Abstract
Microglia, the innate immune cells of the CNS, perform critical inflammatory and noninflammatory functions that maintain normal neural function. For example, microglia clear misfolded proteins, elaborate trophic factors, and regulate and terminate toxic inflammation. In Alzheimer’s disease (AD), however, beneficial microglial functions become impaired, accelerating synaptic and neuronal loss. Better understanding of the molecular mechanisms that contribute to microglial dysfunction is an important objective for identifying potential strategies to delay progression to AD. The inflammatory cyclooxygenase/prostaglandin E2 (COX/PGE2) pathway has been implicated in preclinical AD development, both in human epidemiology studies and in transgenic rodent models of AD. Here, we evaluated murine models that recapitulate microglial responses to Aβ peptides and determined that microglia-specific deletion of the gene encoding the PGE2 receptor EP2 restores microglial chemotaxis and Aβ clearance, suppresses toxic inflammation, increases cytoprotective insulin-like growth factor 1 (IGF1) signaling, and prevents synaptic injury and memory deficits. Our findings indicate that EP2 signaling suppresses beneficial microglia functions that falter during AD development and suggest that inhibition of the COX/PGE2/EP2 immune pathway has potential as a strategy to restore healthy microglial function and prevent progression to AD.
Introduction
Alzheimer’s disease (AD), a neurodegenerative disorder associated with protein misfolding and aggregation in the brain, is the most common memory disorder, and its prevalence is expected to triple by the year 2050 (1). The widely considered “amyloid hypothesis” of AD causation posits that accumulation of amyloid β42 (Aβ42) triggers inflammation, tau hyperphosphorylation, and synaptic and neuronal loss, leading to cognitive decline (2, 3). Recent studies, however, indicate that brain Aβ42 accumulates in subjects that do not exhibit dementia, which suggests that Aβ42 accumulation may be necessary but not sufficient for development of cognitive impairment (4) and that additional factors are required to tip the balance toward progression to AD dementia.
Recent genetic studies of late-onset AD have identified AD-associated genes that are involved in the innate immune response and are expressed in microglia, the resident myeloid cells of the CNS. Microglial genes associated with AD include CD33 (5–7), TREM2 (8, 9), and CR1 (10, 11); together with additional studies (12), these findings are indicative of an important role of microglia in maintaining local brain homeostasis and preventing Aβ42-mediated synaptic and inflammatory injury. Notably, clearance of accumulating Aβ42 is dependent on effective sensing by microglia (mediated by chemokines), followed by Aβ42 degradation. Moreover, prolonged exposure to proinflammatory cytokines or accumulating Aβ42 peptides cause microglia to lose their normal abilities to clear toxic proteins and control inflammation (13, 14), a detrimental phenotype in the context of age-associated Aβ42 accumulation. Thus, microglia are emerging as critical regulators of innate immune responses in AD and, more broadly, in other neurodegenerative disorders, and understanding the molecular and cellular mechanisms that cause microglial dysfunction may help identify strategies to restore healthy microglial function and prevent development of AD.
A longstanding observation in epidemiological studies of normal aging populations has been that NSAIDs, which inhibit cyclooxygenase-1 (COX-1) and COX-2 and prostaglandin (PG) production, prevent development of AD (15–18). In addition, early-stage AD is characterized by increased cerebrospinal fluid levels of PGE2 (19, 20), supporting the hypothesis that inflammatory actions of brain COX/PGE2 may underlie preclinical development of AD. Consistently, studies in AD model mice demonstrate reduced amyloid pathology with global deletion of individual PGE2 G protein–coupled receptors (21–23), and additional studies have shown a suppressive signaling effect of the PGE2 receptor EP2 on Aβ42 phagocytosis (24, 25). These studies, along with the recent demonstration of a broad regulatory function of EP2 signaling on cell cycle, cytoskeletal, and immune genes in quiescent microglia (26), suggest that microglial EP2 signaling may be a general suppressor of immune and nonimmune processes that protect against onset and progression of AD pathology. To investigate this hypothesis, we used in vitro and in vivo mouse models that recapitulate acute and chronic aspects of microglial responses to Aβ peptides. Our findings demonstrate that microglial EP2 signaling suppresses multiple processes critical to microglial maintenance of homeostasis in vivo, notably microglial chemokine generation and chemotaxis, clearance of Aβ peptides, resolution of innate inflammatory responses to Aβ42, and trophic factor generation and signaling. We further demonstrate that ablation of microglial EP2 signaling prevents cognitive impairment and loss of synaptic proteins in AD model mice.
Results
EP2 signaling exerts age-associated and opposing effects on proinflammatory and chemokine gene expression in response to Aβ42 oligomers.
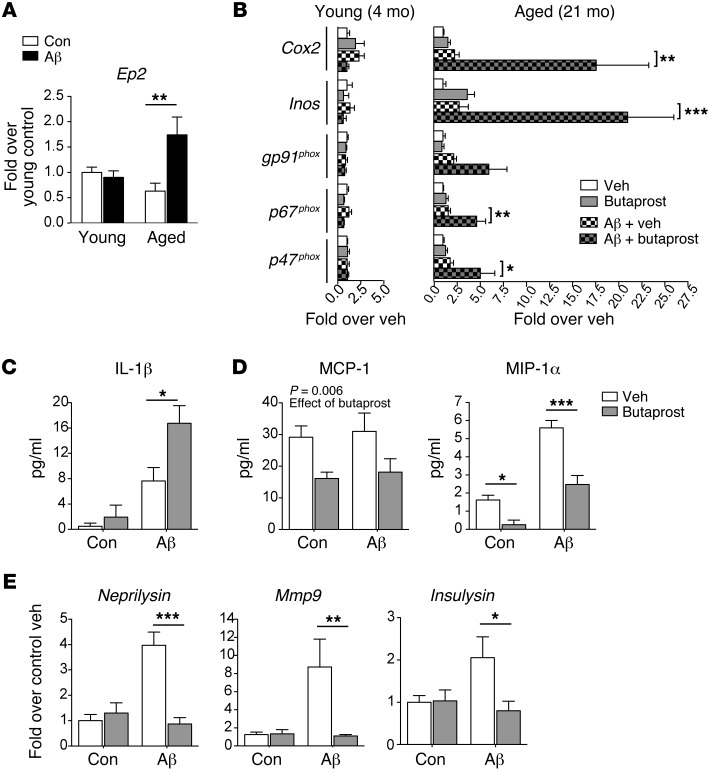
Aβ42 oligomers are early inducers of synaptic and neuronal injury in AD model mice (27). In addition to their direct disruption of synaptic function, Aβ42 oligomers generate a robust NF-κB– and IFN-regulatory factor 1–dependent (IRF1-dependent) inflammatory response (28) that can secondarily injure synapses and neurons. To determine the function of EP2 signaling in young and aged immune responses to oligomeric Aβ42 peptides, we assayed the effects of the selective EP2 agonist butaprost in macrophages stimulated with Aβ42 oligomers; because yields of viable microglia suitable for culture experiments are very low when harvested from adult brain (29), we examined peritoneal macrophages (which share many properties with microglia) harvested from both young (4 months) and aged (21 months) C57B6/J mice. We found that Ep2 mRNA was significantly induced in aged but not young macrophages in response to Aβ42 oligomers (5 μM; Figure 1A). Consistently, Aβ42 oligomers induced a robust inflammatory transcriptional response in aged but not young macrophages that was further increased by costimulation with 1 μM butaprost (Figure 1B). Aβ42-induced increases in IL-1β generation and secretion were further amplified with butaprost (Figure 1C and Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/JCI77487DS1), which suggests that myeloid EP2 signaling increases inflammasome generation of IL-1β. Conversely, expression of the chemokines MCP-1 and MIP-1α, which are involved in myeloid cell recruitment to sites of injury, was suppressed with butaprost both basally and with Aβ42 stimulation (Figure 1D and Supplemental Figure 1, B and C). Finally, expression of Aβ peptide clearance enzymes, notably Neprilysin, Insulysin, and Mmp9, was also suppressed with EP2 activation in Aβ42-stimulated macrophages (Figure 1E). Taken together, these findings demonstrate an age-dependent pattern of gene regulation by EP2 signaling in the context of Aβ42-induced innate immune responses, with induction of proinflammatory factors (including IL-1β, COX-2, iNOS, and NADPH oxidase subunits) and suppression of chemokines and proteases important in microglial migration and Aβ42 oligomer clearance.
Figure 1. EP2 signaling modulates inflammatory responses to Aβ42 oligomers in an age-dependent manner.
(A) Peritoneal macrophages of young and aged mice (4 and 21 months, respectively) were stimulated with Aβ42 oligomers (5 μM). Ep2 mRNA was induced at 4 hours in response to Aβ42 oligomers in aged macrophages (n = 5–6 per group; effect of Aβ42, P = 0.016, ANOVA). (B) Young and aged peritoneal macrophages were stimulated with Aβ42 oligomers (5 μM) or vehicle, with or without the EP2 agonist butaprost (1 μM), and qPCR was performed at 4 hours (n = 5–6 per group). Aged — but not young — macrophages increased expression of inflammatory genes in response to Aβ42 oligomers (effect of Aβ42 in aged macrophages, Cox2, P = 0.013; Inos, P = 0.0056; gp91phox, P = 0.023; p67phox, P = 0.003; p47phox, P = 0.013), and these responses were amplified with butaprost costimulation. (C) Butaprost enhanced Aβ42 oligomer–mediated IL-1β generation in aged macrophages at 6 hours by ELISA (n = 3 per group, effect of Aβ42, P = 0.0006). (D) EP2 agonist suppressed MCP-1 and MIP-1α generation (n = 5 per group; effect of butaprost for MCP-1, P = 0.0062; effect of Aβ42 and butaprost for MIP-1α, P < 0.0001). (E) Expression of Neprilysin, Mmp9, and Insulysin mRNA was induced in aged macrophages with Aβ42 oligomers, but suppressed with butaprost (n = 10–11 per group; for Neprilysin, effect of Aβ42, P = 0.0005, effect of butaprost, P = 0.0014; for Mmp9, effect of Aβ and butaprost, both P < 0.05; for Insulysin, effect of interaction, P < 0.05). (A–E) *P < 0.05; **P < 0.01, ***P < 0.001 as indicated, Bonferroni post-hoc.
EP2 ablation increases microglial chemotaxis to nascent amyloid plaques in the APP-PS1 mouse model.
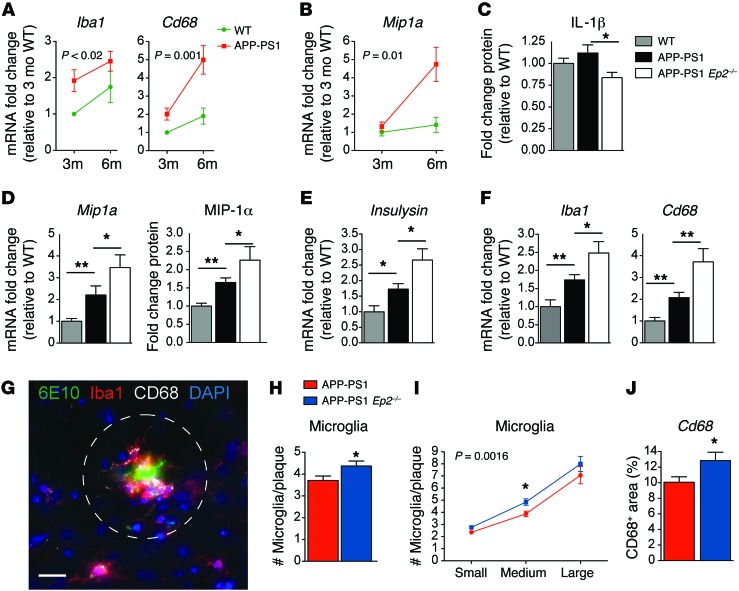
Given that EP2 signaling strongly suppressed generation of chemokines in response to oligomeric Aβ42, we investigated whether EP2 signaling negatively affects microglial chemotaxis in vivo, using the APP-PS1 (APPSwe-PS1ΔE9) mouse model of familial AD. This transgenic line coexpresses the human APPSwe and PS1ΔE9 mutant transgenes and exhibits Aβ peptide plaque deposition beginning at 5 months, with later onset of synaptic loss and spatial memory deficits after 8–9 months of age (30). We found that before significant Aβ plaque accumulation had begun at 3 months, a robust microglial response was already underway, characterized by increased expression of the cytoskeletal protein Iba1 and the lysosomal glycoprotein Cd68 (Figure 2A); the chemokine Mip1a began increasing at 3 months, with a significant rise by 6 months (Figure 2B), presumably in response to accumulating Aβ42 peptide oligomers and fibrils.
Figure 2. Microglial chemotaxis to early accumulating Aβ peptides is enhanced with Ep2 deletion in 4- to 5-month-old APP-PS1 mice.
(A) qPCR demonstrated preplaque increases in hippocampal Iba1 and Cd68 (effect of genotype, Iba1, P < 0.02; Cd68, P = 0.001; n = 5–9 per group). (B) Mip1a was increased early (effect of genotype, P = 0.01; n = 5–9 per group). (C) Decreased cerebral cortical IL-1β levels in APP-PS1 Ep2–/– versus APP-PS1 mice (n = 14–19 per group). Increased (D) Mip1a hippocampal mRNA (n = 8–13 per group) and MIP-1α cerebral cortical protein (n = 13–21 per group) and (E) Insulysin (n = 8–13 per group) in APP-PS1 Ep2–/– versus APP-PS1 brains. (F) Increased Iba1 and Cd68 mRNA in preplaque 4-month-old APP-PS1 Ep2–/– hippocampus (n = 8–13 per group). (G) Representative Aβ plaque with surrounding IBA1+ and CD68+ microglia from 5-month-old hippocampus. Dashed circle indicates area around plaques used to quantify numbers of microglia and CD68 immunofluorescence. Scale bar: 25 μm. (H) Microglia around hippocampal plaques in 5-month-old mice; average IBA1+ microglia number increased with Ep2 deletion (n = 4–7 mice per group, total 102–122 plaques per group). (I) Ep2 deletion enhanced microglial recruitment to early plaques (effect of genotype, P = 0.0016; effect of plaque size, P < 0.0001; post-hoc P < 0.05 for medium-sized plaque). Small, <250 μm2; medium, 250–575 μm2; large, >575 μm2. (J) Increased CD68 staining in periplaque hippocampal microglia of APP-PS1 Ep2–/– mice (n = 5–6 mice per genotype, total 26–48 plaques per genotype). *P < 0.05, **P < 0.01, ANOVA with Bonferroni post-hoc (A, B, and I) or Student’s t test (C–H and J).
Global deletion of Ep2 in APP-PS1 mice (referred to herein as APP-PS1 Ep2–/–) reduced cerebral cortical IL-1β protein at 5 months of age and increased hippocampal Mip1a mRNA, cortical MIP-1α protein, and Insulysin mRNA (Figure 2, C–E), consistent with the in vitro findings shown in Figure 1. Interestingly, APP-PS1 Ep2–/– hippocampus exhibited significantly increased levels of Iba1 and Cd68 compared with control APP-PS1 hippocampus (Figure 2F), suggestive of an altered activation state in microglia lacking Ep2.
We then tested whether EP2-mediated regulation of MIP-1α was associated with altered chemotaxis of microglia to sites of accumulating Aβ peptides in APP-PS1 hippocampus (Figure 2G). At 5 months, a time point at which Aβ peptides begin to accumulate in this model, deletion of Ep2 in the APP-PS1 Ep2–/– mice increased microglial recruitment to nascent Aβ plaques, as assayed by quantification of IBA1+ microglia surrounding newly formed Aβ plaques (Figure 2H). Additional quantification of microglia around small, medium, and large Aβ plaques demonstrated a significant effect of genotype and plaque size (Figure 2I). Levels of CD68 were significantly increased in APP-PS1 Ep2–/– mice (Figure 2J). We did not find differences in levels of Aβ42 between genotypes at this age, presumably because Aβ peptide accumulation is very low at 5 months, in contrast to later ages of 8–9 months, at which APP-PS1 Ep2–/– mice exhibited a reduction in cumulative Aβ peptide load (Supplemental Figure 2). Taken together, these findings suggest that at the earliest stages of pathology in APP-PS1 mice, inhibition of EP2 signaling resulted in beneficial microglial responses to accumulating Aβ peptides, with suppressed proinflammatory IL-1β generation and increased MIP-1α expression and microglial chemotaxis to sites of Aβ peptide accumulation.
Conditional deletion of Ep2 in microglia increases microglial activation and clearance of Aβ peptides.
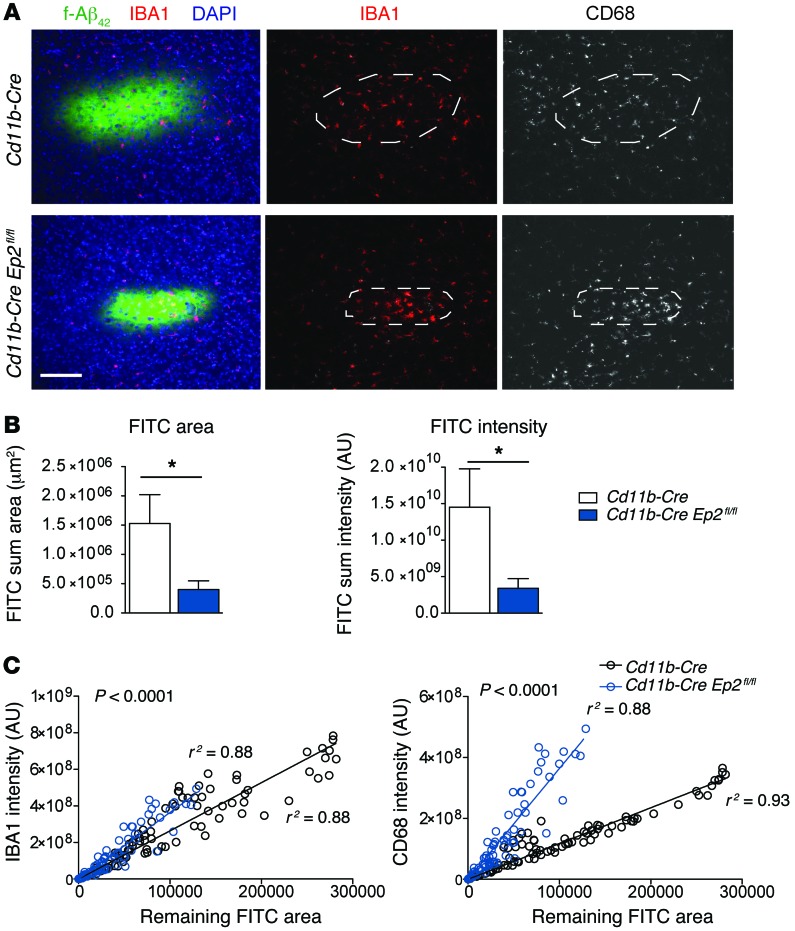
We next used a microglial conditional knockout strategy to directly examine microglial EP2 function in chemotaxis and clearance of Aβ peptide in vivo. The Cd11b-Cre recombinase line leads to excision of floxed sequences in cells of the myeloid lineage, including monocytes, macrophages, and microglia, and has been successfully used to examine neuroinflammatory responses in brain (26, 29, 31). We injected 17-month-old Cd11b-Cre Ep2fl/fl and control littermate Cd11b-Cre mice intracortically with FITC-conjugated fibrillar Aβ42 peptides and examined them 48 hours later to quantify microglial activation and remaining fluorescent Aβ42 peptide (Figure 3A). The remaining FITC+ staining area and intensity, quantified in a blinded fashion (see Methods), indicated that clearance of Aβ42 peptides was significantly higher in Cd11b-Cre Ep2fl/fl mice than in Cd11b-Cre controls (Figure 3B). Although absolute numbers of microglia were not counted, intensities of IBA1 and CD68 were increased in Cd11b-Cre Ep2fl/fl cortex for any given level of remaining FITC+ Aβ42 area (P < 0.0001 between slopes; Figure 3C). These findings support a primary function of microglial Ep2 in suppressing recruitment and activation of microglia that clear Aβ42 peptides chronically in the APP-PS1 model and acutely after intracortical Aβ42 peptide injection.
Figure 3. Conditional deletion of microglial Ep2 accelerates clearance of intracortically injected Aβ peptide.
Cd11b-CreEp2fl/fl and Cd11b-Cre mice (17 months old, n = 6–7 per group) underwent intracortical injection of 185 pmol fibrillar Aβ42 or vehicle, and cortical tissue was analyzed 48 hours later. (A) Representative images of sections at the injection site containing Aβ42 deposits visualized by FITC fluorescence and immunostained for microglial markers IBA1 and CD68. Scale bar: 100 μm. (B) Remaining FITC-labeled Aβ peptide at 48 hours after injection was significantly reduced in Cd11b-Cre Ep2fl/fl versus Cd11b-Cre cerebral cortex (*P < 0.05, Student’s t test). (C) Relationship between intensity of microglial markers IBA1 and CD68 and the remaining FITC+ area showed a significant correlation (Cd11b-Cre, IBA1, r2 = 0.88, CD68, r2 = 0.93; Cd11b-Cre Ep2fl/fl, IBA1, r2 = 0.88, CD68, r2 = 0.88). Correlation of microglial IBA1 and CD68 levels with remaining FITC+ area showed significant difference between genotypes (P < 0.0001), with higher IBA1 and CD68 intensity present in Cd11b-Cre Ep2fl/fl sections per given FITC+ area.
Microglial EP2 signaling regulates inflammatory and noninflammatory pathways in vivo in response to Aβ42 peptides.
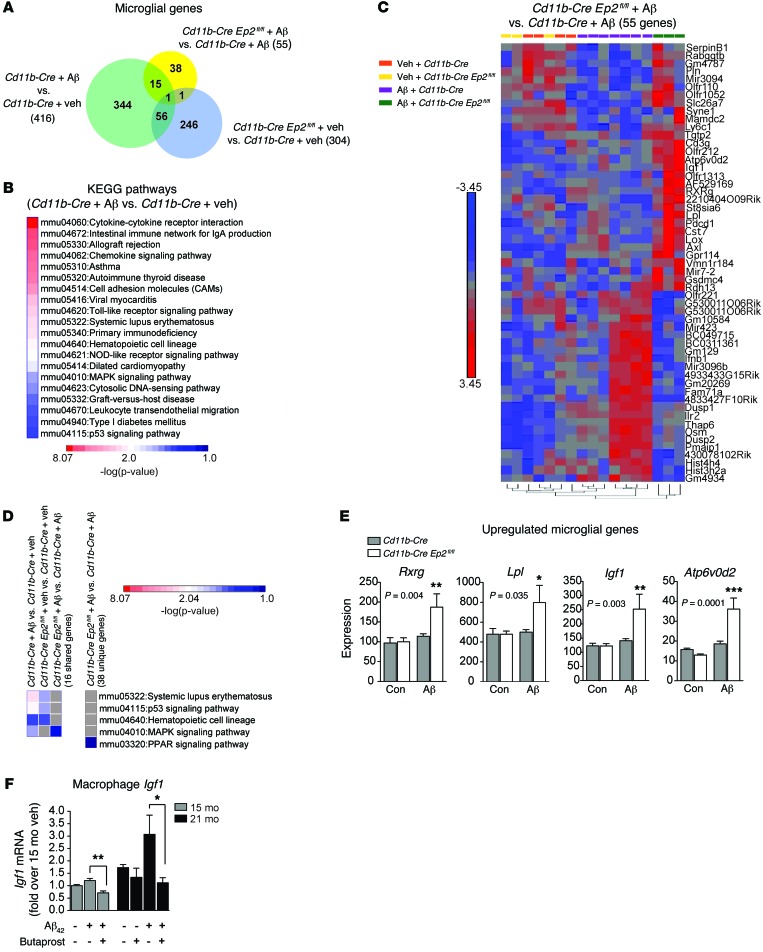
Although microglia function in innate immune brain responses, they are also intimately associated with neurons and synapses and perform essential nonimmune functions important to normal neural function. These include maintaining structural plasticity by pruning and elimination of synapses, clearing apoptotic cells, and generating trophic and neurogenic factors (32). Our findings thus far suggested a harmful function of microglial EP2 signaling both in vitro and in vivo in models of Aβ42 inflammation, with potentiation of proinflammatory responses, suppression of immune cell trafficking to sites of Aβ peptide accumulation, and suppression of Aβ peptide clearance. To identify additional functions of microglial EP2 signaling, we turned to an unbiased approach and examined microglial-specific gene expression in response to i.c.v. injection of Aβ42 peptides. Aβ42 peptide injection i.c.v. not only generates a robust, long-lasting innate immune response to Aβ42 (21), but also disrupts memory consolidation (33), and thus represents a model in which to test effects of microglial EP2 on transcriptional responses and functional outcomes that are altered in response to Aβ42 peptides. We performed microarray analysis on RNA isolated from adult microglia from 8-month-old Cd11b-Cre Ep2fl/fl and Cd11b-Cre mice 48 hours after injection of i.c.v. Aβ42 peptides.
We examined 3 genetic comparisons (absolute fold change ≥1.5, P < 0.05; Figure 4A). Gene Ontology (GO) expression analysis for the comparison between Aβ- versus vehicle-injected Cd11b-Cre mice showed the Immune System Process as the most highly enriched (enrichment score, 94.42). Expression levels of microglial Ep2 were increased 1.30-fold (P = 0.013) at 48 hours after i.c.v. Aβ in the Cd11b-Cre control genotype. Unsupervised hierarchical clustering of differentially expressed genes revealed a striking distinction between the i.c.v. Aβ and i.c.v. vehicle treatment groups (Supplemental Figure 3A). Ingenuity Pathway Analysis (IPA) of upstream regulatory transcription factors demonstrated 2 major nodes of inflammatory gene regulation, Nfkb and Irf7 (Supplemental Figure 3B). In this comparison, Cox2, which is upstream of EP2, was highly induced in vivo in microglia from i.c.v. Aβ42–treated mice (Supplemental Figure 3C). Application of IPA revealed that many Aβ-regulated genes were also regulated by COX-2 and PGE2 (Supplemental Figure 3B).
Figure 4. Microglial EP2 signaling regulates distinct immune and non-immune pathways in response to i.c.v. Aβ42.
Adult microglia were harvested for microarray analysis from brains of 8-month-old Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice 48 hours after i.c.v. administration of either vehicle or Aβ42 fibrillar peptides. (A) Venn diagram of the 3 comparisons. (B) KEGG pathways significantly enriched in Aβ42- versus vehicle-treated Cd11b-Cre mice. (C) Hierarchical clustering of 55 genes differentially regulated in the i.c.v. Aβ42–treated Cd11b-Cre Ep2fl/fl group versus the i.c.v. Aβ–treated Cd11b-Cre group. (D) KEGG pathways shared between comparisons, with the PPARγ pathway represented in 38 uniquely regulated genes in the Cd11b-Cre Ep2fl/fl i.c.v. Aβ42 versus Cd11b-Cre i.c.v. Aβ comparison. Pathways that are not shared are shaded gray. (E) Expression levels for regulated genes compared between Cd11b-Cre Ep2fl/fl and Cd11b-Cre groups treated with i.c.v. Aβ42 include genes encoding PPARγ signaling (Rxrg and Lpl), microglial lysosomal (Atp6v0d2), and trophic (Igf1) proteins. P values for main effect of Aβ42 treatment are shown in the figure (Bonferroni post-hoc between genotypes, *P < 0.05, **P < 0.01, ***P < 0.001; n = 3–7 per group). (F) Butaprost suppressed expression of Igf1 in peritoneal macrophages of 15- and 21-month-old mice stimulated with Aβ42 oligomers at 4 hours (*P < 0.05, **P < 0.01, Student’s t test; n = 5–6 per group).
Functional annotation of the 416 transcripts differentially expressed in Aβ- versus vehicle-treated Cd11b-Cre mice was carried out using the Database for Annotation, Visualization and Integrated Discovery (DAVID; see Methods). This analysis revealed 20 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that were significantly enriched, almost all of which corresponded to inflammatory signaling networks (Figure 4B). Comparison of Aβ-treated Cd11b-Cre Ep2fl/fl versus Cd11b-Cre mice revealed 55 regulated genes (Figure 4A), and hierarchical clustering of these genes across conditions demonstrated a clear segregation of Aβ-regulated genes in Cd11b-Cre Ep2fl/fl mice (Figure 4C). Comparison of KEGG pathways revealed shared pathways between the Aβ-treated Cd11b-Cre and vehicle-treated Cd11b-Cre groups and between the vehicle-treated Cd11b-Cre Ep2fl/fl and vehicle-treated Cd11b-Cre groups (Figure 4D). The complete set of enriched KEGG pathways in the Cd11b-Cre Ep2fl/fl versus Cd11b-Cre comparison included cell cycle, proteolysis, and immune pathways (Supplemental Figure 3D).
Interestingly, the majority of differentially regulated genes in the Aβ-treated Cd11b-Cre Ep2fl/fl versus Aβ-treated Cd11b-Cre comparison were not regulated by Aβ, but were specifically changed with microglial Ep2 deletion (38 genes; Figure 4A). This suggested that rather than simply reversing Aβ42-induced inflammatory changes, Cd11b-Cre Ep2fl/fl microglia engaged alternative response pathways. Functional annotation of these 38 genes using DAVID revealed an enrichment of PPAR signaling pathway genes, including retinol dehydrogenase-13 (Rdh13), retinoid X receptor γ (Rxrg), and lipoprotein lipase (Lpl) (Figure 4, D and E). Rdh13 (1.78-fold increase) participates in the endogenous synthesis of retinoic acid (RA) that binds and activates RXR subunits. PPARγ/RXR heterodimers inhibit proinflammatory gene expression (reviewed in ref. 34) and increase phagocytosis of Aβ peptides (35). Rxrg, along with RXR and LXR heterodimers, increases expression of the cholesterol transporter ABCA1 (36) and apolipoprotein E (ApoE) (37), proteins that enhance proteolytic degradation of soluble Aβ peptides (38, 39); recent studies indicate that administration of the FDA-approved RXR agonist bexarotene (Targretin) reduces interstitial levels of soluble Aβ peptides and rescues behavioral deficits in AD model mice (40, 41). Lpl, which functions in lipoprotein remodeling and cholesterol transport, and whose expression is driven by RA and RXR/LXR transcriptional activity, was increased 1.52-fold with deletion of microglial Ep2. The upregulation of these genes is suggestive of induction of antiinflammatory and Aβ-clearing nuclear hormone receptor signaling genes in the response of EP2-deficient microglia to Aβ42 peptides in vivo. Added to this was the induction of H+ transporting ATPase (Atp6v0d2; 1.92-fold induction; Figure 4E), a proton pump expressed in lysosomes of myeloid cells. Atp6v0d2 participates in degradation of proteins targeted to the lysosome (42), suggestive of a potential role in Aβ42 degradation.
Insulin-like growth factor 1 (IGF1) is upregulated in vivo in microglia derived from Cd11b-Cre Ep2fl/fl brains.
In addition, we found an unexpected increase in Igf1 mRNA levels in microglia derived from i.c.v. Aβ–treated Cd11b-Cre Ep2fl/fl mice (Figure 4E). Whereas at the organismal level, reduced IGF1 signaling increases longevity (43), at the cellular level, IGF1 promotes cell survival through the PI3K/AKT pathway and RasGTPase/RAF-1/MEK pathways, and in brain, IGF1 signaling promotes synaptogenesis, neurogenesis, angiogenesis, and neuroprotection (44). Although IGF1 receptors are expressed on all cell types in the CNS, in general, IGF1 is synthesized in the liver and is transported to the brain bound to IGF1 binding proteins. Exceptions include postnatal brain development, where microglia transiently express IGF1 that supports developing layer V neurons (45), and following brain injury, where microglia express IGF1 and astrocytes and neurons increase IGF receptor expression (44). Validation of the EP2-dependent regulation of IGF1 was carried out in aged primary macrophages, where Igf1 mRNA expression was found to be suppressed by the EP2 agonist butaprost (Figure 4F). Taken together, our unbiased analyses indicated the activation of multiple beneficial pathways in Ep2-deficient microglia in vivo, including antiinflammatory nuclear hormone, Aβ clearing, and trophic pathways. Moreover, these pathways were activated in parallel with suppression of the proinflammatory response (see below).
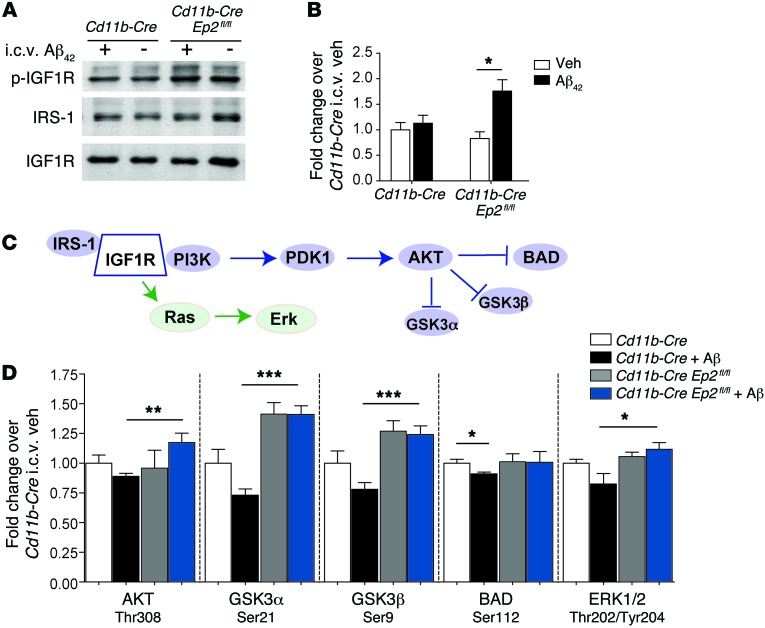
Cd11b-Cre Ep2fl/fl mice stimulated i.c.v. with Aβ42 show increased IGF1 receptor signaling and reduced inflammation.
We next tested whether microglial Ep2 deletion increased IGF1 signaling following stimulation with i.c.v. Aβ42. Binding of IGF1 to its tyrosine kinase receptor (IGF1R) leads to phosphorylation of IGF1R and recruitment of multiple scaffold proteins, including insulin receptor substrates 1–4 (IRS-1–IRS-4) and Src homology 2 domain–containing transforming protein 1 (44), which bind with different time courses to phosphorylated IGF1R to transduce IGF1 signaling. Here, Cd11b-Cre Ep2fl/fl and Cd11b-Cre mice were treated i.c.v. with vehicle or Aβ42 as above, and hippocampi were analyzed 48 hours later for phosphorylation of IGF1R (Figure 5, A and B, and Supplemental Figure 4, A and B). Quantification of immunoprecipitated total and phosphorylated IGF1R (p-IGF1R) demonstrated a significant increase in the p-IGF1R/total IGF1R ratio in Cd11b-Cre Ep2fl/fl versus Cd11b-Cre mice treated with i.c.v. Aβ42 (Figure 5B). We also measured levels of IRS-1, one of several scaffolding proteins that bind to phosphorylated IGF1R, and found a trend toward increased binding in the Cd11b-Cre Ep2fl/fl genotype (Supplemental Figure 4C). No changes in levels of total IGF1R were noted between genotypes (Supplemental Figure 4D).
Figure 5. Inhibition of microglial EP2 signaling increases brain IGF1/PI3K signaling after i.c.v. Aβ42 administration.
(A) Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice were administered vehicle or Aβ42 peptides i.c.v., and hippocampi were examined for IGF1R activation at 48 hours. Hippocampal lysates were immunoprecipitated with anti-IGF1R antibody and blotted against p-Tyr to detect p-IGF1R, IRS-1, and total IGF1R. A representative blot is shown. (B) Increased p-IGF1R/total IGF1R ratio in Cd11b-Cre Ep2fl/fl hippocampus after i.c.v. Aβ42 administration (n = 6–9 per group; *P < 0.05, Student’s t test). (C) Diagram of the IGF1R/AKT pathway. (D) Quantification of phosphoproteins downstream of PI3K/AKT in cerebral cortex showed increased AKT and ERK1/2 signaling in i.c.v. Aβ–treated Cd11b-Cre Ep2fl/fl mice (n = 6–9 per group; *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test).
To examine the effect of increased IGF receptor signaling in Cd11b-Cre Ep2fl/fl mice, we quantified phosphorylation levels of candidate proteins known to be phosphorylated downstream of IGF1R/PI3K in cerebral cortex (Figure 5, C and D, and Supplemental Figure 4, E and F). Levels of AKT phosphorylated by PDK1 at Thr308 and levels of p-ERK1/2 were significantly increased in i.c.v. Aβ–stimulated Cd11b-Cre Ep2fl/fl mice compared with Cd11b-Cre controls. Similarly, phosphorylation of proteins directly targeted by AKT, including GSK3α and GSK3β, also increased in i.c.v. Aβ–treated Cd11b-Cre Ep2fl/fl mice. Phosphorylation of BAD at Ser112 and ERK1/2 at Thr202/Tyr204 was suppressed in i.c.v. Aβ–treated Cd11b-Cre mice, but preserved in i.c.v. Aβ–treated Cd11b-Cre Ep2fl/fl mice. Taken together, these data indicate that increased IGF1R signaling in Cd11b-Cre Ep2fl/fl mice resulted in enhanced AKT/ERK signaling in the setting of Aβ stimulation in vivo.
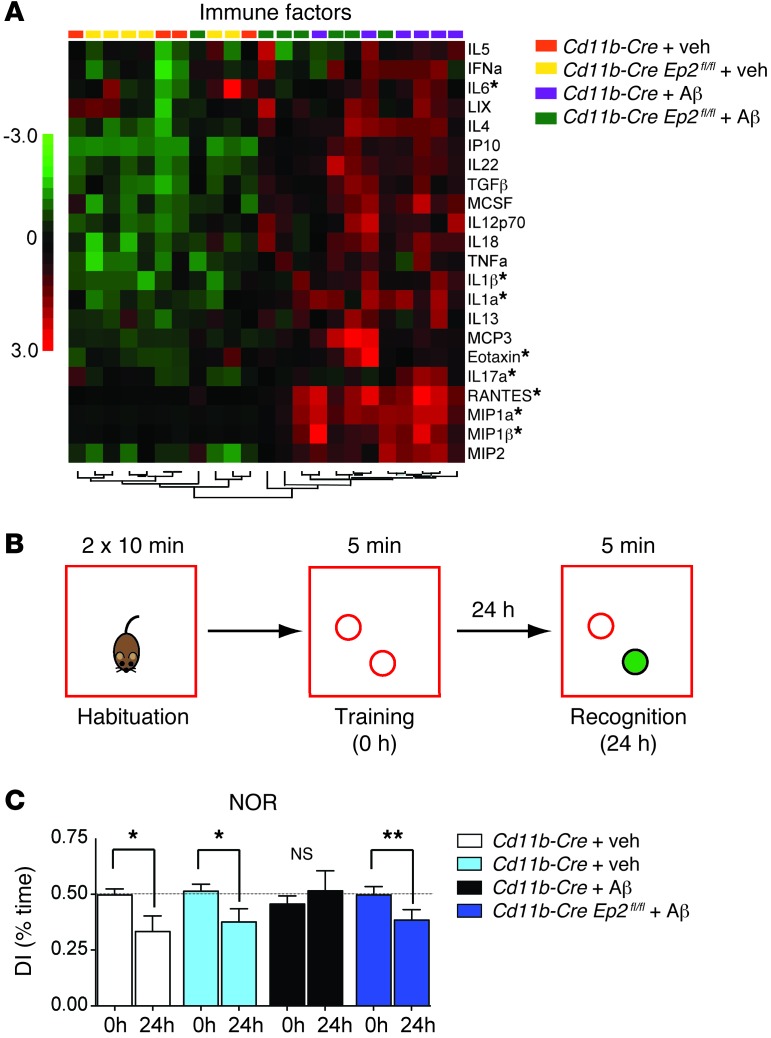
As AKT signaling promotes beneficial antiinflammatory effects, we examined the effect of microglial Ep2 deletion on the neuroinflammatory response to i.c.v. Aβ. Levels of cytokines and chemokines were measured using Luminex multiplex assay in hippocampi isolated from Cd11b-Cre Ep2fl/fl and Cd11b-Cre mice treated or not with i.c.v. Aβ42 at 48 hours. In Cd11b-Cre controls, i.c.v. Aβ42 broadly significantly upregulated 22 factors, compared with 7 factors differentially regulated in Cd11b-Cre Ep2fl/fl mice (Figure 6A and Supplemental Figure 5); these included the proinflammatory cytokines IL-1α, IL-1β, IL-17A, and IL-6. The chemokines MIP-1α, MIP-1β, and RANTES were also highly regulated by EP2 in this model, but in the opposite manner to that found in the APP-PS1 model, which suggests that regulation of chemokines by EP2 in vivo is context specific and may differ between chronic and acute Aβ42 stimulation. In the i.c.v. Aβ42 model, Aβ42 is administered acutely and initiates a robust inflammatory response, in contrast to transgenic expression in the APP-PS1 model, which leads to chronic, low-level production of Aβ peptides. Alternatively, lower chemokine generation in the i.c.v. Aβ42 model may reflect a more benign inflammatory milieu of Cd11b-Cre Ep2fl/fl brain at the 48-hour time point. Taken together, the findings of increased brain IGF1 signaling in combination with reduced production of inflammatory ILs suggest a beneficial effect of microglial Ep2 deletion in the context of Aβ42-generated immune responses.
Figure 6. Inhibition of microglial EP2 signaling decreases inflammation and rescues memory following i.c.v. Aβ42 administration.
(A) Cluster analysis producing separation for genotype and treatment is shown for 22 immune factors in cerebral cortex that were significantly upregulated in hippocampus 48 hours after i.c.v. Aβ42. Asterisks denote factors that were differentially regulated by microglial EP2 (see Supplemental Figure 5). (B) Overview of the NOR memory test, with the novel object shaded green. (C) DI (comparing 0-hour training session with 24-hour recognition session) demonstrated normal memory consolidation in control i.c.v. vehicle–treated Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice, and absence of memory consolidation in the i.c.v. Aβ42–treated Cd11b-Cre cohort, which was significantly rescued by microglial Ep2 deletion in the i.c.v. Aβ42–treated Cd11b-Cre Ep2fl/fl cohort (n = 8–15 mice per group; **P < 0.01, *P < 0.05, paired 1-tailed Student’s t test).
Conditional deletion of microglial Ep2 prevents a functional deficit in novel object recognition (NOR).
Neuroinflammatory responses can significantly impair cognitive function via effects of cytokines, proteases, and oxidative stress on synapses and neurons. We tested whether microglial Ep2 negatively affects memory function in the setting of Aβ42-mediated inflammation. Control experiments examining locomotor, anxiety, and Y-maze performance did not show differences between Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice (Supplemental Figure 6). Next, we used NOR, a memory task that relies on the innate preference of mice to spend more time with a novel rather than a familiar object, which is significantly disrupted in the i.c.v. Aβ42 model (33). NOR requires the normal function of the perirhinal and entorhinal cerebral cortex and the hippocampus. As illustrated in Figure 6B, on day 1 after i.c.v. injection of either vehicle or Aβ42, mice were habituated to an empty arena and later allowed to briefly explore 2 identical objects (training session, 0 hours). After 24 hours, mice were again put in the arena; however, one of the objects used during training was replaced by a novel object. Recognition memory (recognition session, 24 hours) of the old versus new object was assessed as the discrimination index (DI), the ratio of time spent exploring the old object to time spent exploring both objects. A DI of ~50% is characteristic of the training session, where there is no preference for either of the 2 objects; with normal memory consolidation, decreased DI during the recognition session reflects less time spent with the old object and more time exploring the new object, as was shown for the i.c.v. vehicle–injected Cd11b-Cre and Cd11b-Cre Ep2fl/fl groups (Figure 6C). These control mice performed normally (P < 0.05, paired t test), in contrast to i.c.v. Aβ42–injected Cd11b-Cre mice, which were not able to recognize the old from the new object at 24 hours. Importantly, this Aβ42-induced NOR deficit was prevented in Cd11b-Cre Ep2fl/fl mice (P < 0.01, paired t test). Taken together, our findings support a highly beneficial effect of microglial Ep2 ablation, resulting in suppression of proinflammatory responses, increased signaling through the IGF1R pathway, and prevention of NOR memory deficits.
Conditional deletion of microglial Ep2 reduces spatial memory deficits in APP-PS1 mice.
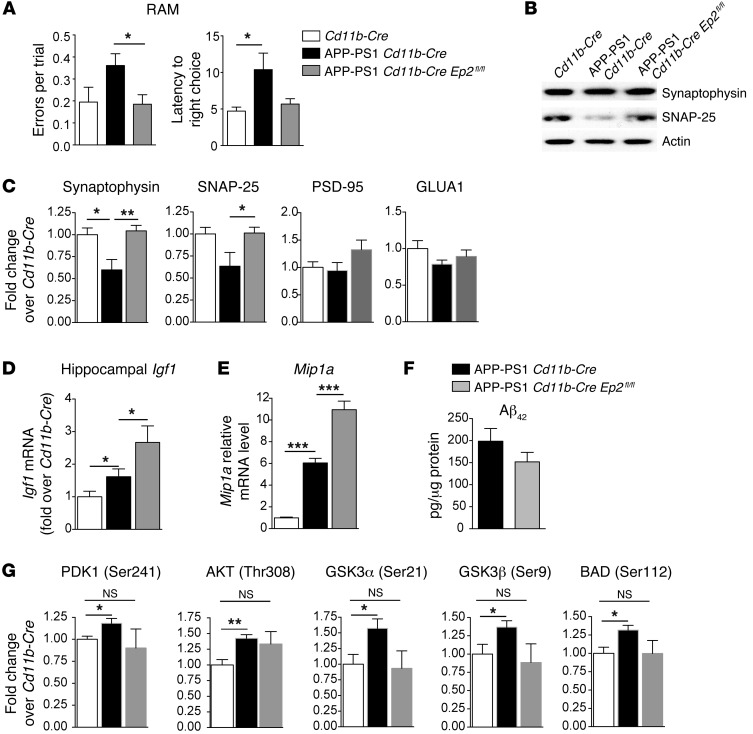
We then tested the effect of deletion of microglial Ep2 in the APP-PS1 model, in which progressive amyloid accumulation and inflammation lead to synaptic loss and hippocampal-dependent memory deficits. Male APP-PS1 Cd11b-Cre Ep2fl/fl mice and APP-PS1 Cd11b-Cre controls were aged to 9 months, the time point at which spatial memory deficits begin in this line. Hippocampal-dependent spatial memory performance in the radial arm maze (RAM) was tested (Figure 7A). Behavior in the RAM was quantified over the last 3 days of a 6-day period in which mice were evaluated for their ability to locate a new rewarded choice arm after visiting a previously rewarded sample arm. For the first 3 days of testing, no significant differences were observed for any genotype. However, the second 3 days of testing showed a significant difference between APP-PS1 Cd11b-Cre and APP-PS1 Cd11b-Cre Ep2fl/fl mice for mean number of errors per trial (P < 0.05, APP-PS1 Cd11b-Cre versus APP-PS1 Cd11b-Cre Ep2fl/fl; P = 0.089, APP-PS1 Cd11b-Cre versus Cd11b-Cre; Figure 7A). APP-PS1 Cd11b-Cre mice also showed increased latency to make a correct choice compared with Cd11b-Cre mice (P < 0.05), and this was partially improved with deletion of microglial Ep2 (P = 0.075, APP-PS1 Cd11b-Cre versus APP-PS1 Cd11b-Cre Ep2fl/fl; Figure 7A).
Figure 7. Effects of microglial Ep2 deletion in 9-month-old male APP-PS1 mice on spatial memory performance, presynaptic protein levels, and PI3K/AKT signaling.
(A) Cd11b-Cre, APP-PS1 Cd11b-Cre, and APP-PS1 Cd11b-Cre Ep2fl/fl cohorts were assessed for spatial memory performance in the RAM, using mean errors per trial and latency to make a correct choice as outcome measures (n = 7–11 per group). Whereas APP-PS1 Cd11b-Cre Ep2fl/fl and Cd11b-Cre animals made similar numbers of errors per trial during the course of testing, APP-PS1 Cd11b-Cre mice made significantly more (*P < 0.05, Mann-Whitney U test). Cd11b-Cre and APP-PS1 Cd11b-Cre Ep2fl/fl mice also made the correct choice more quickly than did APP-PS1 Cd11b-Cre mice (P < 0.05). (B and C) The loss of synaptophysin and SNAP-25 observed in APP-PS1 Cd11b-Cre mice was reversed in APP-PS1 Cd11b-Cre Ep2fl/fl mice (*P < 0.05, **P < 0.01, Student’s t test; n = 5–6 per group). The postsynaptic proteins PSD-95 and GLUA1 were not changed. (D) Increased Igf1 mRNA in APP-PS1 Cd11b-Cre Ep2fl/fl versus APP-PS1 Cd11b-Cre mice and in APP-PS1 Cd11b-Cre versus Cd11b-Cre mice (n = 6–10 per group; *P ≤ 0.05, Student’s t test). (E) Increased Mip1a mRNA expression in APP-PS1 Cd11b-Cre Ep2fl/fl versus APP-PS1 Cd11b-Cre hippocampus (n = 8–13 per group; ***P < 0.0001; Student’s t test). (F) Cerebral cortex from APP-PS1 Cd11b-Cre and APP-PS1 Cd11b-Cre Ep2fl/fl mice was assayed for Aβ42 levels by ELISA (P = 0.17, Mann-Whitney 2-tailed t test; n = 5–6 per group). (G) Quantification of PI3K/AKT phosphoproteins in cerebral cortex of Cd11b-Cre, APP-PS1 Cd11b-Cre, and APP-PS1 Cd11b-Cre Ep2fl/fl mice showed significant induction of the AKT signaling pathway in APP-PS1 Cd11b-Cre mice that was absent in APP-PS1 Cd11b-Cre Ep2fl/fl mice (*P < 0.05, **P < 0.01, Student’s t test; n = 4–5 per group).
We then assessed effects of microglial Ep2 deletion on synaptic integrity by quantifying levels of candidate synaptic proteins (Figure 7, B and C). The loss of synaptophysin correlates with progression of cognitive decline in AD development (46); moreover, studies in transgenic mouse AD models have demonstrated that presynaptic proteins are disrupted early during amyloid accumulation (21, 47), with loss of postsynaptic markers occurring at more advanced stages of pathology (48). At 8–9 months, we found a decrease in levels of the presynaptic proteins synaptophysin and SNAP-25 in APP-PS1 Cd11b-Cre mice compared with Cd11b-Cre controls that was rescued by deletion of microglial Ep2; no changes were demonstrated in the postsynaptic proteins PSD-95 and GLUA1 in either group. Thus, the loss of presynaptic markers was prevented with deletion of microglial Ep2 in the APP-PS1 Cd11b-Cre Ep2fl/fl cerebral cortex.
Consistent with the i.c.v. Aβ42 model, Igf1 mRNA levels were elevated in APP-PS1 Cd11b-Cre Ep2fl/fl versus APP-PS1 Cd11b-Cre hippocampus (Figure 7D). We also assessed levels of chemokine expression and total Aβ42 in APP-PS1 Cd11b-Cre and APP-PS1 Cd11b-Cre Ep2fl/fl mice. For chemokine expression, we found significantly increased Mip1a expression in APP-PS1 Cd11b-Cre Ep2fl/fl versus APP-PS1 Cd11b-Cre 9-month-old male mice (Figure 7E); this was consistent with the increased Mip1a expression observed in APP-PS1 Ep2–/– mice and in vitro data in aged macrophages (Figures 1 and 2). In APP-PS1 Cd11b-Cre Ep2fl/fl cerebral cortex, we found a 23.7% decrease in mean total Aβ42 levels (P = 0.17; Figure 7F), which was not as marked as the effect in APP-PS1 Ep2–/– mice, in which mean cortical levels of Aβ42 were reduced by 36.5% at 8–9 months (P < 0.01; Supplemental Figure 2). The lack of significant decline in Aβ42 levels with microglial Ep2 deletion may be due to incomplete excision of floxed sequences, and we have previously demonstrated that the Cd11b-Cre recombinase line is approximately 50% efficient in excising floxed Ep2 sequences (26). Incomplete excision of floxed sequences is common in many Cre-mediated systems, in which recombinase activity frequently results in cell-specific knockdown of gene expression. Alternatively, it is possible that chronic accumulation of Aβ peptide by 9 months of age in this transgenic model may overwhelm the ability to clear Aβ, in spite of the beneficial effects of microglial Ep2 deletion.
To examine the effect of chronic suppression of microglial EP2 signaling in the APP-PS1 model, we again examined phosphorylation of candidate proteins in the IGF1R/PI3K pathway in cerebral cortex of 9-month-old APP-PS1 Cd11b-Cre Ep2fl/fl, APP-PS1 Cd11b-Cre, and Cd11b-Cre mice (Figure 7G and Supplemental Figure 7). In this chronic model of Aβ stimulation, there was a significant induction of AKT signaling, with increased p-PDK1 (Ser241), p-AKT (Thr308), p-GSK2α (Ser21), p-GSK3β (Ser9), and p-BAD (Ser112) in APP-PS1 Cd11b-Cre versus Cd11b-Cre mice. Interestingly, APP-PS1 Cd11b-Cre Ep2fl/fl mice exhibited abolished AKT signaling induction, which suggests that in the context of microglial Ep2 deletion, IGF1R/PI3K signaling was more similar to Cd11b-Cre levels. Moreover, as many pathways feed into the AKT pathway, the normalization of AKT signaling to Cd11b-Cre levels in APP-PS1 Cd11b-Cre Ep2fl/fl brain may also reflect chronic effects of multiple beneficial microglial functions activated as a result of microglial Ep2 deletion.
Discussion
Microglial activities represent critical lines of defense against the development of neurodegenerative disease; microglia clear misfolded proteins, elaborate trophic and regenerative factors, and regulate and terminate toxic inflammation. Recent studies point to a steady decline of these normal microglial functions in aging and in AD. In AD, microglia not only lose their capacity to clear Aβ peptides but also develop a persistent proinflammatory phenotype that does not resolve, accelerating neuronal and synaptic injury (49, 50). In this study, using in vitro and in vivo genetic strategies, we found that microglial EP2 activity negatively regulates multiple and distinct beneficial functions that are critical in opposing the harmful effects of accumulating Aβ42. Together, our findings in distinct mouse models of Aβ inflammation demonstrated that deletion of microglial Ep2 restores chemotaxis, Aβ clearance, regulation of inflammatory responses, and trophic factor generation and signaling (Figure 8), with behavioral correlates of preventing cognitive deficits and loss of synaptic proteins.
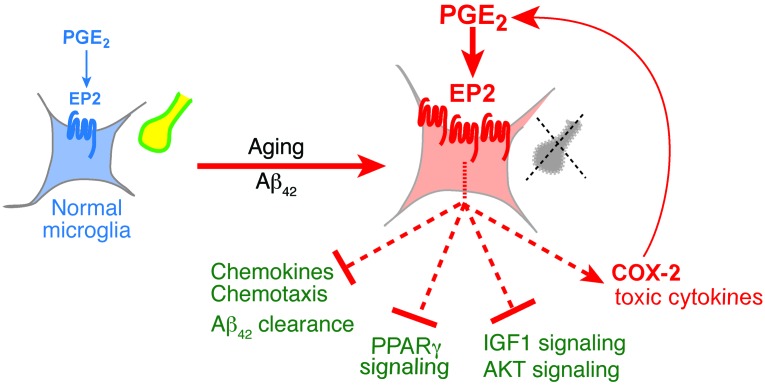
Figure 8. Functions of PGE2 EP2 signaling in healthy and aged/Aβ42-stimulated microglia.
Basal EP2 signaling in healthy microglia regulates normal homeostatic effects on cell cycle, cytoskeletal, and immune functions (Supplemental Figure 3D) and maintenance of healthy synapses (yellow). With aging and/or Aβ42 accumulation, microglial EP2 signaling is upregulated, increasing proinflammatory gene expression and suppressing beneficial chemokine production and chemotaxis, Aβ clearance, nuclear hormone PPARγ signaling, and IGF1/AKT signaling. A major target upregulated by EP2 is COX-2 (Supplemental Figure 3B), which drives additional PGE2 production, perpetuating toxic EP2 inflammatory signaling. In combination, these EP2 regulated effects contribute to synaptic degeneration (gray) and functional memory impairment.
In vitro, aged but not young macrophages increased EP2 expression in response to Aβ42 oligomers. This interesting age-dependent regulation was associated with a robust immune response to Aβ42 that was significantly enhanced with coadministration of EP2 agonist. The observed difference in inflammatory responses to Aβ42 between young and aged macrophages is consistent with observations demonstrating that innate immune responses increase with age, in part through increased activation of NF-κB–driven inflammatory pathways (51). Moreover, the age dependence of EP2 upregulation and EP2-driven inflammatory gene expression is highly relevant to the pathogenesis of AD, for which the primary risk factor is age. We also noted a converse suppressive effect of EP2 signaling on expression of chemokines and Aβ-degrading enzymes, proteins that are beneficial in controlling extracellular levels of Aβ peptides in vivo. Thus, in response to Aβ42 oligomers, EP2 signaling upregulated expression of deleterious oxidative and proinflammatory factors and reduced expression of beneficial chemotactic and proteolytic genes. This dual regulatory function suggests that microglial EP2 signaling may be playing a harmful role in vivo in response to early Aβ42 generation and accumulation. This was confirmed in young 5-month-old APP-PS1 Ep2–/– mice, which exhibited not only increased chemotaxis of microglia to sites of nascent amyloid plaques, but increased levels of Aβ proteases and CD68. Moreover, in Cd11b-Cre Ep2fl/fl mice, microglial deletion of Ep2 significantly accelerated clearance of intracortically injected Aβ peptides.
The opposing regulation of MIP-1α by Aβ peptides and by EP2 signaling is particularly interesting. Inflammatory chemokines such as MIP-1α, MIP-1β, and MCP-1 are β chemokines that are important in recruitment of monocytic cells, including macrophages and microglia. Upon binding to their G protein–coupled receptors (CCR1 and 5 for MIP-1α), these chemokines initiate cytoskeletal reorganization and cellular migration. Of all chemokines, MIP-1α in particular was highly induced by 6 months in the APP-PS1 model, suggestive of a specific role for microglial MIP-1α in the inflammatory response to Aβ peptides. Moreover, in addition to its well-established function of inducing chemotaxis of immune cells, MIP-1α can also activate immune cells to produce inflammatory cytokines. Thus, the suppression of MIP-1α expression coupled to the induction of proinflammatory responses in the context of Aβ stimulation and EP2 signaling is unexpected, but likely suggests a complex transcriptional regulation of MIP-1α and cytokine release. Nonetheless, the functional outcome of EP2 signaling in the context of Aβ stimulation — worsening of the inflammatory response through increased proinflammatory gene expression, as well as suppression of MIP-1α and microglial chemotaxis to sites of Aβ peptide accumulation — is detrimental.
To explore the broader regulatory role of microglial EP2 signaling in response to Aβ42, we used an unbiased approach and identified a class of genes upregulated independently of Aβ42 in Cd11b-Cre Ep2fl/fl mice. Among these genes were the nuclear hormone transcriptional pathway components Rxrg, Rdh13, and Lpl, components of PPAR and RA pathways that promote antiinflammatory, trophic, and phagocytic microglial activities and that are highly beneficial in AD models (35). RXRγ binds the clinical compound bexarotene and increases levels of ApoE4 and ABCA1 lipidator important for Aβ peptide clearance by ApoE; activation of RXR/LXRs by bexarotene may lower interstitial soluble Aβ peptide levels and improve cognitive performance (40, 52). The regulation of RXRγ in microglia is also interesting in the context of the circadian clock, which is modulated by retinoid receptors via regulation of BMAL1 transcription (53). Thus, it is conceivable that EP2 regulation of RXRγ could modulate the forward arm of the clock. Recent studies have linked deficient BMAL:CLOCK/NPAS2 activity to disrupted energy homeostasis, aging, and neurodegeneration (54–56), which suggests that the prominent increase in EP2 signaling in aging myeloid cells shown in Figure 1 may negatively affect myeloid cellular clock function.
Intriguingly, Ep2-deficient microglia responded to Aβ42 peptides by upregulating IGF1; in brain, this growth factor is not only potently neuroprotective, neurogenic, and antiinflammatory, but also enhances synaptic and neuronal plasticity and improves cognitive function (44). Although IGF1 can be produced in most tissues, most of it is generated by the liver and released into the circulation. Circulating IGF1 decreases with age and in AD; moreover, AD is characterized by insulin resistance and decreased IGF1R signaling (57). In mouse models of AD, circulating serum IGF1 can accelerate clearance of Aβ peptides (58). In our studies of mice acutely administered i.c.v. Aβ42, deletion of microglial Ep2 significantly increased microglial IGF1R signaling in hippocampus. Examination of protein phosphorylation downstream of IGF1R and PI3K after Aβ administration revealed marked induction in PI3K/AKT signaling in Cd11b-Cre Ep2fl/fl mice, which suggests that deletion of Ep2 in microglia preserves and enhances the AKT signaling response to a noxious stimulus, in this case Aβ peptides. AKT signaling is beneficial and antiinflammatory; consequently, Cd11b-Cre Ep2fl/fl mice exhibited a reduced inflammatory response and were also able to function normally in the NOR memory task. Taken together, these findings argue for a beneficial protective effect of microglial Ep2 deletion in the context of an acute Aβ stimulus.
Ablation of microglial Ep2 signaling similarly elicited a beneficial effect in the context of chronic Aβ stimulation, notably in aging 9-month-old APP-PS1 Cd11b-Cre Ep2fl/fl mice. Spatial memory testing was improved in APP-PS1 Cd11b-Cre Ep2fl/fl mice, as were levels of presynaptic proteins. APP-PS1 Cd11b-Cre Ep2fl/fl animals also demonstrated increased levels of IGF1 and MIP-1α expression. Interestingly, in this chronic model, AKT signaling was broadly induced in APP-PS1 Cd11b-Cre mice compared with Cd11b-Cre controls; however, deletion of microglial Ep2 normalized the APP-PS1 Cd11b-Cre Ep2fl/fl phosphoprotein levels toward Cd11b-Cre levels. This suggests that chronic microglial Ep2 deletion led to a more benign inflammatory and oxidative environment; this, in addition to the trophic and antiinflammatory effects of chronic IGF1R/PI3K signaling, might be expected to blunt a reactive increase in AKT signaling.
Taken together, our findings suggest a broad effect of conditional deletion of Ep2 in microglia in mouse models of AD. This conclusion was based on the selectivity of the Cd11b promoter–driven expression of Cre recombinase in brain to microglia, as demonstrated by Boillee et al.: crosses of Cd11b-Cre mice to Rosa26-lacZ mice showed Cre recombination in the CNS that was restricted to microglia (31). In a previous study using this Cre recombinase line, we observed approximately 50% reduction of floxed Ep2 sequences in microglia and in peritoneal macrophages (26). Peripherally, CD11b protein is normally expressed in monocytes and tissue-specific macrophages, and to a lesser extent in subpopulations of granulocytes, mature B lymphocytes, and CD4+ T lymphocytes (59, 60). Because Cd11b-Cre recombinase will also reduce levels of peripheral myeloid EP2, we cannot exclude a potential contribution of peripheral myeloid cell Ep2 deletion to our present findings. There is increasing evidence of communication between the periphery and the CNS, particularly in experimental models of aging, where circulating factors can alter hippocampal neurogenesis, synaptic plasticity, and cognitive function (61, 62). Validation with cell-specific Cre recombinase lines that are selectively expressed only in brain microglia or peripherally in macrophages/monocytes will be very helpful in parsing out the relative contributions of these cell types to models of AD and aging.
In conclusion, our data demonstrate that microglial EP2 suppresses multiple beneficial functions that are essential to combat the toxic effects of Aβ42 peptides on synapses and memory function. These findings suggest that microglial EP2 activity hastens pathological progression to AD. By virtue of its broad regulatory effect on beneficial microglial functions, inhibition of inflammatory EP2 signaling may be a promising strategy to restore healthy microglial function, arrest the progression of Aβ42-driven pathology, and prevent development of AD.
Methods
Animals.
All strains were in the C57BL/6 background. APP-PS1 mice (APPSwe-PS1ΔE9; ref. 63) were crossed to Ep2–/– and Ep2+/+ mice (originally from R. Breyer, Vanderbilt University, Nashville, Tennessee, USA; refs. 64, 65). Cd11b-Cre mice (31), provided by G. Kollias (Alexander Fleming Biomedical Sciences Research Center, Vari, Greece) and D. Cleveland (UCSD, La Jolla, California, USA), efficiently excise floxed sequences in microglia and macrophages (26, 29, 31). C57BL/6 Ep2fl/+ mice have been previously described (26). All mice were housed in an environment controlled for lighting (12-hour light/12-hour dark cycle), temperature, and humidity, with food and water available ad libitum.
Macrophage cell cultures.
Peritoneal macrophages were harvested from young (3–5 months) and aged (19–21 months) female mice (except for MIP-1α ELISA, for which males were used). Viable and healthy macrophages can be obtained from both young and aged mice and survive well in culture; this is not the case for adult-derived microglia, which may not survive consistently in culture and yield highly variable data. In addition, macrophage yields are significantly higher per mouse than are microglia yields, thus minimizing the number of mice required for the study. Mice were injected with 1.5 ml 3% (w/v) thioglycollate medium (BD Diagnostic Systems) into the peritoneal cavity, and primary macrophages were isolated 3–4 days later by flushing with ice-cold HBSS (HyClone). Cells were seeded at a density of 2 × 106 cells/well onto 12-well plates for RNA and 8 × 104 cells/well in 96-well plates for ELISA measurements in DMEM supplemented with 10% heat-inactivated FBS (HyClone), 100 U/ml penicillin and streptomycin, and 1 mM sodium pyruvate and maintained at 5% CO2 at 37°C. After overnight culture, cells were washed twice with medium to remove nonadherent cells before treatment.
Primary microglia culture.
Primary microglia were isolated from the brains of postnatal day 7 C57BL/6J mouse pups obtained from Charles River Laboratories. Primary microglia were isolated using the Neural Tissue Dissociation Kit (P), MACS Separation Columns (LS), and magnetic CD11b Microbeads from Miltenyi Biotec. Microglia were grown in culture for 3–5 days before being treated in each experiment as previously described (26).
Preparation of oligomeric and fibrillar Aβ42.
Oligomeric and fibrillar Aβ peptide species were generated as previously described (21, 66).
Quantitative real-time PCR (qPCR).
RNA isolation, cDNA production, and SYBR-Green-based qPCR (QuantiTect SYBR Green Kit; Qiagen) were performed as previously described (29) using the standard curve method and normalizing to 18S and Gapdh. Primers were designed by PrimerQuest (Integrated DNA Technologies) and synthesized by Integrated DNA Technologies. Primer sequences are listed in Supplemental Table 1.
Intracortical and i.c.v. injection of Aβ42 peptides.
FITC-conjugated fibrillar Aβ42 peptides (185 μM; rPeptide) or vehicle were administered intracortically to 17-month-old female and male Cd11b-Cre and Cd11b-Cre Ep2fl/fl littermates (67, 68). Mice were under isoflurane anesthesia during surgery. 1 μl fibrillar Aβ42 (185 pmol) or vehicle was delivered to opposite hemispheres of the cerebral cortex over 10 minutes using a 32-gauge Hamilton syringe; after injection, the needle was left in place for 5 minutes and then withdrawn over 4 minutes to prevent backflow. The stereotaxic injections were placed at the following coordinates from the bregma: mediolateral, 1.2 mm; anteroposterior, 1.2 mm; dorsoventral, 1.5 mm. After injection, each mouse recovered spontaneously on a heated pad. At 48 hours after injection, mice were euthanized, and brains were rapidly harvested. Intracortical Aβ42–injected brains were sectioned coronally on a freezing microtome at 40-μm intervals. From each mouse, 30 sections were chosen for immunostaining with IBA1 and CD68 antibodies, with sections covering the span of the needle injection track and at least 5 sections anterior and posterior to the injection track. FITC+ sections (average, 15 per mouse) were analyzed in a blinded manner by FITC intensity or area covered above a threshold level, and mean IBA1 and CD68 intensities were determined within the FITC+ area. For i.c.v. Aβ peptide injections, fibrillar Aβ peptide species (40 pmol) or vehicle (PBS) were administered to Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice as previously described (69). Stereotaxic injections were placed at the following coordinates from the bregma: mediolateral, –1.0 mm; anteroposterior, –0.3 mm; dorsoventral, –2.5 mm. After injection, each mouse recovered spontaneously on a heated pad.
Microglia quantification.
Images were acquired in the hippocampal area by a blinded experimenter at ×400 magnification of all identifiable hippocampal plaques with surrounding microglia, and with 5 z planes spaced 5 μm apart covering the plaque and microglia. A circle centered on the plaque with diameter of 75 μm was stamped, and the cropped image was analyzed. IBA1+ microglia were manually counted going through the z planes and included if they were touching the plaque with either the cell body or processes. Plaques were grouped into small (<250 μm2), medium (250–575 μm2) and large (>575 μm2) sizes. Percent CD68 coverage area above threshold level was determined in the cropped image for the extended focus, including all z planes. 5 sections (average, 10.6 ± 1.1 plaques) were analyzed per mouse.
Immunostaining.
Immunostaining of paraformaldehyde-fixed mouse sections was carried out as previously described (21). Antibodies used include anti-rat CD68 (1:1,000 dilution; AbD Serotec), anti-rabbit IBA1 (1:2,000 dilution; Wako), and anti-mouse 6E10 for human Aβ (1:2,000 dilution; Covance). Secondary antibodies and detection reagents included donkey FITC-conjugated anti-mouse, Cy3-conjugated donkey anti-rabbit, and DyLight 649–conjugated anti-rat antibody (Jackson ImmunoResearch Laboratories). Biotinylated secondary antibodies (Vector Labs) were used at a dilution of 1:250. Rabbit and mouse sera (Jackson ImmunoLabs) were used as negative controls in place of primary antibodies on adjacent sections.
Image acquisition for immunostaining.
Imaging of immunostained sections was done using a Nikon Eclipse E600 microscope (Nikon Instruments) and a Hamamatsu Orca-ER digital camera (Hamamatsu Photonics). Images were analyzed using the measurements module of Volocity 4.3.2 image analysis software (Improvision).
Western analysis and ELISA and multi-antibody array measurement of phosphoproteins.
For quantification of phosphorylated IGF1R, hippocampi were homogenized in 10 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% Nonidet P-40, 1 mM sodium orthovanadate, and protease inhibitors. After centrifugation to remove insoluble material, supernatants were incubated overnight with bead-conjugated antibody against IGF1R (Santa Cruz), and immunocomplexes were collected with centrifugation; beads were washed in homogenization buffer before separation by SDS-PAGE. Blots were incubated with primary antibodies against phosphotyrosine (4G10, Platinum, Millipore) to detect p-IGF1R; membranes were stripped and reprobed with anti–IGF-I Receptor β (Cell Signalling). Levels of MIP-1α and MCP-1 (R&D Systems) and IL-1β (BD Biosciences) were measured by ELISA. For quantification of phosphoproteins, 36 μg cortical lysates were applied in 60 μl to the PathScan AKT Signaling Antibody Array kit (Chemiluminescent Readout, Cell Signaling Technology); the assay was carried out according to the manufacturer’s instructions. This assay simultaneously detects 16 phosphoproteins, many belonging to the AKT signaling cascade. Capture antibodies specific for the phosphorylated proteins were spotted on nitrocellulose-coated glass slides, and lysates were incubated overnight at 4°C. After washing, arrays were incubated with a biotinylated antibody, followed by chemiluminescent film detection. Spot intensities were quantified using ImageJ.
Aβ peptide quantification.
5M guanidine-extracted Aβ42 peptides were precipitated with ethanol to remove the guanidine, and Aβ42 peptides were measured by ELISA as previously described (22, 70).
Isolation of primary microglia from adult mouse brain.
For isolation of microglia, 8- to 9-month-old Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice were administered i.c.v. injection of Aβ42 fibrils or saline. At 48 hours after surgery, mice were sacrificed by transcardiac perfusion with cold heparinized 0.9% NaCl. Brains were then removed from the mice and pooled, 2 brains of the same genotype per sample, to ensure adequate cell and RNA yield. The brains were then enzymatically dissociated and isolated using magnetic CD11b microbeads (Miltenyi Biotec) according to the manufacturer’s protocol.
RNA isolation and microarray.
RNA purification from primary microglia of adult Cd11b-Cre and Cd11b-Cre Ep2fl/fl mice was performed using TRIzol (Life Technologies) followed by the RNeasy Mini Kit (Qiagen). RNA quality was assessed using a BioAnalyzer (Agilent) and determined to be sufficient for microarray analysis (RNA Integrity Number >7.0 for all samples). cDNA synthesis, labeling, hybridization, and scanning were performed by the Stanford Protein and Nucleic Acid (PAN) Facility using GeneChip Mouse Gene 2.0 ST arrays (Affymetrix). Microarray data were statistically analyzed using Partek software (Partek) to identify differentially expressed genes between groups by ANOVA using an unadjusted P value of <0.05. Genes that had a fold change of >1.5 between genotypes were used for unsupervised hierarchical clustering analysis. Database for Annotation, Visualization and Integrated Discovery (DAVID) functional annotation software (version 6.7; NIAID, NIH) was used to identify KEGG molecular pathways significantly overrepresented among lists of differentially expressed genes. Data were deposited in GEO (accession no. GSE57181).
NOR.
The NOR task, based on the ability of mice to show preference for novel versus familiar objects when allowed to explore freely (71), is disrupted after i.c.v. administration of Aβ peptides and fibrils (33). NOR was performed during the light cycle. Mice were individually habituated to an open arena (50 cm × 50 cm, dim light, 24°C) on day 1. During the subsequent training session, 2 identical objects were placed into the arena, and exploratory behavior was monitored for 5 minutes. On day 2, mice were placed back into the same arena, in which one of the objects used during training was replaced by a novel object of similar dimensions but a different shape/color, and exploratory behavior was monitored for 5 minutes. Digital video tracking (using an infrared camera and vplsi Viewpoint software) of body movements and head position was used to quantify locomotor and exploratory activities around the objects (2-cm zone around the objects). Exploration behavior was assessed by calculating DI, the ratio of time spent exploring the old object to time spent exploring both objects (expressed as a percentage). A DI of ~50% is associated with correct training and no object preference; a significant decrease in DI is characteristic of recognition of the novel object. To evaluate memory, comparisons were made for each group between the recognition (24 hours) and training (0 hours) sessions. Paired t tests were performed between time points. Behavioral testing was performed by experimenters blinded to genotype.
RAM.
The RAM is a spatial memory test that assesses working and reference memory over several consecutive days; the protocol was adopted from that of Clelland et al. (72). Previous experiments demonstrated that no deficits exist in nonmnemonic behaviors (visual and sensory-motor abilities) in APP-PS1 mice (30). Mice were food restricted for 1 week prior to testing and maintained at 85%–90% body weight for the duration of testing, and water was provided ad libitum. All testing occurred at the beginning of the dark phase of the diurnal light/dark cycle or in dim light during the end of the light cycle. The testing apparatus consisted of a wooden 8-arm RAM with Plexiglas walls, elevated 50 cm from the floor and surrounded by visual cues. 3 days before testing, mice were habituated to the maze. Day 1 of the habituation phase consisted of a 10-minute group exposure (by cage) to the RAM, in which all arms were unblocked but no food was provided. The second day, mice were individually exposed twice to the maze for 5 minutes, with each arm baited with food rewards. This was repeated on the third day, but with only 3 doors open. Following habituation, mice were tested for their ability to separate sample (familiar) from choice (new) arms in the RAM. Cage order was randomized throughout testing. Mice received 2 trials per day (consisting of 1 sample phase and 1 choice phase) for 6 consecutive days. During the sample phase, all arms except the start arm and the sample arm were blocked off. The mouse was permitted to visit the sample arm and retrieve the food reward. Mice were retrieved from the maze after either (a) spending 10 seconds in the sample arm after retrieving the pellet or (b) exiting the sample arm. During the choice phase, arms in the start and sample (unrewarded) locations and an additional correct (rewarded) location were open. Mice that entered the correct arm were considered to have made correct choices. Mice that made incorrect choices (i.e., entered the sample arm or reentered the start arm) were allowed to self-correct, enter the correct arm, and retrieve the pellet before being removed from the maze. Sample and correct arms were randomized for each combination, such that sample arm was either to the left or right of the start arm, and for each combination, the start arm was located in 1 of 2 locations perpendicular to either the correct or the sample arm.
Statistics.
Data are expressed as mean ± SEM. Statistical comparisons were made with GraphPad Prism software using Student’s t test (2-tailed unless otherwise indicated; for 2 groups meeting normal distribution criteria by Shapiro-Wilk normality test), Mann-Whitney U test (for 2 groups not meeting normal distribution criteria), or 2-way ANOVA with Bonferroni multiple-comparison tests (for groups across 2 variables, with multiple comparisons between groups). Linear regression was used to analyze microglial markers in the FITC-Aβ intracortical experiment. The linear model was chosen for goodness-of-fit of the dataset (r2 = 0.88 and 0.93). Slopes were compared using F test. For Luminex multiplex analysis, fluorescence intensities that reached statistical significance with i.c.v. Aβ42 were transformed to relative concentrations (median z score). Cluster analysis (Gene Cluster 3.0 and Java TreeView 1.0.13) produced a separation of samples according to treatment and genotype. For NOR analyses, paired t test was used to compare performance between the 0- and 24-hour time points. Data were subjected to Grubbs’ test to identify the presence or absence of outlier data points for exclusion from analysis. For all tests, a P value of 0.05 or less was considered significant.
Study approval.
This study was conducted in accordance with NIH guidelines, and protocols were approved by the IACUC at Stanford University.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1AG030209 (to K.I. Andreasson), NIH grant R21AG033914 (to K.I. Andreasson), Alzheimer’s Association (to K.I. Andreasson), Swedish Research Council (to J.U. Johansson), National Science Foundation (to N.S. Woodling), and NRSA grant F31AG039195 (to N.S. Woodling). The authors thank Damien Colas, Bayara Chuluun, Grace Hagiwara, and Craig Heller for assistance in behavioral experiments; Frank Longo, Theo Palmer, and Tony Wyss-Coray for use of equipment; and Suraj Pradhan and the Stanford Human Immune Monitoring Center for Luminex measurements.
Jenny U. Johansson’s present address is: SRI International, Menlo Park, California, USA.
Nathaniel S. Woodling’s present address is: Institute of Healthy Ageing, University College London, London, United Kingdom.
Holden D. Brown’s present address is: Brains On-Line LLC, South San Francisco, California, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(1):350–364. doi:10.1172/JCI77487.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Bateman RJ, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram L, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83(5):623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerreiro R, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson T, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JC, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert JC, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 12.Raj T, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344(6183):519–523. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krabbe G, et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.in t’ Veld BA, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 16.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48(3):626–632. doi: 10.1212/WNL.48.3.626. [DOI] [PubMed] [Google Scholar]
- 17.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70(19):1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–432. doi: 10.1212/WNL.47.2.425. [DOI] [PubMed] [Google Scholar]
- 19.Montine TJ, et al. Elevated cerebrospinal fluid prostaglandin E2 levels in patients with probable Alzheimer’s disease. Neurology. 1998;53(7):1495–1498. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 20.Combrinck M, et al. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):85–88. doi: 10.1136/jnnp.2005.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, et al. Inflammatory prostaglandin E(2) signaling in a mouse model of Alzheimer disease. Ann Neurol. 2012;72(5):788–798. doi: 10.1002/ana.23677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25(44):10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhen G, et al. PGE2 EP1 receptor exacerbated neurotoxicity in a mouse model of cerebral ischemia and Alzheimer’s disease. Neurobiol Aging. 2012;33(9):2215–2219. doi: 10.1016/j.neurobiolaging.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keene CD, et al. Suppressed accumulation of cerebral amyloid {beta} peptides in aged transgenic Alzheimer’s disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol. 2010;177(1):346–354. doi: 10.2353/ajpath.2010.090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 2005;15(2):134–138. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson JU, et al. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci. 2013;33(40):16012–16032. doi: 10.1523/JNEUROSCI.2203-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodling NS, et al. Suppression of Alzheimer-associated inflammation by microglial prostaglandin-E2 EP4 receptor signaling. J Neurosci. 2014;34(17):5882–5894. doi: 10.1523/JNEUROSCI.0410-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184(12):7207–7218. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18(3):602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 32.Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Figueiredo CP, et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight abeta oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci. 2013;33(23):9626–9634. doi: 10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heneka MT, Landreth GE, Hull M. Drug insight: effects mediated by peroxisome proliferator-activated receptor-γ in CNS disorders. Nat Clin Pract Neurol. 2007;3(9):496–504. doi: 10.1038/ncpneuro0586. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32(48):17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chawla A, et al. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7(1):161–171. doi: 10.1016/S1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 37.Laffitte BA, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98(2):507–512. doi: 10.1073/pnas.98.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Q, et al. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci. 2012;32(30):10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer PE, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science. 2013;340(6135):924-c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han J, Sridevi P, Ramirez M, Ludwig KJ, Wang JY. β-Catenin-dependent lysosomal targeting of internalized tumor necrosis factor-α suppresses caspase-8 activation in apoptosis-resistant colon cancer cells. Mol Biol Cell. 2013;24(4):465–473. doi: 10.1091/mbc.E12-09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontana L, Partridge L, Longo VD. Extending healthy life span — from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13(4):225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 45.Ueno M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 46.Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(8):933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Varo R, et al. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol. 2012;123(1):53–70. doi: 10.1007/s00401-011-0896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao CY, Mirra SS, Sait HB, Sacktor TC, Sigurdsson EM. Postsynaptic degeneration as revealed by PSD-95 reduction occurs after advanced Aβ and tau pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 2011;122(3):285–292. doi: 10.1007/s00401-011-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiala M, et al. Ineffective phagocytosis of amyloid-β by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7(3):221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- 50.Mawuenyega KG, et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007;21(24):3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landreth GE, et al. Response to comments on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science. 2013;340(6135):924-g. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105(7):877–889. doi: 10.1016/S0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 54.Musiek ES, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123(12):5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13(5):325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peek CB, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nat Med. 2002;8(12):1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 59.Dziennis S, et al. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood. 1995;85(2):319–329. [PubMed] [Google Scholar]
- 60.Ferron M, Vacher J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis. 2005;41(3):138–145. doi: 10.1002/gene.20108. [DOI] [PubMed] [Google Scholar]
- 61.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villeda SA, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17(6):157–165. doi: 10.1016/S1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 64.Breyer RM, Kennedy CR, Zhang Y, Guan Y, Breyer MD. Targeted gene disruption of the prostaglandin E2 EP2 receptor. Adv Exp Med Biol. 2002;507:321–326. doi: 10.1007/978-1-4615-0193-0_49. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy CR, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5(2):217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 66.Yang T, et al. Small molecule, non-peptide p75 ligands inhibit Aβ-induced neurodegeneration and synaptic impairment. PLoS One. 2008;3(11):e3604. doi: 10.1371/journal.pone.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Khoury J, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 68.El Khoury JB, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piermartiri TC, et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-β(1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010;226(2):274–284. doi: 10.1016/j.expneurol.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Melnikova T, et al. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience. 2006;141(3):1149–1162. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.