Abstract
This study investigates the role of human agency in the gene flow and geographical distribution of the Australian baobab, Adansonia gregorii. The genus Adansonia is a charismatic tree endemic to Africa, Madagascar, and northwest Australia that has long been valued by humans for its multiple uses. The distribution of genetic variation in baobabs in Africa has been partially attributed to human-mediated dispersal over millennia, but this relationship has never been investigated for the Australian species. We combined genetic and linguistic data to analyse geographic patterns of gene flow and movement of word-forms for A. gregorii in the Aboriginal languages of northwest Australia. Comprehensive assessment of genetic diversity showed weak geographic structure and high gene flow. Of potential dispersal vectors, humans were identified as most likely to have enabled gene flow across biogeographic barriers in northwest Australia. Genetic-linguistic analysis demonstrated congruence of gene flow patterns and directional movement of Aboriginal loanwords for A. gregorii. These findings, along with previous archaeobotanical evidence from the Late Pleistocene and Holocene, suggest that ancient humans significantly influenced the geographic distribution of Adansonia in northwest Australia.
Introduction
The role of humans in shaping crop genetic diversity has always been considered an integral factor in the evolution of agriculture in various regions of the world [1–3]. Interdisciplinary research combining genetics, linguistics, and archaeobotany has further enhanced understanding of the geographic patterns of animal and crop domestication and subsequent diffusion by humans [1–3]. Yet, there is very little comparable research on how anthropogenic agency has influenced the evolution and distribution of uncultivated plants that, nonetheless, have a long history of human use [4–6]. A striking example is that of Adansonia, the baobab tree, an iconic genus endemic to Africa, Madagascar, and the Kimberley region of northwest Australia [7,8]. These giant, long-lived trees hold significant cultural symbolism and multipurpose value as sources of food, medicine, water storage, shelter, and raw material for artisanal products in all these places [9–17]. Although there is no evidence of baobabs being cultivated historically, the distribution of the African baobab species, Adansonia digitata L., has been closely linked to human dispersal and settlement patterns [18,19]. This association is also recognised by the diversity and borrowing of terms for baobabs between language groups in Africa [4, 16]. In contrast, previous research on the evolution and geographic distribution of the Australian baobab, Adansonia gregorii F. Muell., has been based on the assumption of long-term natural processes [7,8] without any significant influence of human agency. This assumption may have stemmed from the long-held view of Aboriginal Australia as a ‘continent of hunter-gatherers’ [20–24] where anthropogenic agency was limited to ‘fire-stick farming’ of landscapes for nomadic foraging and hunting [25,26]. We explore the role of humans in shaping the evolution of A. gregorii by determining whether the geographic distribution of genetic diversity is explained, in part, by patterns of human migration, as inferred from linguistic analysis.
Levels of genetic divergence show that A. gregorii separated from other Adansonia species more recently than the break-up of Gondwana, but before the arrival of humans in Australia [7,8]. Leong-Pock Tsy et al. [27] demonstrated that A. digitata seeds retain their viability in seawater, making oceanic current dispersal feasible. From this it can be inferred that A. gregorii has been in Australia for longer than humans. There is also the possibility, albeit a less parsimonious explanation, that the species arrived more recently from an unknown population which is now extinct. One hypothesis outlining how A. gregorii may have arrived in Australia with humans has been explored in more detail by Pettigrew [28].
Adansonia gregorii, known in Australia as ‘boab’, is mainly distributed across the Kimberley region of northwest Australia, with a small extension eastward into the Victoria River District in the Northern Territory. The distribution of A. gregorii in the Kimberley extends from the northern coastline to the edge of the Great Sandy Desert and the Tanami Desert [17,29–32]. The Kimberley region represents the westernmost extent of the Australian Monsoon Tropics (AMT), which is characterised by highly seasonal rainfall and savanna vegetation [17,33]. The tree has been introduced more recently in urban centers of northern Australia for ornamental purposes [17].
The AMT biome is bounded to the south by arid habitats, which began developing in the Late Cenozoic and contain distinctly different biota [34,35]. The major biogeographical divide in northwest Australia is between the Kimberley to the west and Arnhem Land to the east, with more localized and specific barriers created by major river drainage systems [36–39]. Within the Kimberley, phylogeographic patterns for rock-wallabies (Petrogale spp.) and other species suggest an East-West Divide running through Central Kimberley [34,37,39]. Despite evidence of biogeographic barriers, a previously detailed population genetic analysis of A. gregorii has demonstrated that there is little genetic structure, with F ST values non-significant between most populations [40]. Low geographic structure could be explained by a relatively recent arrival in the Kimberley, a recent genetic bottleneck, or high dispersal rates across the species’ range. For reasons detailed in Bell et al. [40], high dispersal is the most likely explanation.
In this paper we sought to evaluate the latter hypothesis – that the low levels of genetic structure within A. gregorii are due to high levels of gene flow and, specifically, that human-mediated seed dispersal has been an important evolutionary factor in the history of this species. Pollination in A. gregorii might occasionally entail birds or bats, but it appears that hawkmoths are the major pollinating agents [17,29,33,41,42]. Pollination-mediated gene flow is limited to the paternal genome and, in insect pollinated species, is often a less effective mechanism of long-distance gene flow than fruit dispersal [43–45]. Floodwaters could explain some fruit dispersal [7,16], but would probably not spread seeds beyond the edges of seasonal waterways and alluvial flats due to the fragile and dehiscent nature of the A. gregorii pericarp [7,33]. Other seed dispersing agents could be mammals such as rock wallabies (Petrogale spp.), other wallabies and kangaroos (Macropus spp.), which eat the fruit and disperse the seeds in their scat [17]. However, phylogeographic studies of the short-eared rock-wallaby (P. brachyotis), showed strong genetic structure, suggesting that at least this species has limited dispersal ability across biogeographic barriers in the Kimberley [39,46].
Anthropogenic agency has not been formally considered as a gene flow vector for A. gregorii, despite archaeological evidence of its long-term use by Aboriginal groups in the region [28,47–50]. This omission may be due to the fact that the boab was not cultivated historically, or not considered part of the food crops traditionally harvested by Aboriginal groups. Also, the presence of boab fruit pod remains at one or two archaeological sites is not sufficient to demonstrate anthropogenic dispersal across the species’ geographic range. Additional evidence is needed to evaluate whether humans have played a role in boab gene flow. We reasoned that historical linguistics, which has been used in combination with other sources of data to trace geographic patterns of diffusion of domesticated species [1–3], could be applied to a non-domesticated tree species such as A. gregorii [6]. Specifically, in this study, we investigate the role of human agency in the gene flow of A. gregorii by testing for congruence between the spatial distribution of genetic variation in A. gregorii trees and associated word-forms in the Aboriginal languages of northwest Australia. We demonstrate a high level of spatial overlap between the genetic and linguistic data. Our results indicate that, as previously shown for A. digitata in Africa [19,51,52], ancient humans have influenced the distribution of genetic diversity of A. gregorii in northern Australia, probably by acting as seed dispersal agents over long distances.
Materials and Methods
This study makes use of two recently published data sets relating to A. gregorii from different fields of research. The first of these is genetic data from six nuclear microsatellite loci, of 220 A. gregorii individuals [40] (S1 Dataset). Although the number of microsatellite loci is low and can render some quantitative methods inaccurate, we used this data to make qualitative observations on patterns of dispersal and relationship between populations. The second data set consists of the words used for the boab tree in Aboriginal languages from across the species range [53] (S2 Dataset). This study brings these two datasets together, analysing them simultaneously to detect any congruent patterns.
Phylogenetic analysis of microsatellite data
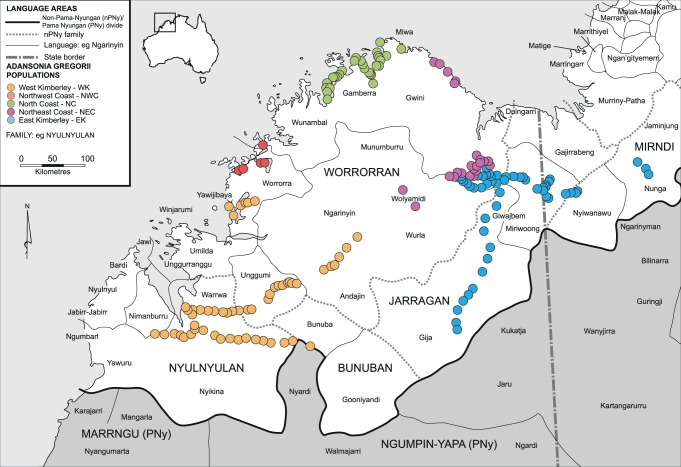
We conducted phylogenetic analysis of the A. gregorii populations shown in Fig. 1, using previously obtained microsatellite data [40]. Collection of plant material, with appropriate permissions, deposition of specimens, and laboratory analyses are described in Bell et al. [40]. This study used the spatial principal components analysis (sPCA) to map and define genetic populations, which identified five genetically differentiated clusters: West Kimberley (WK); East Kimberley (EK); North West Coast (NWC), North Coast (NC), and North East Coast (NEC). Genetic divergence between these clusters was weak, but statistically significant [40], hence these were treated as five populations for subsequent analyses.
Fig 1. Graphical representation of the five inferred Adansonia gregorii populations from the sPCA, shown on a map of Aboriginal language areas of the Kimberley region.
To obtain an estimate of the relationships among the five populations, we first obtained 1000 bootstrap replicates of the microsatellite data. We calculated pairwise genetic distances between populations with the D C method [54] for each bootstrap replicate in Phylip v 3.69 [55]. These distance matrices were then used to construct unrooted neighbour-joining trees, and a bootstrap consensus tree in Phylip. While more complex methods have been found to provide greater accuracy in dating divergence events from microsatellite data, the simple D C method was judged suitable because it performs as well as these methods at phylogenetic reconstruction [56]. The resulting tree was midpoint rooted.
Phylogeny of linguistic data
The Aboriginal languages of Australia are broadly classified into a number of families, Pama-Nyungan (PNy) and ten or more non-Pama-Nyungan (nPNy) families [57,58]. The PNy language groups are most widespread across the continent, while the nPNy families (excluding Tasmania) exist primarily in northwest Australia across the Kimberley and western Arnhem Land [58,59]. The PNy language subgroups and nPNy families have been distinguished by the comparative method [60], including grammatical morphology and measures of difference in vocabulary [57], with sub-groups identified by sets of ‘shared innovations’ [61]. A Neighbour Joining analysis of twenty-one Kimberley languages using the method described in Hudson and Bryant [62] was published by McGregor and Rumsey [63]. The tree generated from that analysis showed the relationships between these Kimberley languages based on lexical resemblances from a basic wordlist of 105 meanings, containing minimum numbers of loanwords [63]. A greater degree of proximity and shared branches in the phylogeny indicates higher lexical similarity between the respective languages. We used this neighbour-joining tree in conjunction with a more general consensus about family classification using the comparative method in discussion of the origins of words for the boab tree [64].
Determining boab proto-words, inherited forms, and loanwords
Data on boab word-forms and related terminology was drawn from both published and unpublished sources and transliterated into a standard orthography [53] (S2 Dataset). The distinction between inheritance (vertical transmission) and borrowing (horizontal transmission) of word-forms is vital to our analysis. Proto-words are word-forms that can be traced back through successive, plausible steps to a common ancestral word-form in an ancestral language (known as the proto-language) [60]. In vertical transmission, proto-words are inherited through nodes in a phylogeny to a number of more recent descendant languages, often changing meaning and form over time. Horizontal transmission, or diffusion, occurs through borrowing, whereby words enter and become adopted in other languages that may or may not be closely related by inheritance. There are several types of evidence used to detect loanwords and their original source [6]. Borrowed words in a language are ones that cannot be connected to plausible proto-words in that language but instead show strong similarity to words in another language. The identification of a loanword is further corroborated when the word can be analyzed into meaningful word-parts in the other language (the putative loan source) but not in a language into which it has been borrowed.
Genetic-Linguistic Analysis
The spatial distribution of each language was defined following Harvey [58] and McGregor and Rumsey [63]. The A. gregorii distribution includes five nPNy families (Worrorran, Nyulnyulan, Bunuban, Jarragan, and Mirndi) and two PNy language subgroups (Marrngu and Ngumpin-Yapa) (Fig. 1). Three sets of relationships of genetic and linguistic data for A. gregorii were examined for evidence of human-mediated dispersal across the plant’s geographic range.
Boab populations and language families
Geographic congruence between A. gregorii populations and language areas was examined by superimposing the genetic data on the phylogenetic tree of the main Kimberley language groups analysed by McGregor and Rumsey [63], and conversely, by superimposing the language group areas [58] in which the boab populations occur on the neighbour-joining tree of the five genetic populations (Fig. 2A and B). A high level of geographic congruence between the A. gregorii genetic populations and the language areas they occupy would be consistent with the idea that people have moved boabs extensively within language areas, but not so much between language areas.
Fig 2. A. Neighbour-joining tree of lexical resemblance among Worrorran and nearby Aboriginal languages of the Kimberley, following McGregor and Rumsey [63].
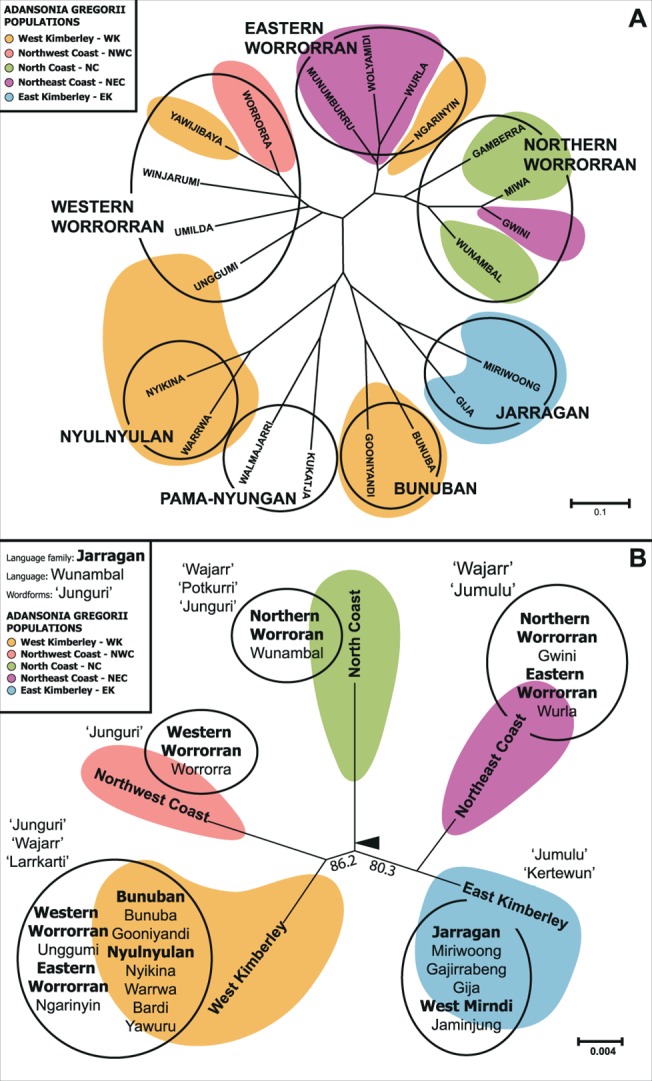
Twenty-one languages (19 belonging to nPNy families and 2 belonging to the Ngumpin PNy sub-group) were chosen for analysis on the basis of availability of relatively reliable information. Distance between nodes indicates degree of dissimilarity between the languages according to the scale shown. The five inferred A. gregorii populations have been superimposed on the tree to visualize spatial congruence with language groups. B. Neighbour-joining phylogeny of the five inferred A. gregorii populations showing their occurrence in language group areas according to Harvey [58]. Distance between nodes indicates genetic divergence between populations calculated with the D C method. Arrow indicates mid-point. The word-forms for boabs associated with each of the five inferred A. gregorii populations are shown in quotation marks.
Analysis of molecular variance (AMOVA [65]) was used to test whether there was a significant broad scale correlation between genetics and language family area. Boab individuals were grouped according to their occurrence in the nPNy family areas and significance was tested with 999 permutations.
A partial Mantel test of genetic distance vs. language family area with geographic distance as a covariate [66] was used to test whether any statistical significance inferred by the AMOVA was a result of isolation by distance in both language and genetic variation. Genetic distance was calculated between boab individuals using the D C method [54], and boab individuals were assigned to language family areas as described above.
We used a permutation test, implemented in Mesquite [67], to test for statistically significant association between language groups and genetic identity. The genetic clusters were scored as single, multistate characters. The length of this character on the language phylogenetic tree was calculated under equally-weighted parsimony. We permuted this character 999 times, and calculated its length on the language phylogenetic tree. We then determined whether the length of the original character was inside the expected distribution based on the randomly permuted character.
Language families and boab word-forms
To examine the geographic relationship between boab word-forms and language families, the numbers of boab word-forms in each of the nPNy language families were examined to identify languages with higher and lower numbers of boab word-forms (Table 1). A greater diversity of word-forms for A. gregorii within language family areas may suggest long-term presence of, and interaction with, boab populations. Conversely, lower diversity of word-forms for the tree could be either due to recent rapid expansion of a language family area, or to a more recent interaction of those populations with boabs.
Table 1. Word-forms for Adansonia gregorii in the Kimberley region.
| Section A: Word-form for boab tree | |||||
| Non-Pama- Nyungan Family, Pama-Nyungan (PNy) subgroup | Language group | Word-Form for boab tree | Reconstructed/loan-source form | Original meaning | |
| N. Worrorran | Wunambal | junguri, jungeri | junguri | ||
| N. Worrorran | Wunambal | po:rkuru | potkurri | ||
| N. Worrorran | Wunambal | potkurri | potkurri | ||
| N. Worrorran | Wunambal | wajer | wajarr | ||
| N. Worrorran | Gwini | jumulu | jumulu | ||
| W. Worrorran | Worrorra | jungura | junguri | ||
| W. Worrorran | Worrorra | jungurim | junguri | ||
| W. Worrorran | Unggumi | la:kaji | larrkarti | ||
| E. Worrorran | Wurla | wajarr | wajarr | ||
| E. Worrorran | Ngarinyin | junguri | junguri | ||
| E. Worrorran | Ngarinyin | jungulan | junguri? | ||
| E. Worrorran | Ngarinyin | larrkarri (larrkari) | larrkarti | ||
| Bunuban | Gooniyandi | wajarri | wajarr | ||
| Bunuban | Bunuba | larrkari | larrkarti | ||
| Nyulnyulan | Nyikina | larrkati | larrkarti | ||
| Nyulnyulan | Warrwa | larrkarti | larrkarti | ||
| Nyulnyulan | Bardi | larrkiti | larrkarti | ||
| Nyulnyulan | Yawuru | larrkarti | larrkarti | ||
| Marrngu (PNy) | Karajarri | larrkarti | larrkarti | ||
| Marrngu (PNy) | Mangarla | larrka-rti | larrkarti | ‘split-prone to’ | |
| Marrngu (PNy) | Nyangumarta | larrkarti | larrkarti | ||
| Ngumpin (PNy) | Walmajarri | larrkarti | larrkarti | ||
| Ngumpin (PNy) | Jaru | larrkarti | larrkarti | ||
| Ngumpin (PNy) | Jaru | jamarlu | jumulu | ||
| Jarragan | Gajirrabeng | kertewun | kertewun | ||
| Jarragan | Miriwoong | katawun | kertewun | ? ‘egg’ | |
| Jarragan | Gija | jumulu-ny | jumulu | ||
| Jarragan | Gija | kuwulu-ny, tyaru-ku-ny | kulpe: ‘tree’, jare: ‘stomach’ | ‘big bellied tree’ | |
| W. Mirndi | Jaminjung | kuruwuny (kuruwan) | kertewun | ||
| Ngumpin (PNy) | Bilinarra | jamula-ng | Jumulu | ||
| Ngumpin (PNy) | Ngarinyman | jamula-ng (jamurlang) | jumulu | ||
| Section B: Word-forms for boab tree parts | |||||
| Non-Pama- Nyungan Family, Pama- Nyungan (PNy) subgroup | Language group | Word-Forms for boab tree parts | Reconstructed/ loan source form | Translation | Original meaning |
| W. Worrorran | Worrorra | yu:ku | yuukun | dry boab fruit pod | |
| W. Worrorran | Unggumi | lakeri | larrkarti | boab fruit pod | |
| E. Worrorran | Ngarinyin | yu:kun | yuukun | dry boab fruit pod | |
| E. Worrorran | Ngarinyin | irrke | ? | pith of the boab tree | |
| E. Worrorran | Ngarinyin | kuranpun | ? | pith of the boab tree | |
| Bunuban | Bunuba | wajarr | wajarr | boab fruit pod | |
| Bunuban | Bunuba | ngipi | ngipi (loanword from Nyikina) | boab seeds | |
| Nyulnyulan | Nyikina | nguja | dry boab fruit pod | ||
| Nyulnyulan | Nyikina | ngipi | boab seeds | ||
| Jarragan | Miriwoong | jang-nge-ng | jang ‘eat’ | edible pith of fruit pod | ‘eating-for’ |
| Jarragan | Gajirrabeng | tijperu-ng | ? | seed inside boab fruit pod | Possibly related to tij- ‘dead’ |
| Jarragan | Gajirrabeng | murl-ng | murl | boab seed | ‘eye’ |
| Jarragan | Gija | larrkarti-m | larrkarti | boab fruit pod | |
| Jarragan | Gija | jililiny | ? | boab seed | |
| Jarragan | Gija | wawang-ku-ny | ? | inside of boab fruit pod | ? |
| Jarragan | Gija | jang-nge | jang-nge | edible pith of fruit pod | ‘eating-for’ |
| W. Mirndi | Jaminjung | jangi | jang-nge | edible pith of fruit pod | |
| Ngumpin (PNy) | Ngarinyman | jangi | jang-nge | edible pith of fruit pod | |
Data on boab word-forms and related terminology were drawn from both published and unpublished sources (S2 Dataset).The two left-hand columns identify the non-Pama-Nyungan language family and Pama-Nyungan (PNy) sub-groups, and the associated language groups. The word-forms are represented in a standardized orthography. A hyphen indicates a morpheme break, e.g., separating a stem from a suffix. The subsequent columns list the reconstructed or loan-source form and the probable original meaning of the reconstructed source form. Section A lists word-forms for the boab tree; Section B lists word-forms for boab tree-parts.
Boab word-forms and genetic population phylogeny
If humans have played a major role in dispersing boabs over the Kimberley, then we might expect congruence between diffusion of loanwords and dispersal of genotypes. Gene flow between A. gregorii populations has previously been examined based on coalescent analysis, using IMa2 [ 40 ]. That analysis yielded estimates of the relative migration rates in two major directions: between western and eastern populations and between coastal and inland populations [40]. These directional patterns of gene flow were compared with the directional movement of boab word-forms, especially loanwords, using the comparative method [6, 64] to trace their original source and examine patterns of geographic congruence. The reconstructed loan source forms for the boab tree in Table 1 were used to identify five words which showed directional movement of borrowing between the languages of the Kimberley region.
Results
Phylogeny of A. gregorii populations
Phylogenetic analysis generated an unrooted tree for the five inferred populations with bootstrap support values greater than 80% for both internal branches (Fig. 2B). Midpoint rooting suggests that the WK and NWC populations are sisters, as are the EK and NEC populations, with the deepest divergence separating the NC population from the other four. However, since the root is close to the node, this conclusion should be treated with caution.
Boab populations and language family areas
The association of the five A. gregorii populations on the language phylogenetic tree (Fig. 2A) and vice-versa, of language families with boab population phylogeny (Fig. 2B), did not reveal strict geographic congruence. Statistical analyses showed that genetic variance between boab individuals from different nPNy family areas was low but statistically significant (AMOVA, 3% of total genetic variation, P = 0.001). A partial Mantel test of genetic distance vs. language family area with geographic distance as a covariate was not significant (R xy = 0.00728; P = 0.285), indicating that the statistical significance of the AMOVA could be due to spatial autocorrelation in both languages and genetic variation. However, it is noteworthy that there seems to be a geographically sharp distinction between the EK and NEC genetic clusters, and this break coincides with the distinction between the Jarragan and Worrorran language families.
The mapping of genetic population assignments onto the language tree required 4 steps for the original, unpermuted data whereas the permuted characters required from 5 to 10 steps, with a mean of 8.55. With 999 permutations, this implies a p-value ≤ 0.001, indicating a strongly non-random pattern of association between the language tree and the genetic clusters. Because geography was not included as a covariate, we cannot rule out the possibility that this association is driven by geography rather than a causal relationship between boab genetics and human language variation.
Language family areas and A. gregorii word-forms
The greatest diversity of word-forms for A. gregorii is found in the northern coastal areas encompassing the Worrorran language family, followed by those in the Jarragan family (Table 1 and Fig. 2A). Each has one dominant word for the tree species that is not borrowed from elsewhere. Worrorran has the term junguri, and Jarragan has jumulu. Both terms are reconstructable as proto-words for boab in these language families. In addition to these proto-words and their descendant forms, the language families have other inherited word-forms for boabs. Worrorran (Northern) has the forms potkurri and wajarr in the Wunambal language; the former word is restricted to this specific language, while the latter is also used in Wurla (Eastern Worrorran). Wajarr has either been borrowed into the Bunuban family or from it: evidence is equivocal at this stage. Forms of the Jarragan proto-word jumulu have been borrowed into northern Worrorran and into the PNy Ngumpin subgroup. Jarragan also has the term kertewun that has been borrowed further east into the nPNy Mirndi language family.
Of particular interest is the boab word-form larrkarti, which is from a language subgroup associated with the desert area south of the main bioregions of A. gregorii distribution. Analyzability of this word into two morphemes (larr-split; karti-side) suggests that it comes from the Karajarri (and/or the closely related and neighbouring Mangarla) languages of the PNy Marrngu subgroup on the southwest periphery of the Kimberley and is of relatively recent origin [53]. While both the elements larr ‘split lengthways’ and the suffix –karti ‘towards; side’ do occur in other languages in the region separately and with somewhat different meanings, their coincidence with Karajarri and Mangarla languages is a strong indication that this is the origin of the new coined term for the boab. As a whole, larrkarti means ‘split side’, which could be an allusion to the hollow trunks of old trees or the dehiscent nature of the boab fruit pod, or more likely to the manner in which the shelled fruit splits into longitudinal segments. The coining of this word may well have come about when the Marrngu speaking people moved from the desert into the west Kimberley areas where they would have encountered these unfamiliar trees in the landscape. This Marrngu word for boab has been borrowed and incorporated into the vocabularies of some of the nPNy Worrorran (Western and Eastern) – Bunuban – Nyulnyulan language families and one Jarragan language alongside other inherited word-forms. Larrkarti has also been borrowed into the PNy Ngumpin language subgroups from desert areas southeast of the Kimberley.
Boab word-forms and genetic population phylogeny
Fig. 2B illustrates the overlap between A. gregorii words-forms and population phylogeny. The NC population, which appears to be sister to the remaining populations, has the inherited Worrorran boab word-forms junguri and potkurri, and possibly wajarr. The NWC population has the inherited form junguri. The WK population, which clusters with NWC and is distributed across a number of nPNy language family areas has three words, junguri, wajarr, and larrkarti. The NEC population has wajarr, jungulan (a probable cognate of junguri) and the Jarragan loanword jumulu. The EK population, which occurs across the Jarragan and Mirndi families, has the word-forms jumulu and kertewun. Each pair of genetic clusters shares at least one boab word-form suggesting extensive word exchange.
Gene flow and diffusion of boab loanwords
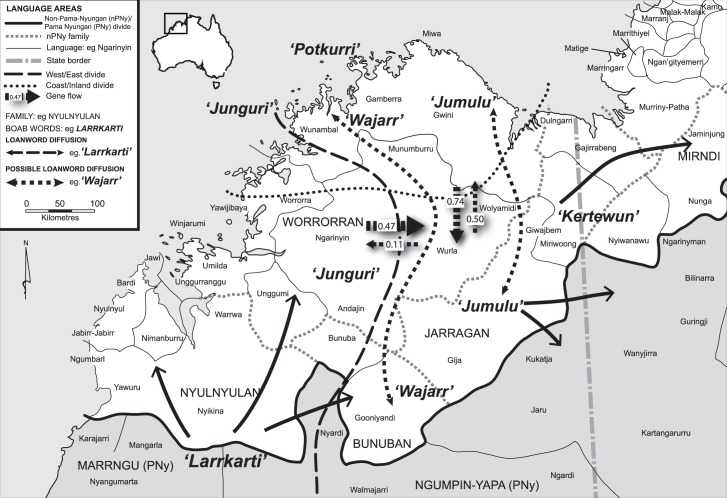
Statistically significant gene flow was recorded across all boab populations in the Kimberley [40]. Migration rates were estimated by grouping the populations across two axes: West (WK, NWC) ↔ East (NC, NEC, EK); and Coast (NWC, NC, NEC) ↔ Inland (WK, EK). The West→East migration rate was 0.47 individuals/ year and the East→West rate was 0.11. The Coast→Inland migration rate was 0.74, and Inland→Coast was 0.50 [40].
We compared these gene flow patterns with inferred loanword diffusion patterns (Fig. 3). The linguistic analysis of boab word diffusion is consistent with the existence of an East-West biogeographic divide [36–39, 40]: jumulu and kertewun have diffused further to the east, but not into west Kimberley, whereas the word junguri remains in the northwest and has not diffused into eastern Kimberley. Larrkarti has diffused widely from its inferred source in the southwest into western and central Kimberley languages, but not further into northern Worrorran languages.
Fig 3. Gene flow between A. gregorii populations and boab loanword diffusion across the Kimberley region.
Approximate locations of inferred East-West and Coast-Inland biogeographic divides are represented by dotted lines. Gene flow between East-West and Coast-Inland is calculated using IMa2 is in units of individuals/year, and shown with dashed block arrows. Unbroken arrows show the direction of borrowing of boab loanwords among the Kimberley languages; broken arrows show possible directions of loanword movements.
If human-mediated dispersal were responsible for the East ↔West and Coast ↔ Inland dispersal, we might expect to see corresponding loanword diffusion along these axes. Furthermore, since there is evidence of much more west-to-east gene flow than east-to-west gene flow across the inferred East-West divide, we might expect more west-to-east diffusion of loanwords than the reverse. These directional patterns accord well with the diffusion of boab loanwords shown in Fig. 3. Larrkarti and kertewun have diffused West→East, but there are no cases of East→West boab loanword diffusion. Thus, the direction and rates of migration of A. gregorii gene flow show correspondence with the inferred diffusion of boab loanwords.
Discussion
Our study supports the hypothesis that human-mediated dispersal has played a role in shaping the geographical distribution of A. gregorii. The limited morphological and genetic divergence of the Adansonia genus in northwest Australia, despite the presence of biogeographic barriers, can be attributed to high gene flow within A. gregorii [40]. Given the lack of other obvious seed dispersal agents, the lack of evidence of barriers to gene flow is most easily explained by a long history of humans moving boab seeds. Concordance between gene flow and loanword diffusion further supports the hypothesis of human-mediated dispersal of A. gregorii. Loanword movements across all boab populations provide an indication of patterns of interaction between nPNy language families and PNy subgroups that would have influenced the geographic distribution of A. gregorii in the Kimberley region.
Additional evidence of anthropogenic agency in facilitating boab gene flow comes from archaeological excavations at Carpenter’s Gap Shelter located in the Napier ranges of the Kimberley, which falls within the Bunuba language area [48,49]. Lithic and macrobotanical remains from this rockshelter site showed continuity of human occupation extending over 40 ka into the late Pleistocene. Carbon dates recorded boab pod fragments at 39 ka, 20 ka, 18 ka and 15 ka [48,49], with substantial increase in pod deposition from 3 ka onwards and peaking at around 650 years ago [49]. Old boab trees have been found near aboriginal middens in western Kimberley, providing further evidence of long-term human consumption of the fruit [48]. Prehistoric rock art in the northern Kimberley also shows possible depictions of the tree, indicating its cultural significance to ancient human groups that may have occupied this region [28].
Based on the above evidence, we postulate that recent boab evolution and geographic distribution have been shaped primarily through ancient human agency. The phylogenetic tree of boab populations and predominant direction of gene flow together imply that the source populations for A. gregorii dispersal were most likely in the extreme northwest Kimberley, potentially overlapping with the inferred NC population area. The range of this source population is likely to have extended beyond the current coastline at the Last Glacial Maximum (LGM, roughly 20 ka) when sea levels were over 120 m below present-day, and the northwest continental shelf was exposed to the maximum extent [68]. The increased land surface exposure of both Sahul and Sundaland shelfs, lower sea surface temperatures (SST), and altered oceanic currents due to closure of several shallow seas and passageways between these continental shelves contributed to a northward shift of the Inter-Tropical Convergence Zone (ITCZ), thereby reducing seasonal precipitation levels [69] and creating semi-arid savanna conditions [70] in which ancestral boab populations would have existed in northwest Australia.
Fig. 4 sketches a possible LGM scenario for this boab population distribution, showing land exposed at 17 ka and at 8 ka in relation to present-day coastlines. With rainfall as much as 30 to 50% below present day levels [69] and higher levels of aeolian landform activity [71], subtropical desert conditions would have prevailed across much of the exposed continental shelf beyond present-day Kimberley [72–75]. These arid climatic conditions, particularly the low levels of seasonal rainfall, would have limited the distribution of A. gregorii, as the current distribution of the species coincides with areas receiving at least 700 mm of annual seasonal rainfall [76]. Therefore, under drier climatic conditions during the LGM due to the northward shift of the ITCZ [69], it is likely that boab populations would have been limited to the extreme northern coast of present-day Kimberley and the exposed continental shelf.
Fig 4. Sketch of LGM scenario showing land exposed at 17 ka and at 8 ka in relation to present-day coastlines, based on Coller [87].
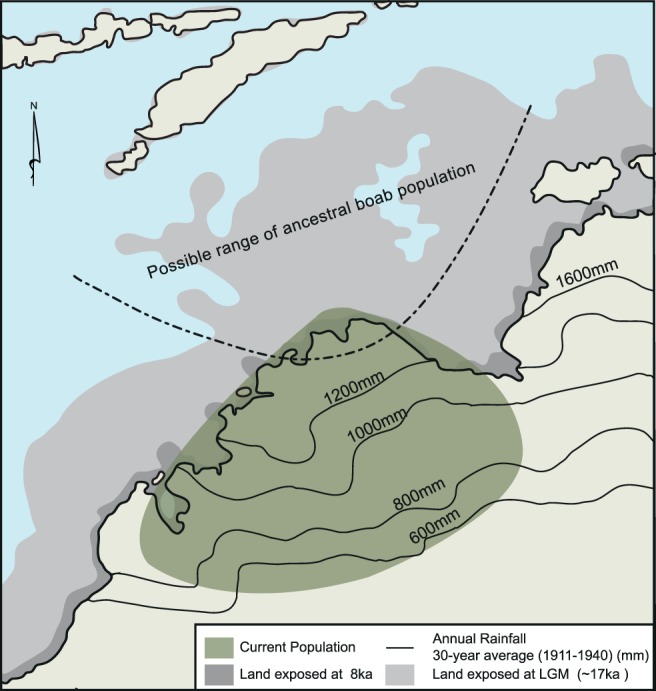
The possible extent of ancestral boab population distribution on the exposed continental shelf is shown by a dashed arc; current boab distribution shown in light grey. Rainfall isohyets (mm) are based on 30-year annual average rainfall (1911–1940) estimates obtained from the Australian Bureau of Meteorology [88].
Subsequent sea-level rise and restoration of monsoonal activity during the post-glacial period and the Pleistocene-Holocene transition between 17 and 6 ka flooded the Sahul shelf and established the present-day coastlines of northern Australia [68,73]. The flooding of the continental shelf beyond the present-day Kimberley coast, along with increased monsoonal rainfall over inland Kimberley, would have altered the distribution of ancestral A. gregorii populations and possibly created a genetic bottleneck from which current populations would have expanded [40]. We propose that ancient human groups that lived on the coast along this previously exposed shelf during the Late Pleistocene would have retreated from inundated areas and carried boab fruit with them as they migrated further south and east.
Late Pleistocene records of boab remnants at the Carpenter’s Gap archaeological site [48,49] may represent sporadic visits of human settlers from the north [77]. The site record of the presence of shells and beads from the Early Holocene suggests movement of high value goods from the coast [78]. In common with other Australian archaeological sites [79], evidence for occupation at Carpenter’s Gap increases sharply from mid- to late Holocene, perhaps reflecting a demographic expansion in southern Kimberley [79]. This population increase, combined with other factors such as climatic and vegetation change [49,50], growth in local boab populations, and more frequent use of the site for cultural ceremonies and exchanges [77] could explain the increase in boab pod remnants along with other food plants and seeds from about 3000 to 650 years ago [48].
The increased mobility of Aboriginal groups in the southern Kimberley during the Late Holocene is likely to have been influenced by greater climate variability in northern Australia. In contrast to the Early Holocene, the Late Holocene (∼1000 BCE – 500 CE) climate in the Australian Monsoon Tropics was marked by periods of increased seasonality and aridity [80–82]. These conditions may have contributed to increased mobility of Aboriginal groups between different parts of the Kimberley and would have contributed to higher A. gregorii gene flow through fruit and seed dispersal, accompanied by diffusion of associated word-forms.
The linguistic data provides additional indication of patterns of migration and social interaction that could have contributed to the spread of A. gregorii (Table 1 and Fig. 3). The periods of aridity during the Late Holocene may have affected the survival of desert-based Marrngu (PNy subgroup) speakers and led to their migration into southern and western Kimberley. The word-form larrkarti was probably coined by Marrngu speakers during this period when they would have encountered the boab tree in the Kimberley landscape. At the same time, the mobility and cultural interactions of these PNy groups with neighbouring nPNy language groups such as Nyikina and Bunuba would have increased. Evidence that the diffusion of the loanword larrkarti is of a relatively recent nature is demonstrated in the way it has been incorporated into other languages, generally in an unchanged form [61]. The Gija language (southern Jarragan) has retained its inherited word jumulu for the tree and adopted larrkarti for the fruit pod, perhaps indicating the salience and portability of the edible seed pod in the more recent borrowing. Likewise, the Jaru language (PNy Ngumpin subgroup) has no inherited words for boab and uses both jamula (modified from Gija) and larrkarti for the tree. Other examples of recent boab expansion and loanword diffusion further east can be found in the Ngarinyman language (Ngumpin) in the Northern Territory, where the words jang-nge (borrowed from Miriwoong, meaning ‘for eating’) and jumulu (borrowed from Gija) are used for the fruit or its edible pith, and the tree respectively.
The possible climatic influences on human migration and boab loanword movement in the Kimberley echoes some aspects of Bostoen et al.’s [6] description of climate-induced dynamics and Bantu expansion in Africa. Although there is no analogous evidence of a large-scale expansion of a single linguistic group into the Kimberley, the PNy Marrngu term larrkarti and other pre-existing nPNy language words such as wajarr, jumulu, and kertewun moved across the Kimberley in patterns corresponding with multi-directional boab gene flow as shown in our study.
The high gene flow in A. gregorii appears similar to the case of A. digitata in Africa, where human agency has been involved in dispersal of the species [19,51,52]. The boab loanword movements in the Kimberley may be compared with Blench’s [4] account of the spread of Bantu words for A. digitata. He notes that the genetic diversity of baobabs in the ecological zones of West Africa and the diversity of vernacular names for the tree in African languages suggests considerable antiquity as well as significant east-west movement along trade routes and exchanges of associated ideas and terminology. However, despite the diversity of baobab names, he points out that two competing Bantu roots, #mbuyu and #muramba, and variations of these, are found in the Bantu languages of southern and eastern Africa. The Bantu expansion from the tropical forest areas of West Africa is said to have begun from around the middle of the first millennium BC (∼ 2500 BP onwards) and reached southern Africa by about 500 CE [6,83]. Blench argues that the Bantu would not have been familiar with the baobab because it does not grow in the tropical forest areas of Cameroon, Gabon and Congo where this proto-language group are thought to have originated. They would have encountered the tree as they expanded eastwards and emerged into the savanna, and developed new terms by either borrowing from resident hunter-gatherer groups or comparing it with some other tree species they already knew. The loan or variations of baobab words mbuyu and muramba in the Bantu languages of eastern and southern Africa would thus indicate the movement of Bantu into these areas [4]. The lower levels of genetic diversity of A. digitata in eastern and southern Africa detected by Leong Pock Tsy et al. [27] may likely be due to the Bantu expansion over this 3000 year period and their contribution to high levels of gene flow in baobabs across these regions.
Conclusion
Our study demonstrates that the limited intraspecific divergence within A. gregorii in Australia is most likely due to high gene flow mediated by human agency, similar to that inferred for A. digitata in continental Africa [27, 51], combined with shifts in suitable habitat and a weak bottleneck following the end of the LGM [40]. Human use of Adansonia over many thousands of years in both continents would have contributed to gene flow over long distances and across biogeographical barriers. In contrast, it could be that the divergence of Adansonia into six species in Madagascar was possible in part because of the lack of humans until ca. 2 ka. However, this hypothesis can only be tested by further investigation of the ecological, physiological and biogeographic processes contributing to speciation within the Adansonia clade from Madagascar.
This study contributes new evidence for the role of ancient humans in influencing the evolution and distribution of non-domesticated plant species in Australia. Australia has long been viewed as a continent of hunter-gatherers [84] where prehistoric and Aboriginal populations prior to European colonisation played minimal roles in selecting and dispersing useful plants [23,24]. However, some recent studies have challenged this assumption by providing evidence for the role of ancient Aboriginal groups in the dispersal of food plants across the continent [77,85,86]. These include bananas (Musa spp.), taro (Colocasia esculenta) and some yams (Dioscorea spp.) in northern Australia [23], Livistona palms in Central Australia [85], and yam daisy (Microseris scapigera) in southeastern Australia [86]. Our findings add new insights regarding the role of ancient human agency in influencing the evolution and distribution of the boab, an important non-cultivated food plant species that has shaped the long-term landscape and environmental history of northwest Australia.
Supporting Information
(XLS)
(XLS)
Acknowledgments
The authors thank Kara Rasmanis for preparing the illustrations; Jack Pettigrew for his generous donation of A. gregorii samples to the Royal Botanic Gardens Melbourne collection; Pat Lowe, Kim Ackerman, Peter Kershaw, Simon Connor, Tim Denham, Joanne Birch, Elizabeth James, Mark McDonnell, and Claudia Vickers for comments and constructive advice; and the anonymous reviewers for their rigorous assessment of the paper. The collections and voucher specimens are lodged at the National Herbarium of Victoria (MEL) and Darwin Botanic Gardens (DNA).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The genetics and biogeographic research in this paper was funded by an Australian Research Council grant (DP1093100, awarded to Rangan, Kull, and Murphy); the linguistic research was supported by a grant from the Kimberley Foundation Australia (awarded to McConvell), a US NSF Grant (HSD 902114, awarded to Bowern, et al., including McConvell), and an AIATSIS grant (G2011/76, awarded to Spronck).
References
- 1. Donohue M, Denham T. Banana (Musa spp.) domestication in the Asia-Pacific region: linguistic and archaeobotanical perspectives. Ethnobotanical Research and Applications. 2009;7: 293. [Google Scholar]
- 2. Perrier X, De Langhe E, Donohue M, Lentfer C, Vrydaghs L, Bakry F, et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc Natl Acad Sci USA. 2011;108(28): 11311–8. 10.1073/pnas.1102001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roullier C, Benoit L, McKey DB, Lebot V. Historical collections reveal patterns of diffusion of sweet potato in Oceania obscured by modern plant movements and recombination. Proc Natl Acad Sci USA. 2013;110: 2205–10. 10.1073/pnas.1211049110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blench RM. The intertwined history of the silk-cotton and baobab In: Cappers RTJ, editor. Fields of change; progress in African ethnobotany. Gröningen, Germany: Barkhuis and Gröningen University, Gröningen; 2007. p. 1–20. [Google Scholar]
- 5. Smith BD. General patterns of niche reconstruction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366: 838–48. 10.1098/rstb.2010.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bostoen K, Grollemund R, Muluwa JK. Climate-induced vegetation dynamics and the Bantu Expansion: Evidence from Bantu names for pioneer trees (Elaeis guineensis, Canarium schweinfurthii, and Musanga cecropioides). Comptes Rendus Geoscience. 2013;345: 336–49. [Google Scholar]
- 7. Baum DA. A systematic revision of Adansonia (Bombacaceae). Ann Mo Bot Gard. 1995;82: 440–71. [Google Scholar]
- 8. Pettigrew DJ, Bell KL, Bhagwandin A, Grinan E, Jillani N, Meyer J, et al. Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Bombacoideae; Malvaceae). Taxon. 2012;61: 1240–50. [Google Scholar]
- 9. Adanson M. Description d'un arbre d'un nouveau genre, appelé baobab, observé au Sénégal [Description of a new tree of a new genus called baobab, observed in Senegal]. Mémoires de l'Académie Royale. 1771;161: 218–43. [Google Scholar]
- 10. De Caluwé E, Halamová K, Van Damme P. Boabab (Adansonia digitata L.): a review of traditional uses, phytochemistry and pharmacology In: Juliani HR, Simon JE, Ho C-T, editors. African natural plant products: new discoveries and challenges in chemistry and quality. Washington DC: American Chemical Society; 2009. p. 51–84. [Google Scholar]
- 11. Gebauer J, El-Siddig K, Ebert G. Baobab (Adansonia digitata L.): a review on a multipurpose tree with promising future in the Sudan. Gartenbauwissenschaft. 2002;67: 155–60. [Google Scholar]
- 12. Livingstone D. A popular account of missionary travels and researches in South Africa London: John Murray, Albemarle Street; 1861. [Google Scholar]
- 13. Patrut A, von Reden KF, Mayne DH, Lowy DA, Patrut RT. AMS radiocarbon investigation of the African baobab: Searching for the oldest tree. Nucl Instrum Methods Phys Res B. 2013;294: 622–6. 10.1016/j.nimb.2012.04.025 [DOI] [Google Scholar]
- 14. Sidibe M, Williams JT. Baobab Adansonia digitata. Southampton, UK: International Centre for Underutilised Crops; 2002. [Google Scholar]
- 15. Swart ER. Age of the baobab tree. Nature. 1963;4881: 708–9. [Google Scholar]
- 16. Wickens GE. The baobab: Africa's upside-down tree. Kew Bulletin. 1982;37: 173–209. [Google Scholar]
- 17. Wickens GE, Lowe P. The baobabs: pachycauls of Africa, Madagascar, and Australia Berlin; New York: Springer; 2008. [Google Scholar]
- 18. Armstrong P, editor. The history, natural history and distribution of Adansonia: a plant genus of the Indian Ocean littoral The Indian Ocean in Focus: International Conference on Indian Ocean Studies, Perth, Western Australia 1979: Section I Environment and Resources; 1979; Perth, Western Australia: Perth Building Society. [Google Scholar]
- 19. Duvall CS. Human settlement and baobab distribution in south-western Mali. Journal of Biogeography. 2007;34: 1947–61. 10.1111/j.1365-2699.2007.01751.x [DOI] [Google Scholar]
- 20. Barker G. The Agricultural Revolution in Prehistory. Oxford: Oxford University Press; 2006. [Google Scholar]
- 21. Bean AR. A new system for determining which plant species are indigenous in Australia. Australian Systematic Botany. 2007;20: 1–43. 10.1071/SB06030 [DOI] [Google Scholar]
- 22. Bellwood P. First Farmers. Oxford: Blackwell; 2005. [Google Scholar]
- 23. Denham T, Donohue M, Booth S. Revisiting an old hypothesis: Horticultural experimentation in northern Australia. Antiquity. 2009;83: 634–48. [Google Scholar]
- 24. Lourandos H. Continent of hunter-gatherers: New perspectives in Australian prehistory Cambridge: CUP; 1997. [Google Scholar]
- 25. Bliege-Bird R, Bird DW, Codding BF, Jones JH. The “fire stick farming” hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc Natl Acad Sci USA. 2008;105: 14796–801. 10.1073/pnas.0804757105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones R. Firestick farming. Australian Natural History. 1969;16: 224–31. [Google Scholar]
- 27. Leong Pock Tsy J-M, Lumaret R, Mayne D, Vall AOM, Abutaba YIM, Sagna M, et al. Chloroplast DNA phylogeography suggests a West African centre of origin for the baobab, Adansonia digitata L. (Bombacoideae, Malvaceae). Molecular Ecology. 2009;18(8): 1707–15. 10.1111/j.1365-294X.2009.04144.x [DOI] [PubMed] [Google Scholar]
- 28. Pettigrew DJ. Iconography in Bradshaw rock art: breaking the circularity. Clin Exp Optom. 2011;94: 403–17. 10.1111/j.1444-0938.2011.00648.x [DOI] [PubMed] [Google Scholar]
- 29.Baum DA, Handasyde T. The boab tree (Adansonia gregorii) in north-west Australia. Perth: Unpublished report in Western Australian Herbarium Library, 1990.
- 30. Brock J. Top End Native Plants. Darwin: Brock; 1988. [Google Scholar]
- 31. Gillison AN. Tropical savannas of Australia and southwest Pacific In: Bourliere F, editor. Tropical savannas. Amsterdam: Elsevier Scientific Publishing; 1983. p. 183–243. [Google Scholar]
- 32. Mueller Fv. Botanical notes from north-west Australia. Victorian Naturalist. 1893;10: 110–1. [Google Scholar]
- 33. Bowman DMJS. Observations on the demography of the Australian boab (Adansonia gibbosa) in the north-west of the Northern Territory, Australia. Australian Journal of Botany. 1997;45: 893–904. [Google Scholar]
- 34. Bowman DMJS, Brown GK, Braby MF, Brown JR, Cook LG, Crisp MD, et al. Biogeography of the Australian monsoon tropics. Journal of Biogeography. 2010;37(2): 201–16. 10.1111/j.1365-2699.2009.02210.x [DOI] [Google Scholar]
- 35. Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MA, et al. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Mol Ecol. 2008;17(20): 4398–417. 10.1111/j.1365-294X.2008.03899.x [DOI] [PubMed] [Google Scholar]
- 36. Eldridge MDB, Potter S, Cooper SJB. Biogeographic barriers in north-western Australia: an overview and standardisation of nomenclature. Aust J Zool. 2011;59: 270–2. 10.1071/ZO12012 [DOI] [Google Scholar]
- 37. Hill KD, Johnson LAS. Systematic studies in the eucalypts. 7. A revision of the bloodwoods, genus Corymbia (Myrtaceae). Telopea. 1995;6: 185–504. [Google Scholar]
- 38. Oliver PM, Adams M, Doughty P. Molecular evidence for ten species and Oligo-Miocene vicariance within a nominal Australian gecko species (Crenadactylus ocellatus, Diplodactylidae). Bmc Evolutionary Biology. 2010;10: 386. doi: 386 10.1186/1471-2148-10-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potter S, Eldridge MDB, Taggart DA, Cooper SJB. Multiple biogeographical barriers identified across the monsoon tropics of northern Australia: phylogeographic analysis of the brachyotis group of rock-wallabies. Mol Ecol. 2012;21: 2254–69. 10.1111/j.1365-294X.2012.05523.x [DOI] [PubMed] [Google Scholar]
- 40. Bell KL, Rangan H, Fowler R, Kull CA, Pettigrew JD, Vickers CE, et al. Genetic diversity and biogeography of the boab Adansonia gregorii (Malvaceae: Bombacoideae). Australian Journal of Botany. 2014; 62: 164–74. [Google Scholar]
- 41. Baum DA. The comparative pollination and floral biology of baobabs (Adansonia—Bombacaceae). Ann Mo Bot Gard. 1995;82: 322–48. [Google Scholar]
- 42. Lowe P. The boab tree Port Melbourne: Lothian Books; 1998. [Google Scholar]
- 43. Aldrich PR, Hamrick JL, Chavarriaga P, Kochert G. Microsatellite analysis of demographic genetic structure in fragmented populations of the tropical tree Symphonia globulifera . Mol Ecol. 1998;7(8): 933–44. [DOI] [PubMed] [Google Scholar]
- 44. Garcia C, Jordano P, Godoy JA. Contemporary pollen and seed dispersal in a Prunus mahaleb population: patterns in distance and direction. Mol Ecol. 2007;16(9): 1947–55. [DOI] [PubMed] [Google Scholar]
- 45. Oddou-Muratorio S, Pettit RJ, Le Gueroue B, Guesnet D, Demesure B. Pollen versus seed-mediated gene flow in a scattered forest tree species. Evolution. 2001;55: 1123–35. [DOI] [PubMed] [Google Scholar]
- 46. Potter S, Eldridge MDB, Cooper SJB, Paplinska JZ, Taggart DA. Habitat connectivity, more than species’ biology, influences genetic differentiation in a habitat specialist, the short-eared rock-wallaby (Petrogale brachyotis). Conserv Genet. 2012;13: 937–52. [Google Scholar]
- 47. Boland DJ, Brooker MIH, Chippendale GM, Hall N, Hyland BPM, Johnston RD, et al. Forest trees of Australia Melbourne: Nelson & CSIRO; 1985. [Google Scholar]
- 48. McConnell K, O'Connor S. 40,000 year record of food plants in the Southern Kimberley Ranges, Western Australia. Australian Archaeology. 1997;45: 20–31. [Google Scholar]
- 49. McConnell K, O'Connor S. Carpenter's Gap Shelter I: A case for total recovery In: Mountain MJ, Bowdery D, editors. Taphonomy: the analysis of processes from phytoliths to megafauna. Canberra, Australia: ANH Publications; 1999. p. 23–34. [Google Scholar]
- 50. Wallis LA. Environmental history of northwest Australia based on phytolith analysis at Carpenter's Gap 1. Quat Int. 2001;83–85: 103–17. [Google Scholar]
- 51. Assogbadjo AE, Glele Kakai R, Kyndt T, Sinsin B. Conservation genetics of baobab (Adansonia digitata L.) in the parklands agroforestry systems of Benin (West Africa). Not Bot Horti Agrobot Cluj Napoca. 2010;38: 136–40. [Google Scholar]
- 52. Leong Pock Tsy JM, Lumaret R, Mayne D, Vall AO, Abutaba YI, Sagna M, et al. Chloroplast DNA phylogeography suggests a West African centre of origin for the baobab, Adansonia digitata L. (Bombacoideae, Malvaceae). Mol Ecol. 2009;18: 1707–15. 10.1111/j.1365-294X.2009.04144.x [DOI] [PubMed] [Google Scholar]
- 53.McConvell P, Spronck S, Saunders T, editors. Linguistic prehistory of the Australian boab. The 43rd meeting of the Australian Linguistics Society Conference Proceedings; 2014.
- 54. Cavalli-Sforza LL, Edwards AWF. Phylogenetic analysis: models and estimation procedures. Am J Hum Genet. 1967;19: 233–57. [PMC free article] [PubMed] [Google Scholar]
- 55. Felsenstein J. PHYLIP: Phylogeny Inference Package 3.69 ed. Seattle, WA, USA: Department of Genome Sciences and Department of Biology, University of Washington; 2009. [Google Scholar]
- 56. Takezaki N, Nei M. Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics. 1996;144: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bowern C, Koch H. Australian Languages: Classification and the Comparative Method. Amsterdam: John Benjamins Publishing Company; 2004. [Google Scholar]
- 58.Harvey MD, cartographer Non-Pama-Nyungan Languages: Mapping Database and Maps: The Aboriginal Studies Electronic Data Archive (ASEDA); 2008.
- 59. Evans N. The Non-Pama–Nyungan Languages of Northern Australia Comparative studies of the continent's most linguistically complex region. Canberra: Pacific Linguistics; 2003. [Google Scholar]
- 60. Campbell L. Historical Linguistics. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 61. McConvell P, Laughren M. Ngumpin-Yapa Languages In: Koch H, Bowern C, editors. Australian Languages: Classification and the Comparative Method. Amsterdam: John Benjamins Publishing Company; 2004. [Google Scholar]
- 62. Hudson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23: 254–67. [DOI] [PubMed] [Google Scholar]
- 63. McGregor W, Rumsey A. Worrorran revisited: the case for genetic relations among languages of the Northern Kimberley region of Western Australia Canberra Pacific Linguistics; 2009;L600: 14. [Google Scholar]
- 64. McGregor W. The Languages of the Kimberley, Western Australia. Abingdon, UK: Routledge Curzon; 2004. [Google Scholar]
- 65. Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics. 1992;131: 479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meirmans PG. The trouble with isolation by distance. Mol Ecol. 2012;21: 2839–46. 10.1111/j.1365-294X.2012.05578.x [DOI] [PubMed] [Google Scholar]
- 67.Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. version 2.75 2009.
- 68. Kershaw P. Environmental change in Greater Australia. Antiquity. 1995;69.265: 656–75. [Google Scholar]
- 69. De Deckker P, Tapper NJ, van der Kaars S. The status of the Indo-Pacific Warm Pool and adjacent land at the Last Glacial Maximum. Glob Planet Change. 2002;35: 25–35. [Google Scholar]
- 70. Bird MI, Taylor D, Hunt C. Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: A savanna corridor in Sundaland? Quat Sci Rev. 2005;24: 2228–42. [Google Scholar]
- 71. Bowler JM, Wyrwoll K-H, Lou Y. Variations of the northwest Australian summer monsoon over the last 300,000 years: the paleohydrological record of the Gregory (Mulan) Lakes System. Quat Int. 2001;83–85: 63–80. [Google Scholar]
- 72. Reeves JM, Bostock HC, Ayliffe LK, Barrows TT, De Deckker P, Devriendt LS, et al. Palaeoenvironmental change in tropical Australasia over the last 30,000 years—a synthesis by the OZ-INTIMATE group. Quaternary Science Reviews. 2013;74: 97–114. 10.1016/j.quascirev.2012.11.027 [DOI] [Google Scholar]
- 73. Hesse PP, Magee JW, van der Kaars S. Late Quaternary climates of the Australian arid zone: a review. Quaternary International. 2004;118–119: 87–102. [Google Scholar]
- 74. Fitzsimmons KE, Cohen TJ, Hesse PP, Jansen J, Nanson GC, May J-H, et al. Late Quaternary palaeoenvironmantal change in the Australian drylands. Quaternary Science Reviews. 2013;74: 78–96. 10.1016/j.quascirev.2012.09.007 [DOI] [Google Scholar]
- 75. Wyrwoll K-H, Miller GH. Initiation of the Australian summer monsoon 14,000 years ago. Quaternary International. 2001;83–85: 87–102. [Google Scholar]
- 76. Beard JS. Some vegetation types of tropical Australia in relation to those of Africa and America. Journal of Ecology. 1967;55(2): 271–90. [Google Scholar]
- 77.McConnell K. Palaeoethnobotanical remains of Carpenter’s Gap Site 1, The Kimberleys, Western Australia. MSc Thesis. Canberra: ANU; 1997.
- 78. O'Connor S, Maloney T, Vannieuwenhuyse D, Balme J, Wood R. Occupation at Carpenters Gap 3, Windjana Gorge, Kimberley, Western Australia. Australian Archaeology. 2014;78: 10–23. [Google Scholar]
- 79. Williams AN. A new population curve for prehistoric Australia. Proceedings of the Royal Society B: Biological Sciences. 2013;280: 20130486 10.1098/rspb.2013.0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lees BG, Clements A. Climatic implications of chenier dates in northern Australia. Radiocarbon. 1987;29: 311–7. [Google Scholar]
- 81. Brockwell C, Marwick B, Bourke P, Faulkner P, Willan R. Late Holocene climate change and human behavioural variability in the coastal wet -dry tropics of northern Australia: Evidence from a pilot study of oxygen isotopes in marine bivalve shells from archaeological sites. Australian Archaeology. 2013;76: 21–33. [Google Scholar]
- 82. Shulmeister J. Australasian evidence for mid-Holocene climate change implies precessional control of Walker Circulation in the Pacific. Quaternary International. 1999;57: 81–91. [Google Scholar]
- 83. Ehret C. Bantu Expansions: Re-envisioning a central problem of early African history. The International Journal of African Historical Studies. 2001;34(1): 5–41. [Google Scholar]
- 84. Denham T, Fullagar R, Head L. Plant exploitation on Sahul: From colonisation to the emergence of regional specialisation during the Holocene. Quat Int. 2009;202: 29–40. [Google Scholar]
- 85. Kondo T, Crisp MD, Linde C, Bowman DM, Kawamura K, Kaneko S, et al. Not an ancient relic: the endemic Livistona palms of arid central Australia could have been introduced by humans. Proc Biol Sci. 2012;279(1738): 2652–61. Epub 2012/03/09. 10.1098/rspb.2012.0103 ; PubMed Central PMCID: PMCPmc3350701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gott B. Murnong—Microseris scapigera: a study of a staple food of Victorian Aborigines. Australian Aboriginal Studies. 1983;2: 2–17. [Google Scholar]
- 87.Coller M. Sahul Time. Available from: http://sahultime.monash.edu.au/explore.html, accessed 5 April 2014
- 88.Australian Bureau of Meteorology. Available: http://www.bom.gov.au/jsp/ncc/climate_averages/decadal-rainfall/index.jsp?maptype=6&period=1140&product=totals–maps. Accessed 2014 April 5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.