Abstract
Spinocerebellar ataxia type 28 (SCA28) is a neurodegenerative disease caused by mutations of the mitochondrial protease AFG3L2. The SCA28 mouse model, which is haploinsufficient for Afg3l2, exhibits a progressive decline in motor function and displays dark degeneration of Purkinje cells (PC-DCD) of mitochondrial origin. Here, we determined that mitochondria in cultured Afg3l2-deficient PCs ineffectively buffer evoked Ca2+ peaks, resulting in enhanced cytoplasmic Ca2+ concentrations, which subsequently triggers PC-DCD. This Ca2+-handling defect is the result of negative synergism between mitochondrial depolarization and altered organelle trafficking to PC dendrites in Afg3l2-mutant cells. In SCA28 mice, partial genetic silencing of the metabotropic glutamate receptor mGluR1 decreased Ca2+ influx in PCs and reversed the ataxic phenotype. Moreover, administration of the β-lactam antibiotic ceftriaxone, which promotes synaptic glutamate clearance, thereby reducing Ca2+ influx, improved ataxia-associated phenotypes in SCA28 mice when given either prior to or after symptom onset. Together, the results of this study indicate that ineffective mitochondrial Ca2+ handling in PCs underlies SCA28 pathogenesis and suggest that strategies that lower glutamate stimulation of PCs should be further explored as a potential treatment for SCA28 patients.
Introduction
SCA28 is a form of dominant cerebellar ataxia characterized by unbalanced standing, gait incoordination, hyperreflexia of the lower limbs, nystagmus, and ophthalmoparesis (1). Several SCA28-causing missense mutations have been identified in the AFG3L2 gene (2–4). Moreover, homozygous mutations in AFG3L2 are responsible for a myoclonic epilepsy-ataxia-polyneuropathy syndrome of childhood (5).
SCA28 is the first autosomal dominant spinocerebellar ataxia (SCA) to be caused by alterations in a mitochondrial protein. Indeed, AFG3L2 belongs to the AAA (ATPases associated with various cellular activities) protease superfamily and assembles with paraplegin in the inner mitochondrial membrane into homo-oligomers and hetero-oligomers, named m-AAA proteases. The m-AAA complexes are crucial components of the quality-control system in the inner membrane, where they mediate the selective degradation of nonassembled and damaged proteins (6). In addition, they exert chaperone-like activity on respiratory chain complexes (7–9) and regulate the processing of the profusion protein OPA1 (10–13) and the subunit of mitochondrial ribosomes MRPL32 (14).
Most of the reported SCA28-causing mutations are heterozygous loss-of-function mutations clustering within the proteolytic domain of AFG3L2. Several pieces of evidence support the hypothesis that haploinsufficiency is the SCA28-causing mechanism. First, functional studies of human mutant AFG3L2 expression in the m-AAA–deficient yeast strain indicate that SCA28 mutations alter the proteolytic competence of AFG3L2 and result in impaired respiration. However, the coexpression of WT and mutant AFG3L2 is able to correct the respiratory defect for most of the mutations, indicating that haploinsufficiency is the disease-causing mechanism (2). Remarkably, a partial AFG3L2 deletion (exons 14–16) has been recently identified as the cause of SCA28 in 2 large Belgian families, and functional studies revealed a reduced number of m-AAA complexes in these patients, demonstrating that SCA28 is due to haploinsufficiency (15). Indeed, the Afg3l2-haploinsufficient mouse resembles the phenotype of SCA28 patients, showing a progressive decline in motor performance caused by dark degeneration of Purkinje cells (PC-DCD) (16). The latter is a specific form of a cell degeneration intermediate between necrosis and apoptosis and is characterized by cell shrinkage, cytoplasm darkening, and chromatin condensation (17). In the SCA28 mouse, this phenomenon is unique, since it originates from mitochondrial dysfunction (16), while in other forms of SCA, it is associated with excitotoxicity and high levels of intracellular Ca2+ (18–20).
PCs are some of the largest and most complex neurons of the mammalian CNS. They present extremely branched dendritic trees with a large number of active spines, which uniquely receive glutamatergic stimulation of ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and metabotropic receptors (e.g., mGluR1) as afferent inputs. PCs are therefore exposed to massive and sudden Ca2+ influx and are especially vulnerable to glutamate-mediated excitotoxicity (21). To control Ca2+ homeostasis, PCs take advantage of several Ca2+ clearance systems: the Ca2+ ATPase and the Ca2+-Na+ exchanger at the plasma membrane, the Ca2+ ATPase at the ER, and Ca2+-binding proteins (22).
Mitochondria accumulate Ca2+ in the matrix via the low-affinity mitochondrial Ca2+ uniporter (MCU) in a process that depends on the electrical potential across the inner membrane (Δψmito) (23). While the capability of mitochondria to internalize Ca2+ is undisputed, their functional role in living cells, and especially in neurons, is less clear. Compelling evidence indicates that mitochondria can act as local Ca2+ buffers in different neuronal cell types (24–26), but the effective impact of this process on neuronal Ca2+ homeostasis and physiology remains to be clarified.
The efficacy of mitochondrial Ca2+ uptake is strictly dependent on the proximity of the organelles to the Ca2+ source (e.g., ER, plasma membrane, dendritic spines) (23, 27). Neurons are therefore particularly reliant on proper trafficking of mitochondria to these sites and consequently on their dynamic properties. Mitochondria continuously fuse and fragment to mix lipids and matrix content and are also actively recruited to subcellular sites, such as the axonal and dendritic processes. Defects in these key features are indeed associated with neurodegenerative diseases (28). Also, alterations in both fusion and fission have been shown to decrease mitochondrial movement (29). Interestingly, loss of AFG3L2 results in increased organellar fragmentation due to enhanced processing of OPA1 (12, 16).
In this study, we have dissected the pathogenetic mechanism of SCA28 in primary PC neurons and provide the first evidence to our knowledge of a pharmacological treatment for this disease in preclinical models. Indeed, we have demonstrated in cultured PCs that Afg3l2 depletion causes defective Ca2+ uptake by mitochondria. The inefficient buffering and shaping of Ca2+ peaks by mutant mitochondria provoke an increase in cytoplasmic Ca2+ concentrations ([Ca2+]cyto), thus triggering PC-DCD. The impaired mitochondrial Ca2+ buffering results from the negative synergism of both depolarization of mitochondria and alteration of the mitochondrial morphology, which also hamper the proper trafficking of the organelles into dendrites.
As a proof of principle of this pathogenetic mechanism, we show that half dosage of mGluR1 (and thus reduced Ca2+ influx into PCs) completely reverses both motor impairment and PC-DCD in Afg3l2+/– mice.
Finally, we obtained the same result by pharmacological treatment with ceftriaxone, an antibiotic able to increase expression of the astrocyte glutamate transporter EAAT2, thereby lowering the glutamatergic stimulation of PCs.
Results
Afg3l2 depletion in PCs leads to increased susceptibility to high Ca2+ concentrations.
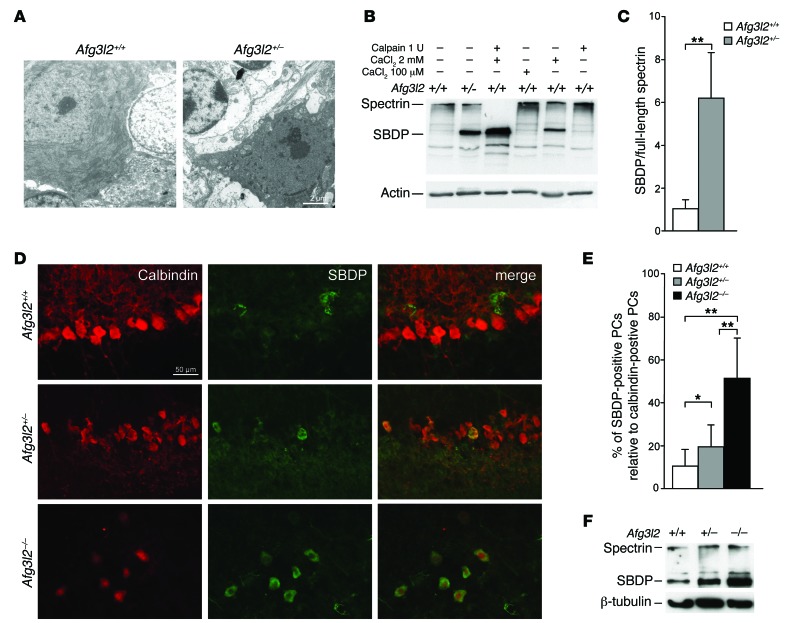
In Afg3l2+/– mice, PCs present morphological features typical of DCD (Figure 1A). DCD in this model represents approximately 20% to 30% of PCs at 8 months, as already reported (16). This phenomenon is caused by an increase in [Ca2+]cyto, thus activating calpains, which are Ca2+-sensitive cysteine proteases that mediate cytoskeletal breakdown (30–32). Looking for molecular evidence of activation of this pathway in SCA28, we assayed calpain-mediated proteolysis of αII-spectrin, which was expected to result in 2 proteolytic fragments of nearly equal electrophoretic mobility (~150 kDa) (33). These fragments were indeed found in the cerebellum of Afg3l2+/– mice by Western blot (WB) analysis and comigrated with the fragments obtained by treating WT cerebellar extracts with recombinant m-calpain plus 2 mM Ca2+, thus demonstrating increased [Ca2+]cyto and calpain activation (Figure 1, B and C).
Figure 1. Reduced dosage of AFG3L2 in PCs induces dark degeneration.
(A) Ultrathin sections of cerebella from 6-month-old Afg3l2+/– and WT mice showing dark degeneration of PCs. Scale bar: 2 μm. (B) WB analysis of cerebellar extracts from Afg3l2+/– and WT mice. When indicated, CaCl2 and/or 1 U calpain were added. (C) Densitometric analysis of WB bands. Data represent the mean ± SD; n = 3; **P < 0.001 by Student’s t test. (D) IF images of organotypic cerebellar slices obtained from 10-day-old Afg3l2–/– and Afg3l2+/– mice and their WT littermates after AMPA exposure. Abs against calbindin and SBDPs were used. Scale bar: 50 μm. (E) Quantitative evaluation of dark-degenerated (SBDP-positive) PCs relative to the total number of PCs in cerebellar slices treated with AMPA. Data represent the mean ± SD; n = 4, with an average of 25 neurons analyzed per genotype per experiment. *P < 0.05 and **P < 0.001 by Student’s t test. (F) WB analysis of cerebellar slices revealing an increased amount of SBDPs in Afg3l2–/– and Afg3l2+/– mice compared with that detected in WT mice.
We then investigated whether PCs in which Afg3l2 expression is reduced or abolished are selectively susceptible to stimuli that increase [Ca2+]cyto. To this end, we used cerebellar sections from Afg3l2-depleted mice and WT littermates and elicited DCDs by a brief exposure to AMPA (34). In fact, PCs express high levels of AMPA receptors, whose activation is able to increase [Ca2+]cyto (21). Upon AMPA treatment, we found that the number of PC-DCDs (identified by immunofluorescence [IF] using an Ab that specifically recognizes the amino-terminal breakdown product of calpain-cleaved spectrin; ref. 30), was increased in Afg3l2+/– PCs compared with the number in WT PCs, and this effect was even greater in Afg3l2–/– cells (Figure 1, D and E, and Supplemental Figure 1), supporting a higher susceptibility of Afg3l2-depleted PCs to stimuli that increase [Ca2+]cyto. We obtained the same result by performing WB analysis of cerebellar slices using an Ab against spectrin (Figure 1B), which revealed an increased amount of cleaved spectrin in Afg3l2–/– and Afg3l2+/– PCs compared with that detected in WT slices (Figure 1F).
Loss of Afg3l2 in PCs results in reduced mitochondrial Ca2+ uptake and increased cytosolic Ca2+ concentration.
In the SCA28 mouse, PC-DCD originates from mitochondrial dysfunction (16). Since PCs are exposed to massive Ca2+ influx (21) and mitochondria can transiently store large amounts of Ca2+ (23, 27), we reasoned that defective Ca2+ buffering operated by Afg3l2-mutant mitochondria in PCs could increase [Ca2+]cyto and cause DCD, thus mimicking excitotoxicity. This hypothesis was also supported by our previous data showing reduced [Ca2+] in the mitochondrial matrix of Afg3l2-depleted fibroblasts (13).
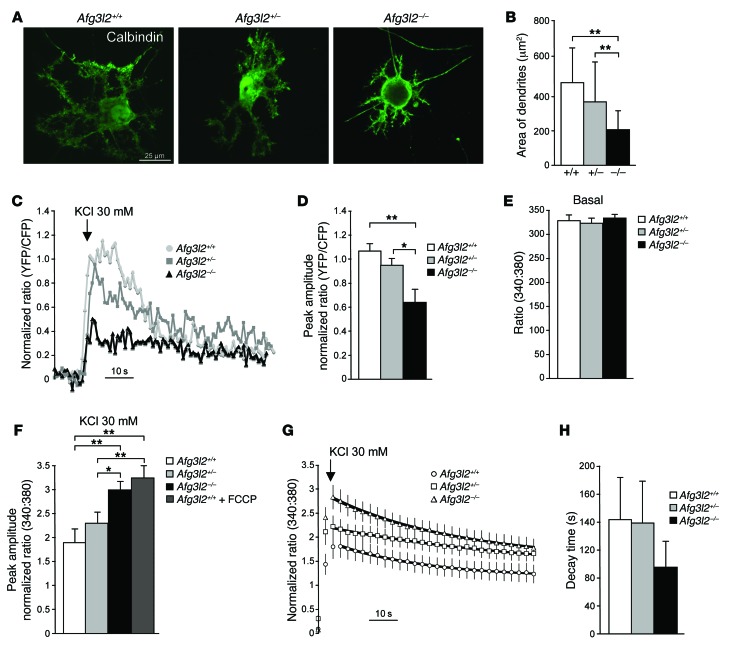
To address this issue, we decided to measure (a) mitochondrial [Ca2+] ([Ca2+]mito), and (b) [Ca2+]cyto in primary cerebellar cultures from Afg3l2-depleted mice. In these cultures PCs are present together with other cerebellar neurons (mainly granule cells) and Bergmann glia (Supplemental Figure 2A), which are required for PC survival and function (35). We first performed a quantitative evaluation of PC morphology by IF using anti-calbindin Ab at day 14 in vitro (14 DIV). Afg3l2+/– PCs appeared comparable to those in WT mice, while in Afg3l2–/– PCs, we found that dendrites were slightly decreased in number and length (Supplemental Figure 2, B and C), but were considerably reduced in terms of area, appearing thinner with shorter side branches (Figure 2, A and B). Spines were visible in the dendrites of PCs of each genotype, with no significant differences in spine density (Supplemental Figure 2D). We also verified that PCs correctly expressed mGluR1 receptors (Supplemental Figure 2A).
Figure 2. Reduced mitochondrial Ca2+ uptake in the absence of AFG3L2 results in increased [Ca2+]cyto.
(A) IF images of primary PCs at 14 DIV using anti-calbindin Ab. (B) Quantification of the area of dendrites. Data represent the mean ± SD; n = 6, with ~20 neurons analyzed per experiment. **P < 0.001 by Student’s t test. (C) Representative traces of evoked [Ca2+]mito responses in PCs expressing 4mtD1cpv. (D) Mean ± SEM of peak [Ca2+]mito responses after KCl stimulation (normalized increase in the YFP/CFP fluorescence ratio measured above the initial value. n = 4, with ~20 traces analyzed per experiment. *P < 0.05 and **P < 0.001 by Student’s t test. (E) Basal values of [Ca2+]cyto evaluated by fura-2 fluorescence at a 340:380 nm ratio. Data represent the mean ± SEM; n = 5, with ~10 traces analyzed per experiment. (F) Mean ± SEM of peak [Ca2+]cyto responses after stimulation with 30 mM KCl (normalized increase measured above the initial value; n = 5, with ~15 traces analyzed per experiment). *P < 0.05 and **P < 0.001 by Student’s t test. (G) Average traces of [Ca2+]cyto responses before and after KCl stimulation. Decays following the peak response were fit with a single exponential (black lines). (H) Mean ± SEM of the decay times measured for each trace of [Ca2+]cyto as obtained by fitting the individual traces with an exponential decay. n = 5, with ~15 traces analyzed per experiment.
To measure [Ca2+]mito in PCs in primary cerebellar cultures, we created an adeno-associated viral vector expressing a mitochondria-targeted version of the GFP-based Ca2+ probe 4mtD1cpv (36) under the synapsin promoter. Transduction of this probe in primary cerebellar cultures gave a neuron-specific signal. Among the various neuronal types, PCs were easily distinguished in bright-field, as they were markedly larger and more ramified than granule cells. Upon challenge with 30 mM KCl, which depolarizes the plasma membrane in neurons by opening potential-sensitive Ca2+ channels, we observed that mitochondrial Ca2+ rises of transduced neurons were significantly smaller in Afg3l2–/– PCs compared with those in Afg3l2+/– and WT cells (Figure 2, C and D).
In parallel, we performed ratiometric measurement of [Ca2+]cyto in PCs by using the high-affinity Ca2+ indicator fura-2. We found that the basal levels of [Ca 2+]cyto were identical in the 3 genotypes (Figure 2E). In contrast, we observed that in Afg3l2–/– PCs, the peak Ca2+ response elicited by acute exposure to 30 mM KCl was significantly higher than that in Afg3l2+/– and WT PCs (Figure 2F). Of note, the peak Ca2+ response observed in Afg3l2–/– PCs was comparable to that measured by pretreating WT PCs with the respiratory chain uncoupler trifluorocarbonylcyanide phenylhydrazone (FCCP) (Figure 2F). Our analysis of the decay kinetics revealed no significant differences among the genotypes, demonstrating that active Ca2+ clearance systems of mutant PCs (e.g., plasma membrane Ca2+ ATPase and ER Ca2+ ATPase) were unaffected (Figure 2, G and H).
We verified that an increased Ca2+ response in the absence of AFG3L2 in PCs occurs not only in the presence of a depolarizing stimulus such as KCl, but also in the presence of the group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG), which is capable of inducing Ca2+ release from the ER (21). We first confirmed the absence of a Ca2+ response in primary PCs lacking mGluR1 (Supplemental Figure 3, A and B). We then treated Afg3l2–/– and WT PCs with DHPG, which elicited an increased peak Ca2+ response in Afg3l2–/– cells compared with that seen in WT cells (Supplemental Figure 3C, Supplemental Results, and Supplemental Methods), in agreement with what we showed in Figure 2F after the administration of a depolarizing stimulus that enhanced Ca2+ influx into the cell from the plasma membrane. This result demonstrates that the inefficient mitochondrial Ca2+ buffering in Afg3l2–/– PCs causes abnormal cytosolic Ca2+ elevation, even in the presence of a stimulus inducing Ca2+ release from the ER.
Loss of Afg3l2 in primary PCs induces mitochondrial morphological alterations and depletion of dendritic mitochondria.
To understand the molecular basis of the decreased mitochondrial Ca2+ buffering in Afg3l2-depleted PCs, we examined the morphology, distribution, and metabolic status of mitochondria in primary PCs.
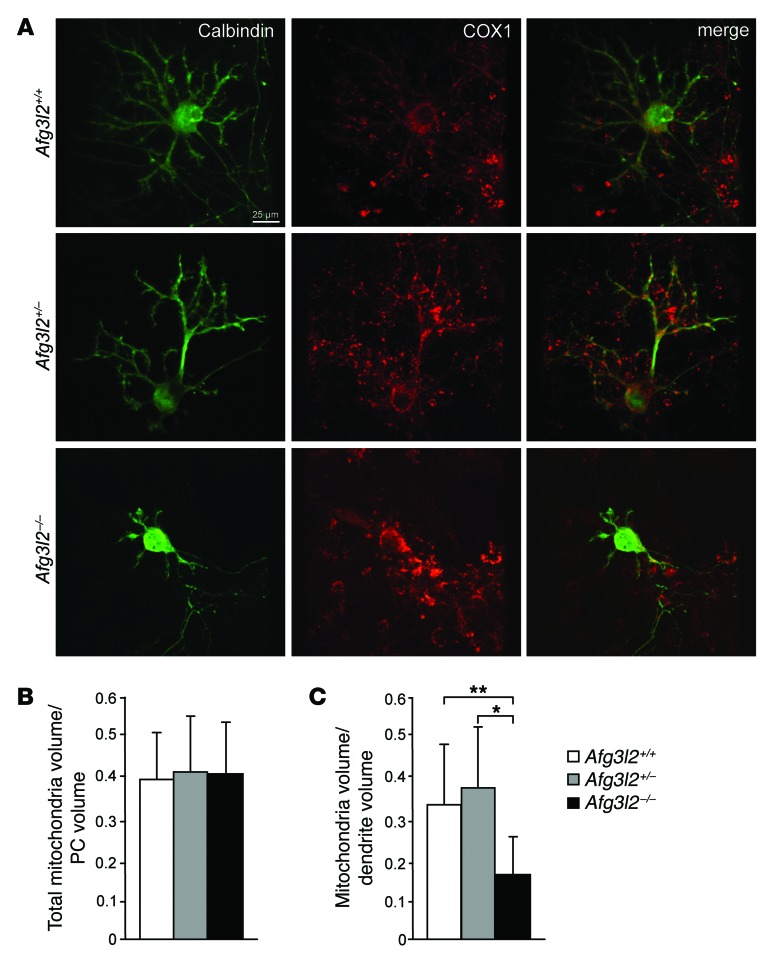
We first performed IF and confocal microscopy using an Ab against both COX1 (mitochondrial marker) and calbindin (PC marker). In Afg3l2–/– PCs, mitochondria were mostly round shaped, as was previously observed in MEFs (13), in contrast to the tubular-shaped organelles we observed in syngenic controls (Figure 3A). We also investigated whether alterations of mitochondrial morphology alter the trafficking of organelles to the distal dendritic branches of PCs. Using 3D reconstruction of confocal stacks of PCs stained with anti-calbindin and anti-COX1 Ab, we analyzed the volume of mitochondria in the whole PC volume and in dendrites. We observed no change in total mitochondrial volume in Afg3l2–/– PCs, though these cells contained fewer mitochondria in their dendrites, indicating an accumulation of organelles in the soma (Figure 3, B and C).
Figure 3. Loss of Afg3l2 in PCs alters dendritic mitochondrial content.
(A) IF images of primary PCs from the indicated genotypes at 14 DIV using Ab against the PC marker calbindin and the mitochondrial marker COX1 analyzed by confocal imaging. Scale bar: 25 μm. (B and C) Quantification of total mitochondria volume relative to total PC volume (B) or relative to PC dendrite volume (C) analyzed by 3D reconstruction of consecutive confocal stacks taken at 0.3 μm. n = 15 PCs analyzed per genotype. *P < 0.05 and **P < 0.001 by Student’s t test.
Alteration of cristae morphology correlates with mitochondrial depolarization in Afg3l2-depleted PCs.
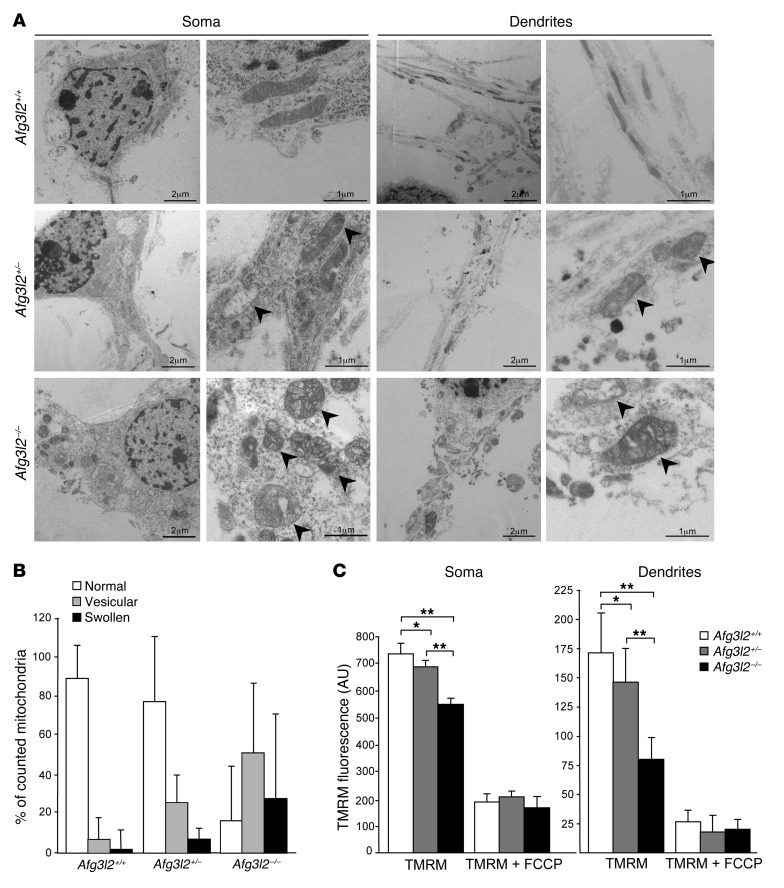
To further address the defective trafficking of mitochondria, we assayed the mitochondrial ultrastructure by electron microscopy (EM). In WT PCs, we observed that mitochondria were evenly distributed in the cell soma and dendrites, had structurally intact inner and outer membranes with well-defined cristae, and were tubular in shape. In contrast, in Afg3l2+/– PCs, we observed a remodeling of the inner membrane conformation, ranging from widening to vesiculation of the cristae, which worsened dramatically in Afg3l2–/– PCs, in which most of the organelles appeared swollen (Figure 4A). Quantification of these alterations is reported in Figure 4B. Consistent with our confocal microscopic observations, in Afg3l2–/– PCs, mitochondria aggregated in the soma, leaving dendrites depleted of organelles. We detected no differences in mitochondrial distribution between Afg3l2+/– and WT PC dendrites, indicating that organellar morphological alterations in the heterozygous mice may not be severe enough to hamper entry of the organelles into the dendritic tree, at least in culture conditions.
Figure 4. Afg3l2 depletion in PCs causes dramatic changes in mitochondrial ultrastructure and reduced membrane potential.
(A) EM of primary PCs at 14 DIV. Arrowheads indicate aberrant mitochondria. Scale bars: 2 μm (left panels, soma and dendrites); 1 μm (right panels, soma and dendrites). (B) Quantification of mitochondrial ultrastructural alterations. Organelles were classified as normal, vesicular, or swollen on the basis of inner membrane remodeling. Graph indicates the mean ± SD of an average of 200 mitochondria from 5 cells in 3 independent experiments. χ2 test (2 degrees of freedom): Afg3l2–/– versus either Afg3l2+/– or WT PCs, P < 0.001, WT versus Afg3l2+/–, P < 0.05. (C) Analysis of Δψmito in primary PCs by live-imaging measurement of TMRM fluorescence intensity in soma and dendrites, respectively. Data represent the mean ± SEM; n = 5, with an average of 40 cells analyzed per experiment. At the end of the experiments, 1 μM FCCP was added. *P < 0.05 and **P < 0.001 by Student’s t test.
These data prompted us to evaluate the metabolic status of the organelles in Afg3l2-depleted conditions. We therefore tested Δψmito by live-imaging measurement of the potentiometric dye tetramethylrhodamine methyl ester (TMRM) in primary PCs. In Afg3l2+/– PCs, and to a greater extent in Afg3l2–/– PCs, we observed a depolarization of mitochondria in the soma that was even more pronounced in dendrites (Figure 4C), indicating that PC mitochondria lacking AFG3L2 or with a halved dosage of AFG3L2 are metabolically dysfunctional.
Reduced excitatory stimulation of PCs rescues the ataxic phenotype of Afg3l2+/– mice.
Considering these pieces of evidence, we designed a rescue strategy based on the rationale that reducing Ca2+ influx into PCs by lowering their glutamatergic stimulation could prevent, or at least ameliorate, the cerebellar phenotype of SCA28 mutants. We addressed this goal using a binary approach, i.e., a genetic and pharmacological rescue.
Activation of the mGluR1 receptor is linked to IP3-mediated Ca2+ release from the ER (21). We thus crossed Afg3l2-haploinsufficient mice with cervelet-4 (crv4) heterozygous mice, which carry a spontaneous mutation causing loss of function of mGluR1 encoded by the Grm1 gene (37). We first verified a halved amount of the mGluR1 receptor (Supplemental Figure 4A) and excluded increased levels of AMPA receptors (Supplemental Figure 4B), signs of gait ataxia and cerebellar morphological alterations in Grm1+/crv4 mice (Supplemental Figure 4, C and D). Also, we found no mGluR5 expression in adult PCs in either WT or Grm1+/crv4 mice (Supplemental Figure 4E).
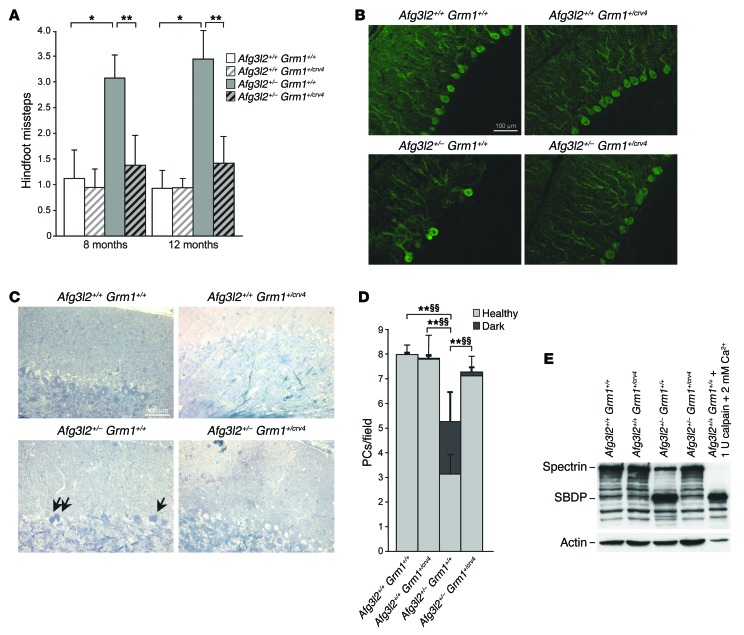
In agreement with the proposed mechanism of pathogenesis, we demonstrated that the SCA28 phenotype is fully rescued by halving the amount of the mGluR1 receptor and thus reducing the release of Ca2+ from the ER. Indeed, the Afg3l2+/– Grm1+/crv4 double mutants displayed motor function and coordination within the normal range, as demonstrated by beam-walking tests (Figure 5A and Supplemental Videos 1 and 2). Accordingly, neuropathological examination indicated that rescue of the ataxic phenotype correlated with decreased PC-DCD in Afg3l2+/– Grm1+/crv4 versus Afg3l2+/– mice (Figure 5, B–E). The molecular link between decreased PC-DCD and lower Ca2+ peaks was complemented by in vitro experiments, which disclosed a significant reduction in peak Ca2+ responses in Afg3l2–/– Grm1+/crv4 versus Afg3l2–/– primary PCs after DHPG stimulation (Supplemental Figure 3C), strongly indicating that mGluR1 haploinsufficiency is protective and able to prevent the exacerbation of glutamate stimulation in Afg3l2–/– PCs.
Figure 5. Half dosage of mGluR1 restores motor performances of Afg3l2+/– mice.
(A) Beam-walking test performed on Afg3l2+/– Grm1+/crv4 mice and their littermates at 8 and 12 months of age. Data represent the mean ± SD of 4 independent tests; n = 13–15. *P < 0.05 and **P < 0.001 by Student’s t test. (B) Cryostat-cut sections of cerebellum from Afg3l2+/– Grm1+/crv4 mice and their littermates at 8 months of age stained with anti-calbindin Ab. Scale bar: 100 μm.(C) Semithin sections of cerebellum from Afg3l2+/– Grm1+/crv4 mice and their littermates at 8 months of age stained with toluidine blue. Arrows indicate dark PCs. (D) Quantification of healthy and dark PCs in 8-month-old Afg3l2+/– Grm1+/crv4 mice. Data represent the mean ± SD; n = 4. P < 0.001 by Student’s t test for both healthy (*) and dark (§) PCs. (E) WB analysis of cerebellar extracts from of Afg3l2+/– Grm1+/crv4 mice and their littermates using anti-spectrin Ab. A cerebellar extract treated with 2 mM CaCl2 and 1 U calpain was used as a positive control. Actin was used to verify equal loading.
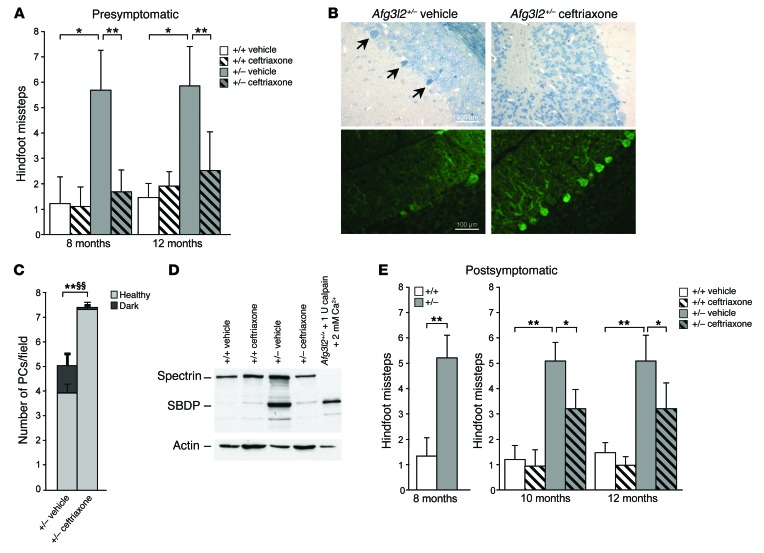
In order to test the rescue of the ataxic phenotype by drug treatment, Afg3l2+/– mice were administered the β-lactam antibiotic ceftriaxone, which is capable of increasing EAAT2 expression and activity (38). We used this drug to specifically reduce postsynaptic glutamatergic stimulation of PCs in Afg3l2+/– mice, and thus Ca2+ influx. We first determined that EAAT2-increased expression was maintained for approximately 7 to 8 weeks in the cerebellum after ceftriaxone treatment (Supplemental Figure 5A). We thus treated Afg3l2+/– and WT mice at 3 months of age (about 1 month before the onset of motor impairment; ref. 16) and repeated the treatment 2 months later. We observed that ceftriaxone administration markedly improved the motor capabilities of SCA28 mice. Indeed, the drug-treated mice made significantly fewer footslips in traversing the beam compared with saline-treated mutants at both 8 and 12 months of age (Figure 6A and Supplemental Video 3 and 4). This indicates that ceftriaxone treatment at presymptomatic stages is able to prevent, and not just delay, the ataxic phenotype in Afg3l2-haploinsufficient mice. In accordance with these findings, semithin sections of the cerebellum revealed a reduced number of PC-DCDs in treated Afg3l2+/– mice (Figure 6, B and C) that was also confirmed by WB, which showed an attenuation of calpain-mediated αII-spectrin cleavage (Figure 6D). Also, we found that PC numbers and dendritic tree arborization were completely recovered in the drug-treated mutants, as shown by IF using calbindin Ab (Figure 6B and Supplemental Figure 5B). Ceftriaxone treatment (as well as a halved dosage of mGluR1) produced neither adverse effects nor body-weight alterations, as demonstrated by the results of the SHIRPA (SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, phenotype assessment) protocol (39), which revealed normal motor, sensory, autonomic, and neuropsychiatric functions (Supplemental Tables 1 and 2).
Figure 6. Presymptomatic treatment with ceftriaxone rescues both motor symptoms and PC-DCD in SCA28 mice.
(A) Beam-walking test performed on Afg3l2+/– and WT mice treated with vehicle or ceftriaxone at presymptomatic stages. Data represent the mean ± SD of 4 independent tests; n = 15. *P < 0.05 and **P < 0.001 by Student’s t test. (B) Semithin (upper panels) and cryostat-cut sections (lower panels) of cerebellum from ceftriaxone-treated versus vehicle-treated Afg3l2+/– and WT mice at 8 months of age stained with toluidine blue and anti-calbindin Abs, respectively. Arrows indicate dark PCs. Scale bars: 100 μm. (C) Quantification of healthy and dark PCs in ceftriaxone-treated versus vehicle-treated Afg3l2+/– mice. Data represent the mean ± SD; n = 4. P < 0.001 by Student’s t test for both healthy (*) and dark (§) PCs. (D) WB analysis of cerebellar extracts from Afg3l2+/– and WT mice treated with vehicle or ceftriaxone using anti-spectrin Ab. A control cerebellar extract treated with 2 mM CaCl2 and 1 U calpain was used as a positive control. (E) Beam-walking test performed on Afg3l2+/– mice and their littermates at postsymptomatic stages (8 months) and after ceftriaxone treatment. Data represent the mean ± SD of 4 independent tests; n = 15. *P < 0.05 and **P < 0.001 by Student’s t test.
Of note, we demonstrated that ceftriaxone treatment is effective after onset of the ataxic phenotype. In fact, Afg3l2+/– mice treated at 8 and 10 months of age showed a significant amelioration of their motor coordination skills (Figure 6E and Supplemental Video 5 and 6).
The genetic and pharmacological rescues of the SCA28 phenotype were both based on the rationale that reducing Ca2+ influx into PCs could ameliorate DCD and ataxia in Afg3l2-haploinsufficient mice. When designing this strategy, we were aware that by limiting the glutamatergic stimulation of PCs, we were intervening downstream of the primary pathological event at the mitochondrial level. Indeed, we interfered with the effects of impaired mitochondrial Ca2+ buffering on cytosolic Ca2+ concentrations. For this reason, it was expected that both genetic and pharmacologic interventions would have no effect on mitochondrial abnormalities, but we could not formally exclude the possibility. In fact, under physiological conditions, a limited Ca2+ increase stimulates mitochondrial metabolism. However, in the presence of an overriding pathological stimulus, Ca2+ concentrations have a detrimental effect on mitochondria, such as the stimulation of ROS production or induction of mitochondrial permeability transition (40). In order to test our hypothesis, we evaluated the possible amelioration of mitochondrial (a) morphological and (b) metabolic abnormalities in Afg3l2+/– Grm1+/crv4 mice and in Afg3l2+/– mice treated with ceftriaxone compared with that in controls. EM analysis of cerebellar sections revealed no significant amelioration of mitochondrial morphology in Afg3l2+/– Grm1+/crv4 mice compared with that seen in Afg3l2+/– mice (Supplemental Figure 6, A and B), nor was this observed in Afg3l2+/– mice treated with ceftriaxone compared with vehicle-treated Afg3l2+/– mice (Supplemental Figure 6, C and D). Also, EM study of primary PCs showed no amelioration of primary PC mitochondrial structures in Afg3l2–/– Grm1+/crv4 mice compared with those observed in Afg3l2–/– mice in both basal conditions and after acute stimulation with DHPG (Supplemental Figure 6E).
We also performed a spectrophotometric assay of respiratory complex I activity on cerebellar protein extracts. As expected, we found a significant reduction in complex I activity in Afg3l2+/– mice compared with that in WT mice. However, this defect was not rescued in Afg3l2+/– Grm1+/crv4 mice or in Afg3l2+/– mice treated with ceftriaxone, consistent with what we observed in the EM studies (Supplemental Figure 7, A and B).
These data indicate that the reduction of Ca2+ concentrations in PCs achieved by our interventions, while rescuing DCD and the clinical phenotype, was not able to recover the alterations in mitochondrial morphology and metabolism in SCA28 mice.
Discussion
In the present study, we provide evidence for a pathogenetic mechanism of SCA28 that involves defective mitochondrial Ca2+ buffering as a primary triggering event. The inefficient shaping of Ca2+ rises operated by mutant mitochondria upon stimulation results in a pathological increase in [Ca2+]cyto, which in turn triggers PC-DCD. Several data support this mechanism. First, we demonstrated in cerebellar slices treated with AMPA that Afg3l2–/– and Afg3l2+/– PCs are more prone to undergo DCD, indicating a reduced ability of mutant cells to cope with stimuli that increase [Ca2+]cyto.
We then determined that the increased sensitivity to high Ca2+ levels was due to reduced mitochondrial Ca2+ handling. We previously reported defective mitochondrial Ca2+ uptake in Afg3l2–/– fibroblasts, but this had no impact on cellular Ca2+ homeostasis (13), as is expected for nonexcitable and nonpolarized cells. We thus tested this hypothesis in primary PCs. The uniqueness of these neurons resides in their large soma and huge dendritic trees, which only receive glutamate excitatory synaptic input from a single climbing fiber and from approximately 200,000 parallel fibers. Thus, PCs require more precise control of Ca2+ homeostasis than do other neurons, implying greater Ca2+-buffering power (27). These features suggest that mitochondrial Ca2+ buffering is crucial to PCs.
We directly measured [Ca2+]mito in primary PCs after KCl induction and found it markedly reduced in Afg3l2–/– PCs. This defect had a marginal involvement in the maintenance of resting Ca2+ levels in PCs, since we determined that [Ca2+]cyto was unchanged in Afg3l2–/– PCs under basal conditions. In contrast, we found that the evoked peak [Ca2+]cyto response was markedly increased in Afg3l2–/– PCs, and this result perfectly matches the one obtained with treating control PCs with FCCP, which inhibits mitochondrial Ca2+ uptake. Of note, [Ca2+]cyto pathological elevation in Afg3l2–/– PCs occurs not only in the presence of a depolarizing stimulus causing massive Ca2+ influx from the plasma membrane, but also in the presence of an mGluR1 agonist, which induces Ca2+ release from the ER. Previous studies showed that massive Ca2+ increases can be substantially buffered by mitochondria in other neuronal cell types, in which the addition of uncoupling agents results in a marked elevation of peak cytosolic Ca2+ levels (24–26). Our data, aside from elucidating the pathogenetic mechanism of SCA28, demonstrate for the first time to our knowledge the impact of defective mitochondrial Ca2+ uptake on local Ca2+ signaling in a physiopathological condition of the nervous system. This concept has been proposed as a potential pathogenetic mechanism of neurological disorders (27, 41) but has never been directly demonstrated and has thus far remained largely speculative.
We also demonstrated that reduced mitochondrial Ca2+ uptake capacity in Afg3l2-mutant PCs is the result of the negative synergy between alterations of mitochondrial morphology and impaired mitochondrial metabolism. Indeed, EM analysis revealed dramatic alterations of organellar ultrastructure in mutant PCs, showing mitochondria with vesiculation of the inner membrane or a completely swollen appearance. We observed aberrant organelles mainly in Afg3l2–/– PCs, but also in Afg3l2+/– PCs, indicating that Afg3l2 haploinsufficiency is sufficient to exert mitochondrial damage in neuronal cells in vitro as well. In addition to these ultrastructural defects, Afg3l2–/– PCs showed increased mitochondrial network fragmentation and faulty mitochondrial distribution in the dendritic tracks. In particular, we observed that the mitochondrial volume associated with dendrites was reduced in the absence of AFG3L2, indicating a skewed distribution of mitochondria toward the cell soma.
AFG3L2 controls the morphology of the mitochondrial network via regulation of OPA1 processing. In particular, the absence of AFG3L2 causes increased mitochondrial fragmentation due to enhanced OPA1 processing (12, 13). Fragmentation of the mitochondrial network can modify organelle Ca2+ uptake by changing the interaction with the Ca2+ source (e.g., the ER and/or plasma membrane) and limiting proper diffusion of the Ca2+ wave along the mitochondrial network (42, 43). Indeed, this was the case in Afg3l2–/– fibroblasts (13).
Defects in both fusion and fission have been shown to affect mitochondrial movement (29). In Afg3l2–/– PCs, the increased diameter of mitochondria due to cristae disruption and swelling may hamper their efficient entry into dendrites, resulting in a lack of mitochondria in these processes. A similar situation was previously observed in mice in which mitofusin 2, a protein involved in mitochondrial fusion, was specifically ablated in PCs (44), indicating that these neurons are particularly sensitive to perturbations of mitochondrial dynamics.
In line with the morphological alterations, mitochondria in Afg3l2-mutant PCs were also metabolically dysfunctional, displaying reduced Δψmito in both soma and dendrites as a consequence of the respiratory chain defect we reported previously (9).
Thus, we propose a pathogenetic mechanism in which loss and haploinsufficiency of Afg3l2 cause fragmentation and mild depolarization of mitochondria, which weakens mitochondrial buffering of Ca2+ elevations in the soma. This scenario is worsened in mutant dendrites due to underpopulation of mitochondria, too.
It is also conceivable that reduced ATP production by mutant mitochondria can locally slow down the kinetics of Ca2+ pumps, affecting extrusion of Ca2+ from the cytosol into the ER and across the plasma membrane in dendrites; however, this does not apply to the soma of PCs, in which the kinetics of Ca2+ recovery after stimulation is unaffected.
According to the proposed mechanism of neurodegeneration, the SCA28 phenotype is fully rescued by halving the amount of the mGluR1 receptor. The Afg3l2+/– Grm1+/crv4 double mutants displaymotor function and coordination within the normal range, and degenerating PCs are remarkably reduced compared with those in Afg3l2+/– mice. Adult PCs express high levels of mGluR1 (but not mGluR5) in the soma and in dendritic spines, and this receptor is essential for synapse plasticity and motor coordination. Indeed, mice lacking mGluR1 display severe ataxia and deficits in spatial and associative learning (37, 45), and homozygous mutations in GRM1 have been recently associated with a recessive form of congenital cerebellar ataxia (46).
Finally, we treated Afg3l2+/– mice with ceftriaxone, a safe and multipotent agent used for decades as an antimicrobial agent. This drug was shown to consistently increase the transcription levels of the astrocyte glutamate transporter EAAT2 (38).
On the basis of the SCA28 pathogenetic mechanism that we defined in this work, we used this drug to reduce the glutamatergic stimulation of PCs in Afg3l2+/– mice. We demonstrated that ceftriaxone treatment at both pre- and postsymptomatic stages was able to consistently reduce the number of PC-DCDs in the SCA28 model.
As expected, we found no amelioration of mitochondrial parameters in Afg3l2+/– mice treated with ceftriaxone or in Afg3l2+/– Grm1+/crv4 double mutants. Indeed, our genetic and pharmacological interventions, by consistently reducing [Ca2+]cyto and thus acting downstream of the primary insulting event at the mitochondrial level, were effective in rescuing DCD and the ataxic phenotype, but not the mitochondrial phenotypes. While further studies aimed at discovering therapies that specifically address SCA28 mitochondrial impairment are needed, administering ceftriaxone downstream in the pathogenic cascade is a potentially effective approach to treating additional forms of SCA that share the final stage of degeneration with SCA28 (i.e., elevated [Ca2+]cyto in PCs).
The data presented in this work disclose important steps in the molecular pathogenesis of SCA28 and provide a strong rationale for a new approach to treating this disease. At present, effective therapies for cerebellar ataxias are lacking, although some improvements in ataxic and nonataxic symptoms have been reported in a few clinical trials (47). We believe our data will contribute to advancing therapeutics for patients with SCA28, as ceftriaxone is a widely used, well-tolerated antibiotic drug that represents an immediately available therapy for SCA28 patients as well as for those affected by other SCAs characterized by PC-DCD.
Methods
Animals, drug administration, and motor tests.
Afg3l2+/– mice (16) were bred on an FVB/N background, and Grm1+/crv4 mice were bred on a BALB/c/ background (37). In the genetic rescue experiments, Afg3l2+/– Grm1+/crv4 mice and their littermates were bred on an FVB/N BALB/c/ mixed background. For the presymptomatic treatment, ceftriaxone (Sigma-Aldrich) was administered to 3-month-old mice by daily i.p. injection at a dose of 200 mg/kg body weight for 5 consecutive days (2 months before the onset of motor impairment in SCA28 mice; ref. 16). The treatment was repeated 2 months later. For the postsymptomatic treatment, ceftriaxone was administered to mice at 8 and 10 months of age by daily i.p. injection at a dose of 200 mg/kg body weight for 5 consecutive days. Beam-walking and SHIRPA tests were performed as previously described (16), and investigators were blinded to the treatment group during assessments.
Abs, drugs, and reagents.
For WB analysis, commercially available monoclonal Abs were used for the detection of rabbit anti-GLT1 (PA3-040A; Thermo Scientific); mouse anti-spectrin (MAB1622; EMD Millipore); mouse anti–β-actin–peroxidase clone AC-15 (A3854; Sigma-Aldrich); and rat anti–PSD-95 (ab2723; Abcam). ECL anti-mouse and anti-rabbit IgG and HRP-linked species-specific whole Abs were purchased from GE Healthcare.
For IF experiments, the following Abs were used: rabbit anti–spectrin–breakdown product (anti-SBDP) (30, 31); mouse anti–GFAP clone G-A-5 (G3893; Santa Cruz Biotechnology Inc.); mouse anti–NeuN clone A60 (MAB377; EMD Millipore); rabbit anti–calbindin 28 kDa (300; Swant); and anti–OxPhos complex IV subunit I (A-6403; Invitrogen). Secondary Abs were conjugated with Alexa 488 and Alexa 596 (A11001 and A11010; Invitrogen).
WB analyses.
Spectrin band patterns were analyzed by standard WB procedure. Briefly, tissues were homogenized in 50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100, and 1 mM DTT using a glass-Teflon homogenizer and incubated for 90 minutes on ice. Cell debris was discarded by centrifugation at 8,000 g for 5 minutes at 4°C. Controls consisted of WT lysates treated with 100 μM or 2 mM CaCl2 or 1 U recombinant rat calpain (EMD Millipore) for 30 minutes at 37°C.
For EAAT2 detection, synaptosomes were isolated as follows: mouse cerebellum was homogenized in sucrose buffer (320 mM sucrose, 5 mM HEPES [pH 7.4], and 2 mM EDTA) and a protease inhibitor cocktail (PIC) (Sigma-Aldrich) using a glass-Teflon homogenizer. Tissue debris was discarded by centrifugation at 1,000 g for 10 minutes at 4°C. Supernatants were centrifuged at 10,000 g for 15 minutes at 4°C, and the pellet was resuspended in 5 mM HEPES (pH 7.4) plus PIC, homogenized with a glass-Teflon homogenizer, incubated for 30 minutes on ice, and centrifuged at 20,000 g for 30 minutes at 4°C. The synaptosome pellet was resuspended in sucrose buffer and analyzed by WB.
Organotypic slices and PC primary cultures.
Organotypic slices from cerebellum were prepared from P10 mice, as previously described (32). Cerebella were removed from newborn Afg3l2 mice. Sagittal cerebellar sections (400-μm thickness) were cut using a chopper tissue slicer (McIlwaintissue Chopper; Stoelting Co.) and placed on Millicell-CM organotypic tissue–culture plate inserts (EMD Millipore) in a medium containing Basal Medium Eagle (Sigma-Aldrich), 25% horse serum (Invitrogen), 0.5% glucose, 2 mM GlutaMax (Invitrogen), 100 U/ml PenStrep (Invitrogen), and 2.5 μM arabinofuranosyl cytidine (Ara-C; Sigma-Aldrich). After 4 to 5 DIV, slices were exposed to 50 μM AMPA (Tocris) for 30 minutes. Controls consisted of identical manipulation for the entire experiment without AMPA. Slices were then fixed in 4% paraformaldehyde solution, transferred to a 10%–20%, and finally a 30%, sucrose solution and then frozen. Sections (16-μM thickness) were cryostat cut with a Leica CM1850.
For primary PC cultures, a modified version of previously described protocols (48) was used. Cerebella from newborn mice were incubated in HBSS 1X (Invitrogen) containing 5 U/ml papain (Sigma-Aldrich), 50 mg/ml cystein-HCl, 0.1 mM EDTA, and 1 mg/ml DNase I (EMD Millipore) for 30 minutes at 37°C. Tissues were then dissociated by mechanical trituration. The reaction was blocked with 10% horse serum, and samples were centrifuged at 100 g for 10 minutes. The cellular pellet was washed twice in HBSS 1X and resuspended in cultured medium containing Neurobasal (Invitrogen), B27 supplement (Invitrogen), 200 mg/ml D-glucose, 2 mM GlutaMax (Invitrogen), 100 U/ml PenStrep (Invitrogen), 1% horse serum (Invitrogen), 3 mM KCl, and 50 ng/ml NGF 2.5S (EMD Millipore). Cells were plated at a density of 1.5 × 104/cm2 on coverslips (14 mm or 24 mm in diameter) or in glass-bottom culture dishes (MatTek) coated with Poly-L-Lysine (Sigma-Aldrich).
Measurement of Δψmito.
Δψmito was measured using the potentiometric dye TMRM (Invitrogen). Primary PCs at 14 DIV were incubated with 50 nM TMRM, 2 mM CsH (Vinci-Biochem), and 2 mg/ml Hoechst 33342 (Invitrogen) in phenol red–free HBSS 1× for 30 minutes at 37°C. Imaging of TMRM fluorescence was performed using an Axio Observer.Z1 inverted microscope (Zeiss). Data represent the average of 4 images acquired every 15 seconds. FCCP (1 μM) was added at the end of acquisition. Images were analyzed using ImageJ software (NIH).
Morphometric analyses of primary PCs.
IF was performed on primary PCs at 14 DIV. Stacks of consecutive confocal images were taken at 0.3-μm intervals using a PerkinElmer UltraVIEW Spinning Disk Confocal Microscope. Analyses of soma and dendrite area and total mitochondria volume were performed using Volocity 3D Image Analysis Software, version 5.5.1 (PerkinElmer). For mitochondrial volume evaluation, a region of interest (ROI) was drawn to cover the profile of each PC (or dendrites only). A threshold for the red signal (mitochondria) and green signal (PCs) was set in order to exclude the background. Mitochondria with a red signal intensity greater than or equal to the green threshold were identified as belonging to PCs. This analysis was extended to the whole cell volume.
Syn-4mtD1cpv rAd construction, production, and titering.
4mtD1cpv pcDNA3 was used as a template in a PCR with the following primers: forward, 5′-TTATCGAAATTAATACGACTCACGTGGAATTCGCCACCATGTCCGTCC-3′; reverse, 5′-CCGCCAGTGTGATGGATATCTGCAGAATTCTTA-3′. The forward primer was designed to introduce an EcoRI restriction site at the 5′ end of 4mtD1cpv and a Kozak sequence to improve translation efficiency. The PCR product was then cloned in the EcoRI site of the pDC511 syn BGH-polyA vector (49). This shuttle plasmid carries the 470-bp human synapsin promoter to guarantee neuron-specific expression of the chameleon probe.
Second-generation rAd was produced by transfecting E2T cells (50) with the genomic helper plasmid (Microbix Biosystems Inc.) that had been modified to contain the E2a deletion and the pDC511 syn 4mtD1cpv-BGH PolyA vector, as previously described (49). Preparation of a high-titer viral stock from crude lysate was performed as described by Ng and Graham (51). The titer was determined by limiting dilution to obtain 3.1 × 107 PFU/ml.
Ca2+ imaging.
[Ca2+]cyto measurements were performed as previously described (52). Fura-2 acetoxymethyl ester (Calbiochem, Merck KGaA) loading was performed at 37°C (4 μM, 40 minutes) in Krebs Ringer HEPES buffer (containing 5 mM KCl, 125 mM NaCl, 2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 6 mM glucose, and 20 mM HEPES [pH 7.4]). When the K+ concentration was increased in the solution, the concentration of Na+ was adjusted to maintain isotonicity. Excitation wavelengths of 340 and 380 nm were used with an emission wavelength of 510 nm. The 340:380 fura-2 ratio was calculated as mean values within ROIs drawn in neuronal soma. The time course of the intensity ratio was analyzed by custom-written routines in MATLAB (The MathWorks Inc.): the code scans each response curve to find its maximum value and fits the subsequent decay with a monoexponential in order to quantify the amplitude of the response and the characteristic time of the decay. To generate the average responses showed in Figure 2G, the curves were first synchronized to the time of maximum fold change, corresponding to the onset of the Ca2+ response to KCl.
For fluorescence resonance energy transfer (FRET) experiments, PCs were transduced at 5 DIV with an MOI of 60 and imaged for Ca2+ measurement 72 hours after infection. The FRET data were acquired on a Leica TCS SP-5 DS confocal microscope equipped with a ×63 NA 1.4 oil-immersion objective lens (Leica Microsystems). The light of a 405-nm solid-state laser was used to excite CFP, and the fluorescence emission from both CFP and YFP was collected simultaneously using 2 separate detection channels (CFP, 470–505 nm and YFP, 525–600 nm, respectively). PCs were easily distinguished from granule cells and neurons, being markedly larger and more ramified. Time series of 100 frames were collected using an imaging time of 1.314 seconds per image. After approximately 20 images, 30 mM KCl was added to the imaging medium as described above to quantify the mitochondrial calcium response. The FRET signal was quantified by measuring the background-subtracted ratio between YFP and CFP channels on pixels corresponding to mitochondria.
EM analysis.
The semithin and ultrathin sections from mouse tissues and primary PCs at 14 DIV were prepared as previously described (16).
Statistics.
Results are reported as the mean ± SD or SEM. Most of the experiments were evaluated by a 2-tailed Student’s t test. P values were adjusted for multiple testing by applying the Holm-Bonferroni method (53). A χ2 test with 2 degrees of freedom was applied for analysis of the ultrastructure of mitochondria. Scores from SHIRPA analysis were analyzed by a nonparametric Mann-Whitney U test. Comparisons were considered statistically significant when P was less than 0.05.
Study approval.
All experiments involving animals were performed in accordance with experimental protocols approved by the IACUC of the San Raffaele Scientific Institute.
Supplementary Material
Acknowledgments
This work was supported by the Italian Telethon Foundation (GGP12235); Fondazione Cariplo (2012 0646); Ministero Italiano dell’Università e della Ricerca (MIUR, 20108WT59Y 001); Associazione Italiana Sindromi Atassiche (AISA); and Max Planck Society (to S.M. Young Jr.). F. Maltecca is a recipient of a Young Investigator Award for SCA research from the National Ataxia Foundation (NAF); I. Drago is a recipient of an EMBO fellowship (ASTF 365.00-2010). We thank Tullio Pozzan and Laura Cassina for critical discussion, Diana Pendin for virus preparation, Laura Corti for histological technical assistance and management of mouse colonies, and Valeria Barili for technical support with cerebellar slice preparation. We thank Alessandro Ambrosi for his support with statistical analyses throughout the study.
Footnotes
Note regarding evaluation of this manuscript: Manuscripts authored by scientists associated with Duke University, The University of North Carolina at Chapel Hill, Duke-NUS, and the Sanford-Burnham Medical Research Institute are handled not by members of the editorial board but rather by the science editors, who consult with selected external editors and reviewers.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(1):263–274. doi:10.1172/JCI74770.
References
- 1.Cagnoli C, et al. SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22-q11.2. Brain. 2006;129(pt 1):235–242. doi: 10.1093/brain/awh651. [DOI] [PubMed] [Google Scholar]
- 2.Di Bella D, et al. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42(4):313–321. doi: 10.1038/ng.544. [DOI] [PubMed] [Google Scholar]
- 3.Cagnoli C, et al. Missense mutations in the AFG3L2 proteolytic domain account for approximately 1.5% of European autosomal dominant cerebellar ataxias. Hum Mutat. 2010;31(10):1117–1124. doi: 10.1002/humu.21342. [DOI] [PubMed] [Google Scholar]
- 4.Edener U, et al. Early onset and slow progression of SCA28, a rare dominant ataxia in a large four-generation family with a novel AFG3L2 mutation. Eur J Hum Genet. 2010;18(8):965–968. doi: 10.1038/ejhg.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierson TM, et al. Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet. 2011;7(10):e1002325. doi: 10.1371/journal.pgen.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppen M, Langer T. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol. 2007;42(3):221–242. doi: 10.1080/10409230701380452. [DOI] [PubMed] [Google Scholar]
- 7.Arlt H, Steglich G, Perryman R, Guiard B, Neupert W, Langer T. The formation of respiratory chain complexes in mitochondria is under the proteolytic control of the m-AAA protease. EMBO J. 1998;17(16):4837–4847. doi: 10.1093/emboj/17.16.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atorino L, et al. Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J Cell Biol. 2003;163(4):777–787. doi: 10.1083/jcb.200304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maltecca F, et al. The mitochondrial protease AFG3L2 is essential for axonal development. J Neurosci. 2008;28(11):2827–2836. doi: 10.1523/JNEUROSCI.4677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvezin-Caubet S, et al. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell. 2007;18(9):3582–3590. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehses S, et al. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltecca F, et al. Respiratory dysfunction by AFG3L2 deficiency causes decreased mitochondrial calcium uptake via organellar network fragmentation. Hum Mol Genet. 2012;21(17):3858–3870. doi: 10.1093/hmg/dds214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell. 2005;123(2):277–289. doi: 10.1016/j.cell.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Smets K, et al. Partial deletion of AFG3L2 causing spinocerebellar ataxia type 28. Neurology. 2014;82(23):2092–2100. doi: 10.1212/WNL.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 16.Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G. Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci. 2009;29(29):9244–9254. doi: 10.1523/JNEUROSCI.1532-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2(8):589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda Y, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38(2):184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 19.Custer SK, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9(10):1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- 20.Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum. 2012;11(3):630–639. doi: 10.1007/s12311-010-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M. The molecular organization of cerebellar long-term depression. Nat Rev. 2002;3(11):896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- 22.Gruol D, Manto M, Haines D. Ca2+ signaling in cerebellar Purkinje neurons — editorial. Cerebellum. 2012;11(3):605–608. doi: 10.1007/s12311-012-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca(2)(+) homeostasis: mechanism, role, and tissue specificities. Pflugers Arch. 2012;464(1):3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- 24.Colegrove SL, Albrecht MA, Friel DD. Dissection of mitochondrial Ca2+ uptake and release fluxes in situ after depolarization-evoked [Ca2+](i) elevations in sympathetic neurons. J Gen Physiol. 2000;115(3):351–370. doi: 10.1085/jgp.115.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca(2+)] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20(19):7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero M, et al. Control of secretion by mitochondria depends on the size of the local [Ca2+] after chromaffin cell stimulation. Eur J Neurosci. 2001;13(12):2247–2254. doi: 10.1046/j.0953-816x.2001.01602.x. [DOI] [PubMed] [Google Scholar]
- 27.Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012;464(1):111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrado M, Scorrano L, Campello S. Mitochondrial dynamics in cancer and neurodegenerative and neuroinflammatory diseases. Int J Cell Biol. 2012;2012:729290. doi: 10.1155/2012/729290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Chan DC. Mitochondrial dynamics — fusion, fission, movement, and mitophagy — in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995;273(2):902–908. [PubMed] [Google Scholar]
- 31.Bahr BA, et al. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol. 2002;174(1):37–47. doi: 10.1006/exnr.2001.7852. [DOI] [PubMed] [Google Scholar]
- 32.Mansouri B, et al. Involvement of calpain in AMPA-induced toxicity to rat cerebellar Purkinje neurons. Eur J Pharmacol. 2007;557(2–3):106–114. doi: 10.1016/j.ejphar.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Czogalla A, Sikorski AF. Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62(17):1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strahlendorf JC, Acosta S, Strahlendorf HK. Diazoxide and cyclothiazide convert AMPA-induced dark cell degeneration of Purkinje cells to edematous damage in the cerebellar slice. Brain Res. 1996;729(2):197–204. doi: 10.1016/0006-8993(96)00367-8. [DOI] [PubMed] [Google Scholar]
- 35.Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12(2):243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 36.Palmer AE, et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13(5):521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Conti V, et al. crv4, a mouse model for human ataxia associated with kyphoscoliosis caused by an mRNA splicing mutation of the metabotropic glutamate receptor 1 (Grm1). Int J Mol Med. 2006;18(4):593–600. [PubMed] [Google Scholar]
- 38.Rothstein JD, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 39.Irwin S, Banuazizi A, Kalsner S, Curtis A. One trial learning in the mouse. I. Its characteristics and modification by experimental-seasonal variables. Psychopharmacologia. 1968;12(4):286–302. doi: 10.1007/BF00401408. [DOI] [PubMed] [Google Scholar]
- 40.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 41.Barrett EF, Barrett JN, David G. Mitochondria in motor nerve terminals: function in health and in mutant superoxide dismutase 1 mouse models of familial ALS. J Bioenerg Biomembr. 2011;43(6):581–586. doi: 10.1007/s10863-011-9392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004;279(21):22704–22714. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 43.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16(1):59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130(3):548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288(5472):1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 46.Guergueltcheva V, et al. Autosomal-recessive congenital cerebellar ataxia is caused by mutations in metabotropic glutamate receptor 1. Am J Hum Genet. 2012;91(3):553–564. doi: 10.1016/j.ajhg.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matilla-Duenas A. Machado-Joseph disease and other rare spinocerebellar ataxias. Adv Exp Med Biol. 2012;724:172–188. doi: 10.1007/978-1-4614-0653-2_14. [DOI] [PubMed] [Google Scholar]
- 48.Tabata T, Sawada S, Araki K, Bono Y, Furuya S, Kano M. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J Neurosci Methods. 2000;104(1):45–53. doi: 10.1016/S0165-0270(00)00323-X. [DOI] [PubMed] [Google Scholar]
- 49.Montesinos MS, Chen Z, Young SM., Jr pUNISHER: a high-level expression cassette for use with recombinant viral vectors for rapid and long term in vivo neuronal expression in the CNS. J Neurophysiol. 2011;106(6):3230–3244. doi: 10.1152/jn.00713.2011. [DOI] [PubMed] [Google Scholar]
- 50.Zhou H, Beaudet AL. A new vector system with inducible E2a cell line for production of higher titer and safer adenoviral vectors. Virology. 2000;275(2):348–357. doi: 10.1006/viro.2000.0515. [DOI] [PubMed] [Google Scholar]
- 51.Ng P, Graham FL. Construction of first-generation adenoviral vectors. Methods Mol Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- 52.Codazzi F, et al. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26(13):3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.