Abstract
Background
Polymorphism in the MBL2 gene lead to MBL deficiency, which has been shown to increase susceptibility to various bacterial, viral and parasitic infections. We assessed role of MBL deficiency in HIV-1 and schistosoma infections in Zimbabwean adults enrolled in the Mupfure Schistosomiasis and HIV Cohort (MUSH Cohort).
Methods
HIV-1, S. haematobium and S. mansoni infections were determined at baseline. Plasma MBL concentration was measured by ELISA and MBL2 genotypes determined by PCR. We calculated and compared the proportions of plasma MBL deficiency, MBL2 structural variant alleles B (codon 54A>G), C (codon 57A>G), and D (codon 52T>C) as well as MBL2 promoter variants -550(H/L), -221(X/Y) and +4(P/Q) between HIV-1 and schistosoma co-infection and control groups using Chi Square test.
Results
We assessed 379 adults, 80% females, median age (IQR) 30 (17–41) years. HIV-1, S. haematobium and S. mansoni prevalence were 26%, 43% and 18% respectively in the MUSH baseline survey. Median (IQR) plasma MBL concentration was 800μg/L (192-1936μg/L). Prevalence of plasma MBL deficiency was 18% with high frequency of the C (codon 57G>A) mutant allele (20%). There was no significant difference in median plasma MBL levels between HIV negative (912μg/L) and HIV positive (688μg/L), p = 0.066. However plasma MBL levels at the assay detection limit of 20μg/L were more frequent among the HIV-1 infected (p = 0.007). S. haematobium and S. mansoni infected participants had significantly higher MBL levels than uninfected. All MBL2 variants were not associated with HIV-1 infection but promoter variants LY and LL were significantly associated with S. haematobium infection.
Conclusion
Our data indicate high prevalence of MBL deficiency, no evidence of association between MBL deficiency and HIV-1 infection. However, lower plasma MBL levels were protective against both S. haematobium and S. mansoni infections and MBL2 promoter and variants LY and LL increased susceptibility to S. haematobium infection.
Introduction
HIV-1 and schistosomiasis co-infections are very common in Africa and have been reported in several studies [1–6]. Sub-Saharan Africa is the region hardest hit by the HIV/AIDS pandemic where 63% of the 33 million infected people live [7]. HIV infection has remained a major public health challenge since its discovery in 1983 [8]. The HIV pandemic is still ravaging most parts of Southern African countries with current prevalence in these countries between 10–20% [7]. Several reports indicate that individuals worldwide differ in their susceptibility to HIV infection and it is widely agreed that genetic polymorphisms in the host genes important in immune regulation can increase or reduce the risk of HIV infections [9, 10]. An understanding of the immunological factors fuelling the HIV-1 epidemic in Africa is very important in an effort to curb the HIV-1 scourge.
Schistosomiasis is one of the neglected tropical diseases, which the World Health Organization is targeting for elimination [11–13]. An estimated 85% of the world’s estimated 200 million people with schistosomiasis live in Sub-Saharan Africa [11, 13]. S. haematobium is associated with urogenital schistosomiasis characterised by severe pathological conditions including hematuria and bladder cancer and S. mansoni causes intestinal schistosomiasis characterised by chronic or intermittent abdominal pain bleeding from gastro-oesophageal varices and bloody stool [14]. Schistosomes are complex multi-cellular helminths with several developmental stages well documented in the human host [14].
Mannose-Binding Lectin (MBL) is a key component of the innate immune system and polymorphism in the MBL2 gene and promoter region lead to MBL deficiency [15–17]. MBL deficiency is associated with impaired function of the innate immune system and leads to increased susceptibility to several infections [18–20]. MBL acts as an opsonin by binding to sugar groups naturally found on the surface of various infectious bacteria, viruses and parasites and activates the complement system [21, 22] through associating with MBL-associated serine proteases MASP-1, MASP-2 and MASP-3 [23–25]. Sub-Saharan Africa has a high burden of viral [26–28], bacterial [27–29] and parasitic infections [12, 13, 27]. Assessment of polymorphisms in the MBL2 gene and promoter region to determine functional MBL deficiency has been carried out in different populations with few studies in Sub-Saharan Africa [17, 30, 31]. Schistosomes carry sugar molecules or glycoconjugates on the surface of all their developmental stages [32, 33] and these glycoconjugates interact with innate immune recognition molecules including MBL [34–36]. In vitro studies have demonstrated compliment mediated killing of all stages of the schistosome parasite [35].
Several studies have been conducted worldwide [37–43] including several African populations [17, 30, 44–48] looking at MBL2 genetic variants and plasma/serum MBL concentration and their association to increased infection susceptibility. The role of MBL deficiency in infections has been shown to be different across different infections [44]. For example MBL deficiency has been shown to increase risk of recurrent respiratory infections [49] and malaria [50] whilst being protective in TB [44], leprosy [51] and leishmaniasis [52, 53]. Results on the role of MBL deficiency in HIV infections are conflicting [54, 55]. In addition, studies conducted in Sub-Saharan Africa, in Mozambique [17, 56, 57], South Africa [48, 58] and Zimbabwe [31] have been done in children and none among adults. One study reports investigations on MBL deficiency and schistosoma infections in Nigeria [59].
In this paper we describe the role of plasma MBL deficiency and MBL2 polymorphism in HIV-1, S. mansoni and S. haematobium infections in Zimbabwean adults enrolled in the MUSH cohort. The infection rates in Zimbabwe are currently 15% for HIV-1 [60] and 40% and 20% for Schistosoma haematobium and Schistosoma mansoni respectively [61]. S. haematobium is the most common species followed by S. mansoni in Zimbabwe [61–64]. In the context of public health, it is important to understand the role of MBL deficiency in these infections in our adult population.
Materials and Methods
Study design and study population
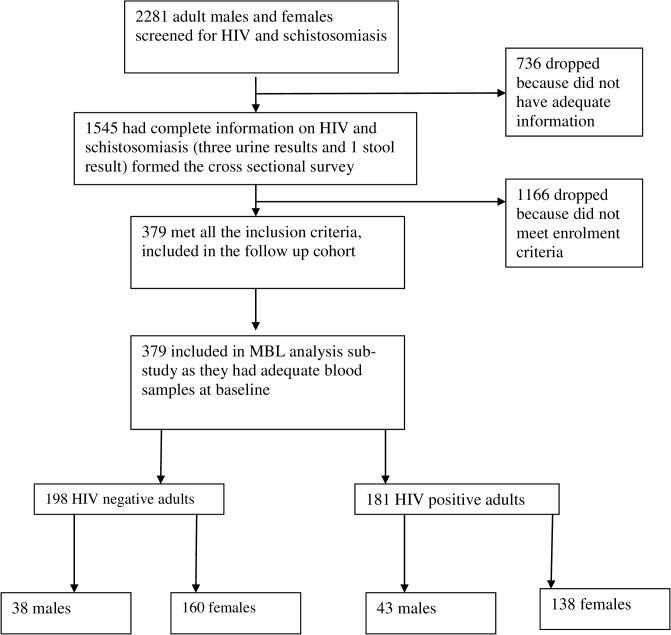
This was a sub-study of the Mupfure Schistosomiasis and HIV Cohort (MUSH Cohort) established in 2001 aimed at studying the immunological interactions between HIV-1 and schistosomiasis infections [35, 36, 38, 44, 45]. We used plasma and whole blood samples collected at baseline in 2001–2002 to determine prevalence of MBL deficiency in this population. Details of the MUSH Cohort and procedures are described elsewhere [1, 2, 65]. Briefly, recruitment into the MUSH study, sample collection and laboratory assays took place between October 2001 and November 2007. A total of 1574 adult males and females were screened for HIV and schistosomiasis infections at baseline (Fig. 1) and 379 met the MUSH eligibility criteria: adult males/females above 18 years old, HIV positive or negative, positive for S. haematobium or S. mansoni or both or uninfected, no active TB infection, non-pregnant women and not currently on schistosomiasis treatment. After screening for HIV-1 and schistosomiasis status, participants were categorised into four groups: HIV-1 only, schistosomiasis only, co-infected with both diseases and controls with none of these two infections.
Fig 1. Participant Flow diagram.
Flow chart showing the recruitment procedures for the adult cohort. A total of 2281 community dwelling rural adult males and females were screened for HIV and schistosomiasis, 379 males and females who met the inclusion criteria were enrolled and their baseline blood samples used for MBL plasma and MBL2 genotype analysis.
This sub-study was approved by the National Research Ethics Committee of the Medical Research Council of Zimbabwe (MRCZ/A/1770) and the University of Witwatersrand Human Research Ethics Committee (M130348). Permission was given by the Provincial Medical Director of Mashonaland Central Province and the District Medical Officer of Shamva District, the village headmen and at village meetings. All individuals in the main MUSH study gave written informed consent for specimen collection, storage and future laboratory studies.
Laboratory procedures
HIV testing
Participants were screened for S. haematobium, S. mansoni and HIV-1 as described earlier (1, 65). Briefly, HIV pre- and post-test counselling was done in the participants’ native language (Shona) by qualified medical personnel. A rapid HIV-1/2 test kit was used on a dry blood spot in the field (Determine, Abbott Laboratories, Tokyo, Japan), followed by two different rapid HIV tests Oraquick (by Orasure) and Serodia (by Fujirebio) for all who tested initially positive. No discrepancies in results were found between the initial Determine HIV test and the two subsequent ELISAs. Strict confidentiality was maintained throughout the study. All those who requested their HIV results were given after post-test counselling from the nursing staff.
Schistosoma parasitology
Microscopic examination of fixed-volume urine samples filtered on Nytrel filters (VesterGaard Frandsen) was used to identify and quantify eggs of S. haematobium by the syringe urine filtration technique [66]. Due to the diurnal and day-to-day variation in egg output, the urine samples were collected on 3 consecutive days [67, 68]. The modified formol-ether concentration technique was used on 1 stool sample from each participant to detect eggs of S. mansoni and other helminth or parasites [69]. Levels of Circulating Anodic Antigen (CAA) were measured in serum samples using an ELISA assay, to complement parasitological detection methods. CAA originates from the parasite gut and is used as a marker of an active schistosome infection [70].
Determination of plasma MBL concentration
Baseline plasma samples stored at -20°C were used for measurement of plasma MBL concentrations using the double enzyme immuno-assay (EIA) (Antibody Shop, Denmark) as described previously [30, 71]. Micro titre plates (Maxisorp, NUNC, Denmark) were coated with a mouse anti-human MBL monoclonal antibody, clone 131–1, IgG1, Kappa (Antibody Shop, Denmark). Plasma samples were diluted 1/25 and 1/400 in Tris/HCL buffer (Bie and Bernsten) containing EDTA (Bie and Bernsten) and 0,05% Tween 20 (Sigma) [71]. A pool of EDTA plasma with a known concentration of MBL was used as the standard. Biotinylated clone 131–1 anti-MBL (Antibody Shop, Denmark) was used as the detection antibody. Horseradish peroxidase labelled streptavidin (Amersham, UK) was added. The substrate uses O-phenylene diamine (OPD) (DakoCytomation) hydrochloride in citrate-phosphate (Bie and Bernsten) buffer pH 5.0, the colour reaction was stopped by addition of sulphuric acid (Sigma). Parallel control plates were coated with equivalent amounts of mouse IgG1 and processed as above. Parallel plates are done in order to reveal binding of rheumatoid factors, anti mouse immunoglobulins and non-specific binding of MBL interfering in the system. Optical density (OD) was read on an ELISA reader (MR5000/7000, Dynatech Laboratories, Denmark) at 490 nm with reference filter 630nm. Final MBL values are given as μg MBL per litre. To determine prevalence of MBL deficiency, plasma MBL concentration was categorised into normal (above 500μg/L), intermediate (100μg/L- 500μg/L) and deficient (below 100μg/L) [30, 72–74].
MBL genotyping
Genomic deoxyribonucleic acid (DNA) was used for MBL2 genotyping. The DNA was extracted from frozen baseline Peripheral Blood Mononuclear Cells (PBMCs) using the standard salting out procedure [75]. The MBL2 genotypes and promoter region alleles were detected by allele-specific oligonucleotide PCR (ASO-PCR) where specific sequences were used for each allele [42]. Briefly, the PCR master mix was made by mixing the following: 400μl sterile water (H/S Apoteket, Rigshospital, Copenhagen), 150μl of 1.5mM MgCl2 (Invitrogen, Denmark), 150μl at 0.07mM final concentration of each deoxynucleotide triphosphate (dNTPs) (Amersham Biosciences, UK Limited), 500 μl PCR Buffer containing 50mM KCL, 10 mM Tris-HCl (pH 8.3), 0.001% (w/v) gelatin p.H.8.3 (Invitrogen, Denmark), 250μl glycerol at 5% final concentration, 50μl cresol red at final concentration 100μg/μl. Cresol red (Invitrogen, Denmark) was added as a colour marker. The PCR Master mix could be kept at 4°C for 2 weeks.
Then a total of 12 primer solutions (DNA Technology) were prepared for each sample (S1 Table). The set of 12 primers for MBL2 variants and promoter region mutations were prepared by mixing 5´ sense and 3´ sense primers with water according to the manufacturer’s instructions. Final concentration of each primer was 0.25uM for each specific primer. Primers for 5 and 3 sense exon 4 of the MBL2 gene (DNA Technology, Denmark) were included in each PCR reaction as an internal positive control. PCR primers were pre-aliquoted in 5μl volumes into the PCR plates and the plates could be kept at 4°C for 1 week.
MBL PCR and sample reaction mixtures were then prepared as 10μl volumes containing 30ng genomic DNA and 0.25μmM of the specific primer, 54 μl of PCR mastermix, 3,5μl sample DNA (if the DNA concentration was 0,3μg/μl), 31μl sterile water, and 0.25 Units of Platinum Taq DNA polymerase (Invitrogen, Denmark) in each 90μl initial reaction mixture volume. PCR reaction mixture was mixed and added to the 12 wells, clearly marked for each sample, which contained 5μl of different sets of primers for the genotyping and promoter typing. Only eight samples could be run on each PCR plate. Plates were put in a plate micro centrifuge at 3000rpm for 1 minute to spin down the well contents. Plates were tightly sealed with foil paper.
DNA was then amplified by PCR in a programmed thermocycler (Gene Amp PCR Systems 9600, Perkin Elmer). All PCRs were initiated by a 2-min denaturation step at 94°C and completed by a 5-min extension step at 72°C. The temperature cycles for the PCR were as follows: 10 cycles of 10 s at 94°C and 60 s at 65°C, and a further 20 cycles of 10 s at 94°C, 50s at 61°C, 30s at 72°C and 2 minutes at 25°C. Gel electrophoresis was done on the PCR products on the same day or kept at 4°C for a maximum 1 week. The PCR products were separated by a 2% agarose gel (Invitrogen) electrophoresis run in an electrophoresis chamber (Sub-cell Biorad) at 150V, 400mA for 32 minutes. The gels were then carefully transferred to the UV Transilluminator (Syngene Synoptics Ltd, Denmark) where a photograph of the gel was taken and used to determine the MBL2 genotype of each sample. MBL2 genotypes A/O, O/O and XAYO/YOYO were classified as MBL deficient producers and the normal MBL2 denoted A/A. MBL2 single nucleotide polymorphisms (SNPs) in the form of structural variants named B (codon 54, rs1800450 A>G), C (codon 57, rs1800451 A>G) and D (codon 52, rs5030737 T>C), as well as the regulatory promoter region variants H/L (-550, rs11003125 G>C), X/Y (-221, rs7096206 C>G) and PQ (+4, rs7095891 T>C) [76, 77] were determined [42]. All the single nucleotide polymorphisms (SNPs) and their reference sequence numbers are found on the online database http://www.ncbi.nlm.nih.gov/snp/.
Statistical analysis
All statistical analysis were done using Stata 11 statistical package (STATA Corp, Timberlake Consultants). Normality of plasma MBL levels was checked graphically. Non-parametric tests (Mann-Whitney and Kruskal-Wallis) were used for comparison of median plasma MBL concentrations by age, gender, HIV-1 and schistosoma infection status. Differences in proportions of MBL deficiency between males and females and infection status were done using Chi Square or Fisher’s exact tests and confidence intervals were calculated using binomial exact methods. The frequencies of the MBL2 alleles were obtained by direct gene counting. Conformation to the Hardy-Weinberg Equilibrium was determined using the Chi Square test in the SHEsis online programme [78, 79]. For MBL2 genotype analysis, normal homozygous MBL2 was denoted as A/A. The variant genotypes A/B (A>G), A/C (G>A), A/D (C>T) were grouped together as A/O heterozygous MBL2 as they all give low plasma MBL levels and all homozygous and compound homozygotes were grouped together as O/O. The differences in frequency of the MBL2 genotypes, promoter region variants and haplotypes between infection status groups were determined using the Chi Square test or Fisher’s exact tests.
Results
A total of 379 plasma and whole blood samples were available for MBL analysis. Median age (IQR) of the participants was 30 (17 to 41) years old and the majority were females (80%). Baseline demographic characteristics of this study population and prevalence of HIV-1, S. haematobium and S. mansoni among the 1545 participants screened during the main MUSH study have been described elsewhere [65].
Prevalence of HIV-1, S. haematobium and S. mansoni in the MBL sub-study
The prevalence of HIV-1, S. haematobium, S. mansoni and co-infection with both schistosma species among the 379 participants used for the MBL sub-study was 52%, 58%, 7% and 10%, respectively (Table 1).
Table 1. Comparison of median plasma MBL concentrations between groups.
| Variable | n | Median MBL levels μg/L | IQR μg/L | p-value |
|---|---|---|---|---|
| Sex | ||||
| Males | 76 | 824 | 159–1968 | |
| Females | 302 | 800 | 200–1936 | 0.561 |
| Age groups | ||||
| <25 years old | 82 | 920 | 209–2608 | |
| >25 years old | 297 | 776 | 166–1864 | 0.202 |
| HIV status | ||||
| Positive | 197 | 688 | 147–1904 | |
| Negative | 181 | 912 | 272–1968 | 0.065 |
| Schistosomiasis infection status | ||||
| No infection | 89 | 656 | 139–1152 | |
| S. haematobium only | 205 | 912 | 213–2320 | |
| S. mansoni only | 24 | 1016 | 258–2192 | |
| Coinfected with both species | 37 | 688 | 297–2432 | 0.036 |
| Schistosomiasis and HIV status | ||||
| S. haematobium+/ HIV+ | 102 | 824 | 151–2160 | |
| S. haematobium+/ HIV- | 103 | 944 | 251–2496 | |
| S. haematobium-/ HIV+ | 44 | 656 | 65–1176 | |
| S. haematobium-/ HIV- | 45 | 707 | 163–1152 | 0.037 |
| S. haematobium infection only | ||||
| S. haematobium positive | 205 | 912 | 213–2320 | |
| S. haematobium negative | 89 | 656 | 139–1152 | 0.006 |
| Circulating anodic antigen | ||||
| Above 40pg/ml (CAA positive) | 293 | 848 | 161–2232 | |
| Below 40pg/ml (CAA negative) | 63 | 784 | 346–1152 | 0.308 |
| Eggs per 10ml urine, S. Haematobium infection | ||||
| 0 (no infection) | 108 | 705 | 145–1264 | |
| <10 (light infection) | 123 | 896 | 230–2112 | |
| 10-<50 (medium infection) | 55 | 1408 | 182–2528 | |
| >50 (heavy infection) | 9 | 295 | 87–2816 | 0.109 |
The concentration of MBL in plasma was measured in μg/L. Differences in median plasma MBL concentration in the different infection status categories were analysed by using non-parametric tests Mann-Whitney for two categories and Kruskal-Wallis for three or more categories.
Plasma MBL levels
Results for plasma MBL concentration are available for 378 individuals; one sample repeatedly gave inconsistent plasma level results and was dropped from this analysis. The median (IQR) plasma MBL concentration in all the investigated individuals was 800μg/L (192–1936μg/L). There was no difference in the median plasma MBL concentration between males and females (p = 0.553) and by to age (p = 0.204) (Table 1).
Association between HIV-1, schistosoma infections and plasma MBL concentration
There was no difference in the median plasma MBL concentration between HIV infected and uninfected individuals (p = 0.065). However, there were 37 participants with plasma MBL levels at the assay detection limit of 20μg/L. This level of plasma MBL was found more frequently among HIV-1 infected 14% (27 of 196, 95% CI 8.4–18.2), compared with HIV-1 uninfected 6% (10 of 181, 95% CI 2.7–9.9) (χ2 = 7.24, p = 0.007), indicating a possible role of MBL deficiency in HIV-1 infection.
There was a significant difference (p = 0.037) in median plasma MBL levels between the four schistosoma infection status groups, namely, no infection, S. haematobium only, S. mansoni only and those co-infected with both species. Those with S. mansoni infection only had the highest median plasma MBL level of 1016μg/L followed by those with S. haematobium only median MBL level 912μg/L, p = 0.037 (Table 1). There were also significant differences in median plasma MBL levels between the four HIV-1 and S. haematobium co-infection groups, with those positive for S. haematobium and negative for HIV having the highest median plasma MBL level (944μg/L, p = 0.037, Table 1). Further comparison was done between the S. haematobium infected and uninfected individuals after excluding those with S. mansoni or co-infected with both species. There was a significant difference in plasma MBL levels, those S. haematobium infected having higher median MBL levels than the uninfected, p = 0.006 (Table 1) indicating protective role low plasma MBL levels. There was no difference in plasma MBL levels when comparison was done between CAA levels groups above or below 40pg/ml (p = 0.381) and four egg count groups among those with S. haematobium infection only (p = 0.114, Table 1).
Association between HIV-1, schistosoma infections and plasma MBL deficiency
Plasma MBL concentration was categorised into normal (above 500μg/L), intermediate (100μg/L- 500μg/L) and deficient (below 100μg/L) (Table 2) (72, 73). The prevalence of plasma MBL deficiency in all participants analysed was 18% (67 of 378, 95% CI: 14–22%), 20% (77 of 378, 95% CI: 16–24%) had intermediate plasma MBL levels and 62% (234 of 378, 95% CI: 56–66%) had normal plasma MBL levels above 500μg/L. There was no difference in proportion of males with MBL deficiency 20% (15 of 76, 95% CI: 11–30%) and females with MBL deficiency 17% (52 of 302, 95% CI: 13–22%).
Table 2. Distribution of participants between three plasma MBL levels, participants stratified according HIV, S. haematobium and S. mansoni infection and co-infection status.
| HIV status | n | Normal MBL levels | Reduced MBL levels | Deficient MBL levels | P value |
|---|---|---|---|---|---|
| HIV positive | 197 | 113 (57%) | 41 (21%) | 43 (22%) | |
| HIV negative | 181 | 121 (67%) | 36 (20%) | 24 (13%) | 0.070 |
| S. haematobium infection | |||||
| S. haematobium positive | 205 | 131 (64%) | 38(19%) | 36 (17%) | |
| S. haematobium negative | 89 | 51 (57%) | 17(19%) | 21 (24%) | 0.446 |
| Schistosoma co-infection | |||||
| No infection (controls) | 89 | 51(57%) | 17 (195) | 21(24%) | |
| S. haematobium only | 205 | 131(64%) | 38(19%) | 36(18%0 | |
| S. mansoni only | 24 | 15(63%) | 6(25%) | 3(13%) | |
| Co-infected with both species | 37 | 23(62%) | 11(30%) | 3(8%) | 0.351 |
| HIV and S. haematobium co-infection | |||||
| HIV+/S. haematobium+ | 102 | 64(63%) | 17(17%) | 21(21%) | |
| HIV+/S. haematobium- | 44 | 23(52%) | 8(18%) | 13(30%) | |
| HIV-/S. haematobium+ | 103 | 67(65%) | 21(20%) | 15(15%) | |
| HIV-/S. haematobium- | 45 | 28(62%) | 9(20%) | 8(18%) | 0.546 |
Prevalence of MBL deficiency, plasma MBL concentration was categorised into normal (above 500μg/L), intermediate (100μg/L- 500μg/L) and deficient (below 100μg/L), analysed by the Chi Square or Fisher’s exact tests, n = 378.
We found no difference in distribution frequency of participants according to HIV (p = 0.070) and among S. haematobium infected and uninfected participants after excluding S. mansoni and co-infections (p = 0.446) between the three plasma MBL levels categories, normal, reduced and deficient (Table 2). There was also no difference when a similar analysis was done among the schistosoma infection status groups (p = 0.351) and among HIV and S. haematobium co-infection groups (p = 0.546).
MBL2 polymorphism
MBL2 genotyping was successfully done on 366 samples out of 379 and these were used in this analysis, the other samples could not be amplified. All the known MBL2 coding alleles, wild-type A/A, B (codon 54, rs1800450 A>G), C (codon 57, rs1800451 A>G) and D (codon 52, rs5030737 T>C), were found in this study population. In the MBL2 coding region, 233/366 (64%) were classified as A/A genotype, 3 (0.8%) were A/B, 117 (32%) were A/C, 1 (0.3%) A/D and 12 (3%) C/C (Table 3). There were neither B/B, C/D, D/D nor B/D variants. The frequency of MBL2 genetic variants was 36% and 64% had the normal homozygous A/A genotype. The normal homozygous MBL2, A/A genotype was found at frequencies of 64% (233 of 366, 95% CI:58.5–68.5%), heterozygous MBL2 A/O variants at 33% (121 of 366, 95% CI: 28.3–38.1) and homozygous O/O variants at 3.3% (12 of 366, 95% CI:1.7–5.7%) (Table 3). All the MBL2 SNPs detected among the HIV negative in this study were in Hardy-Weiburg Equillibrium (structural alleles p = 0.400, -550H/L p = 0.820, -221Y/X p = 0.550 and +4P/Q p = 0.170 (S2 Table).
Table 3. Gene, promoter alleles and haplotype frequencies obtained for MBL2 polymorphism among the enrolled Zimbabwean participants.
| N | Frequency% | |
|---|---|---|
| Exon 1 allele | ||
| A normal | 587 | 80 |
| B(A>G) | 3 | 0.4 |
| C(G>A) | 141 | 20 |
| D(C>T) | 1 | 0.1 |
| Promoter alleles | ||
| -550H | 38 | 5 |
| -550L | 694 | 95 |
| -221X | 134 | 18 |
| -221Y | 598 | 82 |
| +4P | 343 | 47 |
| +4Q | 389 | 53 |
| MBL2 Exon 1 genotype | ||
| AA wild-type | 233 | 64 |
| AB (A>G) | 3 | 0.8 |
| AC(G>A) | 117 | 32 |
| AD (C>T) | 1 | 0.2 |
| CC (C>T) | 12 | 3 |
| Promoter region genotypes | ||
| -550HH | 1 | 0.3 |
| -550HL | 37 | 10.1 |
| -550LL | 328 | 89.6 |
| -221YY | 242 | 66.1 |
| -221XY | 112 | 30.0 |
| -221XX | 12 | 3.2 |
| +4PP | 74 | 20.2 |
| +4QQ | 97 | 26.5 |
| +4PQ | 195 | 53.3 |
| MBL2 Haplotypes | ||
| 1. MBL2*LYPA | 260 | 35.5 |
| 2. MBL2*LYQA | 183 | 25.0 |
| 3. MBL2*LYQC | 108 | 14.6 |
| 4. MBL2*LXPA | 58 | 7.9 |
| 5. MBL2*LXQA | 45 | 6.1 |
| 6. MBL2*HYPA | 41 | 5.6 |
| 7. MBL2*LXQC | 31 | 4.2 |
| 8. MBL2*LXPC | 2 | 0.3 |
| 9. MBL2*LYQB | 2 | 0.3 |
| 10. MBL2*HYPD | 1 | 0.1 |
| 11. MBL2*LYPB | 1 | 0.1 |
MBL2 gene and allele frequencies obtained by direct gene counting, frequencies expressed as percentages.
Frequency of MBL2 genotypes and haplotypes
MBL2 wild-type genotype A/A had the highest frequency of 64% (233 of 366, 95% CI: 77.1–83%) and genotype A/D the lowest frequency of 0.1% (1of 366, 95% CI 0.003–0.76, Table 3). All the currently known MBL2 promoter region alleles, -550H, -550L, -221Y, -221X, +4P and +4Q, were detected. Promoter region allele L had the highest frequency 95% (694 of 732, 95% CI: 92.9–96.3%) compared to 5% (38 of 732, 95% CI: 3.69–7.1%) for the H allele (Table 3). The LL MBL2 promoter region genotype (89%) had the highest frequency and HH (0.3%) the lowest frequency. Eleven different haplotypes were detected in these individuals including three rare haplotypes namely LXQA, LXQC and LXPC. Haplotype LYPA had the highest frequency (35%), followed by LYQA and LYPB, LYPC and HYPD the lowest (0.1%) (Table 3). MBL2 genotypes A/A showed the highest levels of plasma MBL concentrations and O/O genotypes had the lowest (S3 Table).
Association between HIV-1, schistosoma infections and MBL2 genotypes and promoter region genotypes
Analysis was done to determine if MBL2 genetic and promoter region variants were associated with HIV-1 infection. The distribution of the three genotypes (A/A, A/O and O/O) did not differ between the HIV-1 infected and uninfected (p = 0.429, Table 4). The MBL2 haplotypes were further combined and subdivided into three groups namely genotypes that give normal plasma MBL levels (YA/YA, YA/XA), intermediate levels (XA/XA, YA/YO) and deficient levels (XA/YO, YO/YO) (20, 77). There was no difference in distribution frequency when comparison was done among the HIV-1 strata and the three MBL2 genotype groups YAYA/YAXA, XAXA/YAYO and XAYO/YOYO (p = 0.347, Table 4). None of these MBL2 genotypes and promoter variants were associated with HIV-1 infection (Table 4).
Table 4. Distribution of participants between MBL2 genotypes and promoter region haplotypes, participants stratified according to HIV-1 infection status.
| MBL2 genotypes/ haplotypes | n | HIV negative | HIV positive | P value |
|---|---|---|---|---|
| A/A | 233 | 117 (50%) | 116 (50%) | |
| A/O | 121 | 55 (45%) | 66 (55%) | |
| O/O | 12 | 4 (33%) | 8 (67%) | 0.429 |
| YAYA/YAXA | 223 | 113(51%) | 110(49%) | |
| XAXA/YAYO | 131 | 59(45%) | 72(55%) | |
| XAYO/YOYO | 12 | 4(33%) | 8(67%) | 0.347 |
| Promoter genotypes | ||||
| -550HH | 1 | 1(100%) | 0(0%) | |
| -550HL | 37 | 22(59%) | 15(41%) | |
| -550LL | 328 | 153(47%) | 175(53%) | 0.141 |
| -550HY | 31 | 19(61%) | 12(39%) | |
| -550LY | 326 | 154(47%) | 172(53%) | |
| -221LX | 9 | 3(33%) | 6(67%) | 0.229 |
| -221YY | 242 | 120(50%) | 122(50%) | |
| -221XY | 112 | 52(46%) | 60(54%) | |
| -221XX | 12 | 4(33%) | 8(67%) | 0.521 |
| -550HY | 38 | 23(61%) | 15(39%) | |
| -221LY*LX | 328 | 153(47%) | 175(53%) | 0.105 |
| +4C/C(PP) | 74 | 37(50%) | 37(50%) | |
| +4TT(QQ) | 97 | 55(57%) | 42(43%) | |
| +4C/TPQ | 195 | 98(50%) | 97(49%) | 0.545 |
This analysis was done using the Chi Square and Fisher’s exact tests, n = 366
We found no association between MBL2 genotypes and PQ promoter genotypes and schistosoma infections (Table 5). However, there was a significant association between S. haematobium and S. mansoni infections and LL (p = 0.051) and LY*LX (p = 0.026) promoter genotypes. Therefore promoter region genotypes LL, LY and LX which encode for low plasma MBL levels were significantly associated with S. haematobium and S. mansoni infections.
Table 5. Distribution of participants between MBL2 genotypes and promoter region haplotypes according to schistosoma infection status.
| MBL2 Genotype/ Haplotype | N | No infection (controls) | S. haematobium only | S. mansoni only | Coinfected with both species | P value |
|---|---|---|---|---|---|---|
| A/A | 216 | 51(24%) | 128(59%) | 15(7%) | 22(10%) | |
| A/O | 117 | 33(28%) | 62(53%) | 9(8%) | 13(11%) | |
| O/O | 11 | 4(36%) | 6(55%) | 0(0%) | 1(9%) | 0.893 |
| YAYA/YAXA | 208 | 49(23%) | 123(59%) | 14(7%) | 22(11%) | |
| XAXA/YAYO | 125 | 35(28%) | 67(54%) | 10(8%) | 13(10%) | |
| XAYO/YOYO. | 11 | 4(36%) | 6(55%) | 0(0%) | 1(9%) | 0.903 |
| Promoter region genotypes | ||||||
| -550HH | 1 | 0(0%) | 1(100%) | 0(0%) | 0(0%) | |
| -550HL | 34 | 15(44%) | 18(53%) | 0(0%) | 1(3%) | |
| -550LL | 309 | 73(24%) | 177(57%) | 24(8%) | 35(11%) | 0.051 |
| -550HY | 28 | 14(50%) | 13(46%) | 0(0%) | 1(4%) | |
| -221LY | 307 | 72(24%) | 178(58%) | 23(7%) | 34(11%) | |
| -221LX | 9 | 2(22%) | 5(56%) | 1(11%) | 1(11%) | 0.072 |
| -221YY | 228 | 65(29%) | 122(54%) | 14(6%) | 27(12%) | |
| -221XY | 106 | 21(20%) | 68(64%) | 9(8%) | 8(8%) | |
| -221XX | 10 | 2(20%) | 6(60%) | 1(10%) | 1(10%) | 0.351 |
| -550HY | 35 | 15(43%) | 19(54%) | 0(0%) | 1(3%) | |
| -221LY*LX | 309 | 73(24%) | 177(57%) | 24(8%) | 35(11%) | 0.026 |
| +4PP | 69 | 16 (23%) | 43(62%) | 3(4%) | 7(10%) | |
| +4QQ | 88 | 17(19%) | 50(57%) | 8(9%) | 13(15%) | |
| +4PQ | 187 | 55(29%) | 103(55%) | 13(7%) | 16(9%) | 0.381 |
This analysis was done using the Fisher’s exact tests, n = 344
Analysis among S. haematobium infected and uninfected after excluding those with S. mansoni and co-infection with both species showed a significant difference in distribution frequency of the LY promoter region variant, participants with LY promoter genotype being more susceptible to S. haematobium infection (p = 0.048, Table 6).
Table 6. Distribution of participants between MBL2 genotypes and promoter region haplotypes according to schistosoma infection status.
| MBL2 genotype/ haplotype | n | S. haematobium negative | S. haematobium positive | P value |
|---|---|---|---|---|
| A/A | 179 | 51(28%) | 128(72%) | |
| A/O | 95 | 33(35%) | 62(65%) | |
| O/O | 10 | 4(40%) | 6(60%) | 0.466 |
| YAYA/YAXA | 172 | 49(28%) | 123(72%) | |
| XAXA/YAYO | 102 | 35(34%) | 67(66%) | |
| XAYO/YOYO | 10 | 4(40%) | 6(60%) | 0.505 |
| Promoter genotypes | ||||
| -550HH | 1 | 0(0%) | 1(100%) | |
| -550HL | 33 | 15(45%) | 18(55%) | |
| -550LL | 250 | 73(29%) | 177(71%) | 0.132 |
| -550HY | 27 | 14(52%) | 13(48%) | |
| -221LY | 250 | 72(29%) | 178(71%) | |
| -221LX | 7 | 2(29%) | 5(71%) | 0.048 |
| -221YY | 187 | 65(35%) | 122(65%) | |
| -221XY | 89 | 21(24%) | 68(76%) | |
| -221XX | 8 | 2(25%) | 6(75%) | 0.161 |
| -550HY | 34 | 15(44%) | 19(56%) | |
| -221LY*LX | 250 | 73(29%) | 177(71%) | 0.078 |
| +4PP | 59 | 16(27%) | 43(73%) | |
| +4QQ | 67 | 17(25%) | 50(75%) | |
| +4PQ | 158 | 55(35%) | 103(65%) | 0.289 |
This analysis was done using the Chi Square or Fisher’s exact tests, n = 284
Analysis among HIV-1 and S. haematobium co-infections, after excluding S. mansoni infections and schistosoma co-infections showed no difference in distribution frequency of MBL genotypes and promoter genotypes between the four co-infection status groups (Table 7). MBL2 genotypes and promoter region genotypes were not associated with HIV-1 and S. haematobium co-infections.
Table 7. Distribution of participants between MBL2 promoter region haplotypes according to HIV and S. haematobium co-infection status.
| M MBL2 ggenotype/ haplotype | N | HIV-/S. haematobium- | HIV+/S. haematobium+ | HIV-/S. haematobium+ | HIV+/S. haematobium- | P value |
|---|---|---|---|---|---|---|
| A/A | 179 | 27(15%) | 64(36%) | 64(36%) | 24(13%) | |
| A/O | 95 | 16(17%) | 30(32%) | 32(34%) | 17(18%) | |
| O/O | 10 | 1(10%) | 3(30%) | 3(30%) | 330(%) | 0.802 |
| YAYA/YAXA | 172 | 26(15%) | 61(36%) | 62(36%) | 23(13%) | |
| XAXA/YAYO | 102 | 17(17%) | 33(32%) | 34(33%) | 18(18%) | |
| XAYO/YOYO. | 10 | 1(10%) | 3(30%) | 3(30%) | 3(30%) | 0.830 |
| Promoter genotypes | ||||||
| -550HH | 1 | 0(0%) | 0(0%) | 1(100%) | 0(0%) | |
| -550HL | 33 | 8(24%) | 7(21%) | 11(33%) | 17(21%) | |
| -550LL | 250 | 36(14%) | 90(36%) | 87(35%) | 37(15%) | 0.384 |
| -550HY | 27 | 8(30%) | 5(19%) | 8(30%) | 6(22%) | |
| -221LY | 250 | 35(14%) | 89(36%) | 89(36%) | 37(15%) | |
| -221LX | 7 | 1(14%) | 3(43%) | 2(29%) | 1(14%) | 0.295 |
| -221YY | 187 | 32(17%) | 59(32%) | 64(34%) | 32(17%) | |
| -221XY | 89 | 11(12%) | 34(38%) | 33(37%) | 11(12%) | |
| -221XX | 8 | 1(13%) | 4(50%) | 2(25%) | 1(13%) | 0.729 |
| -550HY | 34 | 8(24%) | 7(21%) | 12(35%) | 7(21%) | |
| -221LY*LX | 250 | 36(14%) | 90(36%) | 87(35%) | 37(15%) | 0.227 |
| +4PP | 59 | 7(12%) | 20(34%) | 23(39%) | 9(15%) | |
| +4QQ | 67 | 7(10%) | 26(39%) | 24(36%) | 10(15%) | |
| +4PQ | 158 | 30(19%) | 51(32%) | 52(33%) | 25(16%) | 0.685 |
This analysis was done using the Chi Square or Fisher’s exact tests, n = 284
Discussion
This study showed that plasma MBL deficiency, all MBL genetic and promoter region variants detected were not associated with HIV-1 infection in this population, however participants with plasma MBL levels at the assay detection limit were significantly more frequent among the HIV-1 infected. Higher plasma MBL levels, LY and LL promoter genotypes were associated with increased susceptibility to both S. haematobium and S. mansoni infections. To our knowledge, this is the first study that has investigated the role of MBL deficiency in HIV-1 and schistosoma co-infections and second study that assessed the role of MBL deficiency in schistosoma infections [59]. Our results also confirm previously reported association of MBL polymorphism with MBL levels [15–17].
In view of available literature, we hypothesized that plasma MBL deficiency due to single locus substitutions in MBL2 resulting in varying levels of circulating MBL would have an effect on susceptibility to HIV-1 and schistosoma infections in this study population. The results of our study showed no difference in plasma MBL concentration between the HIV positive and HIV negative individuals, consistent with other reports [80–82]. In contrast, some have reported protective effect of normal plasma MBL levels and increased susceptibility due to low MBL levels [30, 37, 83, 84], but some reported a deleterious effect of normal plasma MBL levels where high MBL levels were associated with acquiring HIV infection [39].
In addition, we investigated the role of plasma MBL deficiency in adults singly infected or co-infected with HIV-1, S. haematobium and S. mansoni and uninfected controls. Our results showed a significant difference in plasma MBL levels with those S. mansoni and S. haematobium positive having significantly higher MBL levels than the uninfected participants. When analysis was done among the S. haematobium infected and uninfected, after excluding those infected with S. mansoni and those co-infected with both schistosoma species, the S. haematobium infected participants had significantly higher plasma MBL levels. The clinical relevance of our findings is that higher plasma MBL levels led to increased susceptibility to schistosoma infections and lower levels were protective. Our results are in contrast to the only similar report available [59] which found lower MBL levels in the infected participants and higher MBL levels were protective against S. haematobium infection in a Nigerian population [59]. The Nigerian study had a sample size of 346 almost similar to our study with 379 participants. The reasons for these contrasting findings on role of plasma levels in schistosomiasis are not clear but may be due to population differences. There have been reports on the possible advantage of evolution of the MBL2 variants and the high prevalence of MBL2 variants resulting in reduced plasma MBL concentrations on the African continent [44, 85]. We can only postulate that selection pressure favouring MBL2 variants and reduced plasma levels that occurred in African populations offers protection against the numerous intracellular infections present in this population at large. Thus in our study population we found a high prevalence of plasma MBL deficiency at 18% and the reduced plasma MBL levels were found to be protective against schistosoma infections.
Our findings of high plasma MBL deficiency are consistent with findings from other African populations [16, 17, 30]. Population surveys have shown that the concentration of MBL in plasma/serum ranges from <20μg/L to 10 000μg/L [42] and plasma/serum MBL concentration less than 100μg/L is considered deficient [72, 73]. The plasma MBL level median of 800μg/L and the wide range of 20–7600μg/L for our study population are consistent with studies in African populations [17, 30]. Plasma MBL concentration did not differ between males and females nor by age as reported by others [30].
When analysis was done comparing the proportions of those with plasma MBL concentration at the assay detection limit, a strong association with being HIV positive was detected, supporting our hypothesis of MBL consumption and reduction during HIV infection, consistent with other findings [82]. The possible explanation for the association between this severe MBL deficiency and being HIV positive in our study could be that the low MBL levels may indicate that there is consumption of the MBL protein molecule when it engages in opsonic clearance of the HIV virus, leading to MBL consumption [82] and not accumulation as proposed by others [39, 86]. Available evidence shows that MBL assists in HIV clearance through activation of the compliment system and MBL bound to HIV can be cleared from the circulation by the CIq receptor, a molecule that has been shown to have high affinity for MBL [87, 88] and MBL binds to and neutralises HIV in vitro [89]. However, other recent studies have conflictingly shown that MBL levels remain relatively stable during the course of HIV infection and does not support the theory of MBL consumption during HIV infection [82]. We also no differences in MBL2 levels between CDC HIV categories, CD4+ T cell count and viral load in our study, similar to findings by others [39], in contrast to other findings [39].
Plasma MBL concentration was categorised into normal (above 500μg/L), intermediate (100μg/L- 500μg/L) and deficient (below 100μg/L) [72, 73]. Our results showed no association between this categorisation of plasma MBL level, HIV-1 and schistosoma infections. Our results are in contrast to a report that showed increased susceptibility to HIV infection in people with MBL deficiency [30]. We could not find any similar studies comparing these MBL categories with schistosoma infections.
Assessment of MBL2 polymorphism showed a high prevalence MBL2 deficient genotypes A/O and O/O at 18%, due to high frequency of the C (G>A) variant allele (20%) and high frequencies of variant promoter alleles X and L. All the currently known MBL2 alleles, wild-type A, B (A>G), C(A>G), and D (C>T) and promoter region alleles -550H, -221Y, -550L, -221X, +4P and +4Q, were found in this population. Presence of a high frequency of the variant C MBL2 allele in our population is consistent with findings from other studies on Africans with frequencies as high as 24% in Mozambicans [17], 27% in Gambian adults [45] and 15–38% in several East and West African countries [44, 45]. The MBL genotypes A/A, A/O and O/O in our study correlated with high, intermediate and deficient plasma MBL levels confirming results on other African populations [17, 30]. Promoter region variants have been reported to significantly affect MBL plasma/serum concentration [16], also confirmed by our results. The HY, LY and LX promoters are associated with high, medium and low MBL expression respectively [17]. In our study, the L allele (94%) had the highest frequency and H allele (5%) had the lowest frequency, consistent with other reports on African populations [16].
We found no association between presence of MBL2 structural variants and promoter variants with HIV infection, in accordance with other findings [38, 90], in contrast to reports of significant associations [37, 83, 84, 91]. Similar results to ours of no association between MBL2 polymorphism and HIV infection, reported in a Colombian population, were explained as being due to existence of redundant constitutive acquired immune defence systems that can complement or take up the innate defence functions provided by MBL, which are likely favoured under conditions of high pathogen exposure [38]. It is indeed true that polyparasitism is common in most rural populations in Zimbabwe [61].
There were no differences in distribution of MBL2 promoter region alleles and variants between the HIV positive and HIV infected participants and no role of MBL promoter region variants in increasing susceptibility to HIV infection, in accord with other reports [92, 93]. In contrast promoter region variants LX/LX have been reported to have higher frequency among HIV-infected adults than controls [94]. The H/Y, L/Y and /LX promoters are associated with high, medium and low MBL2 expression respectively [16]. All the currently known MBL2 promoter region variants H, L, Y, X, P and Q were detected in our study, the L allele (47%) had the highest frequency and H allele (3%) had the lowest frequency, in accord with a report on African populations [16]. The H allele is found predominantly in white populations who also have very low L and X alleles [16]. Our results of no association between presence of MBL2 promoter region variants and HIV infection are in accord with other reports in adults [93] but in contrast and children with promoter type L were protected from HIV rapid progression [95].
The reason for the differences in plasma MBL levels, MBL2 genotypes and HIV association outcomes is not clear [54, 55, 96] but this maybe because studies have been carried out in different populations, homosexual groups [37] and some in heterosexuals [30, 38, 91] and also the methods employed in these studies are different with some measuring plasma MBL levels only [30, 39, 81, 82, 97, 98], others assessing structural alleles only [38, 91, 99], some looked at both [37, 80, 83, 84, 90] others in addition looked at MBL2 promoter region alleles [92, 100]. Our study investigated all three MBL parameters, plasma MBL levels, polymorphism in the MBL2 exon 1 gene and promoter region variants. Therefore the conflicting reports on association of HIV infection with MBL levels and genetic polymorphism may be due to differences in HIV transmission route, sample size, ethnicity, environmental conditions and study design as postulated by others [38].
MBL2 genetic variants A/O and O/O and the other promoter variants were not associated with schistosoma co-infections in our study similar to findings in a Nigerian population [59]. However our findings showed that the promoter genotypes L/Y and /LL which code for low plasma levels, both showed significant association with S. haematobium infection, similar to findings in the Nigerian. In contrast to our findings of no association the promoter genotypes H/L and P/P were reported to be protective and P/Q carriers showed increased susceptibility [59].
Participants were further stratified into six MBL2 haplotype groups which were combined into three groups for analysis namely, haplotypes that give normal plasma MBL levels (YA/YA, YA/XA), intermediate levels (XA/XA, YA/YO) and deficient levels (XA/YO, YO/YO) [20, 77]. None of the three MBL2 haplotype groups were associated with HIV-1 nor schistosoma infections in contrast to a report of increased susceptibility where the XA/XA promoter variant had a detrimental effect in HIV vertical transmission [100]. Our results showed no association between HIV-1 and schistosoma infections and the seven MBL2 haplotypes detected in this study, in contrast to findings of significant association between MBL2*HYPA and S. haematobium infection, those with MBL2*HYPA haplotype were reported to be at a lower risk for S. haematobium infection, presence of MBL2*HYPA was protective [59].
MBL deficiency has been reported in several studies to be strongly associated with increased susceptibility to several other infections like respiratory infections and recurrent infections in adults and children, infections that are highly prevalent in Zimbabwe. Available literature therefore shows evidence of low MBL levels to be therefore protective in some diseases but increases susceptibility in some infections. This varying evidence shows that the clinical significance of the MBL2 variants may possibly depend on the population investigated and the type of disease. We found plasma MBL deficiency and MBL2 gene and promoter region variants to play no role in HIV-1 infection, but high plasma MBL levels and the heterozygous promoter genotype LY and homozygous LL increased susceptibility to schistosoma infections.
The main limitation of our study was inclusion of few men in the main MUSH study. There is a gold panning area in the neighbouring district, most men work away from their rural homes and were not available during our study recruitment phase. This has the potential to bias our findings but every effort was done to enrol all eligible men into the main MUSH study and MBL sub-study.
In conclusion, this study showed a high prevalence of plasma MBL deficiency, high frequency of MBL2 genetic variant C (G>A). These findings are consistent with other observations that the C (G>A) variant allele is the predominant variant allele in Sub-Saharan Africa. We found no evidence of an association between MBL deficiency and HIV-1 infection, however plasma MBL levels at assay detection limit were associated with HIV-1 infection indicating a possible role of MBL deficiency in HIV infection. Lower plasma MBL levels were protective against both S. haematobium and S. mansoni infections and higher plasma MBL levels associated with increased susceptibility to schistosoma infections. All the other MBL gene and promoter region variants detected played no role in both HIV and schistosoma infections but presence of promoter region variants LY and LL increased susceptibility to both S. haematobium and S. mansoni infections. The available evidence on the association of polymorphism in the MBL2 promoter region with HIV-1 infection is still conflicting. In light of all this conflicting evidence it would be difficult to recommend the use of MBL plasma levels, MBL2 structural variants and promoter region mutations as a biomarker of HIV infection and for the monitoring of ARV therapy together with viral load and CD4+ T cell lymphocyte counts in the population represented by our study participants. Our results on role of plasma MBL levels in schistosoma infections are in conflict with the only available report that found lower plasma MBL levels to increase susceptibility to schistosoma infections. There is therefore, need to carry out a much bigger study to verify these results. In addition, future immunological studies looking at the association between MBL deficiency and other diseases are highly recommended for the Zimbabwean population where persistent recurrent bacterial, viral, fungal and other parasitic infections remain prevalent affecting both adults and children.
Supporting Information
Data set showing study variables HIV-1, schistososma, plasma MBL and MBL2 genotyping results.
(XLSX)
MBL2 genotypes were categorized into three functional groups A/A, A/O and O/O. There was a statistically significant difference in median plasma MBL concentration between the 3 genotype groups, with the A/A genotype having the highest median plasma MBL concentration and O/O the lowest (AA: 1552μg/L, A/O: 139μg/L, O/O: 20μg/L, p<0.0001). Differences in median MBL concentration by MBL2 genotype were analysed by Kruskal-Wallis non-parametric tests (n = 366). IQR = Interquartile range.
(TIFF)
HYPA haplotype showed the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC haplotype had the lowest levels, at assay detection limit of 20μg/L. We found varying median MBL concentrations due to the effect of HY, LY and LX promoters.
(TIFF)
The MBL2 genotypes were further combined and subdivided into three groups namely genotypes that give normal plasma MBL levels (YA/YA, YA/XA), intermediate levels (XA/XA, YA/YO) and deficient levels (XA/YO, YO/YO) (20, 77). There was a statistically significant difference in median plasma MBL concentration between the 3 haplotype groups, with the (YA/YA, YA/XA) haplotypes showing the highest median plasma MBL concentration1568μg/L, followed by (XA/XA, YA/YO) (151μg/L) and (XA/YO, YO/YO) (20μg/L) showing deficient levels (p<0.0001).
(TIFF)
These primer sequences and specifications were for identification of the MBL2 and promoter region types, according to manufacturer’s instructions (DNA Technology, Denmark).
(DOCX)
The HWE for the above MBL2 SNPs was determined among the HIV negative participants.
(DOCX)
Gene and allele frequencies were obtained by direct gene counting. The three main MBL2 genotype groups AA, AO and OO were further subdivided according to the MBL2 gene and haplotype combinations detected. Twenty-four different complete MBL2 genotypes were detected as shown above. As expected, the HYPA/HYPA genotype which codes for the homozygous normal A/A MBL2 genotype, had the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC/LYQC had the lowest levels, (MBL 20μg/L). HYPA haplotype showed the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC haplotype had the lowest levels (MBL 20μg/L). We found varying median MBL concentrations due to the effect of HY, LY and LX promoters.
(DOCX)
Acknowledgments
We thank the Mupfure Community, the Village Health Workers, the community leaders and the Environmental Health Technicians for the willing participation and contribution to our study; Mupfure Secondary school for accommodation; many thanks to Bente Fredriksen and Vibeke Weirup for technical assistance on MBL assays in Denmark, the National Institute of Health Research (NIHR) Directorate, the technical team for the MUSH study and in particular its core members E. N. Kurewa, N. Taremeredzwa, W. Mashange, A Makuwaza, C. Mukahiwa, S. Nyandoro, W. Soko, B. Mugwagwa, R Gunda and E. Mashiri for tireless hard work under difficult circumstances, Lowence Gomo for data analysis. Finally special gratitude to all the men and women who agreed to participate in the main MUSH study.
Data Availability
Data are from the MUSH study whose authors may be contacted at UNIVERISTY OF THE WITWATERSRAND, JOHANNESBURG SOUTH AFRICA.
Funding Statement
Funding was provided by the Research Foundation of the Capital Region of Denmark and the Sven Andersen Research Foundation (Peter Garred and Hans O. Madsen), UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) through a grant for R Zinyama-Gutsire, grant No A30670, the Essential National Health Research Fund of the Ministry of Health and Child Welfare of Zimbabwe (P355) and a study fellowship for RBL Zinyama-Gutsire from The Fogarty International Centre, National Institutes of Health (NIH-USA) through the International Clinical, Operational and Health Services and Training Award (ICOHRTA) Programme (2008–2010). Per Kallestrup received funding from the Danish AIDS Foundation (F01-18, F01-19), The Danish Embassy in Zimbabwe (2001), The DANIDA Health Programme in Zimbabwe (2001), The US Centres for Disease Control and Prevention Programme in Zimbabwe. The Letten Research Foundation, University of Oslo Norway/Zimbabwe Collaborative PMTCT BHAMC Programme special mention Professor Letten F. Saugstad. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. An abstract of the results of this study was accepted and presented in Durban at the 6th South Africa AIDS conference as a poster, 18–21 June 2013, funded by the SA AIDS conference, abstract number A2288739 and the Letten Research Foundation, University of Oslo Norway/Zimbabwe Collaborative PMTCT BHAMC Programme.
References
- 1. Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Gerstoft J, et al. Schistosomiasis and HIV in rural Zimbabwe: efficacy of treatment of schistosomiasis in individuals with HIV coinfection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;42: 1781–9. [DOI] [PubMed] [Google Scholar]
- 2. Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. The Journal of infectious diseases. 2005;192: 1956–61. [DOI] [PubMed] [Google Scholar]
- 3. Mwanakasale V, Vounatsou P, Sukwa TY, Ziba M, Ernest A, Tanner M. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. The American journal of tropical medicine and hygiene. 2003;69: 420–8. [PubMed] [Google Scholar]
- 4. Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. The American journal of tropical medicine and hygiene. 1997;56: 515–21. [DOI] [PubMed] [Google Scholar]
- 5. Mwinzi PN, Karanja DM, Colley DG, Orago AS, Secor WE. Cellular immune responses of schistosomiasis patients are altered by human immunodeficiency virus type 1 coinfection. The Journal of infectious diseases. 2001;184: 488–96. [DOI] [PubMed] [Google Scholar]
- 6. Bentwich Z, Maartens G, Torten D, Lal AA, Lal RB. Concurrent infections and HIV pathogenesis. AIDS. 2000;14: 2071–81. [DOI] [PubMed] [Google Scholar]
- 7.Report on the Global AIDS epidemic, Geneva, Switzerland. 2012.
- 8. Gallo RC, Sarin PS, Gelmann EP, Robert-Guroff M, Richardson E, Kalyanaraman VS, et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983;220: 865–7. [DOI] [PubMed] [Google Scholar]
- 9. Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. The New England journal of medicine. 1995;332: 201–8. [DOI] [PubMed] [Google Scholar]
- 10. Tang J, Kaslow RA. The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy. AIDS. 2003;17 Suppl 4: S51–60. [DOI] [PubMed] [Google Scholar]
- 11. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta tropica. 2000;77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nature reviews Microbiology. 2004;2: 12–3. [DOI] [PubMed] [Google Scholar]
- 13. Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta tropica. 2002;82: 139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368: 1106–18. [DOI] [PubMed] [Google Scholar]
- 15. Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40: 37–44. [DOI] [PubMed] [Google Scholar]
- 16. Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155: 3013–20. [PubMed] [Google Scholar]
- 17. Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161: 3169–75. [PubMed] [Google Scholar]
- 18. Turner MW. Deficiency of mannan binding protein—a new complement deficiency syndrome. Clinical and experimental immunology. 1991;86 Suppl 1: 53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunology today. 1996;17: 532–40. [DOI] [PubMed] [Google Scholar]
- 20. Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency—revisited. Molecular immunology. 2003;40:73–84. [DOI] [PubMed] [Google Scholar]
- 21. Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Molecular immunology. 2001; 38: 133–49. [DOI] [PubMed] [Google Scholar]
- 22. Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunological reviews. 2001;180: 86–99. [DOI] [PubMed] [Google Scholar]
- 23. Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. The Journal of Experimental Medicine. 1992;176: 1497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong NK, Kojima M, Dobo J, Ambrus G, Sim RB. Activities of the MBL-associated serine proteases (MASPs) and their regulation by natural inhibitors. Molecular immunology. 1999;36: 853–61. [DOI] [PubMed] [Google Scholar]
- 25. Sekine H, Takahashi M, Iwaki D, Fujita T. The role of MASP-1/3 in complement activation. Advances in experimental medicine and biology. 2013;735: 41–53. [DOI] [PubMed] [Google Scholar]
- 26. UNAIDS. UNAIDS, Joint United Nations Programme on HIV/AIDS (UNAIDS). 2013 Report on the global HIV/AIDS epidemic UNAIDS, Geneva: 2013. [Google Scholar]
- 27. Bhutta ZA, Sommerfeld J, Lassi ZS, Salam RA, Das JK. Global burden, distribution, and interventions for infectious diseases of poverty. Infectious diseases of poverty. 2014;3: 21 10.1186/2049-9957-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das JK, Salam RA, Bhutta ZA. Global burden of childhood diarrhea and interventions. Current Opinion in Infectious Diseases. 2014;27: 451–8 10.1097/QCO.0000000000000096 [DOI] [PubMed] [Google Scholar]
- 29. Corbett EL, Bandason T, Cheung YB, Makamure B, Dauya E, Munyati SS, et al. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2009; 13: 1231–7. [PMC free article] [PubMed] [Google Scholar]
- 30. Garred P, Richter C, Andersen AB, Madsen HO, Mtoni I, Svejgaard A, et al. (1997) Mannan-binding lectin in the sub-Saharan HIV and tuberculosis epidemics. Scandinavian journal of immunology. 1997;46: 204–8. [DOI] [PubMed] [Google Scholar]
- 31. Mhandire K, Pharo G, Kandawasvika GQ, Duri K, Swart M, Stray-Pedersen B, et al. (2014) How Does Mother-to-Child Transmission of HIV Differ Among African Populations? Lessons from MBL2 Genetic Variation in Zimbabweans. OMICS: a journal of integrative biology. 2014;18: 454–60 10.1089/omi.2013.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt J. Glycans with N-acetyllactosamine type 2-like residues covering adult Schistosoma mansoni, and glycomimesis as a putative mechanism of immune evasion. Parasitology. 1995;111: 325–36. [DOI] [PubMed] [Google Scholar]
- 33. Xu X, Stack RJ, Rao N, Caulfield JP. (1994) Schistosoma mansoni: fractionation and characterization of the glycocalyx and glycogen-like material from cercariae. Experimental parasitology. 1994;79(3): 399–409. [DOI] [PubMed] [Google Scholar]
- 34. Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue antigens. 2006;68: 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klabunde J, Berger J, Jensenius JC, Klinkert MQ, Zelck UE, Kremsner PG, et al. Schistosoma mansoni: adhesion of mannan-binding lectin to surface glycoproteins of cercariae and adult worms. Experimental parasitology. 2000;95: 231–9. [DOI] [PubMed] [Google Scholar]
- 36. Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite immunology. 2005;27: 257–64. [DOI] [PubMed] [Google Scholar]
- 37. Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349: 236–40. [DOI] [PubMed] [Google Scholar]
- 38. Malik S, Arias M, Di Flumeri C, Garcia LF, Schurr E. Absence of association between mannose-binding lectin gene polymorphisms and HIV-1 infection in a Colombian population. Immunogenetics. 2003;55: 49–52. [DOI] [PubMed] [Google Scholar]
- 39. Senaldi G, Davies ET, Mahalingam M, Lu J, Pozniak A, Peakman M, et al. Circulating levels of mannose binding protein in human immunodeficiency virus infection. The Journal of infection. 1995;31: 145–8. [DOI] [PubMed] [Google Scholar]
- 40. Chatterjee A, Rathore A, Yamamoto N, Dhole TN. Mannose-binding lectin (+54) exon-1 gene polymorphism influence human immunodeficiency virus-1 susceptibility in North Indians. Tissue antigens. 2011;77: 18–22. 10.1111/j.1399-0039.2010.01563.x [DOI] [PubMed] [Google Scholar]
- 41. Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scandinavian journal of immunology. 2002; 56: 630–41. [DOI] [PubMed] [Google Scholar]
- 42. Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. Journal of immunological methods. 2003;241: 33–42. [DOI] [PubMed] [Google Scholar]
- 43. Lipscombe RJ, Sumiya M, Summerfield JA, Turner MW. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunology. 1995;85: 660–7. [PMC free article] [PubMed] [Google Scholar]
- 44. Mombo LE, Lu CY, Ossari S, Bedjabaga I, Sica L, Krishnamoorthy R, et al. Mannose-binding lectin alleles in sub-Saharan Africans and relation with susceptibility to infections. Genes and immunity. 2003;4: 362–7. [DOI] [PubMed] [Google Scholar]
- 45. Lipscombe RJ, Sumiya M, Hill AV, Lau YL, Levinsky RJ, Summerfield JA, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Human molecular genetics. 1992;1: 709–15. [DOI] [PubMed] [Google Scholar]
- 46. Lipscombe RJ, Beatty DW, Ganczakowski M, Goddard EA, Jenkins T, Lau YL, et al. Mutations in the human mannose-binding protein gene: frequencies in several population groups. European journal of human genetics: EJHG. 1996;4: 13–9. [DOI] [PubMed] [Google Scholar]
- 47. Mombo LE, Ntoumi F, Bisseye C, Ossari S, Lu CY, Nagel RL, et al. Human genetic polymorphisms and asymptomatic Plasmodium falciparum malaria in Gabonese schoolchildren. The American journal of tropical medicine and hygiene. 2003;68: 186–90. [PubMed] [Google Scholar]
- 48. Kuhn L, Coutsoudis A, Trabattoni D, Archary D, Rossi T, Segat L, et al. Synergy between mannose-binding lectin gene polymorphisms and supplementation with vitamin A influences susceptibility to HIV infection in infants born to HIV-positive mothers. The American journal of clinical nutrition. 2006;84: 610–5. [DOI] [PubMed] [Google Scholar]
- 49. Koch A, Melbye M, Sorensen P, Homoe P, Madsen HO, Molbak K, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA: the journal of the American Medical Association. 2001;285: 1316–21. [DOI] [PubMed] [Google Scholar]
- 50. Garred P, Nielsen MA, Kurtzhals JA, Malhotra R, Madsen HO, Goka BQ, et al. Mannose-binding lectin is a disease modifier in clinical malaria and may function as opsonin for Plasmodium falciparum-infected erythrocytes. Infection and immunity. 2003;71: 5245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Messias-Reason IJ, Boldt AB, Moraes Braga AC, Von Rosen Seeling Stahlke E, Dornelles L, Pereira-Ferrari L, et al. The association between mannan-binding lectin gene polymorphism and clinical leprosy: new insight into an old paradigm. The Journal of infectious diseases. 2007;196: 1379–85. [DOI] [PubMed] [Google Scholar]
- 52. Santos IK, Costa CH, Krieger H, Feitosa MF, Zurakowski D, Fardin B, et al. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infection and immunity. 2001;69: 5212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alonso DP, Ferreira AF, Ribolla PE, de Miranda Santos IK, do Socorro Pires e Cruz M, Aecio de Carvalho F, et al. Genotypes of the mannan-binding lectin gene and susceptibility to visceral leishmaniasis and clinical complications. The Journal of infectious diseases. 2007;195: 1212–7. [DOI] [PubMed] [Google Scholar]
- 54. Ji X, Gewurz H, Spear GT. Mannose binding lectin (MBL) and HIV. Molecular immunology. 2005;42(2):145–52. [DOI] [PubMed] [Google Scholar]
- 55. Li H, Fu WP, Hong ZH. Replication study in Chinese Han population and meta-analysis supports association between the MBL2 gene polymorphism and HIV-1 infection. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2013;20: 163–70. 10.1016/j.meegid.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 56. Valles X, Roca A, Lozano F, Morais L, Suarez B, Casals F, et al. Serotype-specific pneumococcal disease may be influenced by mannose-binding lectin deficiency. The European respiratory journal. 2010;36(4): 856–63. 10.1183/09031936.00171409 [DOI] [PubMed] [Google Scholar]
- 57. Valles X, Sarrias MR, Casals F, Farnos M, Piner R, Suarez B, et al. Genetic and structural analysis of MBL2 and MASP2 polymorphisms in south-eastern African children. Tissue antigens. 2009;74: 298–307. 10.1111/j.1399-0039.2009.01328.x [DOI] [PubMed] [Google Scholar]
- 58. Kristensen IA, Thiel S, Steffensen R, Madhi S, Sorour G, Olsen J. Mannan-binding lectin and RSV lower respiratory tract infection leading to hospitalization in children: a case-control study from Soweto, South Africa. Scandinavian journal of immunology. 2004;60: 184–8. [DOI] [PubMed] [Google Scholar]
- 59. Antony JS, Ojurongbe O, van Tong H, Ouf EA, Engleitner T, Akindele AA, et al. Mannose-binding lectin and susceptibility to schistosomiasis. The Journal of infectious diseases. 2013;207: 1675–83. 10.1093/infdis/jit081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimbabwe National AIDS Council. National HIV/AIDS Report. 2014.
- 61. Midzi N, Sangweme D, Zinyowera S, Mapingure MP, Brouwer KC, Munatsi A, et al. The burden of polyparasitism among primary schoolchildren in rural and farming areas in Zimbabwe. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102: 1039–45. 10.1016/j.trstmh.2008.05.024 [DOI] [PubMed] [Google Scholar]
- 62. Mduluza T, Ndhlovu PD, Madziwa TM, Midzi N, Zinyama R, Turner CM, et al. The impact of repeated treatment with praziquantel of schistosomiasis in children under six years of age living in an endemic area for Schistosoma haematobium infection. Memorias do Instituto Oswaldo Cruz. 2001;6 Suppl:157–64. [DOI] [PubMed] [Google Scholar]
- 63. Midzi N, Mtapuri-Zinyowera S, Sangweme D, Paul NH, Makware G, Mapingure MP, et al. Efficacy of integrated school based de-worming and prompt malaria treatment on helminths-Plasmodium falciparum co-infections: A 33 months follow up study. BMC international health and human rights. 2011;11:9 10.1186/1472-698X-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chirundu D, Chimusoro A, Jones D, Midzi N, Mabaera B, Apollo T, et al. Schistosomiasis infection among school children in the Zhaugwe resettlement area, Zimbabwe April 2005. The Central African Journal of Medicine. 2007;53(1–4): 6–11. [DOI] [PubMed] [Google Scholar]
- 65. Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Erikstrup C, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. The Journal of infectious diseases. 2005;191: 1311–20. [DOI] [PubMed] [Google Scholar]
- 66. Mott KE, Baltes R, Bambagha J, Baldassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmedizin und Parasitologie. 1982;33(4): 227–8. [PubMed] [Google Scholar]
- 67. Doehring E, Vester U, Ehrich JH, Feldmeier H. Circadian variation of ova excretion, proteinuria, hematuria, and leukocyturia in urinary schistosomiasis. Kidney international. 1985; 27(4): 667–71. [DOI] [PubMed] [Google Scholar]
- 68. Weber MC, Blair DM, de Clarke VV. The distribution of viable and non-viable eggs of schistosoma haematobium in the urine. The Central African journal of medicine 1969. 15: 27–30. [PubMed] [Google Scholar]
- 69. Knight WB, Hiatt RA, Cline BL, Ritchie LS. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni eggs. The American journal of tropical medicine and hygiene. 1976;25: 818–23. [DOI] [PubMed] [Google Scholar]
- 70. Deelder AM, Qian ZL, Kremsner PG, Acosta L, Rabello AL, Enyong P, et al. Quantitative diagnosis of Schistosoma infections by measurement of circulating antigens in serum and urine. Tropical and geographical medicine. 1994;46: 233–8. [PubMed] [Google Scholar]
- 71. Garred P, Madsen HO, Kurtzhals JA, Lamm LU, Thiel S, Hey AS, et al. Diallelic polymorphism may explain variations of the blood concentration of mannan-binding protein in Eskimos, but not in black Africans. European journal of immunogenetics: official journal of the British Society for Histocompatibility and Immunogenetics. 1992; 19: 403–12. [DOI] [PubMed] [Google Scholar]
- 72. Kruse C, Rosgaard A, Steffensen R, Varming K, Jensenius JC, Christiansen OB. Low serum level of mannan-binding lectin is a determinant for pregnancy outcome in women with recurrent spontaneous abortion. American journal of obstetrics and gynecology. 2002;187: 1313–20. [DOI] [PubMed] [Google Scholar]
- 73. Eisen DP, Dean MM, Boermeester MA, Fidler KJ, Gordon AC, Kronborg G, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;47: 510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Egli A, Schafer J, Osthoff M, Thiel S, Mikkelsen C, Rauch A, et al. Low levels of mannan-binding lectin or ficolins are not associated with an increased risk of cytomegalovirus disease in HIV-infected patients. PloS one. 2013; 8: e51983 10.1371/journal.pone.0051983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research. 1988;16: 1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jha AN, Sundaravadivel P, Singh VK, Pati SS, Patra PK, Kremsner PG, et al. MBL2 variations and malaria susceptibility in Indian populations. Infection and immunity. 2014;82: 52–61. 10.1128/IAI.01041-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vengen IT, Madsen HO, Garred P, Platou C, Vatten L, Videm V. Mannose-binding lectin deficiency is associated with myocardial infarction: the HUNT2 study in Norway. PloS one. 2012;7: e42113 10.1371/journal.pone.0042113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell research. 2009;19: 519–23. 10.1038/cr.2009.33 [DOI] [PubMed] [Google Scholar]
- 79. Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell research. 2005;15: 97–8. [DOI] [PubMed] [Google Scholar]
- 80. Vallinoto AC, Freitas FB, Guirelli I, Machado LF, Azevedo VN, Cayres-Vallinoto I, et al. Characterization of mannose-binding lectin plasma levels and genetic polymorphisms in HIV-1-infected individuals. Revista da Sociedade Brasileira de Medicina Tropical. 2011;44: 1–3. [DOI] [PubMed] [Google Scholar]
- 81. Jambo KC, French N, Zijlstra E, Gordon SB. AIDS patients have increased surfactant protein D but normal mannose binding lectin levels in lung fluid. Respiratory research. 2007;8: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nielsen SL, Andersen PL, Koch C, Jensenius JC, Thiel S. The level of the serum opsonin, mannan-binding protein in HIV-1 antibody-positive patients. Clinical and experimental immunology. 1995;100: 219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sheng A, Lan J, Wu H, Lu J, Wang Y, Chu Q, et al. A clinical case-control study on the association between mannose-binding lectin and susceptibility to HIV-1 infection among northern Han Chinese population. International journal of immunogenetics. 2010; 37(6): 445–54. 10.1111/j.1744-313X.2010.00946.x [DOI] [PubMed] [Google Scholar]
- 84. Tan Y, Liu L, Luo P, Wang A, Jia T, Shen X, et al. Association between mannose-binding lectin and HIV infection and progression in a Chinese population. Molecular immunology. 2009;47: 632–8. 10.1016/j.molimm.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 85. Hoal-Van Helden EG, Epstein J, Victor TC, Hon D, Lewis LA, Beyers N, et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatric research. 1999;45: 459–64. [DOI] [PubMed] [Google Scholar]
- 86. Senaldi G, Peakman M, McManus T, Davies ET, Tee DE, Vergani D. Activation of the complement system in human immunodeficiency virus infection: relevance of the classical pathway to pathogenesis and disease severity. The Journal of infectious diseases 1990; 62: 1227–32. [DOI] [PubMed] [Google Scholar]
- 87. Haurum JS, Thiel S, Jones IM, Fischer PB, Laursen SB, Jensenius JC. Complement activation upon binding of mannan-binding protein to HIV envelope glycoproteins. AIDS. 1993;7: 1307–13. [DOI] [PubMed] [Google Scholar]
- 88. Malhotra R, Sim RB, Reid KB.Interaction of C1q, and other proteins containing collagen-like domains, with the C1q receptor. Biochemical Society transactions. 1990; 18:1145–8. [DOI] [PubMed] [Google Scholar]
- 89. Ezekowitz RA, Kuhlman M, Groopman JE, Byrn RA. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. The Journal of experimental medicine. 1989;169: 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Garcia-Laorden MI, Pena MJ, Caminero JA, Garcia-Saavedra A, Campos-Herrero MI, Caballero A, et al. Influence of mannose-binding lectin on HIV infection and tuberculosis in a Western-European population. Molecular immunology. 2006;43: 2143–50. [DOI] [PubMed] [Google Scholar]
- 91. Pastinen T, Liitsola K, Niini P, Salminen M, Syvanen AC. Contribution of the CCR5 and MBL genes to susceptibility to HIV type 1 infection in the Finnish population. AIDS research and human retroviruses. 1998;14: 695–8. [DOI] [PubMed] [Google Scholar]
- 92. Catano G, Agan BK, Kulkarni H, Telles V, Marconi VC, Dolan MJ, et al. Independent effects of genetic variations in mannose-binding lectin influence the course of HIV disease: the advantage of heterozygosity for coding mutations. The Journal of infectious diseases. 2008;198: 72–80. 10.1086/588712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vallinoto AC, Muto NA, Alves AE, Machado LF, Azevedo VN, Souza LL, et al. Characterization of polymorphisms in the mannose-binding lectin gene promoter among human immunodeficiency virus 1 infected subjects. Memorias do Instituto Oswaldo Cruz 2008;103: 645–9. [DOI] [PubMed] [Google Scholar]
- 94. da Silva GK, Guimraes R, Mattevi VS, Lazzaretti RK, Sprinz E, Kuhmmer R, et al. The role of mannose-binding lectin gene polymorphisms in susceptibility to HIV-1 infection in Southern Brazilian patients. AIDS. 2011;25: 411–8. 10.1097/QAD.0b013e328342fef1 [DOI] [PubMed] [Google Scholar]
- 95. Boniotto M, Crovella S, Pirulli D, Scarlatti G, Spano A, Vatta L, et al. Polymorphisms in the MBL2 promoter correlated with risk of HIV-1 vertical transmission and AIDS progression. Genes and immunity. 2000;1: 346–8. [DOI] [PubMed] [Google Scholar]
- 96. Eisen S, Dzwonek A, Klein NJ. Mannose-binding lectin in HIV infection. Future virology. 2008;3: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prohaszka Z, Thiel S, Ujhelyi E, Szlavik J, Banhegyi D, Fust G. Mannan-binding lectin serum concentrations in HIV-infected patients are influenced by the stage of disease. Immunology letters. 1997;58: 171–5. [DOI] [PubMed] [Google Scholar]
- 98. Heggelund L, Mollnes TE, Ueland T, Christophersen B, Aukrust P, Froland SS. Mannose-binding lectin in HIV infection: relation to disease progression and highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32: 354–61. [DOI] [PubMed] [Google Scholar]
- 99. Maas J, de Roda Husman AM, Brouwer M, Krol A, Coutinho R, Keet I, et al. Presence of the variant mannose-binding lectin alleles associated with slower progression to AIDS. Amsterdam Cohort Study. AIDS. 1998;12: 2275–80. [DOI] [PubMed] [Google Scholar]
- 100. Mangano A, Rocco C, Marino SM, Mecikovsky D, Genre F, Aulicino P, et al. Detrimental effects of mannose-binding lectin (MBL2) promoter genotype XA/XA on HIV-1 vertical transmission and AIDS progression. The Journal of infectious diseases.2008;198: 694–700. 10.1086/590498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data set showing study variables HIV-1, schistososma, plasma MBL and MBL2 genotyping results.
(XLSX)
MBL2 genotypes were categorized into three functional groups A/A, A/O and O/O. There was a statistically significant difference in median plasma MBL concentration between the 3 genotype groups, with the A/A genotype having the highest median plasma MBL concentration and O/O the lowest (AA: 1552μg/L, A/O: 139μg/L, O/O: 20μg/L, p<0.0001). Differences in median MBL concentration by MBL2 genotype were analysed by Kruskal-Wallis non-parametric tests (n = 366). IQR = Interquartile range.
(TIFF)
HYPA haplotype showed the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC haplotype had the lowest levels, at assay detection limit of 20μg/L. We found varying median MBL concentrations due to the effect of HY, LY and LX promoters.
(TIFF)
The MBL2 genotypes were further combined and subdivided into three groups namely genotypes that give normal plasma MBL levels (YA/YA, YA/XA), intermediate levels (XA/XA, YA/YO) and deficient levels (XA/YO, YO/YO) (20, 77). There was a statistically significant difference in median plasma MBL concentration between the 3 haplotype groups, with the (YA/YA, YA/XA) haplotypes showing the highest median plasma MBL concentration1568μg/L, followed by (XA/XA, YA/YO) (151μg/L) and (XA/YO, YO/YO) (20μg/L) showing deficient levels (p<0.0001).
(TIFF)
These primer sequences and specifications were for identification of the MBL2 and promoter region types, according to manufacturer’s instructions (DNA Technology, Denmark).
(DOCX)
The HWE for the above MBL2 SNPs was determined among the HIV negative participants.
(DOCX)
Gene and allele frequencies were obtained by direct gene counting. The three main MBL2 genotype groups AA, AO and OO were further subdivided according to the MBL2 gene and haplotype combinations detected. Twenty-four different complete MBL2 genotypes were detected as shown above. As expected, the HYPA/HYPA genotype which codes for the homozygous normal A/A MBL2 genotype, had the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC/LYQC had the lowest levels, (MBL 20μg/L). HYPA haplotype showed the highest median plasma MBL concentration (median MBL 2464μg/L, IQR 1336–3368μg/L) and LYQC haplotype had the lowest levels (MBL 20μg/L). We found varying median MBL concentrations due to the effect of HY, LY and LX promoters.
(DOCX)
Data Availability Statement
Data are from the MUSH study whose authors may be contacted at UNIVERISTY OF THE WITWATERSRAND, JOHANNESBURG SOUTH AFRICA.