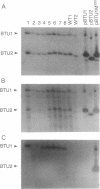
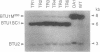
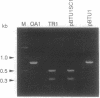
Abstract
Replacement of lysine-350 by methionine in the beta-tubulin gene of Chlamydomonas confers resistance to microtubule-depolymerizing drugs and increased sensitivity to the microtubule-stabilizing drug taxol. This mutation was created in cloned BTU1, one of two coexpressed beta-tublin genes of Tetrahymena thermophila. When introduced by electroporation, the mutated gene transformed Tetrahymena exclusively by gene replacement at the homologous locus. Taxol-sensitive transformants could be retransformed with a wild-type gene and selection for taxol resistance. Analyses of phenotypic assortment and of the mRNA in transformed cells suggest that complete replacement of the BTU1 gene in the polyploid macronucleus can be obtained. These studies demonstrate the utility of this marker for studying tublin gene function and show that electroporation allows facile gene replacement in Tetrahymena.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Barahona I., Soares H., Cyrne L., Penque D., Denoulet P., Rodrigues-Pousada C. Sequence of one alpha- and two beta-tubulin genes of Tetrahymena pyriformis. Structural and functional relationships with other eukaryotic tubulin genes. J Mol Biol. 1988 Aug 5;202(3):365–382. doi: 10.1016/0022-2836(88)90271-9. [DOI] [PubMed] [Google Scholar]
- Bolduc C., Lee V. D., Huang B. Beta-tubulin mutants of the unicellular green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1988 Jan;85(1):131–135. doi: 10.1073/pnas.85.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Deak J. C., Lief J. H. Rate of phenotypic assortment in Tetrahymena thermophila. Dev Genet. 1992;13(2):126–132. doi: 10.1002/dvg.1020130206. [DOI] [PubMed] [Google Scholar]
- Ersfeld K., Wehland J., Plessmann U., Dodemont H., Gerke V., Weber K. Characterization of the tubulin-tyrosine ligase. J Cell Biol. 1993 Feb;120(3):725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaertig J., Gorovsky M. A. Efficient mass transformation of Tetrahymena thermophila by electroporation of conjugants. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9196–9200. doi: 10.1073/pnas.89.19.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Thatcher T. H., McGrath K. E., Callahan R. C., Gorovsky M. A. Perspectives on tubulin isotype function and evolution based on the observation that Tetrahymena thermophila microtubules contain a single alpha- and beta-tubulin. Cell Motil Cytoskeleton. 1993;25(3):243–253. doi: 10.1002/cm.970250305. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A. Genome organization and reorganization in Tetrahymena. Annu Rev Genet. 1980;14:203–239. doi: 10.1146/annurev.ge.14.120180.001223. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Johnson K. A. Preparation and properties of dynein from Tetrahymena cilia. Methods Enzymol. 1986;134:306–317. doi: 10.1016/0076-6879(86)34098-9. [DOI] [PubMed] [Google Scholar]
- Kahn R. W., Andersen B. H., Brunk C. F. Transformation of Tetrahymena thermophila by microinjection of a foreign gene. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9295–9299. doi: 10.1073/pnas.90.20.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. D., Huang B. Missense mutations at lysine 350 in beta 2-tubulin confer altered sensitivity to microtubule inhibitors in Chlamydomonas. Plant Cell. 1990 Nov;2(11):1051–1057. doi: 10.1105/tpc.2.11.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña R. F., Banerjee A., Khan I. A. Tubulin structure and biochemistry. Curr Opin Cell Biol. 1992 Feb;4(1):53–57. doi: 10.1016/0955-0674(92)90058-k. [DOI] [PubMed] [Google Scholar]
- Orias E., Bruns P. J. Induction and isolation of mutants in Tetrahymena. Methods Cell Biol. 1976;13:247–282. [PubMed] [Google Scholar]
- Schibler M. J., Huang B. The colR4 and colR15 beta-tubulin mutations in Chlamydomonas reinhardtii confer altered sensitivities to microtubule inhibitors and herbicides by enhancing microtubule stability. J Cell Biol. 1991 May;113(3):605–614. doi: 10.1083/jcb.113.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprenant K. A., Hays E., LeCluyse E., Dentler W. L. Multiple forms of tubulin in the cilia and cytoplasm of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6908–6912. doi: 10.1073/pnas.82.20.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Transformation of Tetrahymena to cycloheximide resistance with a ribosomal protein gene through sequence replacement. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9493–9497. doi: 10.1073/pnas.88.21.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]