Abstract
Cardiac resynchronization therapy (CRT) is a novel therapy for patients with refractory heart failure (HF). Large clinical trials evaluating CRT have demonstrated significant improvements in cardiac survival, decreases in recurrent HF hospitalization, and improvements in indexes of quality of life. Although numerous mechanisms are involved in CRT’s therapeutic effects, correction of both interventricular and intraventricular mechanical dyssynchrony has been postulated as the key mechanism. To date, most large randomized controlled trials evaluating CRT have identified dyssynchronous patients on the basis of prolongation of the QRS complex from the baseline electrocardiogram. Concerns have been raised regarding the use of this measure for patient selection, stemming from a significant 30% to 40% nonresponse rate to CRT. Because of the cost and invasive nature of CRT, optimal patient selection for this therapy has become a priority for HF specialists and electrophysiologists. Cardiac imaging modalities have attempted to fulfill this need to improve patient selection by identifying mechanical dyssynchrony. Although early echocardiographic studies reported promising results, more recent larger scale studies have curtailed this enthusiasm, with a lack of established selection criteria for CRT in the current practice guidelines. This review summarizes the evidence to date and the potential role of imaging modalities in the selection and care of patients with HF referred for CRT.
Keywords: cardiac computed tomography, cardiac magnetic resonance imaging, cardiac resynchronization therapy, noninvasive imaging, radionuclide imaging
The emergence of cardiac resynchronization therapy (CRT) has provided cardiac caregivers with a novel therapeutic option for patients with refractory heart failure (HF). Large, prospective clinical studies have demonstrated that CRT can provide significant improvement of both cardiac mortality and morbidity (Table 1 [1-6]). However, up to 30% to 40% of patients do not respond to CRT (1,2). CRT is invasive and carries considerable costs related to implantation, serial device monitoring, and requirement for multiple device exchanges over a patient’s lifetime. The recently published Resynchronization/Defibrillation for Ambulatory Heart Failure Trial found the incidence of serious complications in the CRT-defibrillator arm, such as ventricular perforation or device-related infection, to be 13%, which was considerably higher than in the implantable cardioverter-defibrillator arm (7). Furthermore, recent clinical trials have found that even patients with mildly symptomatic HF receive mortality benefit with CRT (7,8) (Table 2 [6-10]).
Table 1.
Landmark CRT Trials
| Study | n | Inclusion Criteria | QRS (ms) | Design | Blinding | Control | Intervention | Follow-Up (months) |
Primary End Point | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| MIRACLE (4) | 453 | NYHA functional class III or IV, LVEF ≤ 35%, LVEDD ≥ 55 mm, SR |
≥130 | Parallel | Double blind |
CRT-Off | CRT-On | 6 | 6MWD, NYHA functional class, MLHFQ score |
↑ 6MWD (p = 0.005), improved NYHA functional class (p < 0.001), ↓ MLHFQ score (p = 0.001) |
| MIRACLE-ICD (5) | 369 | NYHA functional class III or IV, LVEF ≤ 35%, LVEDD ≥ 55 mm, ICD indicated, SR |
≥130 | Parallel | Double blind |
CRT-D (CRT-Off) |
CRT-D (CRT-On) | 6 | Peak VO2, NYHA class, MLHFQ score |
↑ 6MWD (p = 0.36), improved NYHA functional class (p = 0.007), ↓ MLHFQ score (p = 0.02) |
| Contak CD (6) | 490 | NYHA functional class II–IV, LVEF ≤ 35%, ICD indicated, SR |
≥120 | Parallel | Double blind |
CRT-D (CRT-Off) |
CRT-D (CRT-On) | 6 | Composite: all-cause mortality, HF hospitalization, VT/VF requiring intervention |
RRR 15% (p = 0.35) |
| COMPANION (1) | 1,520 | NYHA functional class III or IV, LVEF ≤ 35%, LVEDD ≥ 60 mm, SR |
≥120 | 1:2:2 | — | Medical therapy |
CRT-P, CRT-D | 16.2 (median) | All-cause mortality or hospitalization |
CRT-P HR: 0.81 (95% CI: 0.69−0.96; p = 0.014); CRT-D HR: 0.80 (95% CI: 0.68–0.95; p = 0.010) |
| CARE-HF (3) | 813 | NYHA functional class III or IV, LVEF ≤ 35%, SR |
≥150, 120−150 + echocardiographic dyssynchrony |
Parallel | — | Medical therapy |
CRT-P | 29.4 (mean) | All-cause mortality or CV hospitalization |
HR: 0.63 (95% CI: 0.51−0.77; p < 0.001) |
| CARE-HF Extended (2) |
37.4 | All-cause mortality | HR: 0.60 (95% CI: 0.47−0.77; p < 0.0001) |
CARE-HF = Cardiac Resynchronization–Heart Failure; CI = confidence interval; COMPANION = Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with defibrillator; CRT-P = cardiac resynchronization therapy only; HF = heart failure; HR = hazard ratio; ICD = implantable cardioverter-defibrillator; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction; MIRACLE = Multicenter InSync Randomized Clinical Evaluation; MIRACLE-ICD = Multicenter InSync ICD Randomized Clinical Evaluation; MLHFQ = Minnesota Living With Heart Failure Questionnaire; NYHA = New York Heart Association; RRR = relative risk ratio; 6MWD = 6-minute walk distance; SR = sinus rhythm; VF = ventricular fibrillation; VO2 = oxygen consumption; VT = ventricular tachycardia; ↑ = increased; ↓ = decreased.
Table 2.
CRT Trials in Mildly Symptomatic Heart Failure (NYHA Functional Classes I and II)
| Study | n | Inclusion Criteria | Design | Blinding | Control | Intervention | Follow-Up | Primary End Point | Response |
|---|---|---|---|---|---|---|---|---|---|
| Contak CD (6) | SeeTable1 | ||||||||
| MIRACLE-ICD 2 (9) | 186 | NYHA functional class II, LVEF ≤ 35%, QRS ≥ 130 ms, LVEDD ≥ 55 mm, ICD indicated, SR |
Parallel | Double blind | CRT-D (CRT-Off) | CRT-D (CRT-On) | 6 months | Change in peak VO2 | ↑ Peak VO2 (p = 0.87) |
| REVERSE (10) | 610 | NYHA functional class I or II, QRS ≥ 120 ms, LVEF ≤ 40%, LVEDD ≥ 55 mm, SR |
Parallel | Double blind | CRT-D (CRT-Off) | CRT-D (CRT-On) | 24 months | HF clinical composite score (worsening) |
19% vs. 34% (p = 0.01) |
| MADIT-CRT (8) | 1,820 | NYHA functional class I or II, LVEF ≤ 30%, QRS ≥ 130 ms, SR |
3:2 | Single blind | ICD | CRT-D | 2.4 yr (mean) | All-cause mortality or nonfatal HF |
HR: 0.66 (95% CI: 0.52− 0.84; p = 0.001) |
| RAFT (7) | 1,798 | NYHA functional class II (80%) or III (20%), LVEF ≤ 30%, QRS ≥ 120 ms |
Parallel | Double blind | CRT-D (CRT-Off) | CRT-D (CRT-On) | 40 months (mean) | All-cause mortality or HF hospitalization |
HR: 0.75 (95% CI: 0.64− 0.87; p < 0.001) |
MADIT-CRT = Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy; RAFT = Resynchronization/Defibrillation for Ambulatory Heart Failure Trial; REVERSE = Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction; other abbreviations as in Table 1.
Cumulatively, these factors underscore a need for improved patient selection of this novel therapy to achieve optimal therapeutic cost-effectiveness. Current evidence indicates that cardiac imaging can identify and quantify the severity of mechanical dyssynchrony in patients beyond the presence of a widened QRS complex on electrocardiography. Although novel echocardiographic parameters were reported to be promising in detecting mechanical dyssynchrony in single-center studies, such enthusiasm has been curbed by the negative results from the recent prospective multicenter PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study. We anticipate that new echocardiographic parameters will continue to emerge. However, novel imaging modalities, such as cardiac magnetic resonance (CMR) can assess mechanical dyssynchrony differently from echocardiography from a technical perspective. CMR can also provide the location of myocardial scar and coronary venous anatomy, which influence the likelihood for success of CRT. In this report, we aim to review the evidence in assessing mechanical dyssynchrony by various noninvasive imaging modalities and their clinical relevance.
Mechanism
The main rationale for CRT stems from resynchronization of left ventricular (LV) contraction. Dyssynchronous contraction of the left ventricle over time may contribute to progressive negative remodeling and worsen patient symptoms and has been associated with increased mortality and adverse cardiovascular outcomes.
Patients with a left bundle branch block can have atrioventricular, interventricular, and intraventricular dyssynchrony, each with respective adverse ramifications on ventricular function. Interventricular dyssynchrony results from septal depolarization from the right ventricular bundle, as opposed to physiologic left-sided activation, leading to asynchronous right and LV contraction. Intraventricular dyssynchrony within the left ventricle itself results from septal and anteroseptal activation before the lateral and inferolateral segments, resulting in regional load disparities that can produce increased wall tension. Discoordinated papillary muscle activation during systole leads to abnormal mitral valve closure, with the development or worsening of pre-existing mitral regurgitation (11).
CRT may confer benefits by coordinating right ventricular and LV contraction, synchronizing the LV segments, prolonging the diastolic filling period with improvements of both coronary and LV filling, and restoring atrioventricular synchrony. The Cardiac Resynchronization–Heart Failure trial revealed that CRT can provide up to a 30% improvement in stroke volume and a significant reduction in mitral regurgitation within 3 months of initiating therapy (3). These beneficial effects on cardiac hemodynamic parameters are associated with reduced myocardial energy cost and may indirectly attenuate maladaptive neurohormonal activation and autonomic dysfunction. Recent studies have indicated that correction of intraventricular dyssynchrony, compared with interventricular dyssynchrony, plays a more significant role in the efficacy of CRT (12).
Beyond the Electrocardiogram
Electrocardiography has numerous limitations for identifying patients suitable for CRT. The threshold criteria of a QRS duration of ≥120 ms was not derived from prospective evaluation but rather from inclusion criteria of landmark clinical trials (1,3,4). In fact, the mean and median values of QRS durations of patients enrolled in the largest CRT trials were substantially higher than 120 ms (ranging from 155 to 160 ms) (Table 1). Echocardiographic studies have revealed a significant discordance between electrical and mechanical delay, with up to 30% of patients with QRS durations >150 ms and 50% of those with QRS durations >120 ms demonstrating no evidence of septal to lateral dyssynchrony (13). The QRS pattern may also further complicate patient selection. More than 73% of patients in the CRT arm of the Resynchronization/Defibrillation for Ambulatory Heart Failure Trial had interventricular conduction delays with left bundle branch block patterns (7). Whether patients with right bundle branch block or nonspecific interventricular conduction delays would derive the same benefit from CRT remains unclear. Retrospective examination of landmark CRT trials demonstrated no benefit of CRT in patients with right bundle branch block for any hard clinical end point (14).
Echocardiographic data suggest that up to 30% to 50% of patients with symptomatic HF with compromised LV ejection fractions (LVEFs) and narrow QRS complexes (<120 ms) have evidence of mechanical dyssynchrony (15). The utility of CRT in this patient population was evaluated in the Cardiac Resynchronization Therapy in Patients With Heart Failure and Narrow QRS study, which included patients with QRS durations < 130 ms and echocardiographic evidence of mechanical dyssynchrony. The study demonstrated no difference for the primary end point of peak oxygen uptake at 6 months but found improvement in functional class (16). Although other studies also failed to demonstrate any difference in secondary outcome measures, such as LVEF, no study to date has evaluated hard clinical end points in this population (Table 3 [17-19]).
Table 3.
CRT Trials With Narrow QRS Complex (≤120 ms)
| Study | n | Inclusion Criteria | Dyssynchrony | Blinding | Control/Intervention | Follow-Up (months) |
Primary End Point | Primary End Point | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Yu et al (18) | 51 | NYHA functional class III or IV, LVEF < 40%, QRS < 120 ms |
Yu index ≥ 32.6 ms | Single blind | QRS > 120 ms/QRS < 120 ms | 3 | NYHA functional class, 6MWD, LVESV, LVEF, MR (p = significant for all) | |||
| ESTEEM-CRT (19) |
67 | NYHA functional class III, LVEF ≤ 35%, QRS > 120 ms |
Yu index > 28.7 ms | Single blind | Nil/CRT-D | 12 | NYHA functional class + MLHFQ |
p < 0.0001 | LVEDV, LVESV | p < 0.0001 |
| RethinQ (17) | 172 | NYHA functional class III, LVEF ≤ 35%, QRS < 120 ms |
M-mode echocardiography, pulsed-wave Doppler, color TDI |
Double blind |
CRT-Off/CRT-On | 6 | Peak VO2 | p = NS | NYHA functional class |
29% vs. 54% (p = 0.006) |
| MLHFQ | p = NS | |||||||||
ESTEEM-CRT = Evaluation of CRT in Narrow QRS Patients With Mechanical Dyssynchrony From a Multicenter Study; LVESV = left ventricular end-systolic volume; MR = mitral regurgitation; RethinQ = Cardiac Resynchronization Therapy in Patients With Heart Failure and Narrow QRS; TDI = tissue Doppler imaging; other abbreviations as in Table 1.
These data underscore the importance of other diagnostic modalities that may more precisely identify potential responders of CRT, including patients who not meet current clinical selection criteria.
Echocardiography
Over the past decade, echocardiography has emerged as a potentially useful tool to assess mechanical dyssynchrony. There are numerous advantages of echocardiography, including noninvasiveness, portability, and widespread availability. Numerous echocardiographic techniques have been devised to assess for dyssynchrony, including M mode, pulsed-wave Doppler imaging, tissue Doppler imaging (TDI), speckle tracking, and real-time 3-dimensional (RT3D) imaging (Tables 3 and 4 [12,20-38]).
Table 4.
Echocardiographic Dyssynchrony Assessment
| Method | Threshold (ms) | Studies | Limitations | Attributes |
|---|---|---|---|---|
| Pulsed-wave Doppler | ||||
| IVMD | >44 | Achilli et al. (21): ↑ LVEF ≥ 5% + NYHA functional class |
Requires stable heart rate intervals Affected by RV function |
Highly reproducible |
| LV filling time/R-R interval |
<40% | Requires nearly parallel interrogation of MV inflow |
Highly reproducible | |
| M-mode echocardiography | ||||
| SPWD | >30 | Pitzalis et al. (22): ↑ LVEF ≥ 5% Marcus et al. (23): not predictive |
Difficult in patients with wall motion abnormalities (prior MI or scar) Cannot differentiate active + passive motion |
High temporal resolution High temporal resolution Easily trained No specialized software required |
| TDI: pulsed wave | ||||
| LVDYS | >102 | Penicka et al. (24): ↑ LVEF ≥ 25% Achilli et al. (21): not predictive |
Cannot differentiate active + passive motion Technically challenging Requires optimal image quality Angle dependent Cannot assess multiple segments Cannot assess multiple segments in 1 view |
High temporal resolution No special probe required |
| TPSV-SD (6 segments) | >31.3 | Jansen et al. (31): ↓ LVESV ≥ 15% | ||
| TOSV-DF | >60 | Soliman et al. (32): not predictive | ||
| Color TDI | ||||
| TPSV-DF (2 segments) | >60–65 | Bax et al. (12): ↓ LVESV ≥ 15% | Cannot differentiate active + passive motion Requires technical expertise |
High temporal resolution Multiple segments may be assessed in 1 view |
| TPSV-DF (6 segments) | >105–110 | Knebel et al. (20): ↓ LVESV ≥ 15% | ||
| SPWD | >130 | Bleeker et al. (33): ↓ LVESV ≥ 10% | ||
| Yu index | >31.4–33 | Yu et al. (25,26): ↓ LVESV ≥ 15% Gorcsan et al. (34): death/transplantation/ LVAD implantation |
||
| TSI | ||||
| TPSV-DF | >65 | Van de Veire et al. (35): ↓ LVESV ≥ 15% + NYHA, 6MWD |
||
| Yu index | >34.4 | Yu et al. (36): ↓ LVESV ≥ 15% | ||
| TDI strain | ||||
| TPSS-DF (radial) | >130 | Dohi et al. (37): ↑ SV ≥ 15% | Highly angle dependent Requires technical expertise Requires optimal image quality |
May differentiate between passive + active motion High temporal resolution |
| TPSS-SD (12 segments) | 60 | Mele et al. (38): ↓ LVESV ≥ 15% | ||
| Speckle tracking | ||||
| SPWD (radial) | >130 | Suffoletto et al. (27): ↑ SV ≥ 15% Gorcsan et al. (34): death/transplantation/LVAD implantation STAR study (28): death/transplantation/LVAD implantation Delgado et al. (29): mortality + HF hospitalization |
Requires optimal image quality with high frame rate Requires technical expertise Speckles must remain in imaging plane |
Angle independent May perform offline analysis on routine images May assess radial, longitudinal, and circumferential strain |
| RT3D echocardiography | ||||
| LVDYS index (16 segments) |
>5.6% | Marsan et al. (30): ↓ LVESV ≥ 15% | Slower frame rates Requires optimal image quality Inability to differentiate active + passive motion Lower temporal + spatial resolution Requires regular rhythm Requires special ultrasound probe |
More complete 3D analysis Angle independent Assess radial, longitudinal, and circumferential contraction Accurate LVEF assessment |
DF = difference; IVMD = interventricular mechanical delay; LV = left ventricular; LVAD = left ventricular assist device; LVDYS = left ventricular dyssynchrony; LVEF = left ventricular ejection fraction; MV = mitral valve; NYHA = New York Heart Association; RT3D = real-time 3-dimensional; RV = right ventricular; SPWD = septal to posterior wall delay; STAR = Speckle Tracking and Resynchronization; 3D = 3-dimensional; TPSS = time to peak systolic strain; TPSV = time to peak systolic velocity; TSI = tissue synchronization imaging; other abbreviations as in Tables 1 and 3.
Interventricular dyssynchrony could be identified by traditional 2-dimensional echocardiographic parameters, but intraventricular dyssynchrony is primarily assessed by quantitative echocardiographic methods. The difference of the pre-systolic period, measured from the onset of the QRS complex on the electrocardiogram to the initiation of Doppler flow in the pulmonary artery and the aorta, respectively, may be used to measure interventricular dyssynchrony. A difference of the pre-systolic period of 49 ms, as a marker of interventricular dyssynchrony, has been shown in retrospective analysis of the CARE-HF (Cardiac Resynchronization–Heart Failure) trial to correlate with event-free survival (20), as well as an independent predictor of response in the SCART (Selection of Candidates for Cardiac Resynchronization Therapy) trial (21). Another method of assessing interventricular dyssynchrony uses pulsed-wave tissue Doppler to measure the difference in time to contraction of the right ventricle and the lateral wall of the left ventricle visualized with the apical 4-chamber view. This parameter, however, has not shown any association with CRT outcomes. Other pulsed-wave Doppler techniques for dyssynchrony assessment include quantitation of the physiological intervals of the cardiac cycle, such as filling and isovolumetric contraction times.
M-mode echocardiography
M-mode echocardiography can assess septal to posterior wall motion delay obtained from the short-axis parasternal view of the left ventricle (Fig. 1). More recent color M-mode imaging makes use of TDI (see the following discussion) for quantification of septal to posterior wall motion delay (Fig. 2). Although M-mode echocardiography provides extremely high temporal resolution, there are several limitations, including the depth of ultrasound penetration, angulation issues across acoustic windows of the chest wall, and the requirement for myocardial walls to be as perpendicular to the transducer as possible. Assessment by M-mode imaging is especially challenging if the myocardial segments are akinetic because of prior myocardial infarction or scar, as inward excursion cannot be readily identified relative to the opposing wall. Additionally, this method of interrogation cannot differentiate between passive and active motion, which is a crucial aspect of mechanical dyssynchrony assessment. Early studies demonstrated a correlation of septal to posterior wall motion delay > 130 ms with reverse remodeling (22), but recent studies have failed to demonstrate any association of septal to posterior wall motion delay with clinical outcomes after CRT implantation (23).
Figure 1. Interventricular Mechanical Delay by Pulsed Doppler.
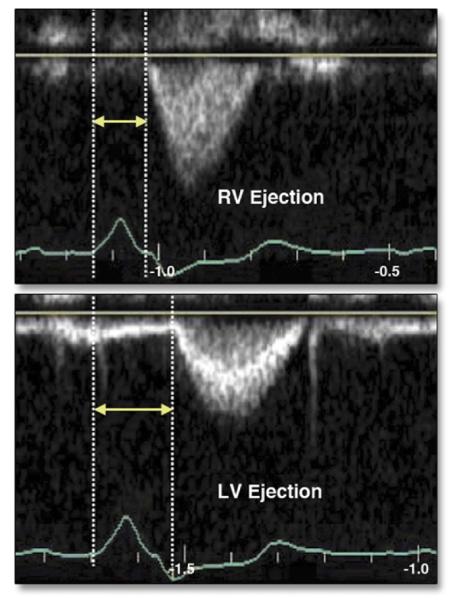
(Top) Time from onset of right ventricular (RV) ejection (arrows), obtained from the parasternal short-axis window. (Bottom) Time from onset of left ventricular (LV) ejection (arrows), obtained from the apical 5-chamber echocardiographic view. Reprinted, with permission, from Gorcsan J III. Echocardiographic assessment of ventricular dyssynchrony. Curr Heart Fail Rep 2008;5:31-7.
Figure 2. Color M-Mode Echocardiography.
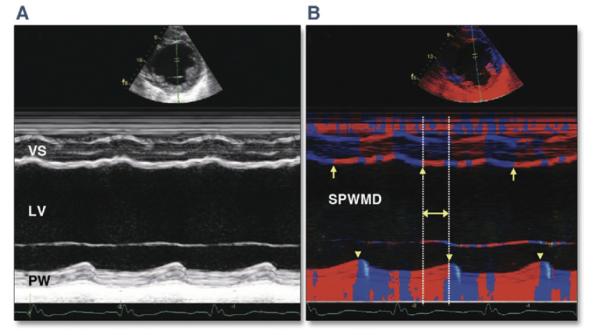
(A) Conventional M-mode echocardiography. Evaluating earliest activation is difficult because of hypokinesis and wall thinning from prior infarction. (B) M-mode echocardiography with color-coded tissue velocity. Activation may be more easily assessed visually as a color change from blue to red (arrows demonstrate change in color denoting myocardial contraction for the ventricular septum [VS], arrow-heads for the posterior wall [PW]). LV = left ventricular; SPWMD = septal to posterior wall motion delay. Reprinted, with permission, from Anderson LJ, Miyazaki C, Sutherland GR, Oh JK. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation 2008;117:2009-23.
TDI
TDI may be used to assess tissue velocity or strain by myocardial deformation. TDI by pulsed-wave Doppler allows the assessment of longitudinal myocardial tissue velocity relative to the ultrasound transducer and has been the most investigated echocardiographic modality for dyssynchrony evaluation. Indexes of dyssynchrony may be generated from differences in the peak velocity, time to peak systolic velocity, or time to onset of peak systolic velocity of the LV walls as assessed in either single or multiple views (2, 4, or 12 segments). Although TDI also enjoys high temporal resolution similar to that of M-mode echocardiography, limitations include the requirement for interrogation of Doppler velocity data perpendicular to the ultrasound beam, limited tissue penetration, the need for adequate acoustic windows, and the inability to simultaneously assess more than 2 LV segments at once. Because of the nature of assessment relative to the ultrasound transducer, measurements are prone to error related to tethering of myocardium to surrounding tissues and translational motion of the heart. Pulsed-wave TDI must be performed online, with preclusion of further offline analysis. Numerous studies have evaluated TDI by pulsed-wave Doppler indexes of dyssynchrony for response to CRT, some demonstrating improvements in echocardiographic parameters (24) and others finding no association with reverse remodeling or clinical outcomes (21).
Color-coded TDI (Fig. 3) and color tissue synchronization imaging provide expansion over pulsed-wave Doppler for regional LV assessment by allowing the simultaneous interrogation of time to peak velocity for multiple myocardial segments in 1 view (13). Tissue synchronization imaging involves the identification of regional mechanical delay by overlaying color-coded temporal velocity data onto 2-dimensional or 3-dimensional images of the left ventricle. This assessment requires proficiency with involved post-processing software, as well as optimal image quality during acquisition of the study. Indexes that incorporate the standard deviation of time to peak systolic velocity for multiple myocardial segments have been proposed and shown to have higher correlation with reverse remodeling over standard color TDI parameters (25). Experienced centers have reported low intraobserver and interobserver variability for color TDI parameters of dyssynchrony (25), whereas other studies, including the PROSPECT trial, have found poor reproducibility (39). Studies evaluating color TDI have demonstrated an association with improved LVEF and reverse remodeling (25). Other parameters may be derived from tissue velocity data, such as myocardial displacement, strain, and strain rate, but these measures have been shown to be inferior in comparison with TDI velocity (26).
Figure 3. Color-Coded Tissue Velocity.
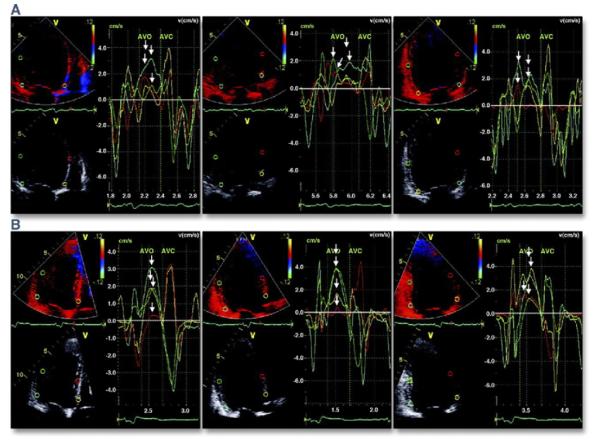
(A) Color-coded tissue velocity data from 12 left ventricular segments in a patient with dyssynchrony. (Left) Apical 4-chamber view, (middle) apical 3-chamber view, and (right) apical 2-chamber view. Arrows mark differences in time to peak velocity during systole for the various segments. (B) The same study after cardiac resynchronization therapy, with reduction in the disparity of time to peak activation. AVC = aortic valve closure; AVO = aortic valve opening. Reprinted, with permission, from Anderson LJ, Miyazaki C, Sutherland GR, Oh JK. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation 2008;117:2009-23.
Strain and strain rate
Myocardial strain, the percentage change of myocardial length from enddiastole to end-systole, and its time derivative, strain rate, may be assessed using TDI (see the previous discussion) or speckle tracking. Speckle tracking involves a post-processing computer algorithm that applies specific acoustic markers to the myocardium, termed “speckles,” to conventional 2-dimensional or 3-dimensional digital echocardiographic images (Fig. 4). Movements of these markers are tracked during the cardiac cycle, frame by frame, to determine regional myocardial strain. Strain is advantageous over the assessment of motion because it measures myocardial deformation and may therefore differentiate between passive translational motion of the myocardium and active systolic contraction.
Figure 4. Two-Dimensional Speckle Tracking.
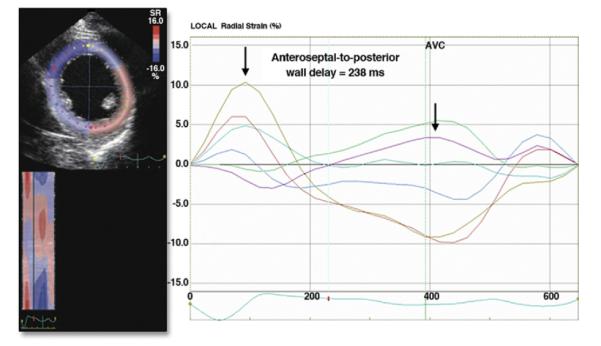
Apical view of the left ventricle demonstrating 2-dimensional speckle tracking for radial strain assessment of dyssynchrony. The time difference between peak strain of the septal segments and the posterolateral segments are shown. AVC = aortic valve closure. Reprinted, with permission, from Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation 2011;123:640-55.
Despite lower temporal resolution, speckle tracking has numerous advantages over TDI, including the ability to assess circumferential and radial strain in addition to longitudinal strain, relatively angle independent image acquisition, and significantly faster post-processing and image analysis. Despite these attributes, speckle tracking is limited by inferior temporal resolution compared with TDI, and similar to TDI, it requires technical expertise with post-processing software and is highly dependent on image quality. Single-center studies that evaluated delays to peak radial strain with speckle tracking have reported a significant association with response to CRT (2,27). The prospective, multicenter STAR (Speckle Tracking and Resynchronization) trial evaluated speckle-tracking assessment of strain in 132 patients referred for conventional CRT indications. Both radial and longitudinal strain were associated with favorable LVEF response and long-term freedom from death, LV assist device implantation, or transplantation over 3.5 years of follow-up (28). A more recent study of 397 patients with ischemic cardiomyopathy evaluated with speckle tracking before CRT found that discordant LV lead position and myocardial scar in the region of the LV pacing lead were independent determinants of all-cause mortality and HF hospitalization, while the extent of baseline LV radial dyssynchrony was associated with improved survival (29).
RT3D echocardiography
RT3D echocardiography can simultaneously assess 3-dimensional ventricular volumes and systolic contraction of all LV segments during the cardiac cycle (Fig. 5). Dyssynchrony indexes may be generated by calculating the standard deviation of the timing of inward systolic excursion of the various LV segments. The acquired data may be post-processed to generate color maps, which can localize regional dyssynchrony in real time as well as in 3 dimensions. This provides advantages over 2-dimensional methods, including angle-independent assessment of strain in all directions during the cardiac cycle. Additional parameters for LV assessment, including function, and volumes may be quantified with high accuracy without the need for any geometric assumptions. Despite these multiple attributes, a number of challenges presently exist for RT3D echocardiography. Data acquisition is more challenging than with 2-dimensional methods, with a high degree of reliance on excellent acoustic windows and stable cardiac rhythms. RT3D imaging has limited temporal and spatial resolution compared with 2-dimensional methods; requires time-consuming, technically challenging post-processing; and suffers from limited reproducibility of time-volume curves for akinetic segments. One study of 60 consecutive patients demonstrated correlation of RT3D findings with LV reverse remodeling (30). Presently, there has not been any clinical study evaluating the role of RT3D echocardiography in guiding CRT or its association with cardiac outcomes.
Figure 5. Three-Dimensional Echocardiographic Evaluation of Dyssynchrony.
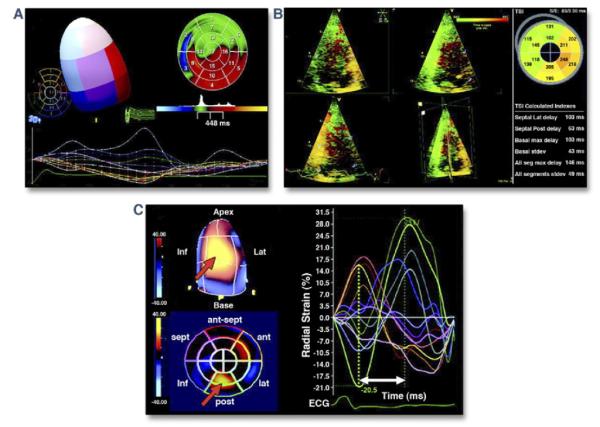
(A) Systolic dyssynchrony index used to evaluate left ventricular (LV) mechanical dyssynchrony calculated from the standard deviation (stdev) of time to minimal systolic regional volume of the 17-segment LV model. (B) Time dispersion map relating relative regional delay to contraction of the left ventricle. The green regions represent earliest myocardial contraction, while orange and red regions are the latest to contract. (C) Three-dimensional speckle tracking for assessment of strain. The time dispersion to peak strain may be calculated (radial strain is shown). A polar map denoting relative regional delay (blue earliest, yellow latest) also provides visual assessment of dyssynchrony. ant = anterior; ECG = electrocardiogram; inf = inferior; lat = lateral; max = maximal; post = posterior; seg = segments; sept = septal; TSI = tissue synchronization index. Reprinted, with permission, from Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation 2011;123:640-55.
The PROSPECT trial
Despite the development of numerous echocardiographic methods to assess dyssynchrony in the past decade, many of the studies evaluating these techniques have fallen under scrutiny for reasons including single-center experience with limited study populations, absence of control arms, and controversial end points with surrogate markers of outcomes. Furthermore, most studies included largely retrospective data from echocardiographic registries, different threshold cutoffs for the same technique, short follow-up, and lack of randomization, blinding, or reporting of intraobserver and interobserver variability. Numerous studies have used the surrogate outcome measure of reverse remodeling, defined as a reduction in LV endsystolic volume of >10% to 15%, as opposed to hard clinical end points. Although some studies have correlated reverse remodeling to clinical outcomes, other studies have demonstrated clinical response in patients without reverse remodeling (40). Post hoc analysis of the Cardiac Resynchronization–Heart Failure study failed to demonstrate an independent association of reverse remodeling at 3 months after CRT with mortality (2). Another concern includes different threshold cutoffs among different studies for the same echocardiographic parameter, some of which have also been shown in asymptomatic, normal subjects (41). Finally, many single-center studies have not reported 95% confidence intervals, suggesting wide boundaries that may limit clinical utility.
To obtain a more comprehensive evaluation of echocardiographic parameters for dyssynchrony, a large, nonrandomized, multicenter trial (PROSPECT) was performed in 498 patients with HF with standard indications for CRT (39). Twelve different echocardiographic measures of dyssynchrony were evaluated. The study included blinded analysis performed by international core laboratories for the outcome measures of clinical composite score and LV reverse remodeling. None of the 12 echocardiographic methods proved to be sensitive or specific enough to be clinically useful for predicting response to CRT. The PROSPECT study demonstrated a high variance of TDI measurements owing to a number of factors inherent to the determination of these values, including operator dependence, angle of interrogation, and acoustic windows. Even the same patient with repeated studies over time had significant variation in the echocardiographic dyssynchrony parameters. Although the PROSPECT study has been criticized for a number of design issues, at the time of the trial, many of the echocardiographic parameters being evaluated were still novel, and most centers did not have extensive experience with their evaluation. More recent analysis of the PROSPECT study revealed an association between the extent of LV dyssynchrony at baseline and the extent of reverse remodeling at 6-month follow-up (40). Whether the results of the PROSPECT trial are generalizable to today’s standard of dyssynchrony assessment remains in contention.
Echocardiography may also play a significant role in serial follow-up of patients for optimization of CRT. In addition to continued noninvasive assessment of remodeling and ventricular function, optimization of pacemaker atrioventricular delay and right ventricular–LV lead intervals may avoid residual dyssynchrony after CRT implantation. Despite early promising data suggesting benefit, the multicenter, prospective SMART-AV (SmartDelay Determined AV Optimization) trial demonstrated no difference in the primary end point of HF-related adverse events at 6 months (42). A second study, which compared an electronic algorithm to echocardiographic optimization of CRT, also found no difference for HF composite score in more than 1,600 patients (43). Although proponents of echocardiographic optimization argue that these studies did not target nonresponders, who may have the greatest potential for benefit, further evaluation is clearly required.
Magnetic Resonance Imaging
Recently, CMR has gained increasing attention for dyssynchrony assessment because of its high tissue and spatial contrast, coupled with highly accurate and reproducible assessment of LV volume and functional indexes. CMR techniques to assess dyssynchrony are rapidly expanding and are presently under evaluation in a number of studies.
MRI dyssynchrony
The advantages of contrast medium–enhanced CMR include high spatial resolution and tissue characterization, in addition to highly accurate quantification of chamber size and ventricular function and 3-dimensional assessment of myocardial strain. Compared with echocardiography, CMR has high reproducibility owing largely to tomographic imaging with less operator dependency. Disadvantages of CMR include long acquisition times, implanted cardiac devices as magnetic resonance hazards, and complex post-processing techniques. In recent years, newer methods have improved the efficiency and standardization of post-processing.
The most basic assessment of dyssynchrony by CMR is achieved with cine MRI, which produces a continuous series of oblique, short-axis slices from the ventricular apex to the base. This allows accurate volumetric assessment of chamber sizes and ventricular function indexes, such as stroke volume and LVEF. Interventricular and intraventricular dyssynchrony may be assessed qualitatively or quantitatively with software that tracks the myocardium during the cardiac cycle (44). Although cine MRI is acquired routinely, it is not particularly optimized for evaluation of regional dyssynchrony. More direct methods have been developed that provide more accurate and direct assessment of intramyocardial deformation, including myocardial tagging, strain-encoded MRI (SENC), phase-contrast MRI, and displacement encoding with stimulated echoes (DENSE), amongst others.
Myocardial tagging
Myocardial tagging is analogous to speckle tracking by echocardiography but with higher spatial resolution and reproducibility. Myocardial tagging is achieved by creating dark tagged lines or a grid over the myocardium through saturation of the magnetization in planes perpendicular to the imaged slice (Fig. 6). Tag lines enable an accurate analysis of myocardial motion and displacement, which has been shown to be a robust method for assessment of both intraventricular and interventricular dyssynchrony (45). Newer methods of tagging, such as spatial modulation of magnetization, have resulted in shorter acquisition times and improved image quality of the myocardial tags (46).
Figure 6. Myocardial Tagging by Cardiac Magnetic Resonance Imaging.
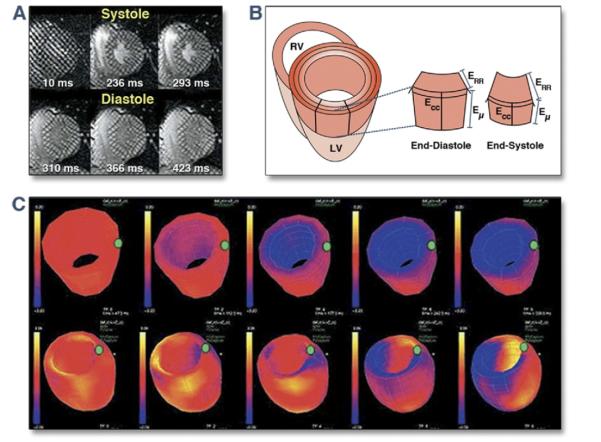
(A) Progressive deformation of the gird over the course of the cardiac cycle. This allows visual estimation of regions of myocardium that are contracting relatively late during systole. (B) Volumetric change of the myocardial segments over the course of the cardiac cycle. (C) Polar map illustrating the time course for contraction of the myocardial segments of the left ventricle (LV). RV = right ventricle. Reprinted, with permission, from Abraham T, Kass D, Tonti G, et al. Imaging cardiac resynchronization therapy. J Am Coll Cardiol Img 2009;2:486-97.
Most echocardiographic techniques, apart from speckle tracking and RT3D imaging, are limited to the assessment of longitudinal strain. Data from animal models, in addition to more recent echocardiographic studies using speckle tracking, suggest that circumferential and radial strain more accurately predict response to CRT (27). Although 2-dimensional myocardial tagging can assess both circumferential and radial strain, the ability to track myocardial motion in 3 dimensions by inclusion of tagged long-axis cine imaging is an attractive extension that would also permit the assessment of longitudinal strain (47). Recently, 3-dimensional tagging with CMR has become more straightforward to analyze with the use of a post-processing technique termed harmonic phase imaging. The significant advantage of harmonic phase imaging relates to almost observer-independent estimation of myocardial strains that permits quick and accurate post-processing of tagged cine images. Data from myocardial tagging may be used to produce myocardial strain-versus-time plots for the various ventricular walls that may be displayed and read similarly to a 12-lead electrocardiogram (48).
By directly tracking intramyocardial motion across the cardiac cycle, myocardial tagging may provide more specific assessment of myocardial deformation than TDI by differentiating regional strain abnormalities due to intrinsic abnormalities of myocardial contraction from passive tethering. TDI is more susceptible to errors from rotational motion of the heart and passive tethering as a result of determining myocardial velocity with respect to the mitral valve annulus or ultrasound transducer as opposed to the myocardium itself. Comparison with TDI has demonstrated higher specificity for dyssynchrony by myocardial tagging (17). Circumferential strain as assessed by tagging has also shown high correlation with LV volume curves assessed by RT3D echocardiography (49). A further advantage of myocardial tagging relates to the derivation of circumferential strain through assessment of the entire myocardium, as opposed to using specific myocardial segments. True 3-dimensional assessment for regional dyssynchrony, in addition to determination of the extent of dyssynchronous myocardium, may be performed to differentiate those patients with diffusely dyssynchronous segments from those with clustered dyssynchronous segments within a particular wall, who may have a very different response to CRT. Use of a dyssynchrony index derived from myocardial tagging with CMR in a single-center study was highly predictive of improved functional class after CRT (50). Accuracy was further improved by the addition of late gadolinium enhancement imaging for the identification of myocardial scar.
SENC
SENC is another method for the assessment of dyssynchrony that involves using sinusoidal “tagged” surfaces that modulate longitudinal magnetization perpendicular to the imaging plane. Myocardial deformation during the cardiac cycle alters the local tag frequency in both the longitudinal and circumferential directions, which may be automatically detected and represented by a color scale as myocardial strain (51). This markedly reduces any required post-processing, as with myocardial tagging, and has further advantages of high temporal and spatial resolution, in addition to extremely short acquisition time. SENC may be used to simultaneously assess regional strain of the right ventricle in addition to the left ventricle. SENC is limited by a requirement for sinusoidal magnetization modulation perpendicular to the image plane, which effectively precludes the assessment of radial strains. Both myocardial viability and regional strain may be simultaneously acquired with composite SENC, correlating regional strain with the extent of scar, which is highly desirable for CRT assessment (52). SENC and a method of performing fast SENC with an acquisition duration of only 1 heartbeat have shown excellent correlation with myocardial tagging (53). However, to date, no clinical outcome studies for CRT have been performed evaluating SENC or any of its derivatives.
Phase-contrast MRI
Phase-contrast velocity mapping uses the shift in phase of the myocardium between 2 opposing pulse gradients, which is proportional to the velocity of the myocardium. A color or gray scale is used to represent the magnitude and direction of tissue displacement in 3 dimensions. This method provides high temporal (2 ms) and spatial resolution and does not require the use of “tags” or segmentation of the myocardium. This is the CMR technique most similar in terms of imaging concept to TDI and has shown high correlation with the echocardiographic method (54). To date, there is a relative paucity of data assessing clinical response to CRT using phase-contrast MRI.
DENSE
DENSE encodes positional information into the phase of each image pixel. Therefore, the spatial resolution of DENSE is limited to only a single pixel (55). Using DENSE imaging, myocardial deformation may be tracked in 3 dimensions over the course of the cardiac cycle and is amenable to automated post-processing. DENSE imaging has been shown to have high correlation with myocardial tagging for regional wall motion abnormalities (56). However, similar to other CMR techniques, DENSE imaging has not been studied in relation to clinical response to CRT.
Presently, the above CMR methods provide a wealth of 3-dimensional data over the entire cardiac cycle. Future directions include a method of integrating this data into a simplified and standardized dyssynchrony index that may be used to efficiently screen and follow patients with CRT over time. One such index proposed by Leclercq et al. (57) uses strain data from the entire myocardium to derive a global dyssynchrony score graded from 0 (pure dyssynchrony) to 1 (pure synchrony). This index has been used both to determine optimal LV lead placement (58) as well as for predicting clinical response to CRT.
Late gadolinium enhancement
One particular attribute of CMR is the potential to provide an integrated assessment for CRT. CMR can delineate chamber size and function with high accuracy, assess prognosis, determine myocardial perfusion, and help guide lead placement through assessment of the extent and location of myocardial scar and coronary venous anatomy, all in addition to dyssynchrony. This holistic evaluation may more accurately and cost-effectively screen patients for CRT. However, the relative contraindication of performing CMR in patients with a device precludes using CMR to evaluate device therapy for serial follow-up.
Multiple observational studies have suggested that the extent of myocardial scar is predictive of response to CRT (59-61). Furthermore, scar in the location of LV lead placement may reduce the effectiveness of CRT (60) (Fig. 7). One study of 40 patients with ischemic cardiomyopathy found lower response rates to CRT and no change in dyssynchrony for those with posterolateral scar (14% vs. 81%, p < 0.05) compared with those without (60). Another study of 23 patients reported that a cutoff value of 15% total LV myocardial scar provided sensitivity and specificity of 85% and 90%, respectively, for clinical response to CRT (59). Other studies have shown that pacing in a site with <50% scar transmurality was associated with response to CRT (61).
Figure 7. Posterolateral Scar by Cardiac Magnetic Resonance Imaging.
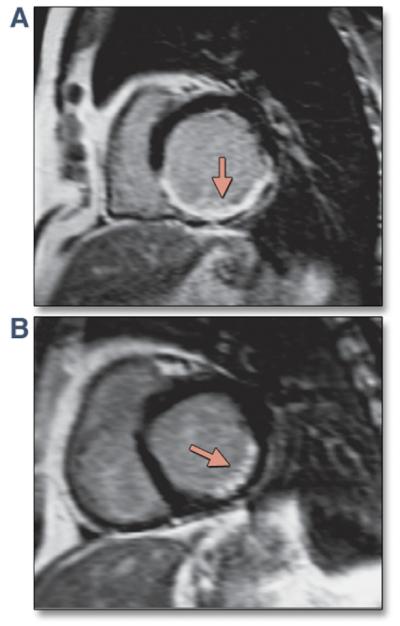
(A,B) Late gadolinium enhancement images by cardiac magnetic resonance imaging demonstrating dense posterolateral scar in the left ventricle. Reprinted, with permission, from Bax JJ, Gorcsan J III. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol 2009;53:1933-43.
Radionuclide Imaging
Similar to CMR, radionuclide imaging represents an attractive option for the selection of CRT in patients with HF because of its potential for comprehensive evaluation of LVEF and degree of myocardial scar, in addition to mechanical dyssynchrony. Abnormalities of resting perfusion assessed by single-photon emission computed tomographic imaging have been associated with reduced improvement of functional and ventricular parameters after CRT (62). In addition to these parameters, phase analysis imaging from routine gated myocardial perfusion single-photon emission computed tomography (GMPS) may be used for dyssynchrony assessment (63). GMPS evaluates the pattern of ventricular activation through determination of regional LV counts during the cardiac cycle. An increase in counts is believed to correlate with regional LV wall thickening and may be used to assess the pattern of systolic contraction. Data from the entire myocardium are used to generate a phase distribution map that may be displayed as a histogram or polar map (Fig. 8).
Figure 8. GMPS Phase Polar Map and Phase Histogram.
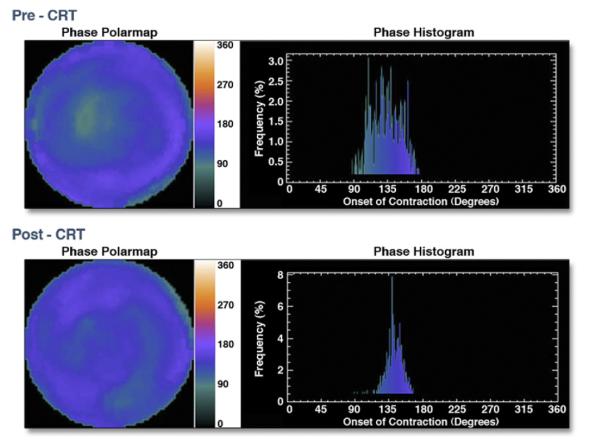
Gated myocardial perfusion single-photon emission computed tomography (GMPS) phase polar map before (top left) and after (bottom left) cardiac resynchronization therapy (CRT). The colored phase polar map demonstrates delayed contraction of the lateral wall compared with the septum before CRT, which corrects after CRT. The phase histogram before CRT (top right) demonstrates a wide bandwidth consistent with delayed contraction of the lateral wall compared with the septum. The phase histogram narrows after CRT (bottom right), consistent with more synchronized contraction. Reprinted, with permission, from Friehling M, Soman P. Newer applications of nuclear cardiology in systolic heart failure: detecting coronary artery disease and guiding device therapy. Curr Heart Fail Rep 2011;8:106-12.
Phase imaging analysis with GMPS for the identification of dyssynchrony has been validated in studies using TDI by echocardiography (63). The degree of LV dyssynchrony as assessed by GMPS was also shown to correlate with response to CRT in a study of 42 patients with severe LV dysfunction (64). A recent study evaluated the use of GMPS to determine the site of latest LV activation for LV lead placement and found a significantly higher response rate for patients with GMPS-guided LV lead placement, defined as a decrease of >15% in LV end-systolic volume (65).
Another method of phase imaging analysis from gated blood-pool ventriculography may also evaluate mechanical dyssynchrony. This method was evaluated in a study of 103 patients with idiopathic dilated cardiomyopathy and was shown to be a significant predictor of adverse clinical outcomes in multivariate analysis (66). However, this technique requires sophisticated post-processing to carefully identify ventricular regions while excluding extracardiac structures and remains largely investigational.
A number of limitations of nuclear dyssynchrony assessment, including sophisticated post-processing techniques, potentially hazardous radiation exposure, and limited spatial resolution, have limited more widespread use. Limited spatial resolution not only affects dyssynchrony assessment but may also lead to overestimation of scar and concomitant underestimation of myocardial viability, particularly in this patient population with dilated cardiomyopathies and thin myocardial walls. Finally, studies to date evaluating GMPS have been small, single-center experiences, which have mostly evaluated secondary outcome measures.
Cardiac Computed Tomography
Mechanical dyssynchrony assessment by cardiac computed tomography (CCT) has previously been limited by poor temporal resolution. However, since the advent of dual-source multidetector computed tomography, both temporal and spatial resolution have significantly improved, redefining the role of CCT for the assessment of patients with HF.
CCT may be especially useful to help guide endocardial LV lead placement for CRT. Apart from prolonged procedure times with resultant increased risk for periprocedural complications and radiation exposure, inadequate LV lead position may lead to poor response to CRT. Pre-procedural use of CCT to characterize venous anatomy may substantially aid in LV lead placement (Fig. 9). One prospective, single-center study of 22 patients (9 with pre-procedural CCT and 13 without) reported decreased procedural times, use of contrast media, and radiation exposure (67).
Figure 9. Volume-Rendered Image of the Coronary Sinus.
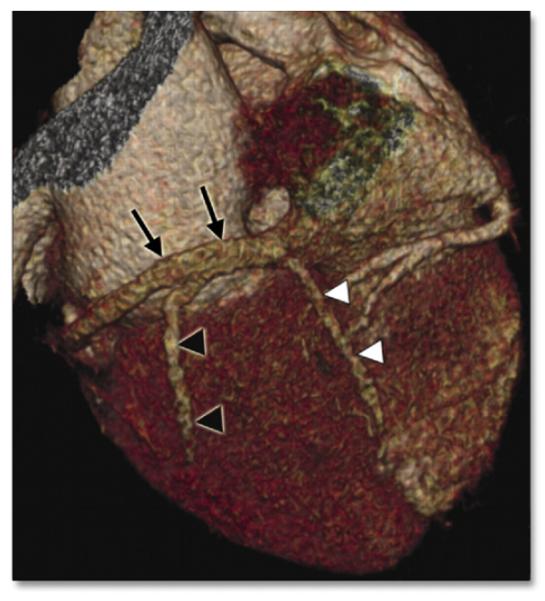
Volume-rendered cardiac computed tomographic image of the coronary sinus (black arrows). The posterior vein of the left ventricle (black arrowheads) and posterior interventricular vein (white arrowheads) may also be visualized. Reprinted, with permission, from Gopalan D, Raj V, Hoey ETD. Cardiac CT: noncoronary applications. Postgrad Med J 2010;86:165-73.
Truong et al. (68) recently evaluated the utility of CCT for dyssynchrony assessment in a study of 38 patients with HF and normal controls. They devised an index of dyssynchrony on the basis of the standard deviation of time from the R-wave of the electrocardiogram to maximal wall thickness as assessed by CCT. Although they found a significant difference for the dyssynchrony index in patients with HF compared with the normal controls, comparison with echocardiographic measures of dyssynchrony demonstrated only a moderate correlation (r = 0.65, p = 0.012). LV structure and function assessment by CCT has also improved, although CCT has not performed as well in patients with LV dysfunction, with a tendency to overestimate LVEF (69). The use of dual-source multidetector computed tomography may identify and quantitate scar burden within the myocardium. This technique has been validated against CMR, although contrast-to-noise ratio is inferior with CCT (70). To date, no clinical studies evaluating CRT response with CCT localization of scar or dyssynchrony have been conducted.
CCT represents a promising tool for the investigation of patients with HF. Similar to radionuclide assessment, CCT is associated with ionizing radiation exposure, particularly in cases in which retrospective electrocardiographic gating is required for acquisition of the entire cardiac cycle.
Conclusions
CRT has demonstrated significant clinical benefits for patients with HF refractory to medical therapy. Despite advances in cardiac imaging over the past decade, there are still no imaging parameters that are routinely indicated to guide CRT therapy. To date, the major modality investigated for dyssynchrony assessment and measuring response to CRT has been echocardiography. Despite the large body of evidence that has quickly accrued, none of the numerous quantitative variables from echocardiography have been shown to achieve adequate robustness to be recommended for guidance of CRT. However, with increasing experience and technical expertise, many echocardiographic laboratories are demonstrating higher reproducibility and accuracy for mechanical dyssynchrony. This has been coupled with studies examining harder clinical end points for outcome measures after CRT. Furthermore, novel imaging methods have shown great promise from both a technical and a physiological standpoint in assessing mechanical dyssynchrony. Future randomized control trials are required to determine whether imaging studies can provide more targeted CRT selection to reduce nonresponsiveness and complications related to CRT and ultimately provide more efficient health care expenditure.
ABBREVIATIONS AND ACRONYMS
- CCT
cardiac computed tomography
- CMR
cardiac magnetic resonance
- CRT
cardiac resynchronization therapy
- DENSE
displacement encoding with stimulated echoes
- GMPS
gated myocardial perfusion single-photon emission computed tomography
- HF
heart failure
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MRI
magnetic resonance imaging
- RT3D
real-time 3-dimensional
- SENC
strain-encoded magnetic resonance imaging
- TDI
tissue Doppler imaging
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J, Freemantle N, Ghio S, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. J Am Coll Cardiol. 2008;52:438–45. doi: 10.1016/j.jacc.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 7.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT, Young JB, Leon AR, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–8. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 10.Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–43. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Hara T, Yamashiro K, Okajima K, Hayashi T, Kajiya T. Posterior shift of the anterior papillary muscle in patients with heart failure: a potential role in the effect of cardiac resynchronization therapy. Int Heart J. 2009;50:773–82. doi: 10.1536/ihj.50.773. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: part 1—issues before device implantation. J Am Coll Cardiol. 2005;46:2153–67. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Bax JJ, Molhoek SG, van Erven L, et al. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;91:94–7. doi: 10.1016/s0002-9149(02)03009-6. [DOI] [PubMed] [Google Scholar]
- 14.Egoavil CA, Ho RT, Greenspon AJ, Pavri BB. Cardiac resynchronization therapy in patients with right bundle branch block: analysis of pooled data from the MIRACLE and Contak CD trials. Heart Rhythm. 2005;2:611–5. doi: 10.1016/j.hrthm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Yu CM, Yang H, Lau CP, et al. Regional left ventricle mechanical asynchrony in patients with heart disease and normal QRS duration: implication for biventricular pacing therapy. Pacing Clin Electrophysiol. 2003;26:562–70. doi: 10.1046/j.1460-9592.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 16.Grimm R, Beshai JF, Nagueh S, et al. The RethinQ Trial Echocardiographic Sub Study: insights from analysis of TDI data on mechanical dys-synchrony in a randomized clinical trial of cardiac resynchronization therapy in narrow QRS patients with heart failure. Circulation. 2008;118:S868. [Google Scholar]
- 17.Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 18.Yu CM, Chan YS, Zhang Q, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–7. doi: 10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Leon AR, Niazi I, Herrman K, et al. Chronic evaluation of CRT in narrow QRS patients with mechanical dys-synchrony from a multi-center study: ESTEEM-CRT. Heart Rhythm. 2008;5:S23–4. [Google Scholar]
- 20.Knebel F, Schattke S, Bondke H, et al. Evaluation of longitudinal and radial two-dimensional strain imaging versus Doppler tissue echocardiography in predicting long-term response to cardiac resynchronization therapy. J Am Soc Echocardiogr. 2007;20:335–41. doi: 10.1016/j.echo.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Achilli A, Peraldo C, Sassara M, et al. Prediction of response to cardiac resynchronization therapy: the Selection of Candidates for CRT (SCART) study. Pacing Clin Electrophysiol. 2006;(suppl):29, S11–9. doi: 10.1111/j.1540-8159.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 22.Pitzalis MV, Iacoviello M, Romito R, et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol. 2005;45:65–9. doi: 10.1016/j.jacc.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Marcus GM, Rose E, Viloria EM, et al. Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2208–14. doi: 10.1016/j.jacc.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 24.Penicka M, Bartunek J, De Bruyne B, et al. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation. 2004;109:978–83. doi: 10.1161/01.CIR.0000116765.43251.D7. [DOI] [PubMed] [Google Scholar]
- 25.Yu CM, Fung JW, Zhang Q, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 26.Yu CM, Zhang Q, Chan YS, et al. Tissue Doppler velocity is superior to displacement and strain mapping in predicting left ventricular reverse re-modelling response after cardiac resynchronisation therapy. Heart. 2006;92:1452–6. doi: 10.1136/hrt.2005.083592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. (3rd) 2006;113:960–8. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Nesser HJ, Buck T, et al. Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur Heart J. 2010;31:1690–700. doi: 10.1093/eurheartj/ehq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado V, van Bommel RJ, Bertini M, et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–8. doi: 10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 30.Marsan NA, Bleeker GB, Ypenburg C, et al. Real-time three-dimensional echocardiography permits quantification of left ventricular mechanical dyssynchrony and predicts acute response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2008;19:392–9. doi: 10.1111/j.1540-8167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 31.Jansen AH, Bracke F, van Dantzig JM, et al. Optimization of pulsed wave tissue Doppler to predict left ventricular reverse remodeling after cardiac resynchronization therapy. J Am Soc Echocardiogr. 2006;19:185–91. doi: 10.1016/j.echo.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Soliman OI, Theuns DA, Geleijnse ML, et al. Spectral pulsed-wave tissue Doppler imaging lateral-to-septal delay fails to predict clinical or echocardiographic outcome after cardiac resynchronization therapy. Europace. 2007;9:113–8. doi: 10.1093/europace/eul149. [DOI] [PubMed] [Google Scholar]
- 33.Bleeker GB, Schalij MJ, Boersma E, et al. Relative merits of M-mode echocardiography and tissue Doppler imaging for prediction of response to cardiac resynchronization therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;99:68–74. doi: 10.1016/j.amjcard.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 34.Gorcsan J, III, Oyenuga O, Habib PJ, et al. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122:1910–8. doi: 10.1161/CIRCULATIONAHA.110.954768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Veire NR, Bleeker GB, De Sutter J, et al. Tissue synchronisation imaging accurately measures left ventricular dyssynchrony and predicts response to cardiac resynchronisation therapy. Heart. 2007;93:1034–9. doi: 10.1136/hrt.2006.099424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu CM, Zhang Q, Fung JW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005;45:677–84. doi: 10.1016/j.jacc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Dohi K, Suffoletto MS, Schwartzman D, Ganz L, Pinsky MR, Gorcsan J. Utility of echocardiographic radial strain imaging to quantify left ventricular dyssynchrony and predict acute response to cardiac resynchronization therapy. Am J Cardiol. (3rd) 2005;96:112–6. doi: 10.1016/j.amjcard.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Mele D, Pasanisi G, Capasso F, et al. Left intraventricular myocardial deformation dyssynchrony identifies responders to cardiac resynchronization therapy in patients with heart failure. Eur Heart J. 2006;27:1070–8. doi: 10.1093/eurheartj/ehi814. [DOI] [PubMed] [Google Scholar]
- 39.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 40.van Bommel RJ, Bax JJ, Abraham WT, et al. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–7. doi: 10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 41.Burri H, Muller H, Vieira I, Lerch R. Poor agreement of echographic measures of ventricular dyssynchrony. Eur J Echocardiogr. 2008;9:235–40. doi: 10.1016/j.euje.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMARTAV) trial: a randomized trial comparing empirical, echocardiographyguided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–8. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 43.Abraham WT, Gras D, Yu CM, Guzzo L, Gupta MS. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159:944–8. doi: 10.1016/j.ahj.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 44.Hor KN, Gottliebson WM, Carson C, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. J Am Coll Cardiol Img. 2010;3:144–51. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Reichek N. MRI myocardial tagging. J Magn Reson Imaging. 1999;10:609–16. doi: 10.1002/(sici)1522-2586(199911)10:5<609::aid-jmri4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–5. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 47.O’Dell, Moore CC, Hunter WC, Zerhouni EA, McVeigh ER. Three-dimensional myocardial deformations: calculation with displacement field fitting to tagged MR images. Radiology. 1995;195:829–35. doi: 10.1148/radiology.195.3.7754016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim ES. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques—pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. doi: 10.1186/1532-429X-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russel IK, van Dijk J, Kleijn SA, et al. Relation between threedimensional echocardiography derived left ventricular volume and MRI derived circumferential strain in patients eligible for cardiac resynchronization therapy. Int J Cardiovasc Imaging. 2009;25:1–11. doi: 10.1007/s10554-008-9339-8. [DOI] [PubMed] [Google Scholar]
- 50.Bilchick KC, Dimaano V, Wu KC, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. J Am Coll Cardiol Img. 2008;1:561–8. doi: 10.1016/j.jcmg.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garot J, Bluemke DA, Osman NF, et al. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101:981–8. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahimel SH, Weiss RG, Stuber M, Spooner AE, Osman NF. Identification of different heart tissues from MRI C-SENC images using an unsupervised multi-stage fuzzy clustering technique. J Magn Reson Imaging. 2008;28:519–26. doi: 10.1002/jmri.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neizel M, Lossnitzer D, Korosoglou G, et al. Strain-encoded (SENC) magnetic resonance imaging to evaluate regional heterogeneity of myocardial strain in healthy volunteers: Comparison with conventional tagging. J Magn Reson Imaging. 2009;29:99–105. doi: 10.1002/jmri.21612. [DOI] [PubMed] [Google Scholar]
- 54.Westenberg JJ, Lamb HJ, van der Geest RJ, et al. Assessment of left ventricular dyssynchrony in patients with conduction delay and idiopathic dilated cardiomyopathy: head-to-head comparison between tissue Doppler imaging and velocity-encoded magnetic resonance imaging. J Am Coll Cardiol. 2006;47:2042–8. doi: 10.1016/j.jacc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 55.Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247–52. doi: 10.1006/jmre.1998.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng L, Donnino R, Babb J, Axel L, Kim D. Numerical and in vivo validation of fast cine displacement-encoded with stimulated echoes (DENSE) MRI for quantification of regional cardiac function. Magn Reson Med. 2009;62:682–90. doi: 10.1002/mrm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–3. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 58.Helm RH, Byrne M, Helm PA, et al. Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation. 2007;115:953–61. doi: 10.1161/CIRCULATIONAHA.106.643718. [DOI] [PubMed] [Google Scholar]
- 59.White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–60. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 60.Bleeker GB, Kaandorp TA, Lamb HJ, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 61.Chalil S, Foley PW, Muyhaldeen SA, et al. Late gadolinium enhancementcardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace. 2007;9:1031–7. doi: 10.1093/europace/eum133. [DOI] [PubMed] [Google Scholar]
- 62.Sciagra R, Giaccardi M, Porciani MC, et al. Myocardial perfusion imaging using gated SPECT in heart failure patients undergoing cardiac resynchronization therapy. J Nucl Med. 2004;45:164–8. [PubMed] [Google Scholar]
- 63.Henneman MM, Chen J, Ypenburg C, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–14. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 64.Henneman MM, Chen J, Dibbets-Schneider P, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11. doi: 10.2967/jnumed.107.039925. [DOI] [PubMed] [Google Scholar]
- 65.Boogers MJ, Chen J, van Bommel RJ, et al. Optimal left ventricular lead position assessed with phase analysis on gated myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2011;38:230–8. doi: 10.1007/s00259-010-1621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fauchier L, Marie O, Casset-Senon D, Babuty D, Cosnay P, Fauchier JP. Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: a prognostic study with Fourier phase analysis of radionuclide angioscintigraphy. J Am Coll Cardiol. 2002;40:2022–30. doi: 10.1016/s0735-1097(02)02569-x. [DOI] [PubMed] [Google Scholar]
- 67.Girsky MJ, Shinbane JS, Ahmadi N, Mao S, Flores F, Budoff MJ. Prospective randomized trial of venous cardiac computed tomographic angiography for facilitation of cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2010;33:1182–7. doi: 10.1111/j.1540-8159.2010.02821.x. [DOI] [PubMed] [Google Scholar]
- 68.Truong QA, Singh JP, Cannon CP, et al. Quantitative analysis of intraventricular dyssynchrony using wall thickness by multidetector computed tomography. J Am Coll Cardiol Img. 2008;1:772–81. doi: 10.1016/j.jcmg.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischbach R, Juergens KU, Ozgun M, et al. Assessment of regional left ventricular function with multidetectorrow computed tomography versus magnetic resonance imaging. Eur Radiol. 2007;17:1009–17. doi: 10.1007/s00330-006-0438-4. [DOI] [PubMed] [Google Scholar]
- 70.Bauer RW, Kerl JM, Fischer N, et al. Dual-energy CT for the assessment of chronic myocardial infarction in patients with chronic coronary artery disease: comparison with 3-T MRI. AJR Am J Roentgenol. 2010;195:639–46. doi: 10.2214/AJR.09.3849. [DOI] [PubMed] [Google Scholar]


