Abstract
Hematopoietic stem cell (HSC) function is regulated by activation of receptor tyrosine kinases (RTKs). Receptor protein tyrosine phosphatases (PTPs) counterbalance RTK signaling; however, the functions of receptor PTPs in HSCs remain incompletely understood. We found that a receptor PTP, PTPσ, was substantially overexpressed in mouse and human HSCs compared with more mature hematopoietic cells. Competitive transplantation of bone marrow cells from PTPσ-deficient mice revealed that the loss of PTPσ substantially increased long-term HSC-repopulating capacity compared with BM cells from control mice. While HSCs from PTPσ-deficient mice had no apparent alterations in cell-cycle status, apoptosis, or homing capacity, these HSCs exhibited increased levels of activated RAC1, a RhoGTPase that regulates HSC engraftment capacity. shRNA-mediated silencing of PTPσ also increased activated RAC1 levels in wild-type HSCs. Functionally, PTPσ-deficient BM cells displayed increased cobblestone area–forming cell (CAFC) capacity and augmented transendothelial migration capacity, which was abrogated by RAC inhibition. Specific selection of human cord blood CD34+CD38–CD45RA–lin– PTPσ– cells substantially increased the repopulating capacity of human HSCs compared with CD34+CD38–CD45RA–lin– cells and CD34+CD38–CD45RA–lin–PTPσ+ cells. Our results demonstrate that PTPσ regulates HSC functional capacity via RAC1 inhibition and suggest that selecting for PTPσ-negative human HSCs may be an effective strategy for enriching human HSCs for transplantation.
Introduction
RTKs regulate the maintenance, differentiation, and malignant transformation of hematopoietic stem cells (HSCs) (1–5). The activity of RTKs is counterbalanced through the action of receptor protein tyrosine phosphatases (PTPs), which dephosphorylate receptor and intracellular kinases (6, 7). The functions of certain intracellular PTPs, such as SHP2, in hematopoiesis are well characterized. SHP2 is required for the maintenance of HSCs and progenitor cells (8). Gain-of-function mutations in SHP2 cause a myeloproliferative disorder, and SHP2 is essential for oncogenic c-KIT transformation to myeloproliferative disease (9, 10). Recently, the intracytoplasmic phosphatase of regenerating liver PRL2 was found to be important for SCF-mediated HSC self renewal (11). In addition to the intracytoplasmic PTPs, there are 21 distinct receptor PTPs. However, the functions of receptor PTPs in hematopoiesis are not well understood (7).
We recently discovered the function of a heparin-binding growth factor, pleiotrophin (PTN), which is secreted by BM endothelial cells (ECs) and promotes the in vitro expansion of murine and human HSCs (12). PTN mediates HSC expansion via binding and inhibition of a receptor PTP, PTPζ (encoded by PTPRZ), on HSCs (12, 13). Deletion of Ptn caused a 10-fold reduction in HSC content in vivo, whereas deletion of Ptprz caused a significant expansion of HSCs in vivo (13). Based on these findings, we sought to determine whether other receptor PTPs might also be expressed by HSCs. We found that PTPσ (encoded by PTPRS) is highly expressed in murine and human HSCs. Interestingly, BM cells from Ptprs–/– mice displayed markedly increased competitive repopulating capacity compared with Ptprs+/+ BM cells. The increased functional capacity of Ptprs–/– HSCs was associated with increased activation of the RhoGTPase RAC1 (14, 15), and inhibition of RAC1 blocked the augmented migration capacity of Ptprs–/– cells. Furthermore, negative selection of human cord blood (CB) HSCs for PTPσ caused a 15-fold increase in repopulating capacity compared with human PTPσ+ HSCs. These data reveal a role for PTPσ in regulating HSC function and suggest that PTPσ inhibition or negative selection for PTPσ can increase HSC repopulation in vivo.
Results and Discussion
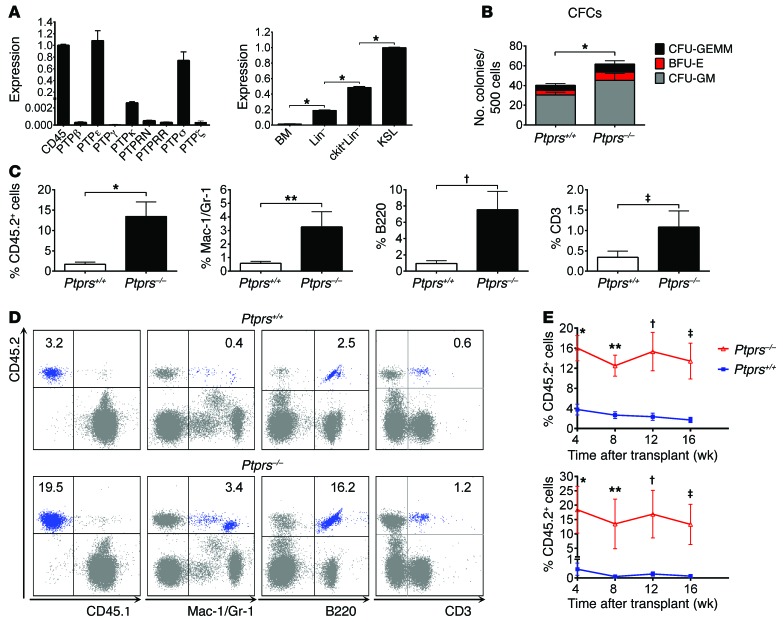
We sought to determine the relative expression of receptor PTPs in murine HSCs. Cd45, Ptprs, and Ptpre were expressed at more than 100-fold higher levels in BM ckit+sca-1+lin– (KSL) stem/progenitor cells compared with other receptor PTPs, including Ptprz (Figure 1A). Since PTPσ has been implicated in regulating the regeneration of neural stem cells (16, 17), we hypothesized that PTPσ might also regulate HSC function. Ptprs expression was increased significantly in HSCs compared with more mature hematopoietic cell populations (Figure 1A). In order to determine whether PTPσ had a functional role in regulating HSC fate, we compared the hematopoietic phenotype and function of Ptprs–/– mice and Ptprs+/+ mice (18). Ptprs–/– mice were viable, and we confirmed decreased PTPσ expression in BM lin– cells from Ptprs–/– mice (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI77866DS1). Adult Ptprs–/– mice had normal peripheral blood (PB) counts and no alterations in total BM cells, KSL cells, SLAM+KSL HSCs, HSC cell-cycle status, or apoptosis compared with Ptprs+/+ mice (Supplemental Figure 1). However, Ptprs–/– mice contained significantly increased myeloid colony-forming cells (CFCs) compared with Ptprs+/+ mice (Figure 1B). Furthermore, mice that were competitively transplanted with limiting doses of BM cells from Ptprs–/– mice had 8-fold increased donor CD45.2+ hematopoietic cell engraftment at 16 weeks compared with mice transplanted with the identical cell dose from Ptprs+/+ mice (Figure 1C). Reconstitution of myeloid, B cell, and T cell lineages was also significantly increased in mice transplanted with Ptprs–/– BM cells compared with recipients of Ptprs+/+ cells (Figure 1, C and D). Secondary competitive transplantation assays demonstrated that Ptprs–/– donor BM cells contained significantly increased long-term HSC function compared with BM cells from Ptprs+/+ mice (Figure 1E). Of note, we observed no differences in the homing capacity of donor BM cells from Ptprs–/– mice versus Ptprs+/+ mice (Supplemental Figure 1).
Figure 1. Deletion of Ptprs augments HSC-repopulating capacity.
(A) Mean expression of receptor PTPs in BM KSL cells by quantitative reverse-transcriptase PCR (qRT-PCR) (left) and expression of Ptprs within hematopoietic cell subsets (right) are shown. n = 3–9/group. *P < 0.0001 for each of the 3 comparisons. (B) Mean (± SEM) numbers of CFCs are shown for 12-week-old Ptprs–/– and Ptprs+/+ mice. *P = 0.002 (n = 6, Mann-Whitney U test). CFU-GEMM, CFU–granulocyte erythroid monocyte megakaryocyte; BFU-E, burst-forming unit–erythroid; CFU-GM, CFU–granulocyte macrophage. (C) Mean levels of donor CD45.2+ hematopoietic cell engraftment are shown in the PB of CD45.1+ mice at 16 weeks following competitive transplantation of 3 × 104 BM cells from Ptprs+/+ or Ptprs–/– mice. *P < 0.0001 (n = 15–18/group, Mann-Whitney U test). Multilineage engraftment of Mac-1/Gr-1+, B220+, and CD3+ donor cells is shown at right. **P = 0.008; †P = 0.0001; ‡P = 0.04 (Mann-Whitney U test). (D) Multilineage flow cytometric analysis of donor hematopoietic cell engraftment in the PB is shown from mice competitively transplanted with BM cells from Ptprs+/+ or Ptprs–/– mice at 16 weeks after transplant. Quadrant numbers represent the percentages of donor lineage cells. (E) In the upper panel, mean donor CD45.2+ cell engraftment in the PB is shown over time following transplantation of BM cells from Ptprs+/+ or Ptprs–/– mice in primary recipient mice. *P < 0.0001; **P = 0.0001; †P = 0.001; and ‡P < 0.0001 for engraftment at 4, 8, 12, and 16 weeks, respectively. In the lower panel, mean donor CD45.2+ cell engraftment in secondary transplanted mice is shown over time. *P = 0.004; **P = 0.01; †P = 0.005; and ‡P = 0.002 for engraftment at 4, 8, 12, and 16 weeks, respectively (n = 7–8/group, Mann-Whitney U test).
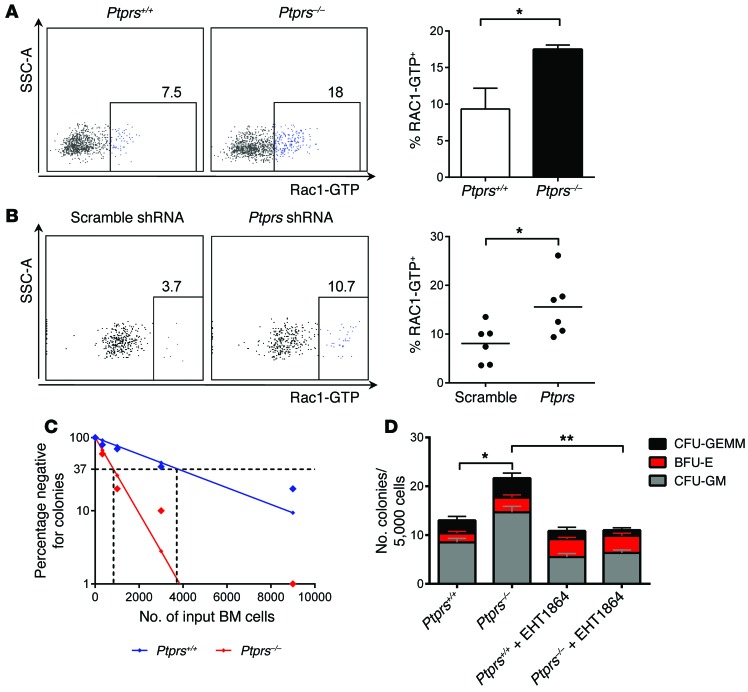
Since Ptprs–/– HSCs displayed increased repopulating capacity in vivo compared with Ptprs+/+ HSCs, this suggested that PTPσ might regulate processes involved in HSC engraftment or self renewal. We thus considered whether RAC proteins, a subset of RhoGTPases that are necessary for normal HSC engraftment capacity (14, 15, 19), might be regulated by PTPσ. In cell lines, it has been shown that PTPσ dephosphorylates and thereby activates p250GAP, a RhoGTPase that inhibits RAC protein activation (20). Interestingly, we found that RAC1-GTP, the activated form of RAC1, was significantly increased in BM KSL cells from Ptprs–/– mice compared with Ptprs+/+ mice (Figure 2A). Treatment of wild-type BM KSL cells with PTPσ shRNA also significantly increased RAC1-GTP levels compared with scramble shRNA–treated BM KSL cells, demonstrating a molecular link between PTPσ and RAC1 (Figure 2B and Supplemental Figure 2). Deletion of Rac1 and Rac2 has been previously shown to decrease the transendothelial migration capacity and cobblestone area–forming cell (CAFC) content of BM cells compared with control BM cells (15). We found that Ptprs–/– BM cells had 4-fold increased numbers of 5-week CAFCs compared with Ptprs+/+ BM cells (Figure 2C). Furthermore, Ptprs–/– BM cells displayed significantly increased transendothelial cell migration capacity compared with Ptprs+/+ BM cells (Figure 2D). Treatment of Ptprs–/– BM cells with EHT1864, a Rac inhibitor, completely abrogated the enhanced transendothelial migration capacity of Ptprs–/– cells (Figure 2D). These data suggest that PTPσ inhibits Rac1 activation in BM HSCs and that the increased HSC engraftment capacity of Ptprs–/– BM cells is dependent, at least in part, on Rac1 activation.
Figure 2. PTPσ regulates RAC1 activation in HSCs, and RAC1 inhibition abrogates the Ptprs–/– BM cell migration capacity.
(A) At left, flow cytometric analysis of RAC1-GTP levels in BM KSL cells from Ptprs+/+ and Ptprs–/– mice is shown. Numbers represent the percentages of Rac1-GTP+ cells. At right, mean percentages of RAC1-GTP+ KSL cells are shown in Ptprs–/– and Ptprs+/+ mice. *P = 0.008 (n = 3, t test). (B) At left, flow cytometric analysis of RAC1-GTP levels in wild-type BM KSL cells treated with scramble shRNA or PTPσ shRNA is shown. Numbers represent the percentages of RAC1-GTP+ cells. At right, scatter plot of percentage of RAC1-GTP+ KSL cells is shown in each group. Horizontal bars represent mean values. *P = 0.01 (n = 6, t test). (C) Poisson statistical analysis of a limiting dilution assay of 5-week CAFCs from Ptprs–/– versus Ptprs+/+ BM cells. The CAFC frequency for Ptprs–/– BM cells was 1 in 839 cells versus 1 in 3,801 cells for Ptprs+/+ BM cells (n = 10/group, P = 0.0001). (D) Mean numbers of CFCs are shown from the lower chambers of transendothelial migration assays containing Ptprs+/+ BM cells and Ptprs–/– BM cells, treated with and without EHT1864. *P < 0.0001 (n = 12, t test) for total CFCs; **P < 0.0001 for total CFCs (n = 6, t test).
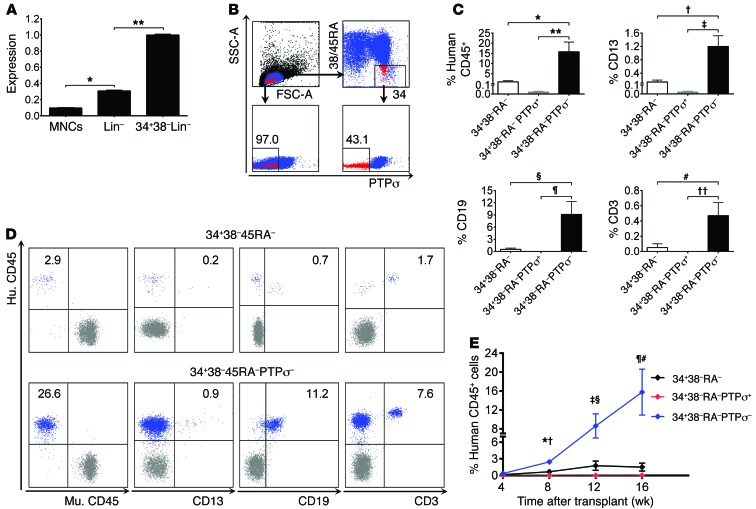
Since deletion of Ptprs increased murine HSC-repopulating capacity, we sought to determine whether the negative selection of human HSCs for PTPσ expression could enrich for HSCs with enhanced repopulating capacity. PTPRS was expressed by a mean of 49.9% of human CB CD34+CD38–lin– stem/progenitor cells (n = 6, Figure 3, A and B). We then performed transplantation assays into NOD/SCID–IL-2 receptor γ chain–null (NSG) mice to assess the repopulating capacity of CB HSCs selected for PTPσ expression. At 16 weeks after transplant, NSG mice transplanted with CD34+CD38–CD45RA–lin–PTPσ– cells displayed 15-fold higher engraftment compared with mice transplanted with parent CD34+CD38–CD45RA–lin– cells and more than 15-fold higher compared with mice transplanted with CD34+CD38–CD45RA–lin–PTPσ+ cells (Figure 3C). NSG mice transplanted with CD34+CD38–CD45RA–lin–PTPσ– cells had significantly increased engraftment of donor myeloid cells, B cells, and T cells compared with mice transplanted with CD34+CD38–CD45RA–lin– cells or CD34+CD38–CD45RA–lin–PTPσ+ cells (Figure 3, C and D). Temporally, the engraftment of PTPσ– CB cells significantly increased between 8 and 16 weeks compared with that of parent CB cells or PTPσ+ CB cells (Figure 3E). Of note, CD34+CD38–CD45RA–lin–PTPσ– cells displayed no difference in cell-cycle status compared with CD34+CD38–CD45RA–lin– cells or CD34+CD38–CD45RA–lin–PTPσ+ cells (Supplemental Figure 3). Surface expression of CXC chemokine receptor type 4 (CXCR4), which regulates HSC homing and retention in the BM microenvironment (21, 22), was not different between CD34+CD38–CD45RA–lin– cells and CD34+CD38–CD45RA–lin–PTPσ– cells, but both populations had higher CXCR4 expression compared with CD34+CD38–CD45RA–lin–PTPσ+ cells (Supplemental Figure 3). We found no differences in CXCR expression between BM KSL cells from Ptprs–/– mice and Ptprs+/+ mice (mean 2.7% CXCR4+ vs. 3.2%, respectively, n = 6).
Figure 3. Selection of PTPσ– CB cells enriches for human HSCs.
(A) Mean expression of PTPRS in subsets of CB cells by qRT-PCR. *P < 0.0001; **P < 0.0001 (n = 3, t test). MNCs, mononuclear cells. (B) Flow cytometric analysis of PTPσ expression on CB cells and on CB CD34+CD38–CD45RA–lin– cells is shown. Numbers represent percentage of PTPσ levels. (C) Mean levels of human CD45+ hematopoietic cell and multilineage engraftment in the PB of NSG mice at 16 weeks following intrafemoral injection of human CB CD34+CD38–CD45RA–lin– cells (34+38–RA–), CD34+CD38–CD45RA–lin–PTPσ+ cells (34+38–RA–PTPσ+), or CD34+CD38–CD45RA–lin–PTPσ– cells (34+38–RA–PTPσ–). Percentage of human CD45+: *P = 0.0002, **P < 0.0001; percentage of CD13+: †P < 0.0001, ‡P < 0.0001; percentage of CD19+: §P = 0.0002, ¶P < 0.0001; percentage of CD3+: #P < 0.0001, ††P < 0.0001 (n = 11–18/group, Mann-Whitney U test). (D) Flow cytometric analysis of human CD45+ cell and multilineage engraftment is shown at 16 weeks in the PB of mice transplanted with CB 34+38–RA– cells or 34+38–RA–PTPσ– cells. Numbers represent the percentages of donor lineage cells. (E) Mean levels of human CD45+ cell engraftment are shown over time after transplant in the PB of NSG mice with parent 34+38–RA– cells, 34+38–RA–PTPσ+ cells, or 34+38–RA–PTPσ– cells. Eight weeks: *P = 0.002 (PTPσ– vs. parent), †P < 0.0001 (PTPσ– vs. PTPσ+); 12 weeks: ‡P = 0.002, §P < 0.0001; 16 weeks: ¶P = 0.0002, #P < 0.0001 (n = 11–18/group).
Our findings reveal several interesting aspects of PTPσ function in hematopoiesis. First, PTPσ negatively regulates HSC engraftment and self renewal in vivo following competitive transplantation. Our findings in hematopoiesis are analogous to the putative role of PTPσ in nerve regeneration, in which PTPσ mediates chondroitin sulfate proteoglycan–driven (CSPG–driven) inhibition of nerve regeneration following spinal cord injury (23–27). Competitive BM transplantation represents a definitive regenerative challenge to HSCs. Our results suggest that PTPσ inhibits HSC regeneration in vivo, perhaps via interaction with CSPGs, which are abundant in the extracellular matrix of the BM (28).
Going forward, it will be important to dissect the precise cellular mechanism through which PTPσ regulates HSC repopulation. We have established that PTPσ regulates RAC1 activation and that RAC activation is responsible for at least some of the augmented function of Ptprs–/– HSCs. RAC proteins regulate several HSC functions, including chemoattraction, homing, proliferation, survival, and endosteal localization (29, 30). Since we found no alterations in HSC apoptosis, cell-cycle status, or homing capacity of PTPσ-deficient HSCs, we propose that the PTPσ-RAC1 axis may regulate HSC localization or “lodgment” in the niche (15, 31). We plan to directly visualize the localization of transplanted Ptprs–/– and Ptprs+/+ progenitor cells in BM niches in vivo utilizing cell-labeling techniques (13) and will interrogate the effect of RAC inhibition on the HSC lodgment process. We will also investigate the role of CXCR4 in this process, since CXCR4 mediates signals via RhoGTPases and RAC1 regulates CXCR4 conformation and function in hematopoietic cells (29, 32).
Translationally, we have shown that the negative selection of human CB CD34+CD38–CD45RA+lin– cells for PTPσ surface expression enriches for human long-term HSCs by approximately 15-fold. This observation has fundamental implications, since the molecular characterization of human HSCs may be improved by utilization of PTPσ to isolate more purified HSCs (33). Surface expression of CD90 (Thy1) has also been utilized to enrich for human HSCs (33, 34). The most effective purification strategy for human HSCs described to date utilized the expression of CD49f (integrin α6) such that a subset of NSG mice transplanted with single CD34+CD38–CD45RA–CD90+lin–RholoCD49f+ cells demonstrated multilineage hematopoietic engraftment (33). It is noteworthy that CD49f and PTPσ are both receptors for the extracellular matrix glycoproteins laminin and chondroitin sulfate/heparin sulfate proteoglycans, respectively. This shared feature suggests that proteoglycan-mediated signaling in the BM microenvironment regulates HSC repopulation in a context-specific manner under the control of integrin- and PTPσ-mediated signaling. Practically, we have provided a method to isolate human HSCs for therapeutic objectives such as gene therapy and allogeneic transplantation. Our results also provide the mechanistic basis for the systemic administration of PTPσ inhibitors (35) as a means to accelerate hematopoietic reconstitution in settings such as adult CB transplantation, in which delayed hematopoietic engraftment remains a major clinical problem (36).
Methods
For more detailed information, see the Supplemental Methods.
Animals.
Mice bearing constitutive deletion of Ptprs in a Balb/c background were provided by Michel Tremblay (McGill University, Montreal, Quebec, Canada). Cby.SJL(B6)-Ptprca/J (CD45.1 Balb/c) and NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratory) were also utilized.
Statistics.
All data are shown as mean ± SEM. We used the Mann-Whitney U test (2-tailed nonparametric analysis) and the 2-tailed Student’s t test for the comparisons shown. P < 0.05 was considered significant.
Study approval.
Animal procedures were performed under protocols approved by Duke University and UCLA animal care and use committees.
Supplementary Material
Acknowledgments
This work was supported, in part, by funding from National Institute of Allergy and Infectious Diseases grant AI067798-09 (to J.P. Chute) and NIH Heart, Lung and Blood Institute grant HL086998-05 (to J.P. Chute). The authors thank Amelia Lorenzo for assistance with cord blood processing.
Footnotes
Note regarding evaluation of this manuscript: Manuscripts authored by scientists associated with Duke University, The University of North Carolina at Chapel Hill, Duke-NUS, and the Sanford-Burnham Medical Research Institute are handled not by members of the editorial board but rather by the science editors, who consult with selected external editors and reviewers.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(1):177–182. doi:10.1172/JCI77866.
References
- 1.Verstraete K, Savvides SN. Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat Rev Cancer. 2012;12(11):753–766. doi: 10.1038/nrc3371. [DOI] [PubMed] [Google Scholar]
- 2.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.De Haan G, et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4(2):241–251. doi: 10.1016/S1534-5807(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 4.Chu S, et al. Flt3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm. Cell Stem Cell. 2012;11(3):346–358. doi: 10.1016/j.stem.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19(3):295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 7.Tonks NK. Protein tyrosine phosphatases — from housekeeping enzymes to master regulators of signal transduction. FEBS J. 2013;280(2):346–378. doi: 10.1111/febs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan G, et al. Essential role for Ptpn11 in survival of hematopoietic stem and progenitor cells. Blood. 2011;117(16):4253–4261. doi: 10.1182/blood-2010-11-319517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D, et al. A germline gain-of-function mutation in Ptpn11 (Shp-2) phosphatase induces myeloproliferative disease by aberrant activation of hematopoietic stem cells. Blood. 2010;116(18):3611–3621. doi: 10.1182/blood-2010-01-265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mali RS, et al. Role of SHP2 phosphatase in KIT-induced transformation: identification of SHP2 as a druggable target in diseases involving oncogenic KIT. Blood. 2012;120(13):2669–2678. doi: 10.1182/blood-2011-08-375873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, et al. PRL2/PTP4A2 phosphatase is important for hematopoietic stem cell self-renewal. Stem Cells. 2014;32(7):1956–1967. doi: 10.1002/stem.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himburg HA, et al. , Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16(4):475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himburg HA, et al. , Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2(4):964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, et al. Loss of the Rho GTPase activating protein p190-B enhances hematopoietic stem cell engraftment potential. Blood. 2009;114(17):3557–3566. doi: 10.1182/blood-2009-02-205815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancelas J, Lee A, Prabhakar R, Stringer K, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11(8):886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham D, Pacey L, Axford M, Siu R, Rotin D, Doering L. Neural stem cells from protein tyrosine phosphatase sigma knockout mice generate an altered neuronal phenotype in culture. BMC Neurosci. 2006;7:50. doi: 10.1186/1471-2202-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ketschek A, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235(2):627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson K, Uetani N, Manitt C, Elchebly M, Tremblay M, Kennedy T. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol Cell Neurosci. 2003;23(4):681–692. doi: 10.1016/S1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 19.Chae H, Lee K, Williams D, Gu Y. Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells. Blood. 2008;111(5):2597–2605. doi: 10.1182/blood-2007-06-093237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chagnon M, et al. Receptor tyrosine phosphatase sigma (RPTPσ) regulates, p250GAP, a novel substrate that attenuates Rac signaling. Cell Signal. 2010;22(11):1626–1633. doi: 10.1016/j.cellsig.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 22.Rosu-Myles M, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci U S A. 2000;97(26):14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner R, Habecker B. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. J Neurosci. 2013;33(17):7175–7183. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pendleton J, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol. 2013;247:113–121. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, et al. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coles C, et al. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332(6028):484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y, Giger R. A new role for RPTPsigma in spinal cord injury: signaling chrondroitin sulfate proteoglycan inhibition. Sci Signal. 2010;3(110):pe6. doi: 10.1126/scisignal.3110pe6. [DOI] [PubMed] [Google Scholar]
- 28.Okayama E, Oguri K, Kondo T, Okayama M. Isolation and characterization of chondroitin 6-sulfate proteoglycans present in the extracellular matrix of rabbit bone marrow. Blood. 1988;72(2):745–755. [PubMed] [Google Scholar]
- 29.Cancelas JA, Jansen M, Williams DA. The role of chemokine activation of Rac GTPases in hematopoietic stem cell marrow homing, retention, and peripheral mobilization. Exp Hematol. 2006;34(8):976–985. doi: 10.1016/j.exphem.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Nayak RC, Chang KH, Vaitinadin N, Cancelas JA. Rho GTPases control specific cytoskeleton-dependent functions of hematopoietic stem cells. Immunol Rev. 2013;256(1):255–268. doi: 10.1111/imr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams GB, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 32.Zoughlami Y, et al. Regulation of CXCR4 conformation by the small GTPase Rac1: implications for HIV infection. Blood. 2012;119(9):2024–2032. doi: 10.1182/blood-2011-06-364828. [DOI] [PubMed] [Google Scholar]
- 33.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 34.Park C, Majeti R, Weissman I. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat Prot. 2008;3(12):1932–1940. doi: 10.1038/nprot.2008.194. [DOI] [PubMed] [Google Scholar]
- 35.Martin K, et al. Identification of small molecular inhibitors of PTPσ through integrative virtual and biochemical approach. PLoS One. 2012;7(11):1–8. doi: 10.1371/journal.pone.0050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues CA, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.