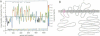Abstract
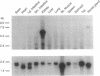
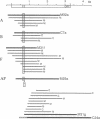
We have used cDNA probes derived from the secretory form of the Na-K-Cl cotransporter to screen both cortical and medullary rabbit kidney cDNA libraries. A sequence of 4750 bases was identified from multiple clones. The DNA encodes a protein containing 1099 amino acids, which is 61% identical over its length to the secretory Na-K-Cl cotransporter from shark rectal gland. From analysis of amino acid hydropathy, we predict that this putative renal Na-K-Cl cotransporter has 12 transmembrane helices and large N- and C-terminal cytoplasmic regions. Two sites for N-linked glycosylation are predicted on an extracellular loop. Three potential sites for modulation by protein kinase A are in the C-terminal cytoplasmic domain. Most of the isolated renal cDNA clones were identical over all regions of overlap; however, there was a 96-bp region for which there were three different but homologous variants (A, B, and F). This region of divergence was identified as an alternatively spliced cassette exon since clones were identified that contained intronic DNA as well as consensus splice acceptor sites that bounded the region. Tissue Northern blot analysis revealed a broad band at approximately 5.1 kb that was unique to the kidney. High-stringency Northern blot analysis of cortical and medullary mRNA using antisense oligonucleotides synthesized over each of the three cassette exons revealed that the isoforms were differentially distributed within the kidney--B almost exclusively in cortex, F almost exclusively in medulla, and A about equally distributed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clapp J. R., Robinson R. R. Osmolality of distal tubular fluid in the dog. J Clin Invest. 1966 Dec;45(12):1847–1853. doi: 10.1172/JCI105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., 3rd, Palfrey H. C. [3H]bumetanide binding to membranes isolated from dog kidney outer medulla. Relationship to the Na,K,Cl co-transport system. J Biol Chem. 1983 Oct 10;258(19):11787–11792. [PubMed] [Google Scholar]
- Friedman P. A., Hebert S. C. Diluting segment in kidney of dogfish shark. I. Localization and characterization of chloride absorption. Am J Physiol. 1990 Feb;258(2 Pt 2):R398–R408. doi: 10.1152/ajpregu.1990.258.2.R398. [DOI] [PubMed] [Google Scholar]
- Gamba G., Saltzberg S. N., Lombardi M., Miyanoshita A., Lytton J., Hediger M. A., Brenner B. M., Hebert S. C. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Haas M., Forbush B., 3rd [3H]bumetanide binding to duck red cells. Correlation with inhibition of (Na + K + 2Cl) co-transport. J Biol Chem. 1986 Jun 25;261(18):8434–8441. [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984 Jun;246(6 Pt 2):F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lytle C., Forbush B., 3rd Na-K-Cl cotransport in the shark rectal gland. II. Regulation in isolated tubules. Am J Physiol. 1992 Apr;262(4 Pt 1):C1009–C1017. doi: 10.1152/ajpcell.1992.262.4.C1009. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M. E. [3H]bumetanide binding in vascular endothelial cells. Quantitation of Na-K-Cl cotransporters. J Biol Chem. 1989 Dec 5;264(34):20326–20330. [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetz S., Lindsey A. E., Ward C. L., Kopito R. R. Functional activation of plasma membrane anion exchangers occurs in a pre-Golgi compartment. J Cell Biol. 1993 Apr;121(1):37–48. doi: 10.1083/jcb.121.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Sun A., Grossman E. B., Lombardi M., Hebert S. C. Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl- to Na+:K+:2Cl- cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991 Feb;120(1):83–94. doi: 10.1007/BF01868594. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Brant S. R., Walker M. S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992 May 5;267(13):9340–9346. [PubMed] [Google Scholar]
- WINDHAGER E. E., GIEBISCH G. Micropuncture study of renal tubular transfer of sodium chloride in the rat. Am J Physiol. 1961 Mar;200:581–590. doi: 10.1152/ajplegacy.1961.200.3.581. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]