Abstract
The current Ebola outbreak in West Africa has already caused substantial mortality and dire human and economic consequences. It continues to represent an alarming public health threat in the region and beyond and jeopardizes the provision of health care and other services in the affected countries. The scale of the epidemic has accelerated research efforts for diagnostics, treatment, and prevention galvanized through increased availability of funding. Our knowledge relating to the virus, disease pathogenesis, risk factors, dynamics of transmission, and epidemic control is increasing, and sociocultural factors have emerged as critical determinants for the success and failure of control efforts. However, there is a long way to go. In this review we summarize the current knowledge, examine the sociocultural context in West Africa, and outline priority areas for future research.
Key words: Ebola virus disease, viral hemorrhagic fever, containment, holistic, West Africa
Abbreviations used: CFR, Case/fatality ratio; CMV, Cytomegalovirus; EV, Ebola virus; EVD, Ebola virus disease; RCT, Randomized controlled trial; VP, Viral protein; WHO, World Health Organization
Discuss this article on the JACI Journal Club blog: www.jaci-online.blogspot.com.
In March 2014, a new outbreak of Ebola virus (EV) was identified in West Africa.1 Cases were first detected on the border of Guinea Conakry with Sierra Leone and Liberia. Subsequently, transmission became intense in these 3 countries, and by the end of January 2015, it is still ongoing. Small numbers of cases linked to the outbreak have also been identified in neighboring countries, such as Senegal (n = 1), Nigeria (n = 20), and Mali (n = 9). Cases have been exported to other countries outside Africa, and person-to-person transmission occurred in Spain (n = 1) and the United States (n = 2). Simultaneously, a small unrelated EV outbreak took place in the Democratic Republic of Congo between July and October 2014.2 By the end of January 2015, the outbreak in West Africa had accounted for more than 22,000 cases and 8,800 deaths.3 Although transmission seems to have slowed down in Liberia and Guinea Conakry, more than 50 new cases are still reported daily in Sierra Leone.3
The current outbreak has unpredictable economic consequences for the 3 deeply affected countries (Guinea Conakry, Sierra Leone, and Liberia) and the region of West Africa as a whole. Even in neighboring countries within the region, where few or no cases have been reported, tourism (an important source of income) has been heavily hampered while facilities for potential Ebola cases have been prepared.
A serious shortage of timely resources in the region is one of the key factors responsible for the disproportionate scale of the ongoing epidemic in West Africa. Although international response eventually occurred, it only arose when the epidemic was already out of control and had been considered an international public health threat.4 An additional trigger for the international response was the appearance of cases in the United States and Europe. Suddenly, it became obvious that Ebola poses an urgent threat not only to West Africa but also to the international community at large.
This review aims to summarize the current scientific knowledge relating to host and pathogen, to analyze drivers of the current epidemic, and to discuss potential mitigation strategies within their ethical and societal context.
Epidemiology of Ebola outbreaks in Africa
The first human EV outbreak occurred in Zaire (now the Democratic Republic of Congo) in 19765 and was named after the nearby Ebola River. The same year, a similar outbreak with a different EV species occurred in Sudan.6 Since 1976, more than 25 known outbreaks of EV have occurred in Africa, and 5 different EV species have been identified. Currently, EV hemorrhagic fever remains a plague for the population of equatorial Africa, with an increase in the numbers of outbreaks and cases since 2000.7
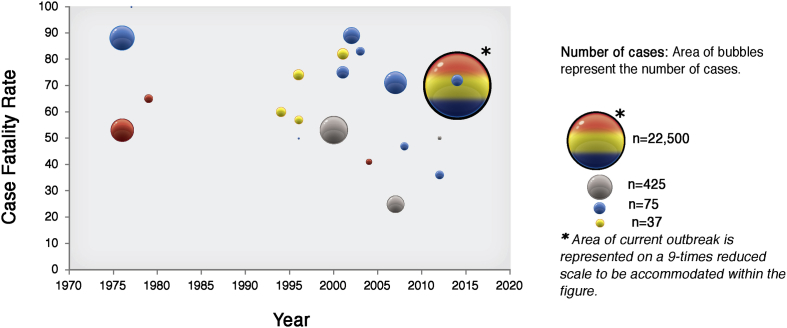
The current EV outbreak in West Africa is the largest ever recorded given the number of affected persons, countries involved, and longest persistent transmission (Fig 1). The previous largest outbreak occurred in Uganda in 2000 and involved 425 persons, less than 2% of the affected subjects in the current outbreak.7 Past outbreaks were confined to relatively rural and isolated areas in Central Africa without spreading to urban sectors, which facilitated the effective intervention of control measures.5 The delay in identifying the outbreak in urban settings in the current outbreak hindered the timely and effective implementation of control efforts in the region.
Fig 1.
Major Ebola outbreaks occurring in Africa from 1976 to 2014. Areas of bubbles represent numbers of cases. Colors of bubbles represent countries: blue, Zaire/Congo; red, Sudan; yellow, Gabon; gray, Uganda; multicolored, current outbreak with several countries involved.
Other clinical and epidemiologic characteristics are similar between past and ongoing EV epidemics. Mortality in patients with EV is very high, with case/fatality ratios (CFRs) that range from 41% to 89% depending on the virus strain.8–11 Although initial numbers seemed to indicate that the CFR in the current epidemic was lower than in previous outbreaks, initial calculations did not account for delays between disease onset and final outcome,9 and the sheer number of cases led to underestimates of the overall mortality. Currently, the CFR among all patients from whom a definitive outcome is recorded is 72%, and it is slightly less for hospitalized patients (CFR, 60%).12 The incubation period has also been similar between outbreaks, ranging from 2 to 21 days.8,13
Several mathematic models have attempted to compare the average number of secondary infections per case (R0) in the past and recent outbreaks. No significant differences in R0 at the start of epidemics have been observed, ranging between 1.35 and 2.22.8,11,13–15 Major limitations of these models are that they use different assumptions and rely on available data where accuracy might be poor under epidemic conditions and can vary between outbreaks and countries.
Characteristics of EV
The genus Ebola are nonsegmented, negative-sense, single-stranded RNA viruses of the Filoviridae family, which is coined from the Latin word “filum,” meaning thread-like. The viral particles form varying shapes of filaments with the RNA molecule encapsulated in a lipid membrane, allowing formation of new particles on the surfaces of their host cells.16,17
Five subtypes of EV are known: Zaire, Sudan, Bundibugyo, Tai Forest (formerly known as Côte d'Ivoire), and Reston. Each subtype was named after the site where it was first isolated. Since 1976, when EV was first described, the first 3 subtypes have been responsible for large outbreaks in Africa, with the Zaire strain causing the most fatalities. The Reston subtype is largely localized to the Western Pacific region. Despite being highly pathogenic in nonhuman primates, it has not been reported to cause illness in human subjects.18
Apart from EV, another member of the Filoviridae family is Marburg virus, which is named after the city in Germany where it was first discovered. EV and Marburg viruses share genome organization and replication mechanisms with rhabdoviruses and paramyxoviruses.16
Molecular structure of EV
The genome of EV consists of a single-stranded RNA approximately 19,000 nucleotides long. The Ebola genome has 7 known nucleotide sequences that code for structural and nonstructural proteins also known as viral proteins (VPs). The core of the virus is made up of RNA genomic molecules comprised of nucleoprotein.19 There are several types of VPs, each with a different function. VP30 plays an important role in RNA transcription activation, which is strongly dependent on the concentration of VP30. VP24, which is the primary matrix protein, is also the most abundant virion component. Its role is unclear. VP35 plays an important role in viral RNA synthesis. It acts as a type of interferon antagonist. There is a very strong possibility that the potency of VP35 could account for the varying degrees of virulence among different strains of EV.20 VP40 is a matrix protein from the negative strand of RNA. It mainly participates in the assembly of lipid-enveloped viruses by providing a link between the surrounding membrane and the nucleocapsid structure. The protein, also known as single-surface transmembrane glycoprotein, forms spikes on virions and plays an important role in viral entry into cells by mediating receptor binding, fusion, and entry into the target cell.19
Immunosuppression caused by EV is largely attributed to a section of the glycoprotein (G1 and G2) that shares a striking homology with another immunosuppressive protein found in oncogenic retroviruses.20 This particular sequence is thought to aid EV in evading the human immune responses in addition to suppressing MHC. The L protein, an RNA-dependent RNA polymerase, plays an essential role in catalyzing transcription.19,21–23
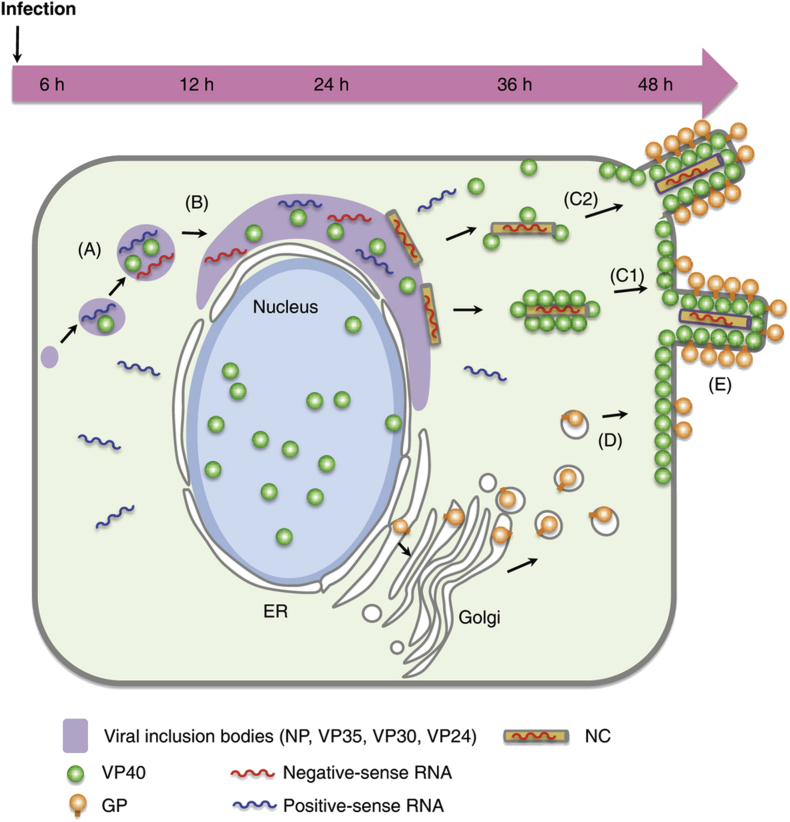
The process of replication and transcription of the viral genome is activated by the VPs that contain the nucleoprotein.24,25 When compared with Marburg virus, the N-terminal region of nucleoprotein defines the inner diameter of the EV nucleocapside, whereas the RNA genome defines its length.26 The details of viral assembly are summarized in Fig 2.
Fig 2.
Viral assembly model of Ebola virus. Viral mRNA transcribed from genomic negative-sense RNA is released into the cytoplasm, where VPs are translated. Nucleoprotein, together with VP35, VP40, VP30, and VP24, forms small inclusions (A), which become larger near the nucleus (B). At the edge of the inclusion bodies, the nucleocapsid (NC) is formed. VP40 associates with the NC, contributing to its transport to the plasma membrane (C1). Alternatively, nucleocapside initially associates with a few VP40 molecules and then moves to the plasma membrane, where it is enveloped with membrane-associated VP40 (C2). Synthesized glycoprotein (GP) is independently transported to the plasma membrane (D). The viral components then assemble, and the progeny virions bud (E). ER, Endoplasmic reticulum. Reprinted with permission from Nanbo et al.21
Transmission and transmission dynamics
Ebola hemorrhagic fever is a classic zoonosis with persistence of EV in a reservoir species thought to be rodents and bats.27 Bats are present in large numbers at the sites of several outbreaks and are known to maintain other pathogenic RNA viruses, such as rabies. EV antibodies have been measured in fruit bats.28 However, the virus has never been isolated from these animals. It is supposed that EV might persist as an asymptomatic or subclinical infection in the reservoir species, with little or no transmission, and is only activated by appropriated stimulus (ie, stress and coinfection).29,30 Apes, humans, and other mammalian species that are susceptible to EV infection are regarded as end hosts.29
An outbreak of Ebola virus disease (EVD) typically begins through human contact with an infected animal.30 These transmissions might be an infrequent event, probably also underreported, given the restricted contact with the reservoir species.29 Once the first human is infected, the disease spreads to other human subjects through direct contact with blood and body fluids of sick patients or persons who have died from EVD.
The most infectious body fluids are blood, feces, and vomit,31 although other fluids, such as urine, saliva, breast milk, semen, and, theoretically, sweat, can also contribute to transmission because EV has been isolated from these fluids. The likelihood of transmission depends on the type of exposure and the viral load. Therefore transmission is unlikely during the incubation period,32 and the risk is highest during contact with very sick patients and dead bodies. Health care workers caring for patients with EVD are at high risk of infection if they do not use appropriate protective measures. Funerals have also played a significant role in the spread of infection in many EV outbreaks.32–34
Other routes of transmission might include contact with contaminated surfaces or objects because the virus can persist for hours or days.35 There is no evidence that EV transmission can spread from human to human through the respiratory route.35
Pathogenesis
Most insights into the pathogenesis of Ebola stem from animal data because the traditionally remote location of the outbreaks and the varying nature of the associated emergency responses and cautious handling of highly contagious specimens in high-containment laboratories make in-depth immunopathogenesis studies in human subjects very difficult.
Serosurveys conducted in the region suggest that EV has been endemic in equatorial Africa at least during the last decades36 and specifically in West Africa for about a decade.37 Therefore it is likely that asymptomatic infections can occur in some subjects.38 However, the factors determining the spectrum and range of clinical symptoms from mild to severe manifestations are not well understood at present. Larger seroprevalence surveys in the affected populations and detailed host studies during the time of an epidemic would be mandatory to understand factors influencing susceptibility and possibly protection.
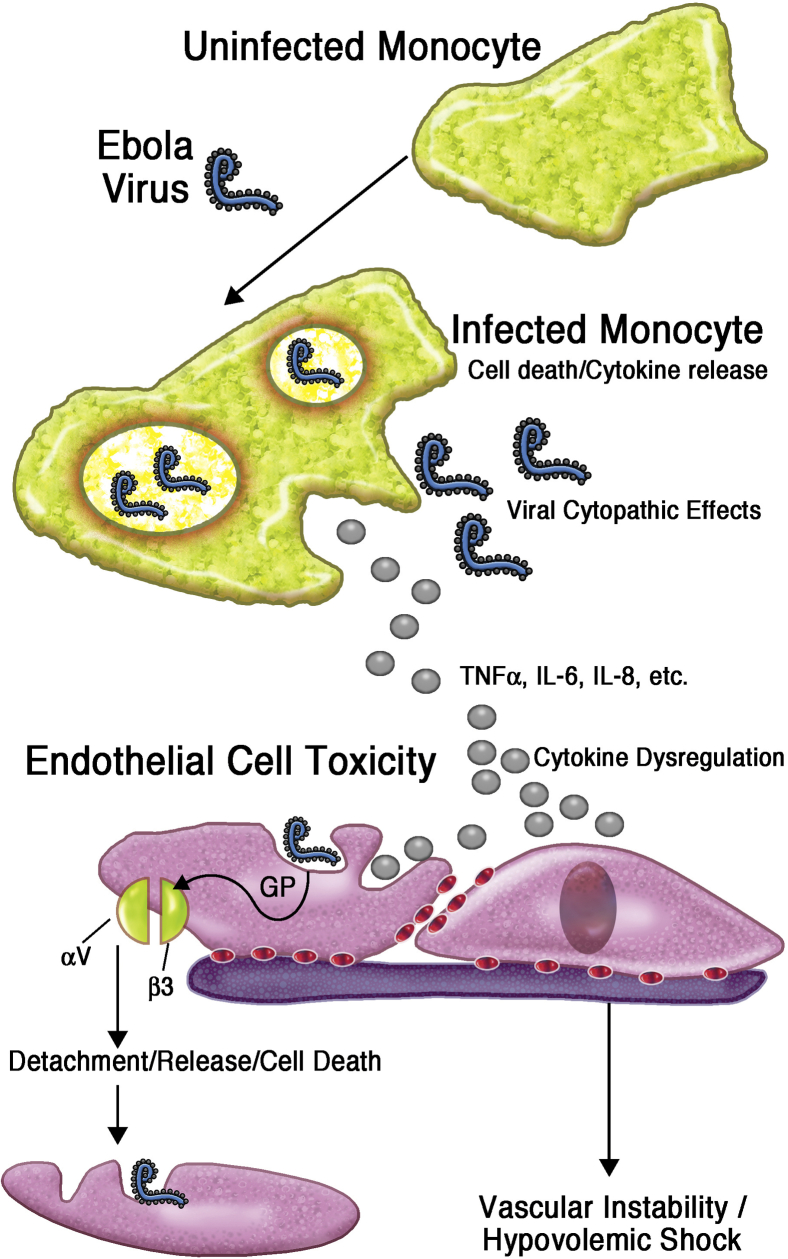
It is well established that the virus enters through mucous membranes or cuts and abrasions in contact with infected materials, such as blood, sweat, urine, and other secretions. Ingestion of contaminated food with high viral titers might also play a role.18 EV can infect a wide range of cell types, with a preference for rapid replication in monocytes, macrophages, and dendritic cells,39 from which the infection spreads through the lymphatic system and hematogenously. Although not a direct target of infection, large numbers of lymphocytes apoptose and release soluble factors, triggering the inflammatory cascade and causing damage to the endothelial system.40,41 The exact mechanisms causing the substantial epithelial damage leading to disseminated intravascular coagulopathy and hypotensive shock are incompletely understood. EV replication and ongoing infection lead to extensive necrosis of adrenocortical cells, with a subsequent effect on steroid synthesis, blood pressure regulation, and loss of sodium, resulting in hypovolemic shock.42
Coagulopathy is a key feature of advanced Ebola disease and is induced by the strong proinflammatory response from monocytes and macrophages, as well as possibly deficiencies in production of liver-derived clotting factors because of liver cell necrosis. The immune mechanisms involved are illustrated in more detail in Fig 3.
Fig 3.
Pathogenesis of Ebola at the cellular level. GP, Glycoprotein.
The infection of antigen-presenting cells with EV triggers a strong inflammatory response, and clinical outcome depends on the extent of the host's counterregulatory response. An increase in nitric oxide levels has been associated with poor outcome.43 According to data from Uganda, HLA alleles B*67 and B*15 were associated with fatal outcomes, whereas B*07 and B*14 were associated with nonfatal outcomes, but this has not been confirmed in West Africa at present.44
Diagnosis
After a median incubation period of 2 to 10 days, the abrupt onset of Ebola hemorrhagic fever is characterized by fever, myalgias, chills, and malaise. The initial diagnosis is often made on clinical grounds in the context of a history of exposure. EV infection is then confirmed in national or international reference laboratories by identifying the viral genome in the blood of patients by using RT-PCR techniques or viral detection ELISAs.45,46 The host response can be measured as the IgM and later IgG antibody response by using ELISA techniques.
In nonfatal cases the increase in antibody titers around day 6 to 11 accompanies the recovery. The development of antigen-specific IgM and then IgG is accompanied by strong inflammatory responses, including TNF-α and IL-6 production. Whether these are markers of disease activity or have protective potential remains to be established. IgG antibodies are known to persist for many years after infection.47,48 Although highly accurate, the currently used diagnostic tests have significant logistic challenges, including the requirements for high-level laboratory biosafety and staff with expertise in using sophisticated machines.
Once in the laboratory, each analysis takes between 2 and 6 hours, and the costs of around US$100 per sample are difficult to meet in resource-constrained West African settings, thus severely limiting testing capacity. Lost time means that infected persons might remain in the community, with a severe risk of unknowingly transmitting the virus to others. Moreover, in the absence of rapid laboratory support, persons with other common infectious diseases, such as malaria or dengue, and similar early symptoms might be unjustifiably held in an Ebola “transit” center as a precautionary measure and are thus at risk of contracting Ebola.
In October 2014, the World Health Organization (WHO) launched an initiative to stimulate diagnostic innovations to bring novel accurate tests to the point-of-care level. Decreasing the turnaround time for diagnosis through such assays would allow triaging and treating appropriate patients as swiftly as possible. The 2 key initiatives aim to rapidly solicit products that meet a prespecified “ideal profile” of the next generation of diagnostic tests and to provide a rapid review process for assessing a diagnostic test's quality, safety, and performance.49
Patients with severe disease and often fatal outcomes progress to multisystem involvement, including gastrointestinal (vomiting, diarrhea) and pulmonary symptoms, such as breathlessness and cough. A maculopapular rash has been reported around day 5 to 7 of the illness. The most feared symptoms of widespread hemorrhages from various mucous membranes and disseminated intravascular coagulopathy accompany the devastating multiorgan failure at the peak of viremia and ultimately lead to the death of the infected patient from hypovolemic shock unless comprehensive supportive therapy can be offered in time.50,51
Abnormal laboratory parameters include leukopenia and lymphopenia, increased liver and pancreatic enzyme levels, abnormal clotting results, and renal failure, all of which are indicative of a severe multisystem disorder with a high rate of fatality.52
Death rates have varied between 40% and 80% at various times during the current epidemic50,51 and might be more reflective of the available support measures than differences in host susceptibility or acquisition of immunity on a larger scale. Delay in diagnosis, presentation at an already advanced stage of disease, and absence of treatment centers were clearly associated with higher death rates than have been reported lately. However, the more recent reports reflect primarily treatment center–associated deaths, whereas there are less survival data from more rural areas, which might not yet have received the same level of support. However, judging by previous epidemics and current observations, it is practically certain that differences in host susceptibility and response to infection and therapy exist, which, if better defined through research conducted in the context of the outbreak situation, might help to develop prognostic markers and tailored interventions.
Time of presentation, viral load, general state of the patient by the time of arrival at a treatment center, and possibly age and comorbidities can all influence the ultimate outcome of the disease in a subject.
Therapeutic interventions
The current recommendations for treatment of Ebola comprise the administration of sufficient fluids (oral or intravenously) to maintain circulatory stability, exclusion or treatment of malaria, and administration of broad-spectrum antibiotics to treat potential concomitant bacterial infections, antipyretics, and analgesia.53
No results from randomized controlled trials (RCTs) comparing different interventions and protocols are available at this point in time. It is widely acknowledged that supportive care has differed between sites and patient populations, varying from basic oral rehydration to intravenous fluid resuscitation. Whether the routine use of antibiotics makes a significant contribution to outcome is unclear. The role of oral potassium supplementation in the absence of close electrolyte monitoring and use of antidiarrheal agents also remains to be established.
The pressure on materials, facilities, and skilled personnel in the endemic areas has limited the supportive care that can be provided in this emergency situation when compared with the care delivered to personnel with Ebola infection who were expatriated to resource-rich settings in Europe and North America. Not surprisingly, death rates in the context of modern intensive care have been much lower, as long as the patients arrived at earlier stages of the disease. Beyond these very basic supportive therapies, some experimental treatments have been used on some patients with various success.54
By December 2014, several hundred patients were expected to take part in 3 separate trials in early 2015 conducted at Médecins Sans Frontières–run Ebola treatment centers in Guinea and Liberia. The different objectives are to test the administration of antibodies from the blood of Ebola survivors to neutralize EV in the patients (passive immunization) or to interfere with transcription and replication of the EV by using antivirals, which have shown promise in other viral diseases.
The 2 leading antiviral candidates are brincidofovir (Chimerix, Durham, NC) and favipiravir (Fujifilm, Toyama Chemical, Tokyo, Japan). Brincidofovir is currently in phase III clinical trials for use in human subjects against cytomegalovirus (CMV) and adenovirus after successful testing for safety in more than 1000 human subjects54 and has received US Food and Drug Administration Fast Track Designation for treatment of CMV, adenovirus, and smallpox.55 On October 6, 2014, Chimerix received a US Food and Drug Administration authorization for emergency investigational new drug applications of brincidofovir for the treatment of EVD, but of late, the company has decided to focus their efforts on its use in CMV and adenovirus infections.
Favipiravir is an experimental antiviral drug being developed by Toyama Chemical of Japan with activity against many RNA viruses, including influenza.56,57 At the time of writing, clinical trials of this drug have started in Guinea, with more than 100 patients already enrolled.
Further therapeutic interventions could also relate to products for correcting the coagulopathy seen in patients with severe Ebola disease,58 but no clinical trials are announced at present.
Prioritized use of experimental drugs in the face of limited supply
Because of the life-threatening situation during EV outbreaks and the absence of known treatments, experimental therapies are being deployed for compassionate use. In line with the principles of reciprocity, health workers infected with EV are currently given higher consideration to access the scarce drugs. Although understandable, this practice can promote distrust in health care systems and potentially undermine the social value of clinical research involving the experimental therapies. Therefore it remains critical that fair selection of beneficiaries of the unproved interventions should be transparently and consistently practiced.59
Vaccines
At the time of writing, there was no licensed vaccine against EV, although clinical trials have now commenced in the United States, Europe, and West Africa, and preliminary results on safety and immunogenicity are becoming available. Despite existence of promising vaccine candidates, the low incidence and sporadic nature of outbreaks of Ebola, largely limited to a few countries in Central and East Africa, have discouraged pharmaceutical companies from making huge investments into their development and testing.60 This situation has dramatically changed with the unprecedented outbreak in West Africa.61 The sheer scale of the global health concerns has finally energized a consortium of pharmaceutical companies, researchers, and funders to prioritize the clinical development of Ebola vaccines. The first goal of this concerted effort is to protect the frontline health workers who are at increased risk of infections and death when providing care for patients.59 This is ultimately planned to be extended to the affected countries in an exceptionally fast-tracked process in which vaccine development, testing, and licensure take place in parallel. Because speed is the goal for Ebola vaccine development and rollout, a number of challenges need to be pragmatically addressed to achieve this lofty objective. Table I62 shows an updated profile of 2 leading candidate Ebola vaccines and others in development.
Table I.
Profile of ongoing and anticipated candidate Ebola vaccines trial
| Vaccine type | Manufacturers | Vaccination approach/target | Progress (including success in animal studies) | Future plans | Issues and concerns |
|---|---|---|---|---|---|
| Current trials on candidate Ebola vaccines | |||||
| ChAd3-ZEBOV: Chimpanzee adenovirus serotype 3 encoding the monovalent Zaire strain of EV glycoprotein | GlaxoSmithKline, Research Triangle Park, NC; National Institutes of Health, Bethesda, Md | Pre-exposure for frontline health workers | Phase I trials are nearing completion in the United Kingdom and Mali. | Parallel phase II/III trials are planned to start in Liberia or Sierra Leone in early 2015. | ChAd3 are genetically modified vectors with biosafety level 2 status. The vaccine is administered intravenously and requires storage at −80°C. |
| ChAd3-EBO: Chimpanzee adenovirus serotype 3 encoding bivalent Sudan and Zaire EV glycoproteins | GlaxoSmithKline, National Institutes of Health | Pre-exposure for frontline health workers | Preliminary findings of phase I trial in the United States showed promising safety and immunogenic profiles.62 | Expanded phase I/Ib trials for dose selection, efficacy evaluation, and MVA-EBO booster regimen are planned for 2015. | Current epidemic in West Africa is caused by the Zaire strain, limiting possible deployment in the event of proved efficacy. |
| rVSV-EBOV: Attenuated version of recombinant vesicular stomatitis virus expressing EV glycoprotein | NewLink Genetics, Ames, Iowa (Public Health Agency of Canada's National Microbiology Laboratory [NML]). | Postexposure | Has demonstrated efficacy in rodents and nonhuman primates; however, it currently trails behind ChAd3-ZEBOV in phase I trials. Report of arthralgia among volunteers in a Swiss trial led to a temporary halt. | Concurrent efficacy trials along with ChAd3-ZEBOV in worst-hit countries | Efficacy is likely to depend on filovirus species and early commencement of intervention after exposure. |
| Anticipated Ebola vaccine trials | |||||
| Ad 25, Ad 35, MVA candidates: Heterologous prime-boost approach using human adenoviruses 25 and 35 and MVA vectors | Johnson & Johnson, New Brunswick, NJ; Bavarian Nordic, Kvistgaard, Denmark | Pre-exposure | Has shown demonstrated efficacy in rodents and nonhuman primates. Phase I trials are planned for early 2015. | Will depend on phase I data | High pre-existing antibody against human adenovirus vectors |
| Recombinant protein–Ebola glycoprotein | Protein Sciences | Pre-exposure | Has demonstrated efficacy in rodents. Human trials are scheduled in 2015. | Will depend on phase I data | Limited safety data in human subjects |
| EBOV GP Vaccine: Recombinant nanoparticle using adjuvant Matrix-M: first Ebola vaccine candidate based on the 2014 Guinea Ebola strain genetic sequence | Novavax, Gaithersburg, Md | Pre-exposure | Robust immune responses demonstrated in preclinical studies; exceptional responses were seen when used with Novavax Matrix-M adjuvant. Nonhuman primate study was initiated. GMP manufacture was initiated. Scaled-up manufacturing is to begin in first quarter of 2015. Phase 1 clinical trial is anticipated to start in December 2014. |
Will depend on phase I data | Limited safety data on Matrix-M adjuvant in human subjects |
| Oral Ad5: Oral tablet vaccine based on human adenovirus | Vaxart, South San Francisco, Calif | Pre-exposure | Protective against challenge in preclinical studies; clinical trials are anticipated in early 2015. | Will depend on phase I data | High pre-existing antibody against human adenovirus vectors |
| rVSV-EBOV: Another vesicular stomatitis vector based vaccine | Profectus Biosciences, Baltimore, Md | Pre-exposure | Safety data are available in this vaccine component expressing HIV gag. Phase I trials are planned for second quarter of 2015. | Will depend on phase I data | Safety issue as vaccine is replication competent |
| DNA-EBOV: Multiagent filovirus DNA vaccine delivered by means of intramuscular electroporation | Inovio, San Diego, Calif | Pre-exposure and postexposure | Phase I trials in 2015 | Will depend on phase I data | Limited safety data and delivery challenges |
| Recombinant rabies EBOV: (chemically inactivated [killed] rabies virus virions containing EBOV glycoprotein) | National Institutes of Health | Pre-exposure and postexposure | Excellent protection in mice against lethal challenge with the mouse adapted EBOV and RABV; phase I trials will occur in 2015. | Will depend on phase I data | No safety data in human subjects |
| Three potential vaccines: Triazoverin based on an EV strain and the other 2 based on recombinant mAbs | Russian Ministry of Health, Moscow, Russia | Preventive and therapeutic | Efficiency is said to range between 70% and 90%. There are plans to send the vaccines to affected West African countries by December 2014. | Will depend on phase I data | No safety data in human subjects |
Changing dogma to accelerate development of vaccines and therapeutics
The utmost urgency required to effectively contain the epidemic has dramatically changed the perception of the need for an Ebola vaccine, and the global response has resulted in substantial funds being released for Ebola.63 This development is accompanied by renewed interest from researchers and pharmaceutical companies. Interestingly, some drug companies have insisted on securing indemnity from government against loss or damages that might follow use of the products developed from fast-tracked clinical trials.64
Removing obstacles to accelerate the pace of Ebola vaccine and treatment trials
The conduct of clinical trials is commonly characterized by complex heterogeneous, expensive, and time-consuming approval processes,65,66 and monitoring usually concentrates on retrospective data verification. However, in the current context a risk-based approach to monitoring of clinical trials with emphasis on centralized monitoring is being encouraged.
In this context regular review of emerging safety data by independent data and safety monitoring committees needs to remain the highest priority, and emerging data should be appropriately shared with regulatory authorities.67 The notion of a single submission point for clinical trial authorization with defined timelines for approval would further support these efforts.
Ethical issues in the search for effective Ebola control
Since the launch of global efforts to accelerate initiatives to identify potent vaccines to control the scourge of Ebola, there have been growing controversies about the appropriate study design to adopt in achieving this.68,69 Although RCTs are widely accepted as the gold standard to provide credible evidence for vaccine efficacy and subsequent licensing, experts have argued that RCTs are ethically inappropriate because of the lack of effective Ebola treatments, very high mortality rate, and almost comatose health systems in the affected countries. The experts proposed a parallel evaluation of different experimental interventions at different sites while concurrently documenting mortality rates after use of “standard” care.69 An emerging body of knowledge advocates a prospective cohort, stepped-wedge design. This design is used when ethical, financial, or logistic constraints prevent the use of individual RCTs.70,71 In this design experimental vaccines/interventional treatments would be sequentially rolled out randomly to communities as they become available. Time periods before the community intervention serve as controls, and efficacy is estimated based on having concurrent preintervention and postintervention follow-up time periods.71 This approach requires close interactions with major stakeholders, including regulators, investigators, affected communities, and the WHO. If backed with robust data collection, this design could be acceptable to regulators to support licensure. It also offers a unique opportunity for governments, manufacturers, Ebola-affected countries, and funders to work harmoniously together to develop a viable vaccine or treatment that could make the present Ebola epidemic the last in medical history.62 Apart from this approach, a ring-vaccination strategy is also planned to evaluate Ebola vaccine in an affected West African country. In line with this strategy, when an Ebola case is confirmed, the affected patient's family members, friends, neighbors, and community will be vaccinated, thereby forming a ring of resistance around the patient to prevent further spread of the infection. This strategy was successfully used to eradicate smallpox globally.72
Because no human data exist on Ebola vaccine efficacy, the adaptive, randomized, observer-blind, controlled trial design still remains attractive because it is the most powerful scientific design for detection of vaccine efficacy and begins with truly randomized groups. This adaptive design allows for discontinuation of the control arm (or vaccination) based on predefined event-driven analyses of near-real time data collected during the trial.73
Anthropologic and health system factors in Ebola control
The role of anthropologic factors in triggering Ebola outbreaks in remote Sub-Saharan Africa settings is well recognized. Because of apparent food insecurity and poverty, wildlife animals, including bats and nonhuman primates, are frequently hunted for subsistence and trade.74 This anthropogenic activity amplifies human exposure to pernicious zoonosis because deadly viruses harbored by these animals can easily be transmitted to human subjects when their carcasses are being processed for human consumption.75 Although the mechanism underlying animal-to-human transmission it is not entirely clear, most Ebola outbreaks to date are traceable to a single index case who (or whose family members) had contact with carcasses of bats or nonhuman primates in impoverished remote African villages with obvious food insecurity.18,76
Once an outbreak has been initiated, spread is often enhanced by an array of cultural beliefs and practices, including adherence to time-honored paradigms of health and illness. Most of these paradigms ascribe diseases to supernatural or evil forces and have specific treatment approaches, including ritualistic practices to appease gods and ancestors.77
In the context of Ebola outbreaks, afflicted communities ascribe the disease to evil spirits, witchcraft, or sorcery. These views lead many to seek care from traditional or spiritual healers, who in turn use unsafe practices to treat patients. In many outbreaks traditional treatment homes have served as epicenters for disease propagation.78,79
Mistrust of governments and foreign workers has helped to fan transmission chains in communities, where containment teams have been blamed for initiating and spreading the disease.80 These misconceptions have rendered dozens of affected villages inaccessible and have led to the destruction of treatment units81 and physical attacks on containment teams, including butchering of staff.6 Additionally, intense disease transmission in hospitals has further amplified mistrust of authorities when communities view hospitals as institutions that kill more than they cure. Fearful patients subsequently absconded from treatment units, and families resisted taking patients to hospitals, thus enhancing disease propagation in the communities. In West African settings unauthorized collections of money for obtaining death certificates has emerged to enable families to bury their loved ones in local graves.82
Burial rites and mourning ceremonies appear to be the most significant drivers of the disease. In some settings they account for roughly two thirds of all new cases.34 The risk is high because these ceremonies, which bring hundreds of persons in close contacts with highly infectious corpses, most often require people to follow long-standing rituals, including keeping corpses for 3 days and communal hand washing in water that was used to bathe corpses.79 In an attempt to limit transmissions during funerals, WHO has developed recommendations on safe burial practices. However, uptake of these recommendations are disappointing because many families decline offers for safe burials.5 The low uptake hindered achieving the United Nations target of safely burying 70% of victims by the end of 2014.
A major obstacle to implementation is the profound view about life after death held in some communities. In many cultures the goal of life is to become an ancestor in the spirit world or to join the creator in heaven. “Proper burials” are mandatory requirements for achieving this goal.83 Therefore the dead must be prepared and buried in a prescribed way, which must be supported by numerous religious rites. According to these beliefs, the person might be subjected to severe torture, rejected by the ancestors, or transformed into wandering ghosts or totems if these rules are not followed. In some cultures improper burials are only reserved for witches and sorcerers, whose bodies are sometime subjected to burning, fed to carnivores, or secretly disposed of in isolated places and away from family graves. In previous outbreaks thoughts of being buried in plastic bags at isolated places by unfamiliar persons and in the absence of relatives and traditional rituals scared patients and families away from seeking health care or even notifying the authorities of Ebola-related deaths in communities.84
To address these issues, cultural paradigms must be acknowledged, and management must be incorporated into Ebola preparedness plans. As seen in Uganda, some of these paradigms contain elements, such as isolation of cases, restrictions on gatherings, and safe burial practices, which are consistent with modern principles of infectious disease control.33 Aligning such containment strategies to existing helpful paradigms has proved useful in controlling deadly epidemics.85 This experience highlights the need for replication of similar studies in other African settings. Such knowledge might prove useful in designing and delivering messages that can inspire cognitive and behavioral changes to time-honored practices.
Health system factors
The significant gaps in each of the 6 building blocks of a functional health care system faced by most Sub-Saharan African nations28 can hamper Ebola containment efforts in several ways. The asymmetric distribution of health services has created significant disparities in health equity and access in many rural areas, where most outbreaks occur. Lack of basic health infrastructure, medical supplies, and trained personnel render the quantity and quality of health service delivery in these areas disproportionate to health needs and affect health-seeking behavior.31
Second, most countries lack laboratory capacity, which can provide rapid confirmation of Ebola.86 During the early phase of an outbreak, samples are often shipped to distant countries for confirmation of Ebola, resulting in delayed response strategies.
Third, surveillance systems and quarantine mechanisms are weak, hindering contact tracing and monitoring efforts. Furthermore, health information systems are failing to deliver timely and reliable information that can guide containment strategies.
Most of these limitations are a result of limited health care resources. In many Sub-Saharan African nations there are less than 0.4 physicians and 1 bed for every 1000 population, and health expenditures as a percentage of gross domestic product hardly exceed 5%.35 This situation is even worse in Ebola-stricken nations because their health systems are plagued by diverse levels of dysfunction, including the inability to maintain adequate infection control. Poor infection control mechanisms have led to the infection and depletion of the nations' already scarce health workforce.87
Control measures
Strengthening health systems and practicing safe burials
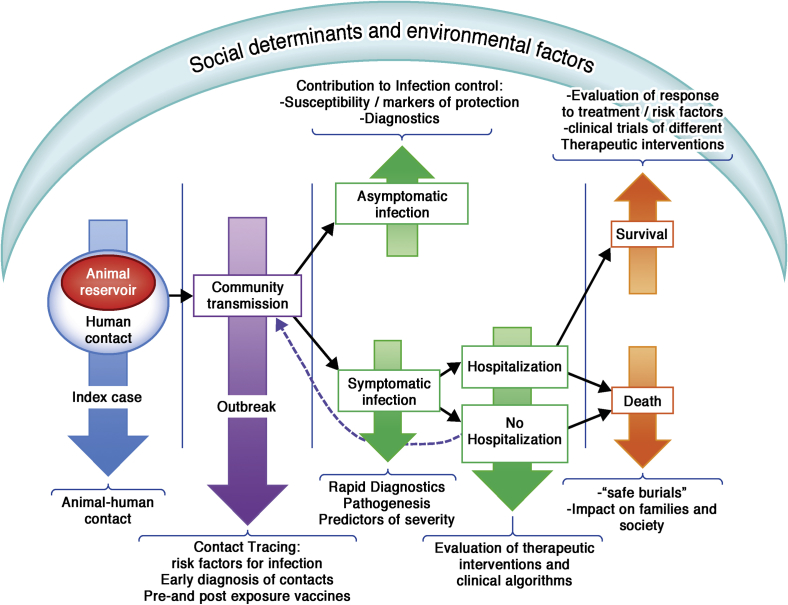
For an outbreak to be effectively controlled and eventually come to an end, control measures need to be rapidly implemented to guarantee that each affected patient infects less than 1 case (R < 1). In the absence of licensed novel treatments and vaccines, control of the epidemic relies on nonpharmaceutical interventions, which include quick identification and isolation of cases, control measures in hospital settings, identification and follow-up of contacts, and, very importantly, safe burials. In the first Ebola epidemic several factors contributed to stopping transmission, including leadership and clear designation of authorities, organization, coordination (assuring international and local support), logistics, and communication.17 Several subsequent epidemics used field teams with experience in containing other outbreaks, such as measles or polio, to trace primary contacts of patients with Ebola because they are trained for rigorous identification and surveillance. Because the virus is not transmitted through air or water, stopping transmission should be feasible when the cases are detected early and managed properly.35 However, burials have effectively served as super-spreader events. Culturally sensitive burials and disposal of cadavers are needed.14 Efforts are ongoing to assess previously successful strategies in the current epidemic. Some mathematic models have quantified the risk of transmission stratified in the different contexts (ie, hospital and community15) and within the community between sick patients and burials.14 In the past, changes in behavior led to a significant reduction both in hospital-to-community and within-community transmission.17 However, reducing transmission in hospitals would not be enough now. The most effective means to control the current epidemic require a combined strategy of intensifying contract tracing to remove infected persons in the community at an early stage of the development of symptoms, provide both isolation and care,15 and achieve sanitary burials.14 Models predict that the epidemic could be stopped by full alignment of these strategies with an efficacy of 60% for each of them if all were implemented.14 If only individual strategies were applied, these would need to achieve greater than 90% efficacy to control the current outbreak in West Africa.14 The experience in developed countries has shown that with appropriate resources, case mortality of EV infection can be decreased.3 Because burials are key in the transmission of the virus, the improvement of clinical care that would lead to decreased mortality would favor control of the epidemic. Improvement in clinical care would also help re-establish the confidence of affected communities in health services. Fig 4 illustrates potential strategies and intervention points to achieve a holistic approach to Ebola control.
Fig 4.
Summary of the potential strategies and intervention points for social and biological scientists to achieve a holistic approach to Ebola control.
Mandatory or self-quarantine of health workers returning from Ebola-affected countries
The heroic efforts of international volunteers working in Ebola-hit countries have been widely acknowledged in the containment of the epidemic. Although many of the personnel sacrifice their lives in the process, others who successfully served in the affected areas face discrimination and stigmatization on return to their home countries. This is fueled by the notion that the returning volunteers might be incubating EV and could potentially be a source of infection to the community. Contrary to well-known evidence, a study suggested that asymptomatic subjects could be a significant threat of infection because EV could persist in the blood of asymptomatic infected subjects for 2 weeks after they were first exposed to an infected person.38 However, a comprehensive analysis of 25 EV outbreaks since its first occurrence in 1976 showed that contact with an asymptomatic subjects is not a risk factor for transmitting the virus.88 Therefore daily reporting of body temperature is considered adequate to detect the onset of disease, and this could be done by the volunteers in the spirit of mutual respect and agreement rather than subjecting them to mandatory quarantine.
Conclusion
Despite the huge potential of proved drugs or vaccines in curbing the menace of EVD, it remains crucial that strengthening of existing health systems and acknowledging and incorporating cultural beliefs and practices in containment strategies in the affected regions take higher priority (Fig 4).89,90 In the longer term only functioning health systems are capable of overcoming the enormous challenges of EV containment and preventing future outbreaks supported by results from international research efforts (Box 1).91
Box 1. Priority areas for future research.
-
•
Identification of surrogate markers for transmission that can be traced through bodily fluids in order to detect infection before presenting symptoms
-
•
Development of new diagnostics that can identify infected patients before the onset of clinical symptoms
-
•
Host immune mechanisms involved in viral shedding rate or disease susceptibility (HIV and other coinfections)
-
•
Asymptomatic infections: correlates of protection/severity
-
•
Effect of genetic modification of the virus on the potential for changes in transmissibility
-
•
Development of appropriate strategies for communicating risk factors and evaluation of the effect of such strategies on mitigating virus transmission
-
•
Contact tracing strategies
Footnotes
Disclosure of potential conflict of interest: B. Kampmann has received research support from the Medical Research Council Unit Core, the Wellcome Trust, and the Bill & Melinda Gates Foundation and has received payment for development of educational presentations from the European Respiratory Society. A. Roca has received travel support from the World Health Organization. M. Afolabi has received research support from the European & Developing Countries Clinical Trial Partnership. Y. Saidu declares no relevant conflicts of interest.
References
- 1.Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 2.Maganga G.D., Kapetshi J., Berthet N., Kebela Ilunga B., Kabange F., Mbala Kingebeni P. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014;371:2083–2091. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- 3.Ebola update in Africa. Available at: https://www.internationalsos.com/ebola/. Accessed December 17, 2014.
- 4.Ebola virus disease update—West Africa. Available at: http://www.who.int/csr/don/2014_08_08_ebola/en/. Accessed August 8, 2014.
- 5.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- 6.Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ. 1978;56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 7.Ebola outbreaks 2000-2014. Available at: http://www.cdc.gov/vhf/ebola/outbreaks/history/summaries.html. Accessed December 20, 2014.
- 8.Chowell G., Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med. 2014;12:196. doi: 10.1186/s12916-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucharski A.J., Edmunds W.J. Case fatality rate for Ebola virus disease in West Africa. Lancet. 2014;384:1260. doi: 10.1016/S0140-6736(14)61706-2. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre A., Fiet C., Belpois-Duchamp C., Tiv M., Astruc K., Aho Glele L.S. Case fatality rates of Ebola virus diseases: a meta-analysis of World Health Organization data. Med Mal Infect. 2014;44:412–416. doi: 10.1016/j.medmal.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Legrand J., Grais R.F., Boelle P.Y., Valleron A.J., Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007;135:610–621. doi: 10.1017/S0950268806007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebola response roadmap—situation report. Available at: http://www.who.int/csr/disease/ebola/situation-reports/en/. Accessed December 20, 2014.
- 13.Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A., Atkins K.E., Medlock J., Wenzel N., Townsend J.P., Childs J.E. Strategies for containing Ebola in West Africa. Science. 2014;346:991–995. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camacho A., Kucharski A.J., Funk S., Breman J., Piot P., Edmunds W.J. Potential for large outbreaks of Ebola virus disease. Epidemics. 2014;9:70–78. doi: 10.1016/j.epidem.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez A., Geisbert T., Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe D., Howley P., editors. Fields virology. 5th ed. Lippincott Williams & Walkins; Philadephia: 2007. pp. 1409-48. [Google Scholar]
- 17.Breman J.G., Johnson K.M. Ebola then and now. N Engl J Med. 2014;371:1663–1666. doi: 10.1056/NEJMp1410540. [DOI] [PubMed] [Google Scholar]
- 18.Leroy E.M., Epelboin A., Mondonge V., Pourrut X., Gonzalez J.P., Muyembe-Tamfum J.J. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 19.Ascenzi P., Bocedi A., Heptonstall J., Capobianchi M.R., Di Caro A., Mastrangelo E. Ebolavirus and Marburgvirus: insight the Filoviridae family. Mol Aspects Med. 2008;29:151–185. doi: 10.1016/j.mam.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Takada A., Kawaoka Y. The pathogenesis of Ebola hemorrhagic fever. Trends Microbiol. 2001;9:506–511. doi: 10.1016/s0966-842x(01)02201-6. [DOI] [PubMed] [Google Scholar]
- 21.Nanbo A., Watanabe S., Halfmann P., Kawaoka Y. The spatio-temporal distribution dynamics of Ebola virus proteins and RNA in infected cells. Sci Rep. 2013;3:1206. doi: 10.1038/srep01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez A., Kiley M.P. Identification and analysis of Ebola virus messenger RNA. Virology. 1987;157:414–420. doi: 10.1016/0042-6822(87)90283-2. [DOI] [PubMed] [Google Scholar]
- 23.Bornholdt Z.A., Noda T., Abelson D.M., Halfmann P., Wood M.R., Kawaoka Y., Saphire E.O. Structural rearrangement of ebola virus VP40 begets multiple functions in the virus life cycle. Cell. 2013;154:763–774. doi: 10.1016/j.cell.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda T. Assembly and budding of Ebolavirus. PLoS Pathog. 2006;2:e99. doi: 10.1371/journal.ppat.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharat T.A. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc Natl Acad Sci U S A. 2012;109:4275–4280. doi: 10.1073/pnas.1120453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modrof J. Phosphorylation of Marburg virus VP30 at serines 40 and 42 is critical for its interaction with NP inclusions. Virology. 2001;287:171–182. doi: 10.1006/viro.2001.1027. [DOI] [PubMed] [Google Scholar]
- 27.Groseth A., Feldmann H., Strong J.E. The ecology of Ebola virus. Trends Microbiol. 2007;15:408–416. doi: 10.1016/j.tim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann H., Geisbert T.W. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray M, Hirsch MS, Mitty J. Epidemiology, pathogenesis, and clinical manifestations of Ebola and Marburg virus disease. Available at: www.uptodate.com. Accessed December 2, 2014.
- 31.WHO. What do we know about transmission of the Ebola virus among humans. Available at: http://www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed December 2, 2014.
- 32.Dowell S.F., Mukunu R., Ksiazek T.G., Khan A.S., Rollin P.E., Peters C.J. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(suppl 1):S87–S91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 33.Hewlett B., Amola R. Cultural contexts of Ebola in northern Uganda. Emerg Infect Dis. 2003;9:1242–1248. doi: 10.3201/eid0910.020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan M. Ebola virus disease in West Africa—no early end to the outbreak. N Engl J Med. 2014;371:1183–1185. doi: 10.1056/NEJMp1409859. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Transmission of Ebola (Ebola virus disease). Available at: http://www.cdc.gov/vhf/ebola/transmission/2014. Accessed September 18, 2014.
- 36.Gonzalez J.P., Nakoune E., Slenczka W., Vidal P., Morvan J.M. Ebola and Marburg virus antibody prevalence in selected populations of the Central African Republic. Microbes Infect. 2000;2:39–44. doi: 10.1016/s1286-4579(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 37.Heffernan R.T., Pambo B., Hatchett R.J., Leman P.A., Swanepoel R., Ryder R.W. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooue-Ivindo region, Northeastern Gabon, 1997. J Infect Dis. 2005;191:964–968. doi: 10.1086/427994. [DOI] [PubMed] [Google Scholar]
- 38.Leroy E.M., Baize S., Volchkov V.E., Fisher-Hoch S.P., Georges-Courbot M.C., Lansoud-Soukate J. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355:2210–2215. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 39.Geisbert T.W., Hensley L.E., Larsen T., Young H.A., Reed D.S., Geisbert J.B. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisbert T.W., Hensley L.E., Gibb T.R., Steele K.E., Jaax N.K., Jahrling P.B. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest. 2000;80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 41.Baize S., Leroy E.M., Georges A.J., Georges-Courbot M.C., Capron M., Bedjabaga I. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher-Hoch S.P., Platt G.S., Neild G.H., Southee T., Baskerville A., Raymond R.T. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola) J Infect Dis. 1985;152:887–894. doi: 10.1093/infdis/152.5.887. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez A., Lukwiya M., Bausch D., Mahanty S., Sanchez A.J., Wagoner K.D. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez A., Wagoner K.E., Rollin P.E. Sequence-based human leukocyte antigen-B typing of patients infected with Ebola virus in Uganda in 2000: identification of alleles associated with fatal and nonfatal disease outcomes. J Infect Dis. 2007;196(suppl 2):S329–S336. doi: 10.1086/520588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towner J.S., Rollin P.E., Bausch D.G., Sanchez A., Crary S.M., Vincent M. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saijo M., Niikura M., Ikegami T., Kurane I., Kurata T., Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol. 2006;13:444–451. doi: 10.1128/CVI.13.4.444-451.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villinger F., Rollin P.E., Brar S.S., Chikkala N.F., Winter J., Sundstrom J.B. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 48.Sobarzo A., Groseth A., Dolnik O., Becker S., Lutwama J.J., Perelman E. Profile and persistence of the virus-specific neutralizing humoral immune response in human survivors of Sudan ebolavirus (Gulu) J Infect Dis. 2013;208:299–309. doi: 10.1093/infdis/jit162. [DOI] [PubMed] [Google Scholar]
- 49.Urgently needed: rapid, sensitive, safe and simple Ebola diagnostic tests. Available at: http://www.who.int/mediacentre/news/ebola/18-november-2014-diagnostics/en/. Accessed December 15, 2014.
- 50.Bah E.I., Lamah M.C., Fletcher T., Jacob S.T., Brett-Major D.M., Sall A.A. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 51.Schieffelin J.S., Shaffer J.G., Goba A., Gbakie M., Gire S.K., Colubri A. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kortepeter M.G., Bausch D.G., Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(suppl 3):S810–S816. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 53.Clark D.V., Jahrling P.B., Lawler J.V. Clinical management of filovirus-infected patients. Viruses. 2012;4:1668–1686. doi: 10.3390/v4091668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop B.M. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49:196–206. doi: 10.1177/1060028014561227. [DOI] [PubMed] [Google Scholar]
- 55.Painter W., Robertson A., Trost L.C., Godkin S., Lampert B., Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56:2726–2734. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oestereich L., Ludtke A., Wurr S., Rieger T., Munoz-Fontela C., Gunther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Wolf T., Kann G., Becker S., Stephan C., Brodt H., de Leuw P. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2014 doi: 10.1016/S0140-6736(14)62384-9. [Epub ahead of print]. doi:10.1016/S0140-6736(14)62384-9. [DOI] [PubMed] [Google Scholar]
- 59.Rid A., Emanuel E.J. Ethical considerations of experimental interventions in the Ebola outbreak. Lancet. 2014;384:1896–1899. doi: 10.1016/S0140-6736(14)61315-5. [DOI] [PubMed] [Google Scholar]
- 60.Kanapathipillai R., Restrepo A.M., Fast P., Wood D., Dye C., Kieny M.P. Ebola vaccine—an urgent international priority. N Engl J Med. 2014;371:2249–2251. doi: 10.1056/NEJMp1412166. [DOI] [PubMed] [Google Scholar]
- 61.Briand S., Bertherat E., Cox P., Formenty P., Kieny M.P., Myhre J.K. The international Ebola emergency. N Engl J Med. 2014;371:1180–1183. doi: 10.1056/NEJMp1409858. [DOI] [PubMed] [Google Scholar]
- 62.Ledgerwood J.E., DeZure A.D., Stanley D.A., Novik L., Enama M.E., Berkowitz N.M. Chimpanzee adenovirus vector Ebola vaccine—preliminary report. N Engl J Med. 2014 doi: 10.1056/NEJMoa1410863. [Epub ahead of print]. doi:10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 63.Farrar J.J., Piot P. The Ebola emergency—immediate action, ongoing strategy. N Engl J Med. 2014;371:1545–1546. doi: 10.1056/NEJMe1411471. [DOI] [PubMed] [Google Scholar]
- 64.Big Pharma seeks legal immunity for damages from experimental Ebola vaccines. Available at: http://www.naturalnews.com/047441_Ebola_vaccines_legal_immunity_Big_Pharma.html. Accessed November 16, 2014.
- 65.Schopper D., Upshur R., Matthys F., Singh J.A., Bandewar S.S., Ahmad A. Research ethics review in humanitarian contexts: the experience of the independent ethics review board of Medecins Sans Frontieres. PLoS Med. 2009;6:e1000115. doi: 10.1371/journal.pmed.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edwards S.J. Ethics of clinical science in a public health emergency: drug discovery at the bedside. Am J Bioeth. 2013;13:3–14. doi: 10.1080/15265161.2013.813597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reith C., Landray M., Devereaux P.J., Bosch J., Granger C.B., Baigent C. Randomized clinical trials—removing unnecessary obstacles. N Engl J Med. 2013;369:1061–1065. doi: 10.1056/NEJMsb1300760. [DOI] [PubMed] [Google Scholar]
- 68.Shaw D. Randomisation is essential in Ebola drug trials. Lancet. 2014;384:1667. doi: 10.1016/S0140-6736(14)61735-9. [DOI] [PubMed] [Google Scholar]
- 69.Adebamowo C., Bah-Sow O., Binka F., Bruzzone R., Caplan A., Delfraissy J.F. Randomised controlled trials for Ebola: practical and ethical issues. Lancet. 2014;384:1423–1424. doi: 10.1016/S0140-6736(14)61734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown C., Lilford R. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hussey M.A., Hughes J.P. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 72.The design and analysis of Phase IIb Ebola vaccine trials. Available at: http://www.who.int/immunization/diseases/ebola/Ebola_Longini.pdf. Accessed January 31, 2015.
- 73.GSK Proposed Phase 2 Program for ChAd3 EBOV Vaccine Candidate. Available at: http://www.who.int/immunization/diseases/ebola/GSK_Phase_2_Program_WHO_29-30_Sep_2014.pdf. Accessed January 31, 2015.
- 74.Schulte-Herbrüggen B., Cowlishaw G., Homewood K., Rowcliffe J.M. The importance of bushmeat in the livelihoods of West African cash-crop farmers living in a faunally-depleted landscape. PLoS One. 2013;8:e72807. doi: 10.1371/journal.pone.0072807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Food and Agricultural Organisation. Bats as bushmeat: implication for global public health. Available at: http://www.fao.org/ag/AGAinfo/programmes/en/empres/news_161110.html. Accessed December 2, 2014.
- 76.Ebola: research team says migrating fruit bats responsible for outbreak. Available at: http://www.theguardian.com/society/2014/aug/23/ebola-outbreak-blamed-on-fruit-bats-africa. Accessed December 21, 2014.
- 77.Skolnik R. Culture and health. In: Riegelman R., editor. Global health 101. Jones & Bartlett Learning; Burlington (MA): 2008. pp. 120–137. [Google Scholar]
- 78.Ebola outbreak in Sierra Leone traced back to a single traditional healer's funeral where 14 women were infected. Available at: http://www.dailymail.co.uk/news/article-2738904/Ebola-outbreak-Sierra-Leone-traced-single-traditional-healer-s-funeral-14-women-infected.html#ixzz3FlB1t4aG. Accessed December 2, 2014.
- 79.Kinsman J. “A time of fear”: local, national, and international responses to a large Ebola outbreak in Uganda. Global Health. 2012;8:15. doi: 10.1186/1744-8603-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nossiter A. Fear of Ebola breeds a terror of physicians. Available at: http://www.nytimes.com/2014/07/28/world/africa/ebola-epidemic-west-africa-guinea.html?_r=2. Accessed November 18, 2014.
- 81.Prewitt KC. Why Liberians raided the Ebola clinic. Available at: http://www.kevinmd.com/blog/2014/08/liberians-raided-ebola-clinic.html. Accessed December 5, 2014.
- 82.The war on Ebola. Available at: http://www.economist.com/news/leaders/21625781-win-it-requires-much-larger-effort-west-africa-outside-world-has-so-far. Accessed November 15, 2014.
- 83.Gire S.K., Goba A., Andersen K.G., Sealfon R.S., Park D.J., Kanneh L. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345:1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.The Rockefeller Foundation. Lessons from previous Ebola outbreaks for improving current risk management. Available at: http://www.rockefellerfoundation.org/newsroom/lessons-from-previous-ebola-outbreaks. Accessed December 2, 2014.
- 85.Omaswa F. Regaining trust: an essential prerequisite for controlling the Ebola outbreak. Lancet Global Health. Available at: http://globalhealth.thelancet.com/2014/08/11/regaining-trust-essential-prerequisite-controlling-ebola-outbreak. Accessed December 2, 2014.
- 86.Gostin L.O. Ebola: towards an International Health Systems Fund. Lancet. 2014;384:e49–51. doi: 10.1016/S0140-6736(14)61345-3. [DOI] [PubMed] [Google Scholar]
- 87.World Health Organisation. Unprecedented number of medical staff infected with Ebola. Available at: http://www.who.int/mediacentre/news/ebola/25-august-2014/en/. Accessed December 12, 2014.
- 88.Nobel Laureates and Ebola virus quarantine. Available at: http://www.virology.ws/2014/11/04/nobel-laureates-and-ebola-virus-quarantine/. Accessed November 28, 2014.
- 89.World Health Organization: Everybody's business: strengthening health systems to improve health outcomes. WHO's Framework for Action. Geneva: World Health Organization; 2007. Available at: http://wwwwhoint/healthsystems/strategy/everybodys_businesspdf. Accessed March 6, 2015.
- 90.Burki T.K. USA focuses on Ebola vaccine but research gaps remain. Lancet. 2011;378:389. doi: 10.1016/s0140-6736(11)61194-x. [DOI] [PubMed] [Google Scholar]
- 91.Fauci A.S. Ebola—underscoring the global disparities in health care resources. N Engl J Med. 2014;371:1084–1086. doi: 10.1056/NEJMp1409494. [DOI] [PubMed] [Google Scholar]