Abstract
Tomato bacterial wilt caused by Ralstonia solanacearum is one of the most destructive soil-borne diseases. Many strategies have been taken to improve soil suppressiveness against this destructive disease, but limited success has been achieved. In this study, a novel bioorganic fertilizer revealed a higher suppressive ability against bacterial wilt compared with several soil management methods in the field over four growing seasons from March 2011 to July 2013. The application of the bioorganic fertilizer significantly (P<0.05) reduced disease incidence of tomato and increased fruit yields in four independent trials. The association among the level of disease incidence, soil physicochemical and biological properties was investigated. The soil treated with the bioorganic fertilizer increased soil pH value, electric conductivity, organic carbon, NH4 +-N, NO3 --N and available K content, microbial activities and microbial biomass carbon content, which were positively related with soil suppressiveness. Bacterial and actinomycete populations assessed using classical plate counts were highest, whereas R. solanacearum and fungal populations were lowest in soil applied with the bioorganic fertilizer. Microbial community diversity and richness were assessed using denaturing gel gradient electrophoresis profile analysis. The soil treated with the bioorganic fertilizer exhibited higher bacterial community diversity but lower fungal community diversity. Redundancy analysis showed that bacterial community diversity and richness negatively related with bacterial wilt suppressiveness, while fungal community richness positively correlated with R. solanacearum population. We concluded that the alteration of soil physicochemical and biological properties in soil treated with the bioorganic fertilizer induced the soil suppressiveness against tomato bacterial wilt.
Introduction
Bacterial wilt caused by the vascular pathogen, Ralstonia solanacearum has resulted in serious damage in agricultural crop production [1]. Disease incidence of bacterial wilt of tomato in southern China is increasing, and has caused severe yield losses. Various strategies have been taken to control bacterial wilt, such as grafting [2], biofumigation [3] and growing resistant crop varieties [4], but limited success has been achieved due to the high surviving capacity in complex environments [5], wide host range [1], and genetic diversity [6] of R. solanacearum. Although soil solarization has been recognized as an effective method to control bacterial wilt [7], a short-term effectiveness of solarization limits its application throughout the year. Therefore, effective measures to suppress bacterial wilt disease are needed to be developed.
Biocontrol and use of organic amendments such as compost, manure and plant residues emerge as environmentally friendly strategies and popular methods to suppress soil-borne disease. The use of organic amendments as a promising alternative to suppress the soil-borne disease less depends on chemicals. Mazzola [8] argued convincingly that organic amendments could increase microbial activity and enrich populations of specific microorganisms or microorganism groups to inhibit the invasion of pathogens. According to the bacterial community profiles after pig slurry application, Gorissen et al. [9] confirmed that the lower population size of R. solanacearum resulted from an alteration of microbial community structure induced by pig slurry. In recent decades, several biocontrol agents of R. solanacearum have been isolated from the rhizosphere soil or plant tissues, such as Bacillus spp. [10], Streptomyces spp. [11] and avirulent mutants of R. solanacearum [12]. However, the ability to competitively colonize and survive in the rhizosphere is still a prerequisite for antagonistic strains in suppressing soil-borne diseases [10]. Therefore, niches and nutrients become important when competition occurs between pathogens and antagonists. However, organic fertilizers can supply adequate energy and nutrients for antagonists to improve the suppressive capacity towards pathogens [13]. Importantly, organic fertilizers from municipalities, industries and farms meet the sustainable development of agriculture by recycling organic wastes and reducing the use of chemical fertilizers and fungicides in crop production [14]. The combination of organic fertilizers and antagonists (termed bioorganic fertilizer in our study), as a novel strategy to control bacterial wilt, showed best results in tobacco [10] and potato [15], and decreased bacterial disease incidences successfully. However, few studies focus on the suppressive capacity of soil treated with bioorganic fertilizers towards tomato bacterial wilt in the field.
Soil suppressive ability against pathogens may be affected by multiple factors, such as soil pH, organic matter content, nutrient availability and enzyme activity, which may in turn affect the interactions between pathogens and antagonists, and microbial resistance against invasion of pathogens in soil [16]. Therefore, analysis of association among the level of disease incidence, physicochemical and biological properties is useful to elaborate the mechanisms of soil suppressiveness of bioorganic fertilizers. The purpose of this study was to evaluate the soil suppressive capacity of a bioorganic fertilizer towards bacterial wilt in two tests over four growing seasons from March 2011 to July 2013. Our results demonstrated that 1) the application of bioorganic fertilizer more efficiently controlled bacterial wilt than other soil amendment methods, 2) the application of bioorganic fertilizer significantly improved soil physicochemical and biological properties, 3) the alteration in soil physicochemical and biological properties after the application of bioorganic fertilizer induced the suppressive capacity towards bacterial wilt.
Materials and Methods
Experimental site
The experiments were conducted in Xiaogang Town, Ningbo, Zhejiang province of China (121°44′ E, 29°56′ N) with a subtropical monsoon climate (no specific permissions were required for soil sampling in this location and the field in this study did not involve endangered or protected species). The average mean minimum and maximum temperatures are 25°C and 35°C, respectively, during the summer period from June to August. The site has 150 rainy days per year, and 1317 mm of mean annual precipitation. Tomato has been cultivated for over six years at this site, and bacterial wilt became a serious problem.
Field experiments
The experimental design was a randomized block with four replicates. Sainto tomato (CTX 201) seedlings were grown in sterile nursery substrate for 25 d. Then, one hundred and twenty seedlings were transplanted into each plot (17.0 m × 1.3 m). Field experiments contained two different tests. Test 1 was conducted for two growing seasons: from March 2011 to July 2011 (Trial 1) and September 2011 to February 2012 (Trial 2). The treatments were shown as follows: (1) CK (NPK fertilizer), (2) O (NPK fertilizer + 3 t organic fertilizer ha-1), (3) B (NPK fertilizer + 3 t bioorganic fertilizer ha-1). After Test 1, a further test (Test 2) was conducted over two growing seasons from September 2012 to February 2013 (Trial 3) and from March 2013 to July 2013 (Trial 4), with the following treatments: (1) CK (NPK fertilizer), (2) S (NPK fertilizer + soil disinfection), (3) S + B (soil disinfection + NPK fertilizer + 3 t bioorganic fertilizer ha-1). The NPK fertilizers consist of chemical fertilizers at the N:P:K ratio of 15:7:12; N was applied as urea, P applied as superphosphate with 7% P and K as potassium sulfate containing 41% K. In order to balance the NPK level in all treatments, different amounts of NPK fertilizers were applied into each plot. The calcium cyanamide containing 17% N was used for soil disinfection at a dosage of 4 kg plot-1. Rape seed meal was applied as organic fertilizer and contained 26.4% organic C, 2.3% N, 1.3% P, 2.5% K and 25% H2O. Bioorganic fertilizer with 24.4% organic C, 3.1% N, 0.6% P, 2.7% K and 42% H2O was supplied by Jiangsu New Ground Bio-fertilizer Engineering Center Co., Ltd. in China. The amounts of N, P and K supplied were 0.60, 0.70 and 2.60 kg plot-1, respectively.
Disease incidence
The incidence and severity of bacterial wilt were observed daily after transplanting tomato seedlings. The disease incidence was evaluated when the disease emerged and calculated as the percentage of diseased plants compared with the total number of growing plants in each plot. We presented the data of disease incidence at 30, 90 and 120 DAT in Trials 1 and 4, and 30, 100 and 150 DAT in Trials 2 and 3.
Soil sampling
Soil samples (5–20 cm) adhering to the roots were taken from each plot in the period of florescence, full fruit and harvest at 30 days after transplanting (DAT), 90 DAT and 120 DAT in Trial 1 and Trial 2, respectively, and at 30 DAT, 100 DAT and 150 DAT in Trial 3 and Trial 4, respectively. The number of culturable microbial community populations in soil was determined immediately after soils were transported to the laboratory. A portion of soil samples were immediately kept at 4°C in 50-mL polypropylene tubes for determining the soil microbial activities and microbial biomass C within 48 h. Another portion of soil samples were kept at −80°C for PCR-DGGE analysis. The remaining soils were air-dried to determine soil physicochemical properties.
Soil physicochemical properties analysis
Soil water content was determined gravimetrically after drying at 105°C for 8 h. Soil pH and electrical conductivity (EC value) were estimated on air-dried samples using a 1:5 soil/water ratio. Soil organic carbon (SOC) was measured by the Walkley-Black method [17], and total N was assayed by Kjeldahl analysis [18]. The NH4 +-N was extracted with 2 M KCl and analyzed colorimetrically. Soil NO3 –-N was extracted with 0.5 M K2SO4 before colorimetric measurement at 220 nm and 270 nm. Available P was determined using the methods outlined by Kuo [19]. Available K in the NH4OAc extracts [20] was estimated using an atomic absorption spectrophotometer.
Soil microbial activity and microbial biomass analysis
The activities of phosphomonoesterase, arylsulfatase, β-D-glucosidase and dehydrogenase were measured according to Tabatabai [21]. The urease activity was determined as described by Tabatabai and Bremner [22]. Fluorescein diacetate (FDA) hydrolysis was assayed according to Adam [23]. Soil respiration was estimated by measuring CO2 evolved from the soil [24]. Microbial biomass carbon was estimated by the fumigation-extraction method [25].
Microbial community populations in soil
The culturable microbes were enumerated by a standard 10-fold dilution method. Briefly, 10 g soil was added to 90 mL sterile distilled water and shaken on a rotary shaker at 200 rpm for 30 min. Soil suspensions at appropriate dilution rates were spread on plates and incubated on suitable media as follows: Beef extract-peptone medium for bacterial population, Martin’s Rose Bengal agar for fungal population, Gaoshi No. 1 agar for actinomycetes population [26] and semi-selective SMSA medium for R. solanacearum population [27]. The inoculated agar plates with bacteria and R. solanacearum were incubated at 28°C for 2 d in the dark, and with fungi at 28°C for 4 d, whereas plates with actinomycetes were incubated in the dark at 30°C for 5 d. Microbial community populations in soil were measured after crop harvest, and R. solanacearum recovery was tested at 30 DAT, 90 DAT and 120 DAT in Trials 1 and 4, and 30, 100 and 150 DAT in Trials 2 and 3 in the field.
Analysis of microbial community diversity in soil by PCR-DGGE
The DNA was extracted from 0.5 g soil samples with the UltraClean Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) according to the manufacturer’s instructions.
Polymerase chain reaction (PCR) targeting the bacterial 16S rRNA used the primer pairs 338f-GC/518r [28]. A 50 μL PCR reaction mixture contained: 5 μL of 10 × PCR Buffer (TaKaRa), 0.3 μL of Tap polymerase (5 units μL-1, TaKaRa), 4 μL of dNTP Mixture (2.5 mM each), 1 μL of DNA template, 2 μL of primers and double-distilled water to a total of 50 μL. Touchdown PCR was conducted under the condition as followed. An initial denaturation step at 94°C for 5 min followed by a thermal cycling programme as follows: 1 min denaturation at 94°C, 1 min primer annealing at an initial 65°C, decreasing 1°C every cycle to a final of 55°C, 3 min primer extension at 72°C. Thirty cycles were run with a final extension step at 72°C for 7 min.
PCR amplifications of the fungi were performed using the ITSF [29] and ITS4 [30] primers, which is the fungal internal transcribed spacer (ITS) rRNA regions. Polymerase chain reaction mixture was the same as the bacterial PCR reaction mixture. Cycle conditions in the fungal PCR were as follows: 94°C for 5 min, then 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by a final extension step at 72°C for 5 min. The first round PCR amplifications were purified using the PCR Purification Kit (Omega, USA) according to the manufacturer’s instructions. The next PCR was performed to produce ITS1 products under the cycling conditions as follows: 94°C for 5 min, then 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by a final extension step at 72°C for 5 min.
DGGE analysis was performed using the Dcode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, USA). Thirty microliter of PCR product samples with 6 mL loading dye were loaded onto 8% (w/v) polyacrylamide gels (37.5:1 40% acrylamide/bis-solution) containing a gradient of 40–60% denaturants for the bacteria DNA and 30–60% for the fungi DNA. Electrophoresis of the bacteria DNA was performed in 1×TAE buffer at 60°C with a constant voltage of 100 V for 10 h, and then gels with fungal DNA were run at 60°C and 75 V for 16 h. After electrophoresis, the gels were stained with SYBR GREEN I (Sigma) and the DNA bands were observed with a Gel-Doc image analyzer (Bio-Rad Laboratories).
DGGE images were analyzed for band detection and intensity with Quantity One computer software (Version 4.6.3, Bio-Rad Laboratories). For bacteria and fungi, the band richness analysis was defined as the number of DGGE bands detected. The Shannon-Weaver index of microbial community diversity (H) characterizes the microbial community diversity in soil [31]. The Shannon-Weaver index was calculated as
where P i was the ratio of the number in a specific group and the total number, n i was the intensity of a band and N was the sum of all band intensities in the densitometry profile.
Statistical analysis
All statistical analyses were performed with the SPSS 13.0 software program (SPSS Inc., Chicago, IL). Differences among the treatments were assessed with a one-way analysis of variance (ANOVA) at the end of each bioassay. A comparison of means was performed by a Fisher’s least significant difference test (LSD) and Duncan multiple range test with a significance level of P<0.05. Principal component analysis (PCA) and redundancy analysis (RDA) were performed by using Canoco 4.5. PCA was performed using a factor analysis model. The associations among soil physicochemical, biological, soil microbial community diversity, R. solanacearum population and disease incidence were performed by RDA.
Results
Control efficiency of bacterial wilt disease
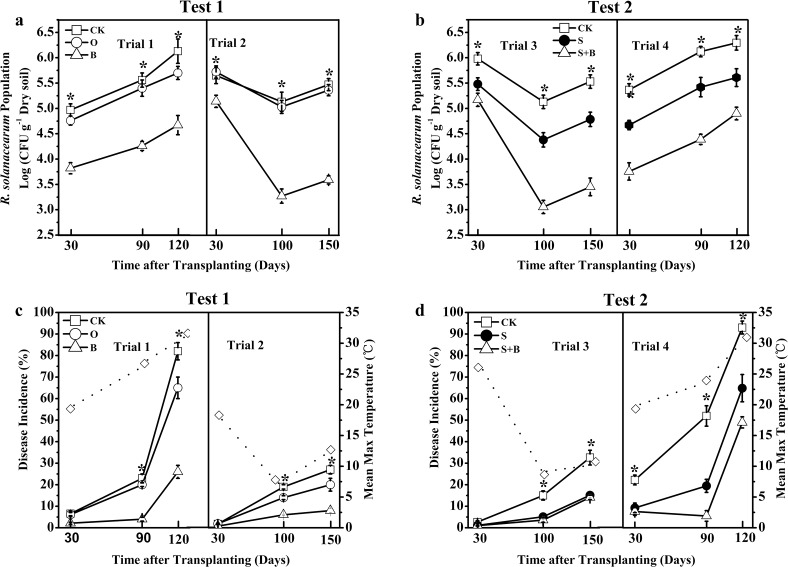
The controlling efficacy of tomato bacterial wilt by different fertilizers/amendments at different growth stages was shown in Fig 1. R. solanacearum population in the B treatment was decreased by 23–24% in Trial 1 and by 8–36% in Trial 2 during the entire experiment period compared with the control (Fig 1A), while no difference was observed between the O and control treatments in both trial periods.
Fig 1. Population of Ralstonia solanacearum in soil and disease incidence over time in Test 1 (a and c) and Test 2 (b and d).
The dotted lines and ◇ represent the mean max temperature in the above time periods in different trials. CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer. * indicates the significant difference between the treatments at the 0.05 probability level according to the Duncan’s test.
The S+B treatment resulted in the lowest R. solanacearum population, which was only 86, 59 and 62% of the control at 30, 100 and 150 DAT, respectively, in Trial 3 (Fig 1B). The S application decreased R. solanacearum population by 8, 15 and 14%, respectively. In Trial 4, the treatment effect on R. solanacearum population displayed the same pattern as observed in Trial 3.
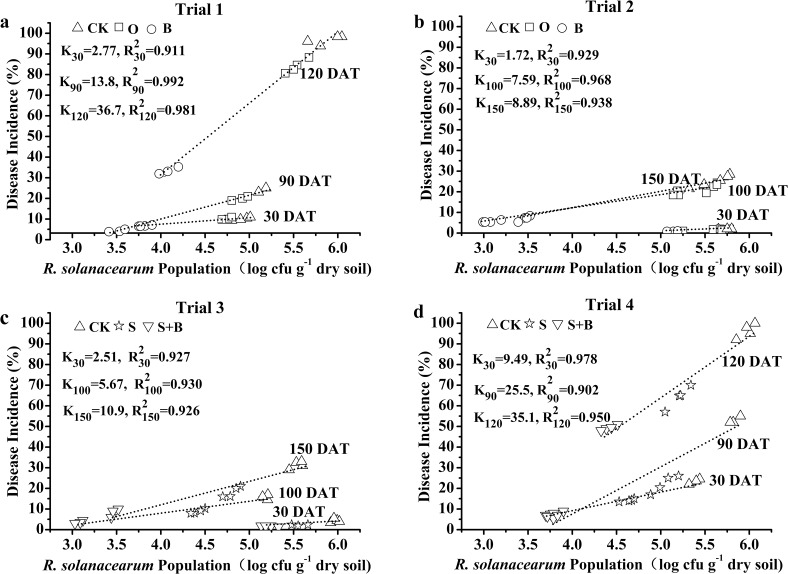
The effects of different treatments on disease incidence were shown in Fig 1C and 1D. The disease incidence in the B treatment was decreased by 67–82% and 55–70%, respectively in Trial 1 and Trial 2 compared with the control (Fig 1C). The treatment effect on disease incidence in Trials 3 and 4 displayed the same pattern as that observed in Trials 2 and 1, respectively (Fig 1D). Interestingly, the disease incidence increased rapidly over the growth stages and exhibited high values in Trials 1 and 4, while it increased slowly and showed lower values in Trials 2 and 3 (Figs 1C and 1D and 2). The difference in disease incidence was related to the temperature variation; high disease incidence was related with high temperatures (Fig 1C and 1D). There were positive relationships between R. solanacearum population and disease incidence with coefficients (R2) over 0.90 in all four trials (Fig 2).
Fig 2. Relationships between population of R. solanacearum and disease incidence in Trial 1 (a), Trial 2 (b), Trial 3 (c) and Trial 4 (d).
CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer. DAT, days after transplanting; K, the slope of line from the relationship between R. solanacearum population and tomato disease incidence; R2, related coefficient for the line from the relationship between R. solanacearum population and tomato disease incidence.
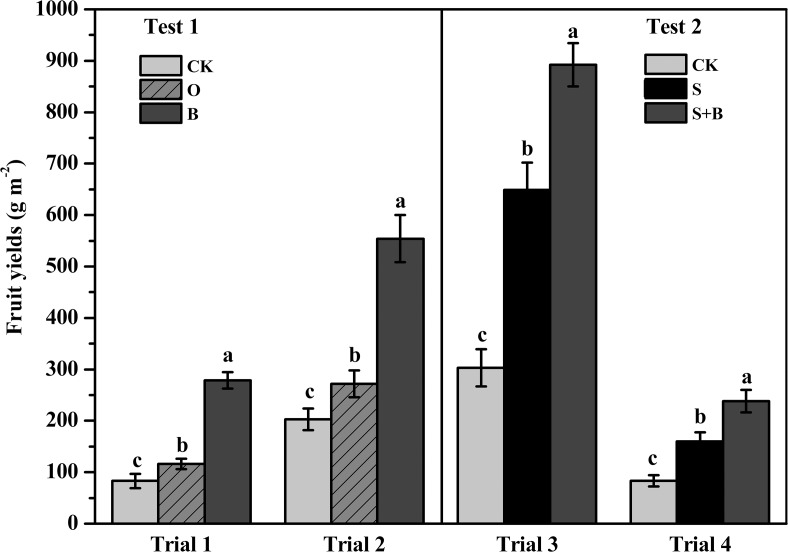
Tomato yields increased by 233% and 173% for the B treatment, and by 39% and 34% for the O treatment compared with the control in Trials 1 and 2, respectively. The yield increased by 194% and 186% in the S+B treatment, by113% and 92% in the S treatment compared with the control in Trials 3 and 4, respectively (Fig 3).
Fig 3. Effect of different treatments on tomato yields.
CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer. Bars with different letters indicate a significant difference between the treatments, as defined by Duncan’s test (P<0.05).
Soil physicochemical properties
Soils collected from different treatments in Test 1 and Test 2 differed substantially in soil physicochemical property values (Table 1). The application of bioorganic fertilizer significantly increased soil pH value, EC value, NH4 +-N, NO3 –-N, available K and SOC contents compared with the O treatment and control in Trials 1 and 2. Organic fertilizer alone only increased NO3 –-N, available K, and SOC content compared with the control.
Table 1. The soil physicochemical properties, and microbial activities and biomass carbon in different treatments.
| pH (1:5 water) | EC (1:5 water) (μS cm-1) | NH4 +-N (μg g-1) | NO3 –-N (μg g-1) | Total N (mg g-1) | Available P (μg g-1) | Available K (μg g-1) | Organic C (mg g-1) | Phosph (μg g-1) | β-gluco (μg g-1) | Aryl (μg g-1) | Urea (μg g-1) | Dehydr (μg g-1) | FDA (μg g-1) | SR (CO2 μL g-1 d-1) | MBC (μg g-1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Trial 1 | CK | 5.47±0.05b | 543±28b | 9.3±0.4c | 321±31c | 2.24±0.18ab | 96±10ab | 75±6c | 26.6±2.3c | 332±24c | 123±24b | z40±4c | 173±13c | 2.43±0.31c | 342±13c | 36.4±3.6d | 186±6d |
| O | 5.62±0.09b | 554±13b | 10.3±0.5b | 413±20b | 2.52±0.15a | 120±15a | 95±7ab | 31.4±1.0ab | 391±24ab | 145±16b | 62±5b | 237±19ab | 3.31±0.35b | 348±27c | 52.2±6.4c | 253±18c | ||
| B | 5.92±0.07a | 712±15a | 12.1±0.2a | 473±20a | 2.75±0.29a | 130±10a | 113±9a | 33.1±1.1a | 420±18a | 198±20a | 91±4a | 286±22a | 4.10±0.22a | 446±24a | 91.7±5.9a | 335±14a | ||
| Trial 2 | CK | 5.54±0.04d | 834±20c | 8.6±0.4b | 446±26c | 1.58±0.06c | 522±21c | 420±21c | 31.2±1.6c | 262±11c | 254±15c | 108±5c | 186±14c | 3.34±0.32d | 262±9c | 29.2±4.3c | 145±12c | |
| O | 5.80±0.06c | 843±32c | 9.3±0.3b | 529±13ab | 1.83±0.12b | 611±19b | 475±28b | 41.0±0.7b | 318±13b | 290±12ab | 150±14b | 241±18ab | 4.03±0.24c | 326±20b | 41.7±5.8b | 224±20b | ||
| B | 6.40±0.07a | 1048±38a | 10.4±0.4a | 562±18a | 2.15±0.04a | 729±30a | 602±30a | 42.8±1.0a | 374±13a | 340±21a | 250±21a | 290±18a | 5.67±0.22a | 395±17a | 73.9±4.6a | 313±12a | ||
| Test 2 | Trial 3 | CK | 5.52±0.06c | 655±14b | 9.7±1.1b | 207±11c | 3.72±0.17c | 723±19b | 543±14b | 41.3±1.6c | 233±30b | 127±19b | 24±2c | 182±14b | 2.76±0.40c | 93±8b | 19.6±3.1c | 79±11c |
| S | 5.99±0.12b | 640±30b | 13.4±1.0a | 254±15b | 4.19±0.12b | 809±20a | 561±13b | 44.4±1.1b | 250±14b | 134±21b | 31±2b | 180±12b | 3.84±0.28b | 87±8b | 26.3±2.0b | 124±10b | ||
| S+B | 6.24±0.04a | 900±25a | 13.7±0.5a | 332±12a | 4.59±0.15a | 816±20a | 613±14a | 53.6±2.4a | 295±15a | 178±16a | 53±4a | 231±14a | 5.77±0.21a | 169±17a | 62.6±6.3a | 248±15a | ||
| Trial 4 | CK | 5.72±0.13c | 476±13c | 10.1±0.3b | 119±17c | 3.48±0.39b | 631±17b | 440±17b | 38.8±2.9c | 303±20b | 153±17b | 48±5b | 112±4c | 2.51±0.32c | 232±18b | 27.6±4.7c | 135±5c | |
| S | 6.12±0.09b | 550±23b | 13.4±1.7a | 169±10b | 3.87±0.45b | 743±20a | 457±20b | 42.8±3.9b | 301±14b | 162±9b | 54±9b | 140±8b | 4.15±0.41b | 240±12b | 42.5±6.4b | 181±5b | ||
| S+B | 6.40±0.12a | 735±32a | 13.7±0.8a | 203±11a | 4.66±0.10a | 754±37a | 734±30a | 49.4±1.5a | 345±12a | 248±13a | 112±6a | 196±10a | 6.09±0.40a | 397±19a | 71.8±5.3a | 352±18a |
Data were expressed as mean ± standard error (n = 4). The data within a column of each trial with a same letter did not differ significantly at Fisher’s least significant difference test (LSD) and Duncan’s significance level 0.05. Phosph, phosphomonoesterase activity; Aryl, arylsulfatase activity; β-gluco, β-D-glucosidase activity; Dehydr, dehydrogenase activity; Urea, urease activity; FDA, fluorescein diacetate hydrolase activity; SR, soil respiration; MBC, microbial biomass carbon. CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer.
The application of bioorganic fertilizer (S+B) resulted in the highest values of pH value, EC value, available K, NO3 –-N, SOC and total N, while the S treatments had higher values of these properties than the control except available K and SOC content (Table 1) in Trials 3 and 4.
Soil microbial activities and biomass carbon
Microbial activity and biomass carbon were chosen as the indicators to assess the soil suppressiveness, as they rapidly respond to the changes of soil environment. Application of bioorganic fertilizer significantly enhanced the activity of phosphomonoesterase, β-D-glucosidase, arylsulfatase, urease and dehydrogenase as well as FDA hydrolysis and soil respiration by 27, 61, 125, 66, 69, 30 and 152%, respectively, in Trial 1, and by 43, 34, 133, 57, 70, 51 and 153%, respectively, in Trial 2 (Table 1). However, the application of organic fertilizer (O) only slightly increased the microbial activity compared with the control. On the other hand, the application of bioorganic fertilizer increased microbial biomass C by 80% and 117% in Trials 1 and 2, respectively.
The S+B treatment exhibited the highest enzyme activities. It significantly increased the activity of phosphomonoesterase, β-D-glucosidase, arylsulfatase, urease and dehydrogenase, FDA hydrolysis and soil respiration by 27, 40, 120, 27, 109, 82 and 220%, respectively, in Trial 3, and 14, 61, 133, 75, 143, 71 and 160%, respectively, in Trial 4 (Table 1). The S application only increased the activity of dehydrogenase but not other enzymes compared with the control. However, the S application increased soil respiration by 34% and 54% in Trials 3 and 4, respectively. Furthermore, the S+B application led to the highest microbial biomass carbon (248 and 352 μg g-1 in Trials 3 and 4, respectively), followed by S (124 and 181μg g-1, respectively) and the control (79 and 135μg g-1, respectively).
Culturable microbial community populations in soil
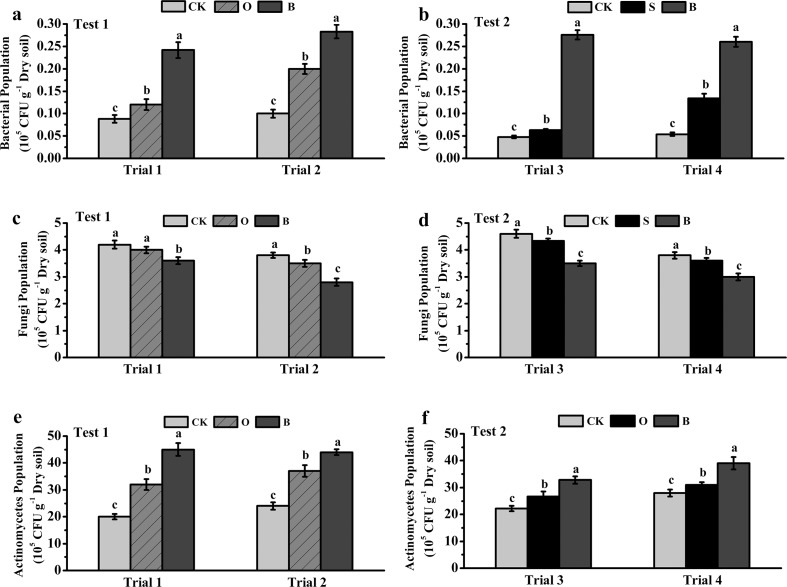
The effects of different fertilizers/amendments on soil microbial community populations were shown in Fig 4. The application of bioorganic fertilizer significantly increased the bacterial and actinomycetes populations, but decreased the fungal population. The bacterial community and actinomycete populations in soil were about 2.5- and 2.0-fold higher in the B treatment than the control in Trials 1 and 2, respectively (Fig 4A and 4E). However, the fungal population was significantly lower in the B treatment than in the O and control treatments (Fig 4C).
Fig 4. The population of bacteria (a and b), fungi (c and d), and actinomycetes (e and f) in soils from different treatments in Test 1 (a, c and e) and Test 2 (b, d and f).
CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer. Bars with different letters indicate a significant difference between the treatments, as defined by Duncan’s test (P<0.05).
The effects of S and S+B treatments on bacterial, actinomycete and fungal populations in Trial 3 and 4 exhibited the same pattern as those of B treatment in 1 and Trial 2 (Fig 4B–4D and 4F).
Microbial community diversity in soil
The DGGE gel profiles obtained with bacterial 16S rRNA and fungal ITS rRNA gene under different treatments were used to analyze microbial community diversity (S1 and S2 Figs). The analysis of Shannon-Weaver diversity and richness showed that the application of bioorganic fertilizer increased bacterial community diversity but decreased fungal community diversity in soil (Table 2). The application of bioorganic fertilizer led to higher values of Shannon-Weaver diversity and richness in bacterial community than the control and O treatments in Trials 1 and 2. The highest Shannon diversity and richness values of soil bacteria were observed in the S+B treatment, followed by the S treatment and lowest in the control (Table 2). Conversely, Shannon-Weaver diversity and richness of soil fungi community were lowest after bioorganic fertilizer application.
Table 2. The richness and Shannon-Weaver index (H) diversity of microbial community under different treatments.
| Bacterial | Fungi | |||||
|---|---|---|---|---|---|---|
| Richness | H | Richness | H | |||
| Test 1 | Trial 1 | CK | 44±2c | 3.77±0.04c | 49±2a | 3.82±0.04a |
| O | 53±1b | 3.96±0.02b | 49±1a | 3.82±0.02a | ||
| B | 66±2a | 4.17±0.03a | 41±2b | 3.62±0.03b | ||
| Trial 2 | CK | 38±1b | 3.63±0.02b | 51±2a | 3.86±0.05a | |
| O | 37±2b | 3.60±0.03b | 53±1a | 3.87±0.03a | ||
| B | 48±2a | 3.88±0.03a | 42±3b | 3.66±0.07b | ||
| Test 2 | Trial 3 | CK | 23±2c | 2.69±0.05c | 50±2a | 3.56±0.03a |
| S | 34±2b | 3.20±0.05b | 43±1b | 3.36±0.03b | ||
| S+B | 44±1a | 3.46±0.03a | 38±2c | 3.24±0.04c | ||
| Trial 4 | CK | 35±1b | 3.53±0.04b | 39±2a | 3.53±0.05a | |
| S | 35±2b | 3.53±0.05b | 39±3a | 3.55±0.07a | ||
| S+B | 57±3a | 3.99±0.05a | 23±2b | 2.99±0.05b | ||
Data were expressed as mean ± standard error (n = 4). The data within a column of each trial with a same letter did not differ significantly at Fisher’s least significant difference test (LSD) and Duncan’s significance level 0.05. CK: NPK fertilizer; O: NPK fertilizer + organic fertilizer; B: NPK fertilizer + bioorganic fertilizer; S: NPK fertilizer + soil disinfection; S+B: soil disinfection + NPK fertilizer + bioorganic fertilizer.
Association among R. solanacearum population, disease incidence and soil biochemical properties
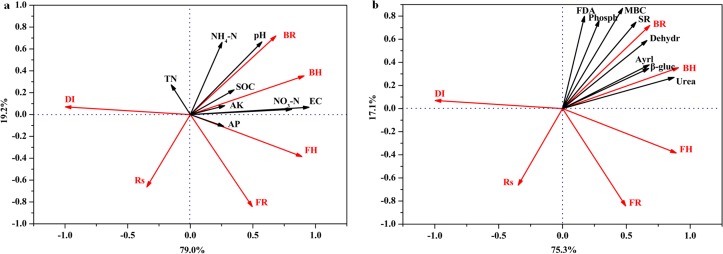
Associations of microbial diversity, R. solanacearum population and disease incidence with soil biochemical and with biological properties explained 98.2% and 92.4% variability of datasets, respectively (Fig 5A and 5B). There was a positive relationship between R. solanacearum population and disease incidence. Whereas bacterial community diversity was negatively related to the R. solanacearum population and disease incidence, R. solanacearum population correlated positively with the richness and diversity of fungal community. Soil pH value, EC value, SOC, NH4 +-N, NO3 —N and available K correlated positively but R. solanacearum population and disease incidence correlated negatively with bacterial community diversity. A close association also existed among soil microbial activities, microbial biomass and bacterial community diversity.
Fig 5. Association among level of bacterial wilt disease, microbial community diversity, soil physicochemical (a) and biological (b) properties.
Monte Carlo permutation tests carried out for all canonical axes confirmed the significance of the relationship between the two datasets (a: F = 4.07, P = 0.03; b: F = 8.67, P = 0.04). TN, total N; AP, available P; AK, available K; DI, tomato disease incidence; Rs, R. solanacearum population; BR, bacterial population richness; BH, microbial diversity of bacterial community; FR, fungal population richness; FH, microbial diversity of fungal community; Phosph, phosphomonoesterase activity; Aryl, arylsulfatase activity; β-gluco, β-D-glucosidase activity; Dehydro, dehydrogenase activity; Urea, urease activity; FDA, fluorescein diacetate hydrolase activity; MBC, microbial biomass carbon; SR, soil respiration.
Discussion
This present study examined the suppressive capacity of a bioorganic fertilizer on tomato bacterial wilt compared with other soil management methods across four seasons under field conditions. Our results demonstrated that the application of bioorganic fertilizer as a novel strategy provided the greatest ability to control bacterial wilt caused by R. solanacearum in tomato. The application of bioorganic fertilizer (B and B+S treatments) altered the microbial community structure, significantly decreased the population of R. solanacearum and showed the lowest disease incidence, which were consistent with the effect of bioorganic fertilizer on the suppressiveness against bacterial wilt of potato [15] and tobacco [10]. Furthermore, it was noted that there was a close association among disease incidence, R. solanacearum population and biochemical properties (Fig 5). These findings suggest that the enhancement of soil suppressiveness against bacterial wilt by the bioorganic fertilizer may be induced by multiple aspects, particularly the alteration of soil physicochemical and biological properties.
The application of bioorganic fertilizer improved soil physicochemical properties in all four trials (Table 1). The redundancy analysis (RDA) showed a close relationship between physicochemical properties and soil suppressiveness (Fig 5A). Previous studies showed that soil pH value [32], organic matter [33], NO3 –-N and NH4 +-N content [16] were negatively correlated with disease incidence. Michel and Mew [16] believed that ammonium toxicity of R. solanacearum was one mechanism of suppression. In addition, the application of organic amendments, which are rich in nitrogen, may reduce soil-borne diseases by allelochemicals generated during their storage or by subsequent microbial decomposition [34]. Furthermore, our results showed that soil pH value, EC value, SOC, NH4 +-N, NO3 —N and available K contents correlated positively with bacterial community diversity, which was negatively associated with R. solanacearum population and disease incidence. We suggest that the changes in soil properties by the bioorganic fertilizer strongly affect the interactions between pathogens and antagonistic populations, and contribute to the establishment of beneficial microbial community population, and subsequently influence the soil suppressiveness.
The application of bioorganic fertilizer alone or together with other amendments methods (B and S+B) significantly increased soil microbial activities (soil enzymes, FDA hydrolysis and soil respiration) and microbial biomass carbon (Table 1). Recently, Yin et al. [35] indicated that the severity level of upland rice seedling wilt negatively correlated with microbial biomass carbon and microbial activities in 19 soils from nine counties under various treatments. Conversely, Knudsen et al. [36] found no correlation between microbial activity and disease suppression of brown foot rot in soils from different sites under organic, integrated and conventional management. However, our present study clearly showed that microbial activities and microbial biomass were positively related with bacterial richness and diversity (H) (Shannon-Weaver index), but negatively with R. solanacearum population and tomato disease incidence (Fig 5B). Such a discrepancy between the studies could be due to the specific effect of different soil amendments on pathogens [37] or a stimulation of antagonists [38]. Moreover, a large biomass could create a competitive environment against the viability of pathogens in soils by organic amendments [39]. Increased microbial biomass in soil amended with compost was negatively associated with disease incidence or severity of Pythium root-rot in bulbous Iris [39]. We suggest that the decreased disease incidence was related to the poor competitive ability of the pathogens with native microorganisms in the soil amended with the bioorganic fertilizer.
The application of bioorganic fertilizer also altered microbial community structure as revealed by the plate count method (Fig 4) and PCR-DGGE profiles analysis (Table 2). Increased populations of bacteria and actinomycetes in soil are important to suppress soil-borne disease [40], whereas decrease of fungal community population might be beneficial for controlling soil disease, because most soil-borne crop diseases were caused by fungi, such as wilts, root rot, clubrot, and blight [41]. In addition, soil microbial diversity plays a key role in controlling bacterial pathogens [42]. Fargione and Tilman [43] reported that competition for nutrients with dominant microorganisms may limit invasion of pathogens in highly diverse communities. In the bioorganic fertilizer treatment (B and S+B), the diversity of bacterial community increased, but the fungal community decreased (Table 2), indicating that alteration of microbial diversity in soil with bioorganic fertilizer application promoted soil defensive capability against invasion of R. solanacearum. Our study further found that R. solanacearum population and disease incidence negatively related to the richness and diversity of bacterial community, but positively with fungal community richness (Fig 5). These results suggest that there was a close association between the improvement of soil suppressiveness and enhancement of microbial diversity in the bioorganic fertilizer treatment.
In our study, the application of bioorganic fertilizer significantly decreased population of R. solanacearum and thus inhibited the invasion of plant roots by the pathogens (Fig 1A and 1B). These results are consistent with the findings of Yuan et al. [10], showing a significantly lower population of R. solanacearum in the soils amended with bioorganic fertilizer compared to the control. The mechanism by which bioorganic fertilizer controlled tomato bacterial wilt is mainly through an antagonism by the antagonistic bacteria together with organic fertilizer, as a component of bioorganic fertilizer, suppressing R. solanacearum in the rhizosphere of tomato plants. In addition, the organic fertilizer provided antagonistic strains with additional carbon and nutrients to survive and hence to suppress R. solanacearum for a longer period of time (Fig 1A and 1B). Furthermore, where the bacterial wilt disease could be controlled efficiently, R. solanacearum populations were below 6.0 log cfu g-1 dry soil (Fig 2). Therefore, decreased R. solanacearum population in the soil is important for soil suppressiveness against the bacterial wilt.
The efficiency of the bioorganic fertilizer controlling tomato bacterial wilt was not consistent across the four trials, which may be resulted from different climatic conditions. The greater disease incidence in Trials 1 and 4 than Trials 2 and 3 (Figs 1C and 1D and 2) could be explained by unique epidemiology of R. solanacearum under different temperature regimes. Some studies reported that temperature fluctuation affected the viability of R. solanacearum [44]. Guo et al. [45] indicated that the bacterial wilt developed immediately and biocontrol efficiency decreased when the temperature was greater than 30°C. Other environmental factors, such as moisture may also cause the inconsistent controlling efficiency of biocontrol agents due to a lack of display of biocontrol traits (e.g., production of siderophores and antibiotics), which are regulated by genetics as well as many environmental factors [46]. We suggested that appropriate farming time with low temperature, low humidity and good ventilation should be considered to ensure the beneficial environmental conditions for tomato growth.
It was also noted that soil-borne pathogens could not be effectively controlled when a single management strategy was used, e.g. in the management of Fusarium wilt in banana [47]. Therefore, bioorganic fertilizer integrated with other agricultural measures may further decrease R. solanacearum population in soil and efficiently control the bacterial wilt. These agricultural measures include soil solarization [48], crop rotation [49], use of other amendments such as lime and calcium cyanamide with deep ploughing [50].
Conclusions
The use of bioorganic fertilizers is a promising alternative strategy to controlling bacterial wilt caused by R. solanacearum. An alteration in soil physicochemical and biological properties caused by the bioorganic fertilizer contributes to soil suppressiveness towards bacterial wilt. It is worth noting that the application of bioorganic fertilizer in appropriate farming conditions, such as low temperature, low humidity and good ventilation and integration with other agricultural methods should be considered to effectively suppress R. solanacearum survival. However, in our study, the specific microorganisms responsible for suppressing bacterial wilt in diverse soil microflora after bioorganic fertilizer application are unknown. Further research is needed to detect the specific microorganisms and to study their functions in improving soil suppressiveness.
Supporting Information
(TIF)
(TIF)
Acknowledgments
We thank Ningbo Haohai Luye Fruit and Vegetable Cooperatives for great help in tomato seedling plantation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the Chinese Ministry of Agriculture (201103004), the National Key Project on Science and Technology of China (2012BAC17B02) and the Project of Scientific Emissary of Zhejiang Province (2012T2T209). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Swanson JK, Yao J, Tans-Kersten J, Allen C. Behavior of Ralstonia solanacearum race 3 biovar 2 during latent and active infection of geranium. Phytopathol. 2005;95: 136–143. [DOI] [PubMed] [Google Scholar]
- 2. Rivard CL, O'Connell S, Peet MM, Welker RM, Louws FJ. Grafting Tomato to Manage Bacterial Wilt Caused by Ralstonia solanacearum in the Southeastern United States. Plant Dis. 2012;96: 973–978. [DOI] [PubMed] [Google Scholar]
- 3. Pradhanang P, Momol M, Olson S, Jones J. Effects of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis.2003;87: 423–427. [DOI] [PubMed] [Google Scholar]
- 4. Dalal N, Dalal S, Golliwar V, Khobragade R. Studies on grading and pre-packaging of some bacterial wilt resistant brinjal (Solanum melongena L.) varieties. J Soil Crop. 1999;9: 223–226. [Google Scholar]
- 5. King SR, Davis AR, Liu W, Levi A. Grafting for disease resistance. HortSci. 2008;43: 1673–1676. [Google Scholar]
- 6. Wang JF, Hanson P, Barnes JA. Worldwide evaluation of an international set of resistance sources to bacterial wilt in tomato In: Prior P., Allen C., Elphinstone J. (eds). Bacterial wilt disease: Molecular and ecological aspects. INRA Editions, Paris, France; 1998. pp. 269–275. [Google Scholar]
- 7. Tamietti G, Valentino D. Soil solarization as an ecological method for the control of Fusarium wilt of melon in Italy. Crop Prot. 2006;25: 389–397. [Google Scholar]
- 8. Mazzola M. Assessment and management of soil microbial community structure for disease suppression1 . Ann Rev Phytopathol. 2004;42: 35–59. [DOI] [PubMed] [Google Scholar]
- 9. Gorissen A, Van Overbeek L, Van Elsas J. Pig slurry reduces the survival of Ralstonia solanacearum biovar 2 in soil. Can J Microbiol. 2004;50: 587–593. [DOI] [PubMed] [Google Scholar]
- 10. Yuan S, Wang L, Wu K, Shi J, Wang M, Yang X, et al. Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol. 2014;75: 86–94. [Google Scholar]
- 11. Boukaew S, Chuenchit S, Petcharat V. Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili pepper. Biocontrol. 2011;56: 365–374. [Google Scholar]
- 12. Wei Z, Huang J, Tan S, Mei X, Shen Q, Xu Y. The congeneric strain Ralstonia pickettii QL-A6 of Ralstonia solanacearum as an effective as an effective biocontrol agent for bacterial wilt of tomato. Biocontrol. 2013; 65: 278–285. [Google Scholar]
- 13. Sullivan P (2001) Sustainable management of soil-borne plant diseases ATTRA,USDA’s Rural Business Cooperative Service, Available: https://www.attra.org. [Google Scholar]
- 14. Padel S, Röcklinsberg H, Schmid O. The implementation of organic principles and values in the European Regulation for organic food. Food Policy. 2009;34: 245–251. [Google Scholar]
- 15. Ding C, Shen Q, Zhang R, Chen W. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil. 2013;366: 453–466. [Google Scholar]
- 16. Michel VV, Mew T. Effect of a soil amendment on the survival of Ralstonia solanacearum in different soils. Phytopathol. 1998;88: 300–305. [DOI] [PubMed] [Google Scholar]
- 17. Nelson D, Sommers L. Total carbon, organic carbon, and organic matter In: Page A.L., Miller R.H., Keeney D.R. (Eds.), Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Soil Science Society of America, Madison, WI, USA; 1982. pp. 539–594. [Google Scholar]
- 18. Bremner J, Breitenbeck GA. A simple method for determination of ammonium in semimicro‐Kjeldahl analysis of soils and plant materials using a block digester. Commun Soil Sci Plant Anal. 1983;14: 905–913. [Google Scholar]
- 19. Kuo S. Phosphorous In: Sparks D.L. (Ed.), Methods of Soil Analysis: Part 3.Chemical Methods. Soil Science Society of America Book Series, No. 5. Soil Science Society of America, Madison, WI, USA; 1996. pp. 869–919. [Google Scholar]
- 20. Helmke PA, Sparks DL. In: Bigham JM (ed) Methods of soil analysis: Part 3 Chemical methods. Soil Science Society of America, American Society of Agronomy, Madison; 1996. pp. 551–574. [Google Scholar]
- 21.Tabatabai M. Soil enzymes. In: RW Weaver, JS Angle, Bottomley, D Bezdicek, S Smith, A Tabatabai, A Wollum (eds) Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; 1994. pp. 775–833.
- 22. Tabatabai M, Bremner J. Assay of urease activity in soils. Soil Biol Biochem. 1972; 4: 479–487. [Google Scholar]
- 23. Adam G, Duncan H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem. 2001;33: 943–951. [Google Scholar]
- 24. Gong W, Yan X, Wang J, Hu T, Gong Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat-maize cropping system in northern China. Geoderma. 2009;149: 318–324. [Google Scholar]
- 25. Vance E, Brookes P, Jenkinson D. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987;19: 703–707. [Google Scholar]
- 26. Wollum AG. Cultural methods for soil microorganisms In: Page A L, et al. (Eds.). Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed ASA, Madison, WI, USA; 1982. pp. 781–802. [Google Scholar]
- 27. French EB, Aley P, Elphinstone J. Culture media for Ralstonia solanacearum isolation, identification and maintenance. Fitopatologia. 1995;30: 126–130. [Google Scholar]
- 28. Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microb.1993;59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2: 113–118. [DOI] [PubMed] [Google Scholar]
- 30. White T, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (Eds.) PCR Protocols: A Guide to Methods and applications Academic Press, New York; 1990. pp. 315–322. [Google Scholar]
- 31. Shannon C. The mathematical theory of communication University of Illinois Press, Urbana (USA); 1963. [Google Scholar]
- 32. Höper H, Alabouvette C. Importance of physical and chemical soil properties in the suppressiveness of soils to plant diseases. Eur J Soil Biol. 1996;32: 41–58. [Google Scholar]
- 33. Davis J, Huisman O, Everson D, Schneider A. Verticillium wilt of potato: a model of key factors related to disease severity and tuber yield in southeastern Idaho. Am J Potato Res. 2001;78: 291–300. [Google Scholar]
- 34. Bailey K, Lazarovits G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Till Res. 2003;72: 169–180. [Google Scholar]
- 35. Yin S, Dong Y, Xu Y, Huang Q, Shen Q. Upland rice seedling wilt and microbial biomass and enzyme activities of compost-treated soils. Biol Fertil Soil. 2001;47: 303–313. [Google Scholar]
- 36. Knudsen IMB, Debosz K, Hockenhull J, Jensen DF, Elmholt S. Suppressiveness of organically and conventionally managed soils towards brown foot rot of barley. Appl Soil Ecol. 1990;12: 61–72. [Google Scholar]
- 37. Pankhurst C, Hawke B, McDonald H, Kirby C, Buckerfield JC, Michelsen P, et al. Evaluation of soil biological properties as potential bioindicators of soil health. Aust J Exp Agric. 1995;35: 1015–1028. [Google Scholar]
- 38. Chet I, Hadar Y, Elad Y, Katan J, Henis Y. Biological control of soil-borne plant pathogens by Trichoderma harzianum Soil-borne plant pathogens, Academic Press, London; 1979. pp. 585–591. [Google Scholar]
- 39. Van Os G, Van Ginkel J. Suppression of Pythium root rot in bulbous Iris in relation to biomass and activity of the soil microflora. Soil Biol Biochem. 2001;33: 1447–1454. [Google Scholar]
- 40. Boulter JI, Trevors JT, Boland GJ. Microbial studies of compost: bacterial identification, and their potential for turfgrass pathogen suppression. World J Microbiol Biotechnol. 2002;18: 661–671. [Google Scholar]
- 41. Brussaard L, De Ruiter PC, Brown GG. Soil biodiversity for agricultural sustainability. Agric Ecosyst Environ. 2007;121: 233–24. [Google Scholar]
- 42. van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A. 2012;109: 1159–1164. 10.1073/pnas.1109326109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fargione JE, Tilman D. Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett. 2005;8: 604–611. [Google Scholar]
- 44. Scherf JM, Milling A, Allen C. Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum Race 3, Biovar 2, in infected geranium, tomato, and potato plants. Appl Environ Microb. 2010;76: 7061–7067. 10.1128/AEM.01580-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo J, Qi H, Guo Y, Ge H, Gong L, Zhang L, et al. Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biocontrol. 2004;29: 66–72. [Google Scholar]
- 46. Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3: 307–319. [DOI] [PubMed] [Google Scholar]
- 47. Akila R, Rajendran L, Harish S, Saveetha K, Raguchander T. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biocontrol. 2011;57: 175–183. [Google Scholar]
- 48. Yilmaz S, Celik I, Zengin S. Combining effects of soil solarization and grafting on plant yield and soil-borne pathogens in cucumber. Int J Plant Prod. 2011;5: 95–104. [Google Scholar]
- 49. Ghorbani R, Wilcockson S, Koocheki A, Leifert C. Soil management for sustainable crop disease control: a review. Environ Chem Lett. 2008;6: 149–162. [Google Scholar]
- 50. Shi K, Wang L, Zhou Y, Yu Y, Yu J. Effects of calcium cyanamide on soil microbial communities and Fusarium oxysporum f. sp. Cucumberinum .Chemosphere. 2009;75: 872–877. 10.1016/j.chemosphere.2009.01.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.