancy, rather than MenC-CRM, induces more robust bactericidal antibodies that are still persistent at 24 months of age
Protection provided by just one MenC glycoconjugate dose at 12 months of age is not sustained and will rely on herd immunity
We thank all the children who took part in the study as well as their parents/guardians; the study staff in the research centres at Bristol, London, Malta, Oxford, and Southampton; and Philip de Whalley for his help with recruiting participants in Oxford. The research team acknowledges the support of the National Institute for Health Research Clinical Research Network. AJP and MDS are Jenner Institute Investigators.
Contributors: DP, AmK, AF, SNF, PTH, MDS, and AJP formulated the study design, and performed the data collection, analysis, interpretation, and writing of the manuscript. JM, DC, SAM, CW, EM, and ALK collected data collection and revised the manuscript. RB carried out the laboratory assays and revised the manuscript. JB and MV analysed the data and revised the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. AJP is guarantor.
Funding: The study was funded by the NIHR Oxford Biomedical Research Centre, UK, the NIHR Medicines for Children Network South West and London (now NIHR Clinical Research Network: Paediatrics), the Southampton NIHR Wellcome Trust Clinical Research Facility and NIHR Respiratory Biomedical Research Unit, GlaxoSmithKline Biologicals, Belgium, and European Society of Paediatric Infectious Diseases. This study was conducted independently from the funders. The funders were not involved in study design, data collection and analysis, interpretation of the data or in the writing of the report or its submission for publication. The final manuscript was reviewed and approved by GlaxoSmithKline Biologicals.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: ALK, AmK, and DP have received travel grants from vaccine manufacturers to attend scientific meetings. AJP has previously conducted studies on behalf of Oxford University funded by vaccine manufacturers but does not receive any personal payments or travel support. AJP chairs the UK Department of Health’s (DH) Joint Committee on Vaccination and Immunisation (JCVI); the views expressed in this manuscript do not necessarily reflect the views of JCVI or DH. MDS, AF, SNF, and PH act as investigators for clinical trials conducted on behalf of their respective Universities and NHS Hospital Trusts sponsored by vaccine manufacturers and have participated in advisory boards but receive no personal payments from these activities. MDS and SNF have had travel and accommodation expenses paid by vaccine manufacturers to attend international conferences related to paediatric infectious disease. RB performs contract research on behalf of Public Health England for Baxter Bioscience, GlaxoSmithKline, Pfizer, Sanofi Pasteur, Sanofi Pasteur MSD, and Novartis Vaccines. PTH has conducted studies on behalf of St George’s University of London, funded by vaccine manufacturers but does not receive any personal payments or travel support. AmK and JM have received grants from the NIHR Oxford Biomedical Research Centre UK, GlaxoSmithKline Biologicals, and the European Society of Paediatric Infectious Diseases.
Ethical approval: The study was approved by the respective research ethics committees and medicinal regulatory agencies in each country (UK NRES REC No 10/H0604/7 and Malta HEC No 24/10). Informed written consent was obtained from the parent or legal guardian of all infants before recruitment.
Transparency: DP affirms that the manuscript is an honest, accurate, and transparent account of the study; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing: No additional data available.
Cite this as: BMJ 2105;350:h1554
Web Extra. Extra material supplied by the author
Appendix: Supplementary tables A-C
References
- 1.Ali A, Jafri RZ, Messonnier N, et al. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health 2014;108:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow R, Abad R, Trotter C, et al. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013;31:4477-86. [DOI] [PubMed] [Google Scholar]
- 3.Campbell H, Borrow R, Salisbury D, et al. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 2009. Jun 24;27(suppl 2):B20-9. [DOI] [PubMed]
- 4.Borrow R, Andrews N, Goldblatt D, et al. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 2001;69:1568-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richmond P, Borrow R, Miller E, et al. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis 1999;179:1569-72. [DOI] [PubMed] [Google Scholar]
- 6.Fairley CK, Begg N, Borrow R, et al. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J Infect Dis 1996;174:1360-3. [DOI] [PubMed] [Google Scholar]
- 7.Trotter CL, Andrews NJ, Kaczmarski EB, et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004;364:365-7. [DOI] [PubMed] [Google Scholar]
- 8.Pollard AJ, Green C, Sadarangani M, et al. Adolescents need a booster of serogroup C meningococcal vaccine to protect them and maintain population control of the disease. Arch Dis Child 2013;98:248-51. [DOI] [PubMed] [Google Scholar]
- 9.Southern J, Crowley-Luke A, Borrow R, et al. Immunogenicity of one, two or three doses of a meningococcal C conjugate vaccine conjugated to tetanus toxoid, given as a three-dose primary vaccination course in UK infants at 2, 3 and 4 months of age with acellular pertussis-containing DTP/Hib vaccine. Vaccine 2006;24:215-9. [DOI] [PubMed] [Google Scholar]
- 10.Southern J, Borrow R, Andrews N, et al. Immunogenicity of a reduced schedule of meningococcal group C conjugate vaccine given concomitantly with the Prevenar and Pediacel vaccines in healthy infants in the United Kingdom. Clin Vaccine Immunol 2009;16:194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow R, Goldblatt D, Finn A, et al. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United Kingdom. Infect Immun 2003;71:5549-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findlow H, Borrow R, Andrews N, et al. Immunogenicity of a single dose of meningococcal group C conjugate vaccine given at 3 months of age to healthy infants in the United Kingdom. Pediatr Infect Dis J 2012;31:616-22. [DOI] [PubMed] [Google Scholar]
- 13.Maslanka SE, Gheesling LL, Libutti DE, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 1997;4:156-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003;10:780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health Agency of Canada. Provincial and Territorial web sites and immunization schedules. www.publichealth.gc.ca.
- 16.Centers for Disease Control and Prevention (CDC). Immunization schedules. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- 17.Pan American Health Organization. Regional Office for the Americas of the World Health Organization. www.paho.org.
- 18.Australian Government Department of Health. National Immunisation Program Schedule. www.immunise.health.gov.au.
- 19.European Centre for Disease Prevention and Control (ECDC). Vaccine schedule. http://vaccine-schedule.ecdc.europa.eu.
- 20.Khatami A, Clutterbuck EA, Thompson AJ, et al. Evaluation of the induction of immune memory following infant immunisation with serogroup C Neisseria meningitidis conjugate vaccines—exploratory analyses within a randomised controlled trial. PLoS One 2014;9:e101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach A, Twumasi PA, Kumah S, et al. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis 1997;175:200-4. [DOI] [PubMed] [Google Scholar]
- 22.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J 2007;26:716-22. [DOI] [PubMed] [Google Scholar]
- 23.Diez-Domingo J, Planelles-Cantarino MV, Baldo-Torrenti JM, et al. Antibody persistence 12 months after a booster dose of meningococcal-C conjugated vaccine in the second year of life. Pediatr Infect Dis J 2010;29:768-70. [DOI] [PubMed] [Google Scholar]
- 24.Borrow R, Goldblatt D, Andrews N, et al. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United Kingdom. J Infect Dis 2002;186:1353-7. [DOI] [PubMed] [Google Scholar]
- 25.Khatami A, Snape MD, John T, et al. Persistence of immunity following a booster dose of Haemophilus influenzae type b-Meningococcal serogroup C glycoconjugate vaccine: follow-up of a randomized controlled trial. Pediatr Infect Dis J 2011;30:197-202. [DOI] [PubMed] [Google Scholar]
- 26.Borrow R, Andrews N, Findlow H, et al. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and Haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccine Immunol 2010;17:154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaaijk P, van der Ende A, Berbers G, et al. Is a single dose of meningococcal serogroup C conjugate vaccine sufficient for protection? Experience from the Netherlands. BMC Infect Dis 2012;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpkins D, Wood N, Jelfs J, et al. Modern trends in mortality from meningococcal disease in Australia. Pediatr Infect Dis J 2009;28:1119-20. [DOI] [PubMed] [Google Scholar]
- 29.De Wals P, Deceuninck G, Lefebvre B, et al. Effectiveness of serogroup C meningococcal conjugate vaccine: a 7-year follow-up in Quebec, Canada. Pediatr Infect Dis J 2011;30:566-9. [DOI] [PubMed] [Google Scholar]
- 30.Committee on Infectious Diseases. Updated recommendations on the use of meningococcal vaccines. Pediatrics 2014;134:400-3. [DOI] [PubMed] [Google Scholar]
- 31.Stoof SP, van der Klis FR, van Rooijen DM, et al. Timing of an adolescent booster after single primary meningococcal serogroup C conjugate immunization at young age; an intervention study among Dutch teenagers. PLoS One 2014. 25;9:e100651. [DOI] [PMC free article] [PubMed]
- 32.Pollard AJ, Riordan A, Ramsay M. Group B meningococcal vaccine: recommendations for UK use. Lancet 2014. 29;383:1103-4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Supplementary tables A-C