Abstract
Background
Immunoglobulin class-switch recombination defects (CSR-D) are rare primary immunodeficiencies characterized by impaired production of switched immunoglobulin isotypes and normal or elevated IgM levels. They are caused by impaired T:B cooperation or intrinsic B cell defects. However, many immunoglobulin CSR-Ds are still undefined at the molecular level.
Objective
This study's objective was to delineate new causes of immunoglobulin CSR-Ds and thus gain further insights into the process of immunoglobulin class-switch recombination (CSR).
Methods
Exome sequencing in 2 immunoglobulin CSR-D patients identified variations in the INO80 gene. Functional experiments were performed to assess the function of INO80 on immunoglobulin CSR.
Results
We identified recessive, nonsynonymous coding variations in the INO80 gene in 2 patients affected by defective immunoglobulin CSR. Expression of wild-type INO80 in patients' fibroblastic cells corrected their hypersensitivity to high doses of γ-irradiation. In murine CH12-F3 cells, the INO80 complex accumulates at Sα and Eμ regions of the IgH locus, and downregulation of INO80 as well as its partners Reptin and Pontin impaired CSR. In addition, Reptin and Pontin were shown to interact with activation-induced cytidine deaminase. Finally, an abnormal separation of sister chromatids was observed upon INO80 downregulation in CH12-F3 cells, pinpointing its role in cohesin activity.
Conclusion
INO80 deficiency appears to be associated with defective immunoglobulin CSR. We propose that the INO80 complex modulates cohesin function that may be required during immunoglobulin switch region synapsis.
Key words: Chromatin remodeling, class-switch recombination defect, CSR synapse, cohesin
Abbreviations used: AID, Activation-induced cytidine deaminase; ChIP, Chromatin immunoprecipitation; CSR, Class-switch recombination; CSR-Ds, CSR defects; DAPI, 4′,6-Diamidino-2-phenylindole; GLT, Germ-line transcript; MMR, Mismatch repair; NHEJ, Non-homologous end joining; TCR, T-cell receptor; wt, Wild type
Immunoglobulin CSR defects (CSR-Ds) are rare primary immunodeficiencies characterized by impaired production of switched immunoglobulin isotypes and normal or elevated IgM levels.1 Indeed, the analysis of CSR-Ds caused by impaired T:B cooperation2 or intrinsic B cell defects has provided a better understanding of the complex mechanisms underlying antibody maturation in humans. The description of patients with an activation-induced cytidine deaminase (AID) deficiency revealed this enzyme's master role in both CSR and somatic hypermutation.3 The identification of a CSR-D caused by mutations in the uracil-N glycosylase gene also demonstrated that AID had DNA editing activity.4 Furthermore, the identification of mutations in CSR-D patients has shown that several proteins involved in DNA repair—such as non-homologous end joining (NHEJ) factors and mismatch repair (MMR) enzymes—also have roles in CSR.5-7 However, many immunoglobulin CSR-Ds remain still undefined at the molecular level, and their delineation, now possible through the use of whole exome (or genome) sequencing, affords a better understanding of the complex mechanisms involved in CSR.
In the present study, we report the identification of 2 CSR-D patients with recessive, nonsynonymous coding variations in the INO80 gene and show that in vitro downregulation of INO80 complex subunits impairs CSR. Our results also suggest that INO80 is involved in the conformational modification of the immunoglobulin locus required for the S-region-specific recombination process in CSR, possibly through modulation of cohesin activity. We also found that the INO80 complex subunits Reptin and Pontin interact with AID—suggesting that AID's known role in S-region synapsis8,9 occurs through its interaction with the INO80 complex.
A role for a chromatin remodeling complex in CSR is not unexpected, because CSR is achieved by a DNA recombination between two S regions. The S regions need to be accessible and transcribed, and DNA's interactions with most nuclear factors is restricted when the chromatin is highly condensed, suggesting the requirement of chromatin modification. Chromatin dynamics are regulated by (i) post-translational modifications of the core histones and (ii) ATP-dependent chromatin remodeling.10 Histone phosphorylation, ubiquitination, methylation and acetylation have all been implicated in immunoglobulin CSR.11-15
Four structurally related families of ATP-dependent chromatin remodeling complexes (SWI/SNF, INO80, CHD, and ISWI) have been described, each being defined by its characteristic catalytic core ATPase from the SWI2/SNF2 superfamily.16 The complexes' biological functions include the disruption of histone-DNA contact within nucleosomes and the cis and trans movements of histone octamers that facilitate access to nucleosomal DNA for transcription factors and restriction endonucleases.
The INO80 chromatin remodeling complex has 3′-5′ helicase activity and contains the SNF/SWI2 ATPase INO80.17 The INO80 ATPase binds to actin, 3 actin-related proteins (ARPs, with ARP5 and ARP8 specifically present in the INO80 complex), and 2 AAA+-ATPases (RUVBL1 and RUVBL2, also known respectively as Reptin and Pontin).18 The INO80 complex is conserved from budding yeasts through to humans and has functional roles in DNA replication, DNA repair, the regulation of transcription, chromosomal segregation, and telomere maintenance.19
Methods
A detailed description of materials and methods is provided in this article's Online Repository available at www.jacionline.org. The study was performed in accordance with the precepts of the Declaration of Helsinki.
Results
Immune system defects in CSR-deficient patients
Patient 1 (P1) was the unique child born from a Turkish nonconsanguineous family. He presented with severe, recurrent bacterial infections at the age of 5 years. No opportunistic infections were noticed. A serum immunoglobulin assay revealed normal IgM levels (0.7 g/L) but decreased IgG (4.7 g/L) and IgA (0.09 g/L) levels. P1 received prophylactic antibiotics with no immunoglobulin substitution. During follow-up, the IgG levels (including IgG isotypes) and IgA levels rose progressively but remained lower than normal at 10 years of age (Table I). No specific antibody response to antigenic challenge could be studied.
Table I.
Patients' B cell phenotype and function at time of last examination
| Age (y) |
In vivo CSR |
In vitro CSR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Immunoglobulin levels (g/L) |
Lymphocytes/μL |
In vitro IgE production (ng/mL) |
|||||||
| IgM | IgG | IgA | B | Total memory B cells | Switched B cells | Nonstimulated | Stimulated | ||
| P1 | 5 | 1.04 | 5.62 | 0.09 | 399 | 88 | 5 | 0.42 | 159 |
| P2 | 67 | 0.7 | NE | <0.07 | 180 | 11 | 1 | 0.09 | 52.6 |
| Control | 5 | 0.6-1.3 | 6.8-11.8 | 0.5-1.2 | 250-594 | 55-118 | 45-101 | <0.07-12 | 270->1800 |
| Control | >50 | 1.4-2.6 | 9.2-14.8 | 0.9-1.9 | 84-210 | 25-81 | 12-31 | <0.07-6.7 | 1170->1800 |
Under immunoglobulin substitution, IgG = 0.70 g/L at diagnosis (18 years).
Total memory B cells (CD19+CD27+).
Switched B cells (CD19+CD27+IgM−IgD−).
NE, Not evaluable.
Patient 2 (P2) was an English-born male not related to P1 who first suffered from severe and recurrent upper respiratory infections at the age of 18. No susceptibility to opportunistic infections was reported. At diagnosis, he presented with depressed IgG levels (0.70 g/L) and IgA levels (0.03 g/L) but had normal IgM levels (0.87 g/L). At the time of evaluation, P2 was 67 years old and had chronic obstructive pulmonary disease following 35 years of smoking. Immunoglobulin assays revealed a lack of serum IgA but a slight decrease of serum IgM; IgG (and specific antibody response) could not be evaluated because of the patient's regular immunoglobulin replacement therapy (Table I). The patient responded well to immunoglobulin replacement therapy, with the exception of the chronic obstructive pulmonary disease.
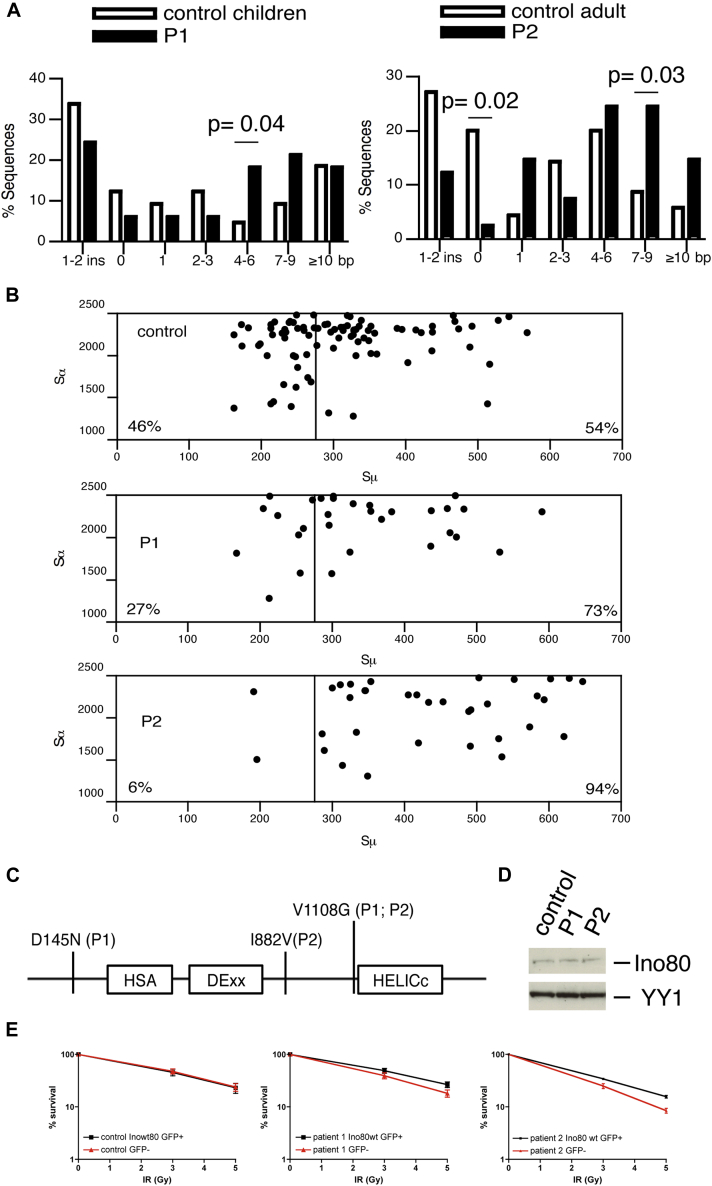
In both patients, the presence of mutations in genes already known to be involved in CSR was ruled out through sequence analysis (AID and UNG) or the observation of normal expression (CD40L and CD40) and CD40-mediated B cell proliferative responses. Total B cell counts were normal, but the number of IgM− IgD− CD19+ CD27+–switched memory B cells was low. T cell counts were within the normal range. Likewise, the T-cell receptor (TCR) beta chain and the BCR repertoires were within the normal range, as assessed by amplification of V-J rearrangements (data not shown). Analysis of B cell function revealed a normal frequency of somatic hypermutations in the VH3-23 region of IgM in P1. The nucleotide substitution pattern was normal, suggesting that AID activity was unaffected (data not shown). In vitro CD40L+ IL4-induced CSR to IgE was consistently found impaired in peripheral blood lymphocytes from both patients, when compared with age-matched controls (Table I). An ex vivo analysis of Sμ-Sα junctions revealed that blunt junctions were less frequent in P1 and P2 than in age-matched controls. In contrast, junctions based on 4 to 9 nt microhomologies were more frequent in the patients (Fig 1, A). In agreement with the preferential usage of microhomology, both patients displayed a significantly higher portion of Sμ-Sα junction breakpoints in the distal part of the Sμ region (which has the highest degree of homology with Sα) (Fig 1, B).
Fig 1.
A, Abnormal switch junction repair in 2 patients carrying INO80 gene variations. Analysis of Sμ–Sα recombination junctions. White bars indicate control sequences (65 and 70 for children and adult controls, respectively, recently published37). Black bars indicate patient sequences (33 and 41 for P1 and P2, respectively). B, Scatterplot analysis of Sμ and Sα breakpoints. Vertical line at position 275 indicates the start of the Sμ region with highest degree of homology with Sα1 and Sα2. C, INO80 protein structure. D, Immunoblot analysis of INO80 and YY1. Radiosensitivities of lentivirally infected patients and control fibroblast cell lines (E) co-expressing wt INO80 and GFP; P value from paired Student t test of percentage survival at 5 Gy for patients' INO80wt GFP+ cells versus patients' GFP− cells for P1: .02 and for P2: .04.
Altogether, these results indicate that these 2 patients presented a CSR deficiency likely associated with defective repair of switch junctions.
INO8O gene variations
By whole exome sequencing of DNA from P1, no abnormalities of genes involved in CSR were noted (eg, AID, UNG, CD40, CD40L). However, we identified 2 nonsynonymous, compound heterozygous single nucleotide variants in INO80 (G433A and T3323G, leading respectively to D145N and V1108G amino acid substitutions). The D145N variant was inherited from a healthy father, and V1108G was inherited from a healthy mother. Additional screening using Sanger sequencing identified P2 as carrying 1 of the variants seen in P1 (V1108G) and a further, nonsynonymous A2644G variant (leading to an I882V amino acid substitution) (Fig 1, C). By cloning and sequencing the messenger RNA, we found that P2's variants were located on different alleles. The variant leading to the D145N amino acid substitution has not been reported as a single nucleotide polymorphism. The amino acid position D145 of human INO80 appears to be generally conserved as a D in mammals and vertebrates, although an N followed by an insertion of 1, 2, 4, or 9 amino acids is found in some mammalian species (see Fig E1 in this article's Online Repository, available at www.jacionline.org). The nucleotide variation leading to the V1108G amino acid substitution has been observed in the 1000 Genome Project data (May 2011) with a low prevalence in the general population (allele frequency 0.004; rs34178030). The amino acid position 1108 in human INO80 is conserved as a V in mammals (with the exception of the rat, with an I) and an L in other vertebrates (Fig E1). The nucleotide variation leading to I882V amino acid substitution has been observed in the 1000 Genome Project data (May 2011) with a slightly higher prevalence in the general population (allele frequency 0.009; rs34153025). From an evolutionary standpoint, the amino acid position I882 in human INO80 is not highly conserved, since several mammalian species have a V at this position (Fig E1). Thus, the I882V substitution could be regarded as an allele with potentially mild functional consequences. These gene alterations did not alter the expression of INO80 protein in patient-derived EBV B cell lines, relative to cells from healthy controls (Fig 1, D).
Our results indicate that INO80 gene variations can be associated with a CSR defect with switch junctions' DNA repair abnormality.
DNA repair deficiency in INO80
In view of INO80's previously described roles in the repair process18,20,21 and the abnormal repair of switch junctions in patients' B cells, we derived fibroblastic cell lines from patients and tested their sensitivity to γ-radiation with or without lentiviral transduction of a vector coding for wild-type (wt) INO80. After exposure to 5 Gy of γ-radiation, cell survival was lower in the non-transduced cells from both patients compared with the cells transduced with the lentiviral vector coding for wt INO80 in the same culture. This difference was not observed in a control fibroblast cell line nor in both patients' fibroblast cell lines transduced with an empty vector (data not shown; Fig 1, E). We conclude that the CSR deficiency observed in both patients is associated with a mild DNA repair defect that is corrected by wt INO80 overexpression.
CSR is impaired in CH12-F3 cells in which the INO80 complex is downregulated
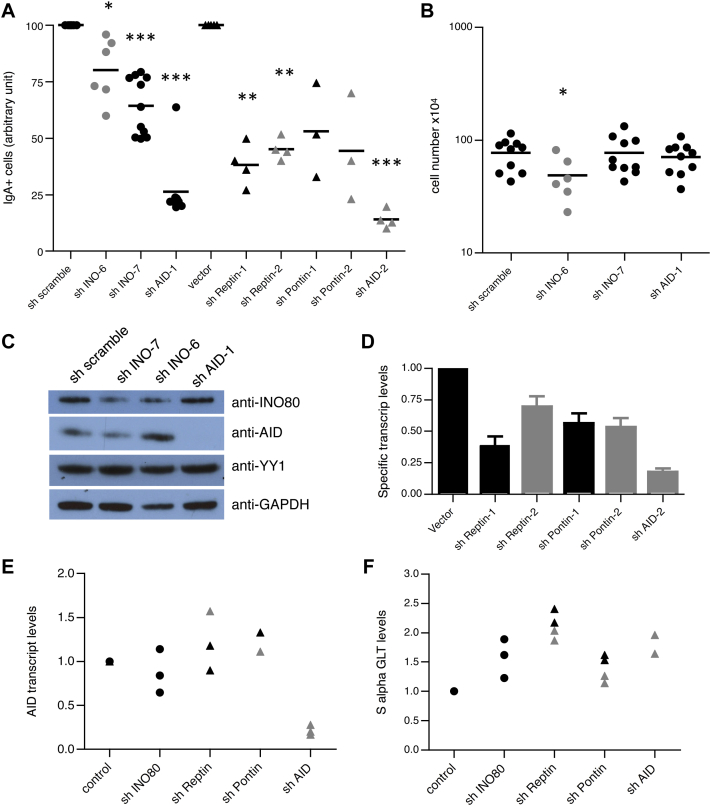
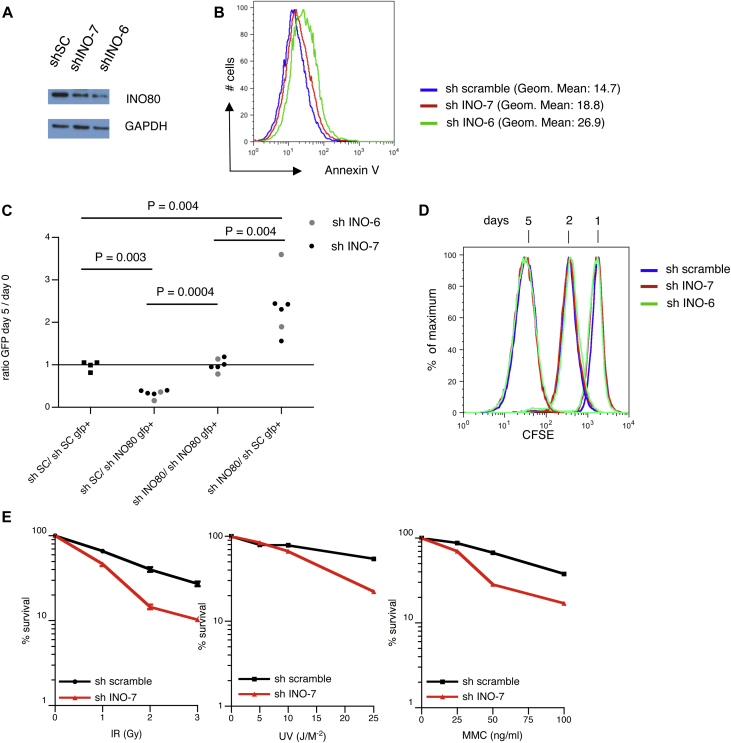
We next determined whether the INO80 complex plays a role in CSR via an in vitro CSR assay in the CH12-F3 B cell line. These cells undergo efficient CSR to IgA when stimulated with anti-CD40, TGF-β and IL-4. To limit possible effects of impaired cellular viability on CSR, expression of IgA was determined by flow cytometry as soon as 48 hours post-stimulation. As expected, AID knockdown resulted in a robust reduction of CSR to IgA (Fig 2, A). Both lentiviral constructs encoding shINO-6 and shINO-7 reduced the percentage of IgA+ cells by an average of 30% (compared with control cells transduced with lentiviral vectors expressing a scrambled shRNA). The effect on CSR after INO80 knockdown was very likely not caused by impaired survival, because similar numbers of living cells were recovered on Day 2 (Fig 2, A-C). Next, we downregulated the expression of the INO80 complex subunits Reptin and Pontin AAA+ ATPases. In agreement with the INO80 knockdown results, the presence of shRNAs against Reptin and Pontin inhibited switching to IgA, relative to controls (Fig 2, A and D). Given that (i) INO80 regulates transcription22,23 and (ii) CSR is dependent on both germ-line transcript (GLT) and AID expression, we analyzed the levels of AID and IgA sterile transcripts in CH12-F3 cells in which INO80, Reptin and Pontin were knocked down. Neither AID nor IgA GLT expression were affected (Fig 2, C, E, and F). Similarly, the level of IgM sterile transcripts was not modified by downregulation of INO80 (data not shown). Hence, INO80 involvement in CSR appears not to be related to a defect in cell proliferation, AID, or GLT expression. Furthermore, we observed that levels of YY1 protein (a central regulator of the germinal centre B-cell-specific transcriptional programme, INO8018,24,25 interactor, and also described to function in CSR) were similar in CH12-F3 cells transduced with either scrambled shRNA or INO80 shRNA (Fig 2, C). Altogether, our data suggest that the INO80 complex plays a role in CSR that is independent of its function as a transcriptional regulator.
Fig 2.
INO80 complex knockdown impairs immunoglobulin CSR in CH12-F3 cells. Quantification of CSR to IgA (on day 2 after activation) in CH12-F3 cells expressing indicated shRNA (black and grey indicate different shRNA) (A). P values from a paired Student t test: *(P < .05); **(P < .01); and ***(P < .001) are indicated. Number of viable CH12-F3 cells at the end of CSR cultures (2 days) (B) described in A. Statistically significant P value from an unpaired Student t test is indicated as *(P < .05). Immunoblot analysis of INO80, AID, YY1, and GAPDH (on day 2 after stimulation) in whole-cell extracts of CH12-F3 cells expressing indicated shRNA (C). Relative levels of specific shRNA target transcripts in CH12 cells transduced with the indicated shRNA (D). Results are presented relative to empty vector and normalized against levels of GAPDH mRNA. Real-time quantitative PCR analysis of AID transcripts (E) and S alpha GLT (F) in RNA from CH12-F3 cells expressing indicated shRNA. Results are presented relative to controls and were normalized against levels of GAPDH mRNA.
INO80 downregulation affects cellular viability and DNA repair in CH12-F3 cells
The function of INO80 is important for cellular viability, because INO80 knockout mice die during the early stages of embryonic development26 and INO80 knockout in different cell lines results in growth arrest and cell death after a small number of cell passages.21,26 Therefore, we determined the effect of diminished INO80 protein expression on cellular viability and proliferation of CH12-F3 cells following transduction of either scrambled shRNA or shINO80. INO80 knockdown cultures contained a higher proportion of annexin-V positive cells compared with control cultures, which correlated with the degree of Ino80 protein downregulation (see Fig E2, A and B, in this article's Online Repository at www.jacionline.org). After 5 days, CH12-F3 cells in which INO80 was downregulated were negatively selected as compared with CH12-F3 cells expressing scrambled shRNA (Fig E2, C). This effect was not the consequence of defective cell proliferation (as assessed by the loss of carboxyfluorescein succinimidyl ester intensity [Fig E2, D]) nor of a defective cell cycle (as shown by propidium iodide incorporation; data not shown), and therefore was likely the result of increased apoptosis in the absence of Ino80 protein (as assessed by the annexin V staining; Fig E2, B). Next, we studied the CH12-F3 cells' sensitivity to several DNA-damaging agents, because a contribution of INO80 function in the repair of different types of DNA damage has been suggested by several studies.20,21 CH12-F3 cells transduced with INO80 shRNA-7 were more sensitive to γ-radiation, UV light, and mitomycin C than cells expressing scrambled shRNA (Fig E2, E). Together, these results indicate that INO80 knockdown in CH12-F3 cells alters cell survival and DNA repair.
INO80 downregulation affects sister chromatid cohesion in CH12-F3 cells
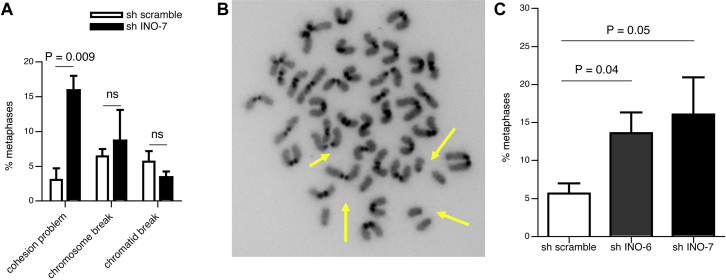
Because INO80 deficiency impairs DNA repair and INO80 functions likely at the DNA repair step in CSR, we checked for genome instability manifestations in stimulated CH12-F3 cells transduced with either INO80 shRNA or a control scrambled shRNA. Metaphase analysis with 4′,6-diamidino-2-phenylindole (DAPI) and telomere fluorescence in situ hybridization showed that chromosome/chromatid break frequency was not altered following INO80 downregulation (Fig 3, A). Interestingly, we observed a higher frequency of separated sister chromatids in both nonstimulated and stimulated CH12-F3 cells in which INO80 expression was downregulated, when compared to controls (Fig 3, A-C). This result suggests that INO80 plays a role in sister chromatid cohesion. Consistent with this, data obtained in yeast has shown that the INO80 chromatin-remodeling complex contributes to sister chromatid cohesion through the establishment of cohesin activity.27
Fig 3.
Early separation of sister chromatids in CH12-F3 INO80 knockdown cells. Chromosomal aberrations in metaphase spreads hybridized with a telomere probe and counterstained with DAPI from activated CH12-F3 cells expressing INO80-specific shRNA (sh INO-7; black bar) or a nonspecific control shRNA (sh scramble; white bar) (A); 39 to 62 metaphases were analyzed. P values from unpaired Student t test are quoted; ns, not significant. A representative metaphase spread with early separation of sister chromatids (indicated by an arrow), from nonstimulated CH12-F3 cells expressing INO80-specific shRNA (B). Frequency of metaphases with abnormal separation of sister chromatids in nonstimulated CH12-F3 cells expressing INO80-specific shRNAs (sh INO-6 and sh INO-7; grey and black bars, respectively) or a nonspecific control shRNA (sh scramble; white bar) (C). In 2 independent experiments, 41 to 49 metaphases were analyzed (mean and SEM). The P value from an unpaired Student t test is quoted.
INO80 and cohesin localize at the Ig locus in CH12-F3 cells
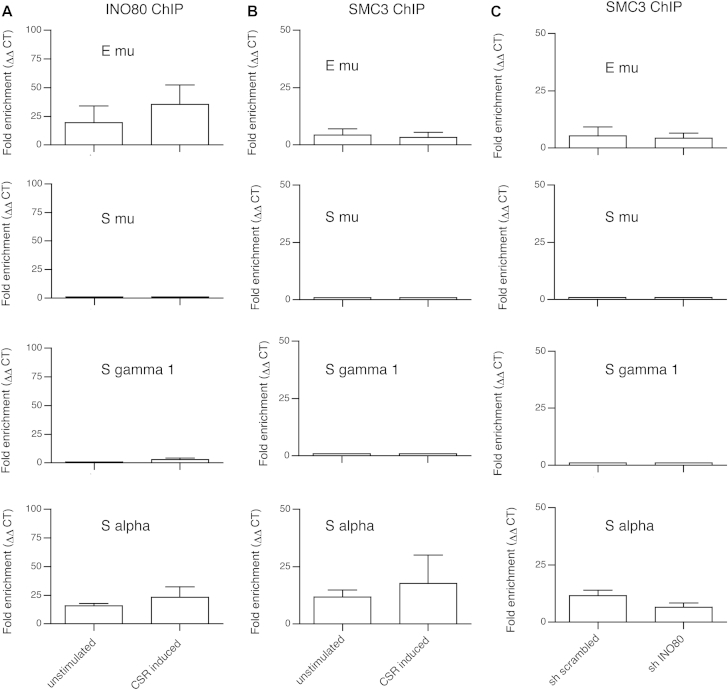
Cohesin was recently found to exert a role in intrachromosomal interaction during gene expression and T cell receptor alpha gene rearrangement.28,29 Moreover, recent evidence suggests that a cohesin-dependent interaction between the 3′ regulatory region of the immunoglobulin locus and specific I region promoters (which enable sterile transcript expression) could be involved in CSR-induced formation or maintenance of synapsis.30 We therefore used chromatin immunoprecipitation (ChIP) to determine the extent to which INO80 and cohesin associate with the S and enhancer (E) μ regions in CH12-F3 cells. Indeed, INO80 and SMC3 (1 of the subunits of the cohesin complex) were found to accumulate at both the Sα and Eμ regions (Fig 4, A and B). Strikingly, there was no detectable binding to Sγ1, Sγ3, or Sμ regions in CH12-F3 cells (Fig 4, A and B; data not shown). INO80 and cohesin were bound to Sα and Eμ in both nonstimulated CH12-F3 cells and cells stimulated with anti-CD40+TGF–β+IL−4. Furthermore, the degree of association between the cohesin SMC3 subunit and the Sα region was similar in INO80-knockdown CH12-F3 cells and control cells (Fig 4, C).
Fig 4.
INO80 and cohesin (SMC3) binding to Sα and Eμ regions in CH12-F3 cells. ChIP analysis of lysates of nonstimulated and stimulated (for 48 hours with anti-CD40, TGF-β, and IL-4) CH12-F3 B cells. Lysates were immunoprecipitated with anti-INO80 antibody (A) or anti-SMC3 antibody (B), followed by quantitative real-time PCR analysis (in triplicate) to assess the presence of Sα, Eμ, Sμ, Sγ1, and Sγ3. Data are represented as fold-enrichment calculated with ΔΔCT method of qPCR data analysis. Presented are the mean and SEM of 2 independent ChIP experiments. ChIP analysis of lysates of nonstimulated cells expressing an INO80-specific shRNA (sh INO-7) or a control shRNA in the presence and absence of anti-SMC3 antibody, followed by PCR analysis to assess the presence of Sα, Eμ, Sμ, and Sγ1 (C). Data are represented as fold-enrichment calculated with ΔΔCT method of qPCR data analysis. Presented are the mean and SEM of 2 independent ChIP experiments.
Our results suggest that INO80 controls cohesin activity rather than modulating cohesin deposition at the immunoglobulin locus.
Reptin and Pontin interact with AID
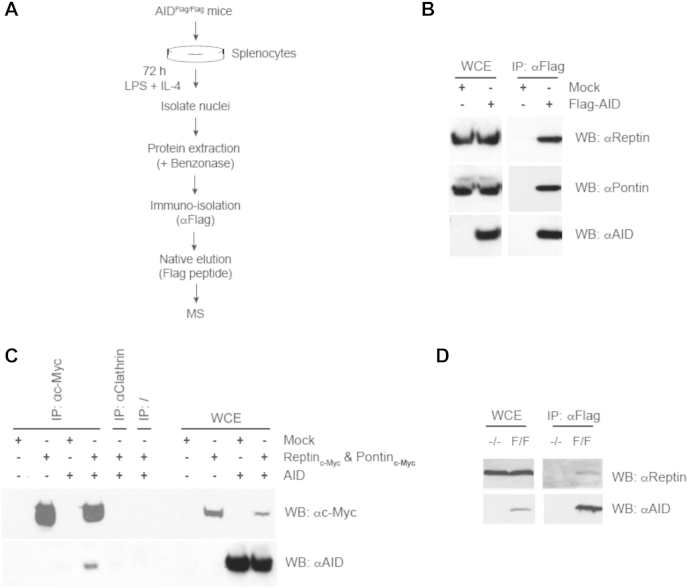
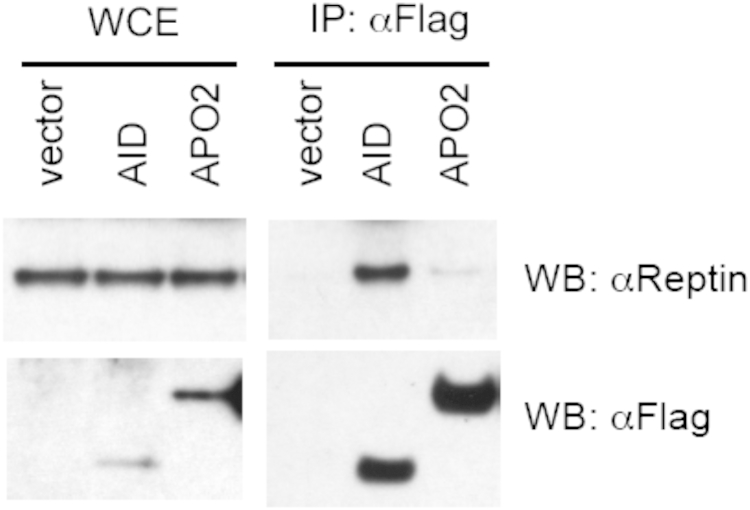
We adopted a complementary experimental approach by using mass spectrometry to study AID-containing complexes in AIDFlag/Flag splenocytes stimulated with LPS and IL-4 for 3 days (Fig 5, A). Reptin was found to co-purify with AID. To confirm that Reptin indeed interacts with AID, we transfected the 293 cell line derivative BOSC23 with Flag-AID. Cell lysates were then immunoprecipitated with anti-Flag antibodies. Endogenous Reptin was readily detected in anti-Flag immunoprecipitates from AID-transfected (but not mock-transfected) cells (Fig 5, B). Specific binding was confirmed in lysates from Flag-AID but not in lysates from control or Flag-Apobec2-transfected cells (see Fig E3 in this article's Online Repository, available at www.jacionline.org). Pontin was also immunoprecipitated by anti-Flag antibodies in lysates from Flag-AID transfected cells (Fig 5, B). We confirmed the interaction by performing the reverse pull-down in BOSC23 cells co-transfected respectively with c-myc-tagged Reptin (Reptinc-myc), Pontin (Pontinc-myc) and AID. Indeed, AID was seen to co-immunoprecipitate with Reptin and Pontin (Fig 5, C). Furthermore, Reptin also interacted with AID in AIDFlag/Flag splenocytes stimulated with LPS and IL-4 for 3 days (Fig 5, D). These results indicate that the INO80 complex ATPases Reptin and Pontin interact with AID.
Fig 5.
Reptin and Pontin interact with AID in vivo. A, A schematic representation of the immune-isolation protocol for AID-interacting proteins. B, AID and co-purifying proteins were isolated from Flag-AID-transfected BOSC23 cell extracts by immunoprecipitation with anti-Flag antibodies and then analysed by Western blotting with the indicated antibodies. C, c-myc-tagged Reptin (Reptinc-myc) and Pontin (Pontinc-myc) were immunoprecipitated with anti-c-myc antibody from lysates of BOSC23 cells expressing Reptinc-myc, Pontinc-myc, and AID and then analyzed by Western blotting with the indicated antibodies. Anti-Clathrin and “beads-only” (‘/’) immunoprecipitations were used as negative controls. Reptinc-myc and Pontinc-myc were always co-transfected because these proteins stabilize each other41 (Fig E4). D, Endogenous Reptin interacts with AID in switching splenocytes. Anti-Flag immunoprecipitates from lysates of IL-4- and LPS-stimulated AID−/− (−/−) and AIDFlag/Flag (F/F) splenocytes were analyzed with an antibody against endogenous Reptin.
Discussion
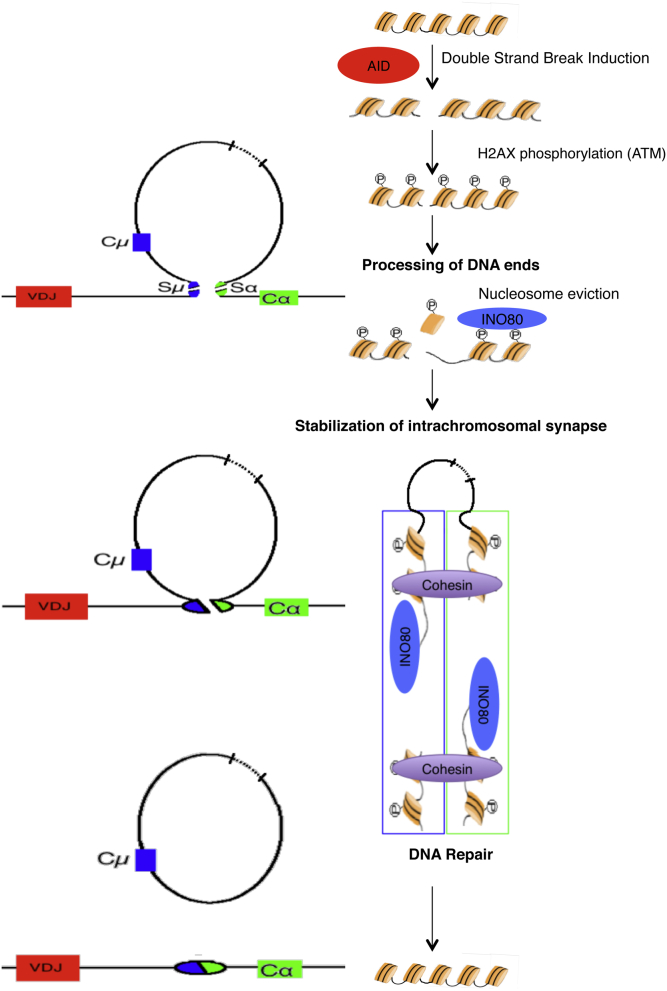
Our present results suggest that the INO80 chromatin-remodeling complex is involved in immunoglobulin CSR. In 2 CSR-deficient patients, 3 INO80 genetic variants were identified. Although the D145N mutation has never been reported, the allele frequency of the INO80 variations I882V and V1108G (0.009 and 0.004, respectively) in the 1000 Genome project data (May 2011) could suggest that both are predisposition variations rather than truly causative of the disease. However, because the mild γ-radiation sensitivity of patients' fibroblasts was corrected by transduction of wt INO80, impaired INO80 function appears to be involved in the pathogenesis of the disease. Moreover, in vitro experiments indicate an actual role for INO80 in CSR (Fig 6): Downregulation of INO80 and its partners Reptin and Pontin in CH12-F3 cells induced a significant decrease in immunoglobulin CSR. In ChIP experiments, INO80 was found to be bound to Sα and Eμ regions in murine CH12-F3 cells. Although the INO80 complex is a major transcriptional regulator, its downregulation did not affect S region transcription or expression of AID or YY1. However, our results do not completely rule out a role for the INO80 complex in the transcriptional regulation of other genes involved in CSR. CH12-F3 cells in which INO80 expression was downregulated were less viable, negatively selected in long-term cultures and more sensitive to DNA-damaging agents. These observations are consistent with previously reported data on the role of INO80 in DNA repair.20,21 However, we did not observe an increase in the frequency of chromosomal or chromatid breaks in metaphase of CH12-F3 cells expressing downregulated INO80; this result contrasts with the chromosomal instability seen in DNA-repair-deficient mice.31 Interestingly, we consistently observed an abnormal separation of sister chromatids following INO80 downregulation. These results suggest an effect of INO80 on cohesin recruitment and/or function. In addition, we detected the presence of both INO80 and the cohesin subunit SMC3 on Sα and Eμ regions. Cohesin deposition was not affected by INO80 knockdown, in agreement with yeast data showing that INO80 is essential for cohesin's function rather than its deposition.27 Collectively, these results suggest that INO80 modulates cohesin activity in B cells and may thus be involved in S-region synapsis during CSR. Furthermore, INO80 might promote switch synapsis formation by enhancing the flexibility of the chromatin fiber through local chromatin remodeling and nucleosome eviction, as recently described in Saccharomyces cerevisiae.32
Fig 6.
Putative INO80 function in immunoglobulin CSR. The different steps involved in immunoglobulin CSR are depicted. The induction of DNA double strand breaks by AID is followed by phosphorylation of histones H2AX through the kinase ataxia telangiectasia mutated (ATM). INO80 mediates nucleosome eviction, followed by the stabilization of intrachromosomal switch synapse together with cohesin, and subsequent switch region DNA repair.
Cohesin is also known to have a role in DNA repair. In yeast, the cohesin complex subunit NIPBL binds directly to double-strand breaks through interaction with γ-H2AX.33 This process requires molecules known to be involved in CSR, such as RNF168, the lack of which is responsible for a CSR deficiency in humans.34 Thus, poor survival of CH12F3 cells following INO80 downregulation may be caused (at least in part) by defective cohesin activity.
Our data suggest that INO80 potentially contributes to CSR at several steps—including S-region synapsis and DNA repair. These steps appear to be intimately connected during CSR. First, GLTs allow AID to exert its catalytic activity on single-stranded S region DNA.35 Second, it has been suggested that the C terminal portion of AID is involved in DNA repair.36,37 Third, functional involvement of AID in synapsis formation8 may be related to the INO80 complex, since we found that the INO80 complex subunits Reptin and Pontin interact with AID in switching primary B cells. These observations are further supported by a recent report describing interaction of AID with INO80, YY1, Reptin, Pontin, condensin, and cohesion complex proteins,38 possibly as a large molecular weight complex. Likewise, cohesin exerts a role at several steps in CSR, including switch synapsis30 and DNA repair.39 Defective CSR has been observed in some patients affected by Cornelia de Lange syndrome (caused by hemizygous mutations in genes encoding molecules of the cohesin complex)40 and in CH12-F3 cells after knockdown of cohesin (or its regulatory subunits).38 B lymphocytes from Cornelia de Lange syndrome patients presented with a defective in vitro CSR and an increased frequency of Sμ-Sα junctions with microhomologies,40 as observed in both patients carrying INO80 gene variations. These observations therefore suggest that the INO80 complex could mediate CSR by promoting cohesin activity through its chromatin remodeling activity.
Our data indicate that INO80 variations are associated with CSR-D. As IgA appears to be more affected than IgG, at least in P1, it is possible that it could also be associated with sIgAD. In contrast, more drastic mutations could be lethal or cause a more severe immunodeficiency associated with multiple additional developmental defects.
Key messages.
-
•
INO80, Reptin and Pontin function in immunoglobulin class-switch recombination.
-
•
Reptin and Pontin interact with activation-induced cytidine deaminase.
-
•
INO80 plays a role in sister chromatid cohesion, thus in cohesin activity.
-
•
Human INO80 deficiency appears to be associated with defective immunoglobulin class-switch recombination.
Acknowledgments
We thank T. Honjo (University of Kyoto) for CH12-F3 cells, J. Chaudhuri (Memorial Sloan Kettering Cancer Center, New York) for the anti-mouse AID antibody, David Root (RNAi Platform, Broad Institute) for shRNAs against Reptin and Pontin and C. Wu (US National Cancer Institute) for the anti-INO80 antibody.
Footnotes
This study was supported by the National Institute of Health and Medical Research, le fonds de recherche clinique du Ministère de la Santé, the European Union's Seventh Research and Technological Development Framework Programme (EUROPAD contract 201549 and ERC advanced grant PID-IMMUNE contract 249816), Association Contre Le Cancer, the Fondation pour la Recherche Médicale (grant number: ING20130526624), la Ligue Contre le Cancer (Comité de Paris) and the Agence Nationale de la Recherche (grant: 2010-CSRD) and a government grant managed by the French Agence Nationale de la Recherche as part of the Investments for the Future program (ANR-10-IAHU-01). This work in the Epigenetics group at the International Agency for Research on Cancer was supported by the La Ligue National contre le Cancer (France), Fondation ARC, France, and Institut National du Cancer (INCA), France. This study was also funded by the German Federal Ministry of Education and Research (grant: BMBF 01 EO 0803) and by the Canadian Gene Cure Foundation and Finding of Rare Disease Genes (FORGE) Canada. This work was supported in part by National Institutes of Health grant AI037526 to M.C.N. M.C.N. is a Howard Hughes Medical Institute investigator. S.K. is a Centre National de la Recherche Scientifique researcher.
Disclosure of potential conflict of interest: S. Kracker has received research support from EUROPAD contract 201549, Agence Nationale de la Recherche (grant: 2010-CSRD), Fondation pour la Recherche Médicale (grant no. ING20130526624), and la Ligue Contre le Cancer (Comité de Paris). M.-C. Deau has received research support from Fondation pour la Recherche Médicale (grant no. ING20130526624). S. Seneviratne is employed by the Royal Free Hospital. B. Grimbacher has received research support from Bundesministerium für Bildung und Forschung, the European Union, and Helmholtz; is employed by University College London and University Medical Center-Freiberg; and has received payment for lectures from CSL, Baxter, and Biotest. A. Fischer has received research support from the European Research Council: ERC advanced grant PID-IMMUNE contract 249816. A. Durandy has received research support from the European Union seventh framework program: EUROPAD contract 201549, Agence Nationale de la Recherche (grant: 2010-CSRD), and Association contre le cancer. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Patients
Blood samples were obtained after provision of informed consent by P2 and by P1's parents. The study was performed in accordance with the precepts of the Declaration of Helsinki.
Whole-exome sequencing
We followed standard manufacturer's protocols to perform targeted capture of DNA from P1 with the Agilent SureSelect 30 MB exome enrichment kit (Agilent, Santa Clara, Calif) and then sequencing of 76 bp single-end reads on an Illumina GAII system (Illumina, San Diego, Calif). This generated 2.3 Gb of sequence data and gave >20-fold mean coverage of consensus coding sequence bases.
Reads were aligned to hg19 for each sample with BWA 0.5.9 software.E1 Single nucleotide variants and short insertions and deletions were called using a SAMtools pileup procedureE2 and were then quality-filtered to require at least 20% of the reads supporting the variant call. Variants were annotated to determine the functional effect, the “1000 Genomes” allele frequency, and the dbSNP ID using Annovar.E3 Genes with homozygous or multiple heterozygous variants were studied for possible involvement in CSR-Ds.
Switch junction analysis was performed as described previously.E4 Statistical analyses were performed with the χ2 test, and statistically significant differences from control junctions are indicated.
Immunoblotting was performed using anti-AID (against mouse AID; a gift from J. Chaudhuri), anti-β-actin (Sigma-Aldrich, St Louis, Mo), anti-YY1 (H-414 from Santa Cruz Biotechnology, Santa Cruz, Calif), and anti-INO80 (Proteintech Group, Chicago, Ill) antibodies.
Knockdown with lentiviral shRNA
Lentiviral supernatants were produced by transiently transfected 293T cells. Cells were transfected with a packaging plasmid, a vector encoding the VSV-G envelope protein and the pLKO.1 shRNA vector via the use of Lipofectamine 2000 in accordance with the manufacturer's protocol (Invitrogen, Carlsbad, Calif). Viral supernatants were filtered through a 0.45 μm filter and mixed with polybrene (8 μg/mL). CH12-F3 cells were then resuspended in the viral supernatant at a concentration of 0.25 × 106 per mL. Cells were spin-infected in 12-well plates at 800 g for 2 hours at room temperature. The viral supernatant was removed, and cells were resuspended in fresh medium. Puromycin selection was initiated the next day, and the cells were cultured in puromycin-containing medium for the remainder of the experiment. The INO80-specific shRNAs (sh INO-7: TRCN0000096377; sh INO-6: TRCN0000096376) were obtained from Open Biosystems (Huntsville, Ala). AID-specific shRNAs were obtained from Sigma (sh AID-1: TRCN0000112032) and the Broad Institute RNAi Platform (sh AID-2: TRCN0000112031). Reptin- and Pontin-specific shRNAs and viral supernatants were supplied by the Broad Institute RNAi Platform (shReptin-1: TRCN0000115256; shReptin-2: TRCN0000115259; shPontin-1: TRCN0000115241; and shPontin-2: TRCN0000115244). The Q-PCR primers for measuring specific transcript levels were: 5′-CACCAAAGTCCCTGAGATCC-3′ (forward) and 5′-TTCCCTTCTCGGATCATCTC-3′ (reverse) for Reptin; 5′-TCAGGGTGGGCAAGATATTC-3′ (forward) and 5′-TGTGGACCTCATCGACAAAA-3′ (reverse) for Pontin.
Survival assay
CH12-F3 cells at a concentration of 2.5 × 104 per mL were treated with indicated doses of γ-radiation, UV light, or DNA damaging agents and cultured for 4 days. The proportion of viable cells was assessed by flow cytometry analysis by excluding dead cells (topro-3 positive) and by using counting beads (Invitrogen). For the complementation of INO80's function in the patients' fibroblast-derived cell lines, cells were infected with a lentiviral vector encoding wt INO80 and driven by the EF1α promoter (EF.PGK.GFP from Addgene, Cambridge, Mass). Because the vector also expresses green fluorescent protein (GFP) driven by the PGK promoter, infected cells were identified as GFP-positive in the flow cytometry analysis and their survival compared with that of untransfected cells (GFP-negative) in the same culture.
Stimulation of CH12-F3 cells for CSR
CH12-F3 cells (a gift from T. Honjo) were stimulated with anti-CD40 (1 μg/mL; HM40-3 from BD Bioscience, San Jose, Calif), murine IL-4 (10 ng/mL: Peprotech, Rocky Hill, NJ) and human TGF-β (2 ng/mL: R&D Systems, Abingdon, United Kingdom) at a concentration of 5 × 104 per mL.
Analysis of germ-line transcripts and AID messenger RNA
Total RNA was extracted from CSR-stimulated CH12-F3 cells after 2 days of stimulation with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and in accordance with the manufacturer's instructions. The cDNA was synthesized with Superscript II (Invitrogen) and an equal mixture of oligo(dT) and random hexamers, in accordance with the manufacturer's instructions. Primers for quantitative PCR analysis of AID and Sμ and Sα GLTs are described elsewhere.E5
Telomere fluorescence in situ hybridization
CH12-F3 cells were incubated in colcemid (KaryoMAX, GIBCO/Life Technologies, Paisley, United Kingdom), swollen in prewarmed 30 mM sodium citrate for 25 minutes at 37°C, and then fixed with freshly prepared, ice-cold methanol/acetic acid (3/1). Metaphase preparations were hybridized with a Cy3-labelled peptide nucleic acid telomeric probe in 70% formamide and covered with DAPI-containing mounting medium (Vectashield, Vector Laboratories, Burlingame, Calif).
Chromatin immunoprecipitation
Chromatin immunoprecipitation with anti-INO80 (a gift from C. Wu) and anti-SMC3 antibody (ab9263, Abcam, Cambridge, United Kingdom) was performed as described elsewhere.E6 The PCR primers for Eμ were 5′-CCCCCTAAAGCAATGACTGA-3′ (EmuF1) and 5′-GACTCTGGACCTCTCCGAAA-3′ (EmuR1). Those for the S alpha regionE7 and Sμ, Sγ1, and Sγ3 have been described elsewhere.E8
Immuno-isolation of AID-interacting proteins
In order to avoid overexpression of artefacts and to study AID and its interacting proteins in their true physiological context, we used mice bearing a Flag-tagged AID allele (AIDFlag/Flag),E5 because AIDFlag is expressed, induces CSR, and is phosphorylated in the same way as wt AID (E5). A total of 1010 splenocytes were isolated from AIDFlag/Flag mice by anti-CD43 magnetic cell sorting bead depletion (Miltenyi Biotech, Bergisch Gladbach, Germany). B cells were cultured in LPS and IL-4 for 72 hours, and nuclei were purified on a sucrose cushion as described.E9 To obtain chromatin-associated AID and avoid nonspecific interactions due to co-precipitation of DNA/RNA, lysates were treated with Benzonase nuclease (Sigma) in low-salt buffer (20 mM HEPES, 10 mM KCl, 1 mM MgCl2, 10% glycerol, 1% NP40) and sonicated. The supernatant was adjusted to 150 mM NaCl, cleared with mouse IgG and protein G agarose, immunoprecipitated with anti-Flag agarose (Sigma) and eluted with Flag peptide (Fig 6, A). The resulting co-purifying proteins were identified by mass spectrometry. To confirm interaction, co-immunoprecipitation analyses were performed as described previously.E5 The antibodies used for immunoprecipitation and Western blot analysis were anti-AID,E10 anti-c-Myc (9E1, Santa Cruz Biotechnology), anti-clathrin HC (TD.1, Santa Cruz Biotechnology), anti-Flag M2 (Sigma), anti-Reptin (Abcam), and anti-Pontin (Proteintech Group).
Fig E1.
Evolutionary conservation analysis of the INO80 protein at the amino-acid substitution positions identified in 2 CSR-D patients. Protein sequences from the indicated mammalian and vertebrate species were aligned with ClustalW2. The positions of amino acid substitutions identified in the CSR-D patients are highlighted in yellow.
Fig E2.
Evidence for a defect in cell survival and sensitivity to DNA-damaging agents in CH12-F3 INO80-knockdown cells. Immunoblot analysis of INO80 and GAPDH in whole-cell extracts of CH12-F3 cells expressing indicated shRNA (A). Analysis of apoptosis; geometric mean for annexin V staining is indicated (B). Dead cells were excluded by topro-3 incorporation. Counter selection of CH12-F3 cells expressing INO80-specific shRNA (C). Proliferation profile of CH12-F3 cells expressing INO80-specific or control shRNAs (D). Increased sensitivity to γ-radiation, UV radiation, and mitomycin C (MMC) treatment in CH12-F3 cells expressing INO80-specific shRNA (sh INO-7), compared with a nonspecific, control shRNA (sh scramble) (E).
Fig E3.
Specific interaction of AID with Reptin. Whole-cell extract (WCE) from BOSC23 cells transfected with Flag-AID (AID) or Apobec2 (APO2) were immunoprecipitated with anti-Flag antibodies and blotted with an anti-Reptin antibody. The AID:Apobec2 transfected DNA ratio was 10:1, in order to bring down Apobec2 protein expression level within the range for AID.
Fig E4.
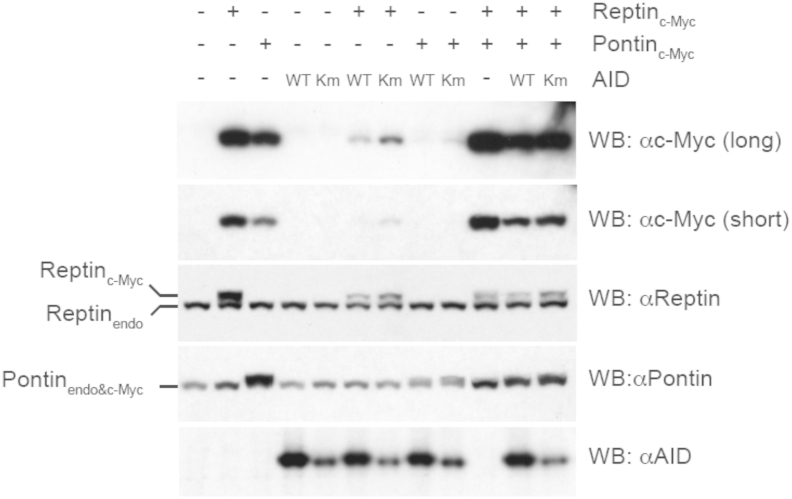
Co-expression of Reptin and Pontin is required to stabilize Pontin protein levels during co-transfection with AID. Total cell lysates from BOSC23 cells transfected with c-Myc tagged-Reptin (ReptincMyc), -Pontin (PontincMyc), and/or either wild-type (WT) or mutated Kozak (Km) AID were analyzed by Western blotting with the indicated sera. “Reptin/Pontin-endo” indicates endogenous protein. The 2 upper panels represent long and short exposure times of the same anti-cMyc blot. Reptin and Pontin proteins both have molecular weights of around 50 KDa and migrate very closely to each other on SDS-PAGE gels.
References
- 1.Durandy A., Taubenheim N., Peron S., Fischer A. Pathophysiology of B-cell intrinsic immunoglobulin class switch recombination deficiencies. Adv Immunol. 2007;94:275–306. doi: 10.1016/S0065-2776(06)94009-7. [DOI] [PubMed] [Google Scholar]
- 2.Korthauer U., Graf D., Mages H.W., Briere F., Padayachee M., Malcolm S. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 3.Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 4.Imai K., Slupphaug G., Lee W.I., Revy P., Nonoyama S., Catalan N. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 5.Pan Q., Petit-Frere C., Lahdesmaki A., Gregorek H., Chrzanowska K.H., Hammarstrom L. Alternative end joining during switch recombination in patients with ataxia-telangiectasia. Eur J Immunol. 2002;32:1300–1308. doi: 10.1002/1521-4141(200205)32:5<1300::AID-IMMU1300>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Peron S., Metin A., Gardes P., Alyanakian M.A., Sheridan E., Kratz C.P. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J Exp Med. 2008;205:2465–2472. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan-Hammarstrom Q., Jones A.M., Lahdesmaki A., Zhou W., Gatti R.A., Hammarstrom L. Impact of DNA ligase IV on nonhomologous end joining pathways during class switch recombination in human cells. J Exp Med. 2005;201:189–194. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuerffel R., Wang L., Grigera F., Manis J., Selsing E., Perlot T. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabouri S., Kobayashi M., Begum N.A., Xu J., Hirota K., Honjo T. C-terminal region of activation-induced cytidine deaminase (AID) is required for efficient class switch recombination and gene conversion. Proc Natl Acad Sci U S A. 2014;111:2253–2258. doi: 10.1073/pnas.1324057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs J.A., Nussenzweig M.C., Nussenzweig A. Chromatin dynamics and the preservation of genetic information. Nature. 2007;447:951–958. doi: 10.1038/nature05980. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran S., Chahwan R., Nepal R.M., Frieder D., Panier S., Roa S. The RNF8/RNF168 ubiquitin ligase cascade facilitates class switch recombination. Proc Natl Acad Sci U S A. 2010;107:809–814. doi: 10.1073/pnas.0913790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celeste A., Petersen S., Romanienko P.J., Fernandez-Capetillo O., Chen H.T., Sedelnikova O.A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schotta G., Sengupta R., Kubicek S., Malin S., Kauer M., Callen E. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev. 2008;22:2048–2061. doi: 10.1101/gad.476008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang F.L., Luo Z., Scharff M.D. H3 trimethyl K9 and H3 acetyl K9 chromatin modifications are associated with class switch recombination. Proc Natl Acad Sci U S A. 2009;106:5288–5293. doi: 10.1073/pnas.0901368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanlie A., Aida M., Muramatsu M., Honjo T., Begum N.A. Histone3 lysine4 trimethylation regulated by the facilitates chromatin transcription complex is critical for DNA cleavage in class switch recombination. Proc Natl Acad Sci U S A. 2010;107:22190–22195. doi: 10.1073/pnas.1016923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusser A., Kadonaga J.T. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003;25:1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- 17.Shen X., Mizuguchi G., Hamiche A., Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 18.Conaway R.C., Conaway J.W. The INO80 chromatin remodeling complex in transcription, replication and repair. Trends Biochem Sci. 2009;34:71–77. doi: 10.1016/j.tibs.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Morrison A.J., Shen X. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat Rev Mol Cell Biol. 2009;10:373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gospodinov A., Vaissiere T., Krastev D.B., Legube G., Anachkova B., Herceg Z. Mammalian Ino80 mediates double-strand break repair through its role in DNA end strand resection. Mol Cell Biol. 2011;31:4735–4745. doi: 10.1128/MCB.06182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y., Wang X., Bao S., Guo R., Johnson D.G., Shen X. INO80 chromatin remodeling complex promotes the removal of UV lesions by the nucleotide excision repair pathway. Proc Natl Acad Sci U S A. 2010;107:17274–17279. doi: 10.1073/pnas.1008388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park E.J., Hur S.K., Kwon J. Human INO80 chromatin-remodelling complex contributes to DNA double-strand break repair via the expression of Rad54B and XRCC3 genes. Biochem J. 2010;431:179–187. doi: 10.1042/BJ20100988. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y., Jin J., Yao T., Gottschalk A.J., Swanson S.K., Wu S. YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol. 2007;14:872–874. doi: 10.1038/nsmb1276. [DOI] [PubMed] [Google Scholar]
- 24.Green M.R., Monti S., Dalla-Favera R., Pasqualucci L., Walsh N.C., Schmidt-Supprian M. Signatures of murine B-cell development implicate Yy1 as a regulator of the germinal center-specific program. Proc Natl Acad Sci U S A. 2011;108:2873–2878. doi: 10.1073/pnas.1019537108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaprazna K., Atchison M.L. YY1 controls immunoglobulin class switch recombination and nuclear activation-induced deaminase levels. Mol Cell Biol. 2012;32:1542–1554. doi: 10.1128/MCB.05989-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min J.N., Tian Y., Xiao Y., Wu L., Li L., Chang S. The mINO80 chromatin remodeling complex is required for efficient telomere replication and maintenance of genome stability. Cell Res. 2013;23:1396–1413. doi: 10.1038/cr.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogiwara H., Enomoto T., Seki M. The INO80 chromatin remodeling complex functions in sister chromatid cohesion. Cell Cycle. 2007;6:1090–1095. doi: 10.4161/cc.6.9.4130. [DOI] [PubMed] [Google Scholar]
- 28.Kagey M.H., Newman J.J., Bilodeau S., Zhan Y., Orlando D.A., van Berkum N.L. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seitan V.C., Hao B., Tachibana-Konwalski K., Lavagnolli T., Mira-Bontenbal H., Brown K.E. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature. 2011;476:467–471. doi: 10.1038/nature10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S., Ju Z., Hassan R., Volpi S.A., Emelyanov A.V., Birshtein B.K. Dynamic changes in binding of immunoglobulin heavy chain 3′ regulatory region to protein factors during class switching. J Biol Chem. 2011;286:29303–29312. doi: 10.1074/jbc.M111.243543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franco S., Gostissa M., Zha S., Lombard D.B., Murphy M.M., Zarrin A.A. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Neumann F.R., Dion V., Gehlen L.R., Tsai-Pflugfelder M., Schmid R., Taddei A. Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev. 2012;26:369–383. doi: 10.1101/gad.176156.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oka Y., Suzuki K., Yamauchi M., Mitsutake N., Yamashita S. Recruitment of the cohesin loading factor NIPBL to DNA double-strand breaks depends on MDC1, RNF168 and HP1gamma in human cells. Biochem Biophys Res Commun. 2011;411:762–767. doi: 10.1016/j.bbrc.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Stewart G.S., Panier S., Townsend K., Al-Hakim A.K., Kolas N.K., Miller E.S. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri J., Tian M., Khuong C., Chua K., Pinaud E., Alt F.W. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 36.Ta V.T., Nagaoka H., Catalan N., Durandy A., Fischer A., Imai K. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 37.Kracker S., Imai K., Gardes P., Ochs H.D., Fischer A., Durandy A.H. Impaired induction of DNA lesions during immunoglobulin class-switch recombination in humans influences end-joining repair. Proc Natl Acad Sci U S A. 2010;107:22225–22230. doi: 10.1073/pnas.1012591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas-Claudepierre A.S., Schiavo E., Heyer V., Fournier M., Page A., Robert I. The cohesin complex regulates immunoglobulin class switch recombination. J Exp Med. 2013;210:2495–2502. doi: 10.1084/jem.20130166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorsett D., Strom L. The ancient and evolving roles of cohesin in gene expression and DNA repair. Curr Biol. 2012;22:R240–R250. doi: 10.1016/j.cub.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enervald E., Du L., Visnes T., Bjorkman A., Lindgren E., Wincent J. A regulatory role for the cohesin loader NIPBL in nonhomologous end joining during immunoglobulin class switch recombination. J Exp Med. 2013;210:2503–2513. doi: 10.1084/jem.20130168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venteicher A.S., Meng Z., Mason P.J., Veenstra T.D., Artandi S.E. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracker S., Imai K., Gardes P., Ochs H.D., Fischer A., Durandy A.H. Impaired induction of DNA lesions during immunoglobulin class-switch recombination in humans influences end-joining repair. Proc Natl Acad Sci U S A. 2010;107:22225–22230. doi: 10.1073/pnas.1012591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavri R., Gazumyan A., Jankovic M., Di Virgilio M., Klein I., Ansarah-Sobrinho C. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. 2010;143:122–133. doi: 10.1016/j.cell.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodinov A., Vaissiere T., Krastev D.B., Legube G., Anachkova B., Herceg Z. Mammalian Ino80 mediates double-strand break repair through its role in DNA end strand resection. Mol Cell Biol. 2011;31:4735–4745. doi: 10.1128/MCB.06182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U., Meng F.L., Keim C., Grinstein V., Pefanis E., Eccleston J. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144:353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong B.Q., Lee M., Kabir S., Irimia C., Macchiarulo S., McKnight G.S. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat Immunol. 2009;10:420–426. doi: 10.1038/ni.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride K.M., Gazumyan A., Woo E.M., Barreto V.M., Robbiani D.F., Chait B.T. Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc Natl Acad Sci U S A. 2006;103:8798–8803. doi: 10.1073/pnas.0603272103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride K.M., Barreto V., Ramiro A.R., Stavropoulos P., Nussenzweig M.C. Somatic hypermutation is limited by CRM1-dependent nuclear export of activation-induced deaminase. J Exp Med. 2004;199:1235–1244. doi: 10.1084/jem.20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]