Abstract
OBJECTIVE
The objective of this article is to discuss optimal imaging strategies for the evaluation of cardiac masses. The advantages and disadvantages of echocardiography, cardiac MRI, gated cardiac CT, and nuclear imaging will be discussed and specific techniques presented.
CONCLUSION
Multimodality imaging plays a pivotal role in the diagnosis and surgical planning of cardiac masses. Clinical features, such as patient age, location, and imaging characteristics of the mass will determine the likely differential diagnosis.
Keywords: cardiac, cardiac CT, cardiac MRI, fibroma, myxoma, pericardium, thrombus
Multiple imaging modalities are available for evaluation and characterization of primary and secondary tumors involving the heart. Cross-sectional imaging with CT, MRI, and 18F-FDG PET/CT work synergistically with echocardiography. The article deals with specific protocols used for cardiac imaging. The relative merits of each modality will be identified to provide the reader with a strategy for approaching a cardiac mass.
Echocardiography
Echocardiography is typically the primary modality used for diagnosis or evaluation of a suspected cardiac mass because of its availability, noninvasiveness, absence of contrast material or radiation exposure, and ability to make dynamic assessment of cardiac masses. Echocardiography can be used to guide biopsy of cardiac tumors. Intraoperative transesophageal and 3D echocardiography can be helpful in guiding surgical planning. Real-time 3D echocardiography improves the diagnostic capabilities of cardiac echocardiography in the assessment of the location, composition, size, and relationship to adjacent structures of intracardiac masses (Table 1) [1]. The proximity of the probe to the posterior aspect of the cardiac chambers in transesophageal echocardiography permits better evaluation of cardiac masses, particularly in the atria, and differentiation of benign from pathologic structures (for example, lipomatous hypertrophy of the interatrial septum, which is benign but can have a masslike appearance). Echocardiography may be limited in obese patients and those with poor acoustic windows. Echocardiography is also limited in its ability to make a global assessment of cardiac and extracardiac structures. Soft-tissue characterization is less specific with echocardiography than with MRI.
TABLE 1.
Protocol for Cardiac MRI of Tumors at Our Institution
| Pulse Sequence | Technical Parameters | Utility |
|---|---|---|
|
| ||
| Bright-blood, cine SSFP (Figs. 1 and S1) | Planes of acquisition: two-, three-, and four-chamber with TR/ TE, 36.27/1.22; FOV, patient size dependent between 340 and 400 mm; matrix, 162 × 192; slice thickness, 8 mm; flip angle, 50°; segments, 12 with goal of temporal resolution of 40 ms | Advantages: Good SNR and CNR; functional and anatomic assessment; T2 > T1 weighting; fast single breath-hold acquisitions and disadvantage of off-resonance artifact at 3 T |
| Bright-blood cine spoiled gradient- echo (Figs. 2 and S2) | Planes of acquisition: two-, three-, and four-chamber with TR/ TE, 40/2–43; FOV, 340–400 mm; matrix, 125 × 192; slice thickness, 8 mm; flip angle, 12° | Advantages: good CNR but weaker than SSFP; less susceptible to off-resonance artifact; T1 weighting Disadvantage: turbulent flow can cause signal loss and overestimation of size of lesion |
| T1-weighted black-blood double inversion recovery fast spin-echo (Figs 3A and 4) | Planes of acquisition: two-, three-, and four-chamber with TR/ TE, 1000 or one R-R interval (depending on heart rate)/9–11; FOV, 300–400 mm; matrix, 256 × 256; slice thickness, 6 mm; echo-train length, 10; inversion time, 340; flip angle, 60° | Black-blood effects give good SNR and CNR and allow delineation of the mass relative to adjacent structures; T1 tissue characterization |
| T2-weighted black-blood double inversion recovery fast spin-echo (Fig. 3B) | Planes of acquisition: two-, three-, and four-chamber with TR/ TE, two R-R intervals–2000/120; FOV, 300–400 mm; matrix, 256 × 256; slice thickness, 6 mm; flip angle, 60° | Black-blood effects give good SNR and CNR allow delineation of the mass relative to adjacent structures; T2 tissue characterization |
| Black-blood triple inversion fast spin-echo) (Fig. 5) | Planes of acquisition: two-, three-, and four-chamber with FOV, 300–400 mm; matrix, 256 × 256; slice thickness, 6 mm; T1/T2-weighted with additional inversion pulse between non–spatially selective and spatially selective pulse to null signal from fat | Visualization of presence of fat can help diagnose a lesion; elimination of signal from adjacent high-signal epicardial fat can allow better definition of the lesion |
| T1 fat-saturated 3D volumetric unenhanced and contrast-enhanced imaging (Fig. 6) | Planes of acquisition: two-, three-, and four-chamber with TR/ TE, minimum full/minimum; FOV, typically full chest; flip angle, 12–15°; contrast-enhanced imaging acquired in equilibrium phase | Permits evaluation of the presence and extent of contrast enhancement of the mass; provides fast global assessment of the chest (20–25 s) |
| Late gadolinium enhancement imaging (T1 turboFlash) (Fig. 7) | Planes of acquisition: two-, three-, and four-chamber; cumulative dose of 0.1–0.2 mmol/kg gadolinium IV; first-pass perfusion imaging can be acquired dynamically during contrast injection and subsequently, late gadolinium-enhanced images acquired 10 minutes after contrast injection, with a null time of myocardium typically 350 ms; high inversion times, such as 600–800 ms, can be used to null signal in thrombus | Provides definition of the presence and pattern of enhancement of the mass, presence of myocardial scar, or invasion of coronary arteries; can be used to differentiate thrombus from a mass |
| Tagging (Fig. S4) | Spatial presaturation pulse is applied with a line thickness of 1–2 mm and a grid tag distance of 6 mm, with the orientation of the lines perpendicular to the axis of the mass/myocardium; tagging can be in a linear or grid pattern | Can determine whether a mass is inseparable from myocardium with loss of normal separating epicardial fat plane in the case of an extracardiac mass; can determine the presence of a noncontractile intramyocardial mass, such as a rhabdomyoma |
Note—Using ECG-gated Trio 3-T MR scanner manufactured by Siemens Healthcare. SSFP = steady-state free precession, SNR = signal-to-noise ratio, CNR = contrast-to-noise ratio.
Cardiac MRI
Cardiac MRI is the most important modality in differentiating tumor from thrombus and distinguishing benign from malignant cardiac masses, and it helps to determine the extent of myocardial and pericardial invasion in cardiac masses [2]. At our institution, cardiac MRI is performed using both 1.5- and 3-T MRI units with a standard scanning time of approximately 1 hour. The goals of the cardiac MRI sequences selected are to define the extent and nature of the mass by its T1, T2, and T2* effects; to use cine sequences to determine mobility of the mass; to determine compromise of valvular or cardiac function secondary to the mass; and to determine enhancement pattern of the mass and invasion of the mass into coronary vasculature [3]. That MRI does not require the use of ionizing radiation makes it the modality of choice along with echocardiography for evaluation of pediatric patients with cardiac masses.
Detection and Localization
The protocol for cardiac MRI for tumors at our institution is listed in Table 1. First, dynamic multiplanar (four-chamber, vertical long axis, and short axis) cine bright-blood imaging is performed, which can localize the mass and provide both T1 and T2 characterization depending on the sequence used. Typically a cine steady-state free precession (SSFP) gradient-echo sequence is used first (for example, single-shot true fast imaging with steady-state precession [FISP], Siemens Healthcare) (Figs. 1 and 2), but cine spoiled gradient-echo sequences can be used as an alternative (for example, turboFLASH, Siemens Healthcare). The SSFP sequence has very high spatial and temporal resolution. SSFP has both T1 and T2 effects (both fat and water will be hyperintense) but is predominantly T2-weighted. The combined T1 and T2 contrast often allows differentiation of tissue characteristics from adjacent myocardium before (based on T2 effect) and after contrast injection (primarily on the basis of the T1 effect). The signal-to-noise and contrast-to-noise ratios are higher for this sequence because the sequence depends on intrinsic contrast properties and not flow effects. Nondependence on flow effects allows the use of extremely short TR times, leading to good temporal resolution, making this an extremely useful sequence in patients with difficulty holding their breath. This dynamic cine SSFP sequence permits determination of the mobility of a lesion, valvular function, and cardiac function. The addition of fat saturation may help with tissue characterization. Although cine fast gradient-echo imaging may be more prone to artifacts and reduced signal to noise [3], it is often useful to characterize any blood flow turbulence due to outflow obstruction or associated valvular dysfunction. The advantage of cine spoiled gradient-echo sequences is less susceptibility to off-resonance artifacts, which can be problematic when using steady-state precession sequences at magnet strengths of 3 T. The disadvantage of cine spoiled gradient-echo sequences is that mass size may be difficult to delineate because of poorer contrast resolution and mobile masses may cause turbulent flow causing signal loss because of the sensitivity of this sequence to inflow effects [3]. Also, lesion size can be overestimated because of adjacent turbulent flow and signal loss with these spoiled gradient-echo techniques. Spoiled gradient-echo bright-blood sequences can provide T1 signal characteristics of masses, but the adjacent bright-blood signal may be a limitation.
Fig. 1.
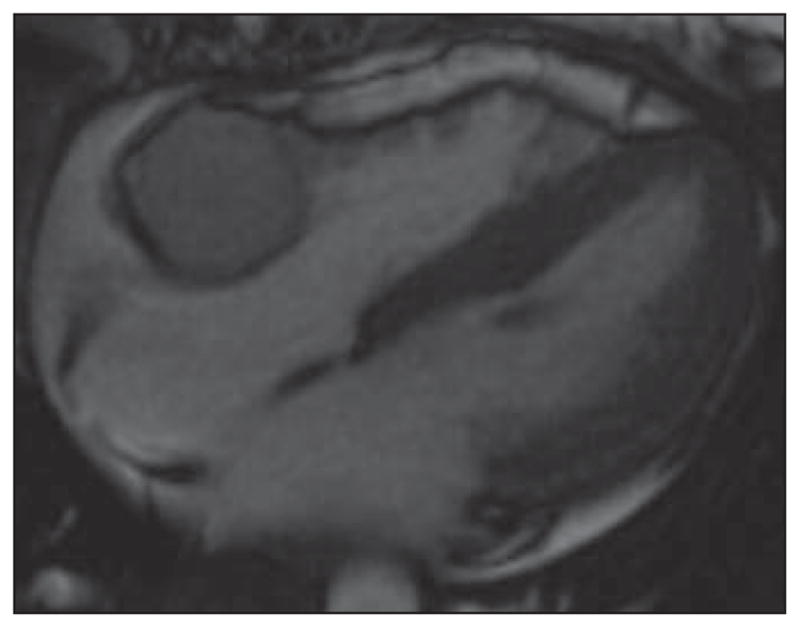
Steady-state free precession (SSFP) four-chamber image of heart in 37-year-old woman provides good anatomic information about cardiac morphology and can be used for functional assessment of cardiac motion and is typically acquired in multiple planes including two-, three-, and four-chamber cardiac planes. This is bright-blood imaging sequence with good endocardial-to-blood pool contrast ratio. In this patient, there is round mass in right atrioventricular groove that is isointense to myocardium of SSFP images, which have both T1 and T2 weighting that on subsequent imaging was confirmed to represent paraganglioma of atrioventricular groove. See also Figure S1, cine loop, in supplemental data online.
Fig. 2.
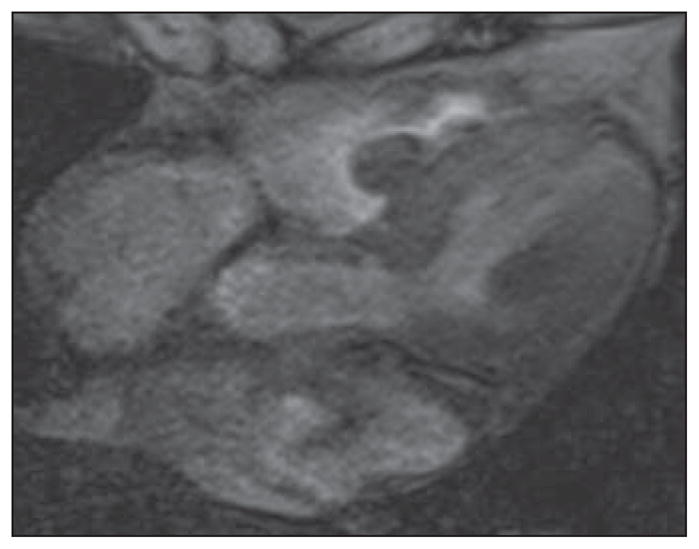
Gradient-echo MR images in four-chamber plane of heart in 82-year-old woman. These bright-blood images show lobulated mass in right ventricle subsequently resected and confirmed to represent myxoma. Contrast between endocardium and blood pool is less than on steady-state free precession (SSFP) cine sequences, and flow-related artifacts may be seen but this sequence is less susceptible to flow-related artifacts. See also Figure S2, cine loop, in supplemental data online.
Size and Extent
Next in our protocol, dark-blood, or black-blood imaging with T1- and T2-weighted turbo spin-echo double inversion recovery single breath-hold is performed (Fig. 3). Double inversion recovery sequences specifically null the signal from the blood in the selected slice by the application of a spatially nonselective 180° pulse followed by a spatially selective 180° pulse to the standard fast spin-echo sequence. These T1 turbo spin-echo double inversion recovery images allow excellent contrast resolution, which is particularly useful for determining the size and extent of invasion of a lesion relative to adjacent structures (Fig. 4). Fast spin-echo T1-weighted sequences are used at some institutions with similar effect, but the black-blood effect from nulling of signal from moving blood is better when double inversion recovery sequences are used. By adding a third slice-selective inversion pulse to saturate signal from fat (typically between the spatially nonselective pulse and the spatially selective pulse), a triple inversion pulse sequence is acquired, which is useful in detection of fat within a lesion (Fig. 5). The presence of T1 shortening by hemorrhage or melanin can be identified by T1-weighted double inversion recovery or gradient-echo sequences. Melanin causes T1 and T2 shortening, and the signal of a melanoma metastasis is determined by the extent of melanin in the lesion [3]. Melanoma metastasis also has a tendency to bleed, which can also cause T1 shortening because of the presence of methemoglobin [3]. Melanin can also cause T2* effects with susceptibility effect secondary to the presence of metal ions [3]. T2-weighted black-blood sequences can be acquired either using standard fast spin-echo or double inversion recovery sequences. Before gadolinium contrast material is administered, T2-weighted triple inversion fast spin-echo technique is useful to characterize for any myocardial edema. Both T1- and T2-weighted black-blood double inversion recovery or triple inversion recovery sequences are acquired with a single breath-hold. In patients who cannot hold their breath, free breathing fast spin-echo black-blood imaging can be used.
Fig. 3.
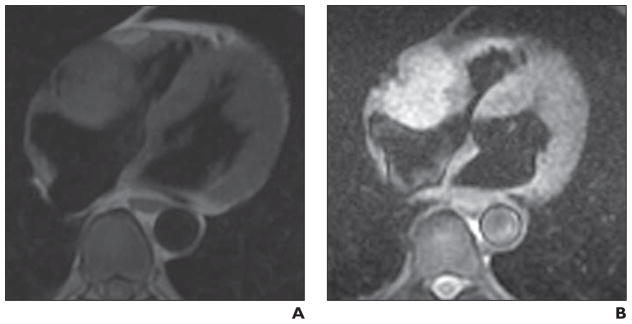
Imaging characteristics of phaeochromocytoma.
A and B, Four-chamber black-blood T1-weighted (A) and T2-weighted (B) double inversion recovery images in 62-year-old man. These are high-resolution images that show mass relative to key adjacent structures. Mass is T1 isointense and T2 hyperintense to adjacent myocardium. Mass has imaging characteristics of phaeochromocytoma and was resected and confirmed to represent this diagnosis.
Fig. 4.
T1-weighted double inversion recovery four-chamber image of heart in 47-year-old man shows large epicardial mass adjacent to left ventricular lateral wall, which was metastasis from known germ cell tumor. Utility of high-resolution T1-weighted double inversion recovery imaging is high spatial resolution that allows determination that epicardial fat has not been invaded by tumor (arrow). When there is doubt, adding tagging sequences (Fig. S4, cine loop in supplemental data online) may better depict whether there is independent motion of a mass from myocardium.
Fig. 5.
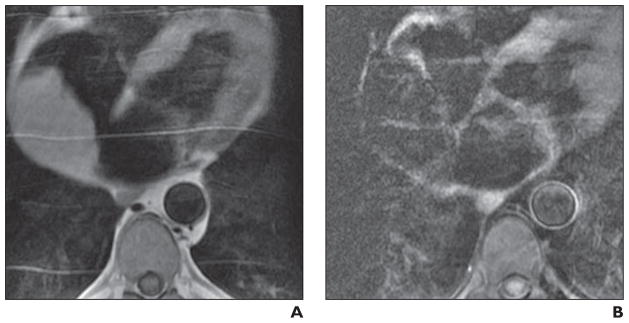
Large right atrial mass.
A and B, Four-chamber T1-weighted double inversion recovery (A) and triple inversion recovery (B) cardiac MR images in 60-year-old man show mass is isointense on T1-weighted double inversion recovery images, and when fat saturation is applied to generate triple inversion recovery, mass loses signal homogeneously, consistent with lipoma.
Vascularity and Perfusion
Vascularity of the tumor mass can be assessed using a T1-weighted single phase fast gradient-echo or SSFP sequence by comparing signal intensity of the mass before and after contrast administration. In addition, dynamic FLASH imaging can assess the first-pass perfusion of the tumor by a gadolinium bolus, which is often useful in distinguishing thrombus from vascular tumor. Unenhanced 3D volumetric breath-hold T1 fat-saturated sequences (volumetric interpolated breath-hold examination [VIBE], liver acceleration volume acquisition [LAVA]) allow further T1 characterization of tissue and are then used to determine enhancement when contrast-enhanced 3D volumetric breath-hold T1 fat-saturated sequences are acquired in the equilibrium phase after the administration of gadolinium (0.1–0.2 mmol/kg) (Fig. 6). Ten minutes after this contrast injection, late gadolinium enhancement single-shot, slice selective breath-hold images are acquired. An inversion time is set to achieve appropriate nulling of the normal myocardium (typically in the region of 200–300 milliseconds at 1.5 T) to allow delineation of the extent of the enhancing mass. The myocardiac delayed enhancement technique is useful in characterizing inflammation, fibrosis, and scar in the tumor (Fig. 7). Often, we also perform late gadolinium-enhanced imaging using a long inversion time of 600 milliseconds to detect thrombus in appropriate cardiac locations. Using this technique, thrombus is characteristically dark because of its long T1. In cases in which there is a concern for invasion into the myocardium of a pericardial or epicardial tumor, myocardial tagging with balanced SSFP MRI can be helpful. Tagging techniques are useful for evaluation of regional myocardial contraction, definition of tissue planes, differentiation between contractile and noncontractile tissue (tumor), and differentiation of a true intramyocardial mass versus a pseudotumor (such as focal asymmetric hypertrophic cardiomyopathy). Break (deflection) of tag lines during different phases of the cardiac cycle facilitates delineation of tissue planes and differentiates contractile (myocardial) from noncontractile (tumor) tissue.
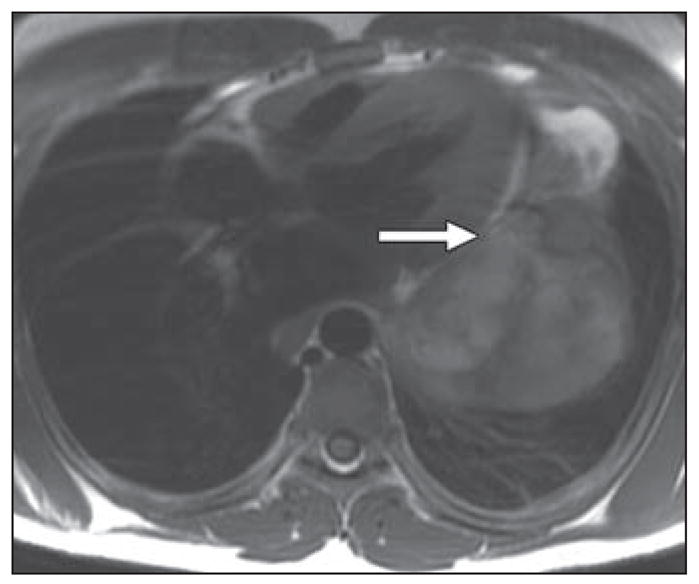
Fig. 6.
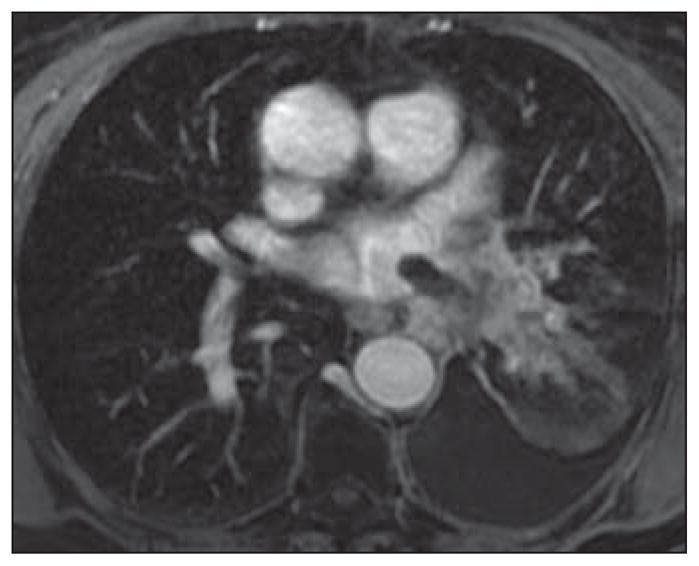
Contrast-enhanced volumetric interpolated breath-hold examination (VIBE) axial image of chest in 78-year-old woman with known primary adenocarcinoma of lung and worsening shortness of breath. There is extension of tumor along superior pulmonary vein with thumblike projection into vein and left atrium.
Fig. 7.
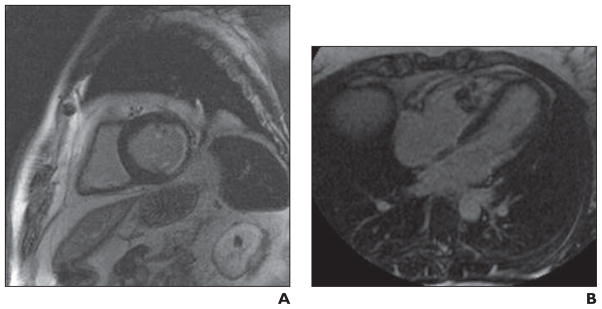
Myocardiac delayed enhancement imaging. Delayed enhancement cardiac MRI is typically acquired 10 minutes after weight-based dose of IV gadolinium with inversion time selected on turboFLASH (Siemens Healthcare) imaging to null signal from myocardium (typically in region of 300 milliseconds). Nulling myocardial signal is performed to better delineate areas of abnormal enhancement of myocardium.
A, Myocardiac delayed enhancement imaging was initially described in imaging of myocardial scar after infarct. In this short-axis myocardiac delayed enhancement image there is subendocardial nontransmural delayed enhancement of myocardium in inferolateral basal myocardial segment, consistent with nontransmural infarct. In imaging of cardiac masses, myocardiac delayed enhancement imaging can be used to determine enhancement characteristics of mass and to differentiate mass from thrombus.
B, Myocardiac delayed enhancement image shows heterogeneous enhancement of right ventricular mass in woman with history of sarcoma in calf. Right ventricular mass was resected and confirmed to represent metastasis.
Gated Cardiac CT
Gated cardiac CT can act in a complimentary role to the other modalities in the evaluation of a cardiac mass. CT can provide anatomic information, functional assessment, and tissue characterization. A global assessment of the chest can be acquired that can be useful, particularly in patients in whom a metastatic cardiac mass is suspected. CT may be useful in patients who are short of breath and unable to tolerate the prolonged supine positioning and repeated breath-holds required for adequate assessment by cardiac MRI. CT has inherent advantages for tissue characterization, such as in the detection of calcifications within a mass that may provide diagnostic information (prominent heterotopic mitral annular calcification can be mistaken at echocardiography and MRI for a mass or the presence of calcifications can differentiate a fibroma from a rhabdomyoma). Also patients with pacemakers, very obese patients, and very claustrophobic patients may be unsuitable for cardiac MRI. In cases of renal impairment in which gadolinium is contraindicated, iodinated contrast material may be used in certain clinical scenarios, such as patients with end-stage renal disease who are undergoing dialysis. Gated cardiac CT angiography can be used to determine the presence of vascular invasion by a mass—neovascular recruitment by the mass. Also, the presence of coronary artery disease may be incidentally discovered, which may guide surgical technique and intervention if clinically indicated. At our institution, single-heartbeat cardiac CT angiography is performed using 320-MDCT. The kilovoltage is adapted to patient body mass index and altered between 80–130 kVp; tube current modulation monitors tube current and exposure time. The protocol is tailored to the requirements of the study; if dynamic imaging is being performed then full R-R interval imaging is required and a dose modulated technique is performed. Where appropriate, prospective image acquisition is performed with tube output confined to typically the end diastolic phase for evaluation of the coronary arteries. However, functional analysis and generation of ventricular volumes are not possible when the acquisition is acquired prospectively. Typically up to 75 mL of non-ionic contrast material (370 mmol/L adapted according to patient weight and renal function) is administered at a rate of 6 mL/s.
Nuclear Imaging
Nuclear imaging including FDG PET/CT and other techniques, such as octreotide imaging, can be helpful in detection and diagnosis of cardiac and paracardiac tumors. Normal myocardial uptake of FDG may obscure small myocardial or pericardial lesions. Potential pitfalls exist, including uptake within benign lipomatous hypertrophy of the interatrial septum because it contains brown fat rich in mitochondria and may show a higher standardized uptake value than subcutaneous fat mimicking a mass. Secondary metastases to the heart are more commonly seen, especially in adult populations, and the use of PET CT may not only detect a cardiac mass but define the extent of disease by identifying further distant lesions.
Supplementary Material
References
- 1.Plana JC. Added value of real-time three-dimensional echocardiography in assessing cardiac masses. Curr Cardiol Rep. 2009;11:205–209. doi: 10.1007/s11886-009-0029-5. [DOI] [PubMed] [Google Scholar]
- 2.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radio Graphics. 2005;25:1255–1276. doi: 10.1148/rg.255045721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


