Abstract
Our objective was to study the diagnostic performance of regadenoson 82Rb myocardial perfusion PET imaging to detect obstructive coronary artery disease (CAD).
Methods
We studied 134 patients (mean age, 63 ± 12 y; mean body mass index, 31 ± 9 kg/m2) without known CAD (96 with coronary angiography and 38 with low pretest likelihood of CAD). Stress left ventricular ejection fraction (LVEF) minus rest LVEF defined LVEF reserve. The Duke score was used to estimate the anatomic extent of jeopardized myocardium.
Results
Regadenoson PET had a high sensitivity, 92% (95% confidence interval [CI], 83%–97%), in detecting obstructive CAD, with a normalcy rate of 97% (95% CI, 86%–99%), specificity of 77% (54/70 patients; 95% CI, 66%–86%), and area under the receiver-operator-characteristic curve of 0.847 (95% CI, 0.774–0.903; P < 0.001). Regadenoson PET demonstrated high sensitivity to detect CAD in patients with single-vessel CAD (89%; 95% CI, 70%–98%). The mean LVEF reserve was significantly higher in patients with normal myocardial perfusion imaging results (6.5% ± 5.4%) than in those with mild (4.3 ± 5.1, P = 0.03) and moderate to severe reversible defects (−0.2% ± 8.4%, P = 0.001). Also, mean LVEF reserve was significantly higher in patients with a low likelihood of CAD (7.2% ± 4.5%, P < 0.0001) and mild or moderate jeopardized myocardium than in those with significant jeopardized myocardium (score ≥ 6), −2.8% ± 8.3%.
Conclusion
Regadenoson 82Rb myocardial perfusion imaging is accurate for the detection of obstructive CAD. LVEF reserve is high in patients without significant ischemia or significant angiographic jeopardized myocardium.
Keywords: regadenoson, 82Rb, diagnostic accuracy, coronary angiography, ejection fraction
Regadenoson is a selective adenosine A2A receptor agonist aproved for use with SPECT myocardial perfusion imaging (MPI). Regadenoson is not inferior to adenosine for diagnosing reversible perfusion defects in patients undergoing 99mTc and 201Tl SPECT (1–3). The rapid onset of maximal hyperemia (<1 min), short duration of action, and ease of use (fixed-dose bolus administration) are the advantages of regadenoson (4). These features translate into a short stress protocol and rapid throughput, especially when used in conjunction with short-acting radiotracers such as 82Rb. The use of regadenoson as an intravenous bolus makes it particularly well suited for stress imaging inside a scanner gantry, as with MR imaging and PET.
82Rb PET MPI is being widely used in the management of symptomatic patients with known or suspected coronary artery disease (CAD) (5). The diagnostic value of 82Rb MPI using dipyridamole (6–11), adenosine, and dobutamine stress (12) is well documented. However, the diagnostic accuracy of regadenoson stress with 82Rb PET MPI has not been reported. Myocardial uptake, clearance, and biodistribution of various radiotracers can vary with the type of stressor used (13) and affect diagnostic accuracy. Therefore, to independently establish the diagnostic value of 82Rb MPI with regadenoson stress remains paramount. The primary objective of this study was to investigate the diagnostic value of vasodilator stress testing with regadenoson in conjunction with perfusion defects on 82Rb MPI to detect obstructive CAD.
MATERIALS AND METHODS
Patient Population
All patients underwent clinically indicated regadenoson 82Rb MPI between December 2008 and July 2010. Patients were evaluated for suspected CAD because of chest pain or nonclassic symptoms and multiple coronary risk factors. The study sample consisted of 134 patients without known CAD, including 96 consecutive stable patients who underwent invasive coronary angiography within 6 mo after the index PET/ CT study and, during the same period, 38 patients with a low pretest likelihood of CAD (without coronary angiography; <10% likelihood of CAD based on Diamond and Forrester method; included to calculate the normalcy rate) (14). Patients with a known history of angiographic CAD, pathologic Q waves on resting electrocardiography, or prior coronary revascularization were excluded. Patients with a left bundle branch block, hemodialysis, active wheezing, or oxygen-dependent lung disease who could not receive regadenoson were also excluded. This study was approved by the Partners Human Research Committee, and the need for informed consent from the patients was waived.
Acquisition and Analysis of PET Myocardial Perfusion Images
All patients were studied using a whole-body PET/CT scanner (Discovery Lightspeed VCT 64; GE Healthcare) after an overnight fast and a 12-h cessation of caffeine- or methylxanthine-containing substances. Images were acquired and processed as described in Figure1. The average radiation dose for this protocol was 4.6 mSv (15). Symptoms consistent with regadenoson (flushing, chest pain, headache, or dyspnea) were observed in 12 patients (8.9%), abdominal discomfort was observed in 3 patients (2.2%), and 3 patients (2.2%) received 100 mg of intravenous aminophylline for symptomatic relief.
FIGURE 1.
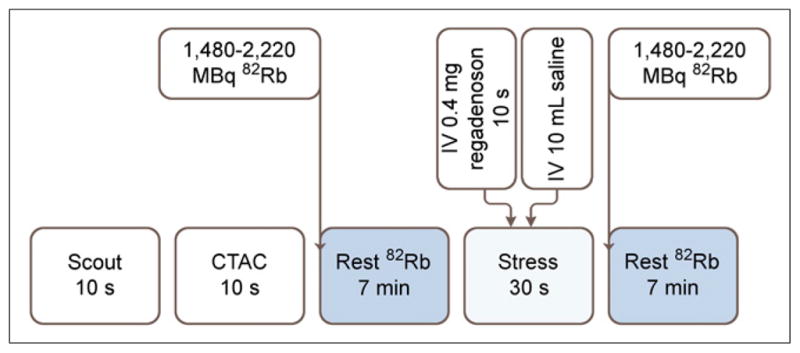
Rest–stress regadenoson 82Rb PET/CT protocol. After scout CT acquisition (120 kVp, 10 mA), CT transmission scan (CTAC) (140 kVp, 10 mA, pitch of 1.35) was acquired. Patients received 1,480–2,220 MBq of 82Rb intravenously at rest, and emission images were acquired in 2-dimensional list mode. After rest imaging, patients remained in scanner gantry for stress imaging. Stress was induced with 0.4 mg of regadenoson given intravenously over 10 s followed by 10-mL flush with normal saline. Immediately after saline flush, second dose of 1,480–2,220 MBq of 82Rb was administered intravenously approximately 30 s after regadenoson injection and emission images were acquired as previously described. Ordered-subsets expectation maximization (30 iterations and 2 subsets) and 3-dimensional PET filtering (Butterworth filter, cutoff frequency of 10, order of 5) were used for reconstruction of images.
Images were interpreted semiquantitatively and independently by 4 experienced observers using a standard 17-segment model and a 5-point (0–4) scoring system, without knowledge of the angiographic results. Global summed stress score (reflecting the magnitude of scar and ischemia), summed rest score (reflecting the magnitude of scar), and summed difference score (reflecting the magnitude of ischemia) (the difference between summed stress score and summed rest score) were computed. A summed stress score of more than 0 was considered abnormal. Rest and stress left ventricular volumes and ejection fraction were calculated using 4DM SPECT software (Invia; University of Michigan). The left ventricular ejection fraction (LVEF) reserve was computed as stress minus rest LVEF in a subset of 115 patients without gating errors, atrial fibrillation, atrial flutter, severe valvular heart disease, or prior valve replacement.
Coronary Angiography
Cineangiograms were obtained in multiple projections using an Integris BH3000 angiographic system (Philips). A visually determined stenosis diameter of at least 70% and at least 50% (both thresholds tested) for the left anterior descending, left circumflex, and right coronary arteries or their major branches and at least 50% for the left main coronary segment were considered significant. The anatomic extent of jeopardized myocardium was calculated using the Duke Jeopardy Score, which takes into account not only stenosis severity but also stenosis location. Calculation of the angiographic score was based on the location and distribution of obstructive CAD as described previously (16). The Duke Jeopardy Score reflects the amount of myocardium in the distribution of severe CAD and was categorized as tertiles of ≤1, 2–6, >6.
Statistical Analysis
Continuous variables are reported as mean ± SD, and binary or ordinal variables as proportions. Sensitivity and specificity were calculated using 2 by 2 tables and standard definitions. An online binomial calculator (http://statpages.org/confint.html) was used to calculate the 95% confidence interval (CI). Overall, of 995 patients without a prior coronary artery bypass graft, percutaneous coronary intervention, or Q-wave myocardial infarction who underwent regadenoson PET MPI during this time, 96 underwent coronary angiography and were clinically stable between the PET study and the coronary angiogram. The rate of coronary angiography was 3.8% for normal PET MPI findings and severalfold higher, at 24.7%, for abnormal PET MPI findings. Because of this referral bias to angiography based on scan results, specificity was calculated including subjects with a low pretest likelihood of CAD. To account for posttest referral bias, in 38 patients with a low pretest likelihood of CAD (without coronary angiography) we reported the normalcy rate (rate of scans with normal findings). Overall diagnostic accuracy was calculated using receiver-operator-characteristic curves and PASW statistics (version 18.0; SPSS Inc.).
RESULTS
The baseline characteristics of the study cohort are shown in Table 1. The mean age of the patient cohort was 63 ± 12 y, and the mean body mass index was 31 ± 9 kg/m2. On average, regadenoson increased the heart rate by 20 ± 12 beats/min and decreased systolic blood pressure by 9 ± 20 mm Hg. The median duration between the regadenoson PET study and invasive coronary angiography was 3 d (range, 0–123 d).
TABLE 1.
Baseline Characteristics of Study Cohort
| Patient characteristics | Coronary angiography (n = 96) | Low-likelihood (n = 38) |
|---|---|---|
| Age (y) | 66.5 ± 11.0* | 56.7 ± 11.0 |
| Body mass index (kg/m2) | 30.6 ± 7.3 | 29 ± 9 |
| Male | 62.5 (60) | 65.8 (25) |
| Hypertension | 84.4 (81)* | 0 |
| Diabetes | 29.2 (28)* | 0 |
| Dyslipidemia | 70.8 (68)* | 7.9 (3) |
| Tobacco | 9.4 (9) | 9.4 (2) |
| Family history | 15.6 (15) | 13.2 (5) |
| β-blockers | 67.7 (63)* | 21.7 (5) |
| Calcium blockers | 26.9 (25)† | 4.3 (1) |
| ACE inhibitors | 36.6 (34)† | 4.3 (1) |
| Nitrates | 9.7 (9) | 0 |
| Chest pain | 46.9 (45)* | 0 |
| Dyspnea | 33.3 (32)* | 0 |
| ST depression | 10.4 (10) | 2.6 (1) |
| Rest HR (bpm) | 71 ± 13 | 74 ± 13 |
| Peak stress HR (bpm) | 90 ± 15† | 97 ± 15 |
| Rest systolic BP (mm Hg) | 146 ± 27 | 137 ± 24 |
| Peak stress systolic BP (mm Hg) | 134 ± 23 | 128 ± 20 |
| Rest diastolic BP (mm Hg) | 76 ± 13 | 75 ± 11 |
| Peak stress diastolic BP (mm Hg) | 68 ± 12 | 67 ± 9 |
| Summed rest score | 2 ± 5* | 0.1 ± 0.6 |
| Summed stress score | 8.7 ± 8.3† | 0.1 ± 0.6 |
| Summed difference score | 6.8 ± 7.1† | 0.0 ± 0.0 |
P < 0.001 vs. low likelihood.
P ≤ 0.05 vs. low likelihood.
ACE = angiotensin-converting enzyme; HR = heart rate; BP = blood pressure.
Qualitative data are expressed as numbers, followed by percentages in parentheses; continuous data are expressed as mean ± SD.
Regadenoson PET MPI Results
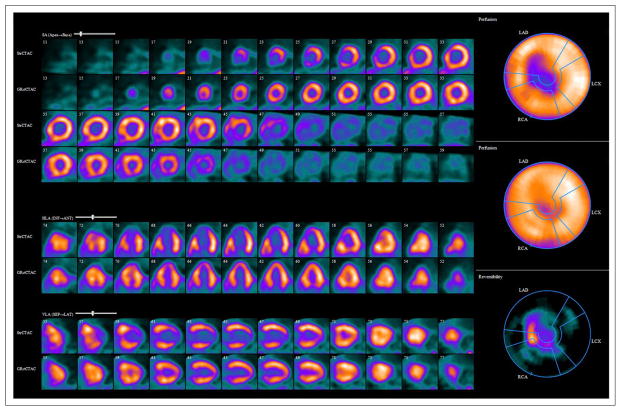
Overall, on the clinical read of 96 patients with invasive coronary angiography, 22 patients (23%) had a normal regadenoson PET MPI result and 74 patients (77%) had an abnormal result. Four (4.2%) of the 96 patients had fixed defects, whereas 15 (15.6%) had mild reversible defects and 55 (57.3%) had moderate to severe reversible defects (Figs. 2 and 3).
FIGURE 2.
Rest and regadenoson stress 82Rb PET myocardial perfusion images demonstrate medium-sized region of severe reversible perfusion defects in mid anterior wall, septum, apical anterior wall, apical septum, apical inferior wall, and apex, with transient cavity dilation. Coronary angiogram confirmed severe obstructive CAD in left anterior descending, left circumflex, and right coronary arteries.
FIGURE 3.

Rest and regadenoson stress 82Rb PET myocardial perfusion images demonstrate large region of severe reversible perfusion defect in entire inferior and inferolateral walls and basal inferoseptal region. Coronary angiogram demonstrated occluded left circumflex and right coronary arteries, without significant disease in left anterior descending coronary artery.
Diagnosis of Obstructive CAD by Regadenoson 82Rb PET
Overall, regadenoson 82Rb PET MPI correctly identified obstructive CAD in 59 of the 64 patients (sensitivity, 92%; 95% CI, 83%–97%) with evidence of significant stenosis on invasive angiography (defined as ≥50% stenosis in the left main coronary artery or ≥70% stenosis in other coronary arteries) (Table 2). Among patients with single-vessel CAD, regadenoson PET MPI had a sensitivity of 89% (23/26 patients; 95% CI, 70%–98%), and in patients with multivessel disease, the test sensitivity was 91% (32/35 patients; 95% CI, 77%–98%). The sensitivity was similar in men (95%) and women (88%) and in obese (94%) and nonobese (85%) individuals. Using a threshold of at least 50% in any coronary artery for significant stenosis (Table 3), the sensitivity for diagnosis of obstructive CAD was 90% (95% CI, 80%–96%).
TABLE 2.
Summary of Regadenoson Stress PET MPI Diagnostic Accuracy
| Regadenoson 82Rb PET | Significant CAD | No significant CAD | Total |
|---|---|---|---|
| Abnormal | 59 | 15 | 74 |
| Normal | 5 | 17 | 22 |
| Total | 64 | 32 | 96 |
Thirty-seven of 38 patients with low-likelihood CAD and 17 of 32 patients with nonobstructive CAD on invasive angiography were identified correctly. Significant CAD was defined as ≥70% CAD stenosis or ≥50% left main stenosis.
TABLE 3.
Summary of Regadenoson Stress PET MPI Diagnostic Accuracy
| Regadenoson 82Rb PET | Significant CAD | No significant CAD | Total |
|---|---|---|---|
| Abnormal | 61 | 13 | 74 |
| Normal | 7 | 15 | 22 |
| Total | 68 | 28 | 96 |
Significant CAD was defined as ≥50% CAD stenosis or ≥50% left main stenosis.
Regadenoson PET MPI correctly identified the absence of disease in 37 of the 38 patients with a low likelihood of CAD (normalcy rate, 97%; 95% CI, 86%–99%) and in 17 of the 32 patients without obstructive CAD (specificity, 53%; 95% CI, 34%–71%). Overall specificity, including low-likelihood patients, was 77% (54/70 patients; 95% CI, 66%–86%). For overall diagnostic accuracy, the area under the receiver-operator-characteristic curve was 0.847 (95% CI, 0.774–0.903; P < 0.001).
Gated Regadenoson PET
The mean end-diastolic volume and end-systolic volume were significantly higher and LVEF significantly lower in the abnormal than in the normal regadenoson MPI group (P < 0.0001 for each comparison) (Fig. 4). The mean LVEDV increased from rest to stress in both groups (Fig. 4). The mean LVESV decreased from rest to stress in the normal group but increased from rest to stress in the abnormal group (Fig. 4). As a consequence, LVEF increased from rest to stress in the normal group and did not change in the abnormal group (Fig. 4).
FIGURE 4.
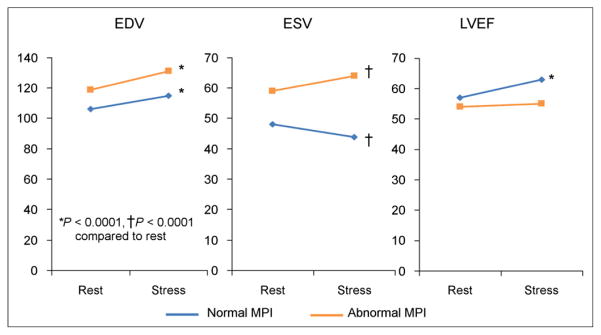
Changes in left ventricular volumes and ejection fraction from rest to regadenoson stress 82Rb MPI. EDV = end-diastolic volume; ESV = end-systolic volume.
LVEF Reserve and Extent of Jeopardized Myocardium
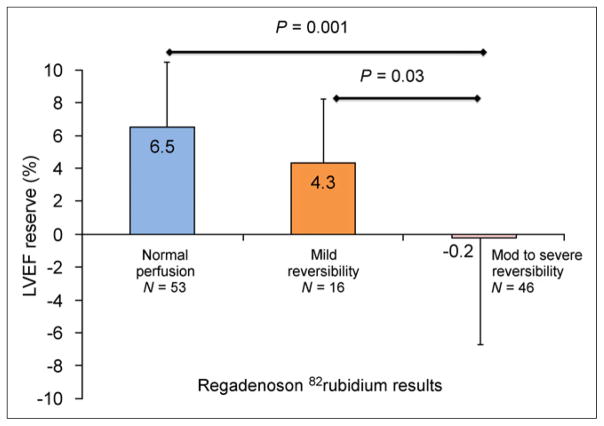
Overall, the extent and severity of reversible stress defects (summed difference score) was inversely proportional to the measured LVEF reserve (R = −0.5, P < 0.0001). The mean LVEF reserve was significantly higher in patients with normal regadenoson PET MPI findings than in those with abnormal findings (mean LVEF reserve, 6.5% ± 5.4% vs. 0.9% ± 7.8%; P < 0.0001). Patients with mild reversible defects (4.3 ± 5.1, P = 0.03) and moderate to severe reversible defects (−0.2% ± 8.4%, P = 0.001) (Fig. 5) showed a significantly lower mean LVEF reserve than did those with normal regadenoson PET findings (6.5% ± 5.4%).
FIGURE 5.
Regadenoson LVEF reserve as function of relative MPI results. Mod = moderate.
LVEF Reserve and Extent of Jeopardized Myocardium on Coronary Angiography
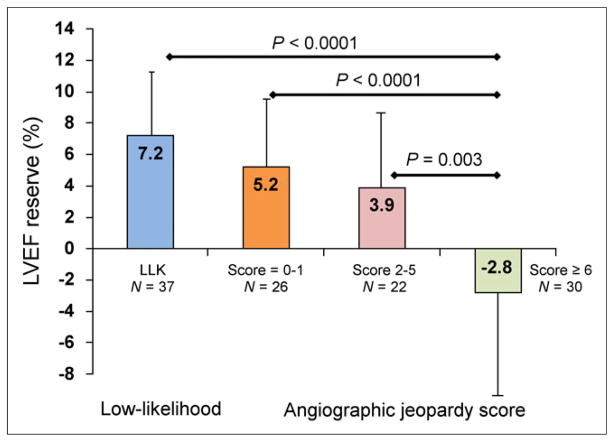
The Duke Jeopardy Score was inversely related to LVEF reserve (r = 0.4, P < 0.001). As shown in Figure 6, LVEF reserve was lowest, −2.8% ± 8.3%, in patients with significant jeopardized myocardium (score ≥ 6); by comparison, LVEF reserve was much higher in patients with a low likelihood of CAD (7.2% ± 4.5%, P < 0.0001), no or minimal jeopardized myocardium (score ≤ 1, 5.2% ± 5.5%; P < 0.0001), or moderate jeopardized myocardium (score of 2–5, 3.9% ± 6.6%; P = 0.003).
FIGURE 6.
Regadenoson LVEF reserve as function of Duke Jeopardy Score. LLK = low likelihood.
DISCUSSION
Regadenoson is logistically better suited for PET MPI than is infusion-based vasodilator stressors because of the bolus administration and fast throughput (15). Thus, although initially tested with SPECT radiotracers, regadenoson is clinically used with 82Rb MPI (17). Adenosine and dipyridamole were also initially tested with SPECT radiotracers (12) and clinically used with PET MPI, but several clinical studies support their diagnostic accuracy with PET MPI (5). However, to the best of our knowledge, there are no published clinical studies illustrating the diagnostic accuracy of regadenoson stress with PET MPI for the detection of obstructive CAD. Clinical diagnostic accuracy studies are paramount for supporting the continued use (or not) of regadenoson in clinical PET practice. Our study findings confirm that regadenoson stress with 82Rb MPI has a high sensitivity (92%) for the detection of obstructive CAD and a high diagnostic accuracy (area under the curve, 0.847). Importantly, high diagnostic sensitivity was maintained irrespective of age, sex, and body mass index. Also, the sensitivity for the detection of obstructive CAD in patients with single-vessel CAD and in patients with underlying multivessel CAD was equally high. The normalcy rate for excluding the presence of significant CAD was 97%.
When comparing our study findings with prior reports, it is notable that despite high sensitivity for detecting obstructive CAD, the specificity of regadenoson 82Rb PET MPI was somewhat low (8,12). The probable cause was posttest referral bias, as there was a 7-fold higher referral to coronary angiography in patients with abnormal PET MPI findings than in those with normal findings. Also, on invasive angiography 15 patients with abnormal PET MPI findings had no obstructive CAD. Coronary angiography is an imperfect gold standard for a functional test of ischemia, and in these 15 cases, the discordant findings between perfusion and coronary angiography were related to hemodynamically significant disease (coronary aneurysms with ectasia and no ≥70% stenosis, n = 1; nonobstructive and <70% CAD, n = 1), real scarring without obstructive epicardial CAD (scar on delayed hyperenhancement on cardiac MR imaging, n = 1), severe left ventricular hypertrophy (n = 1), likely real perfusion abnormalities (micro-vascular dysfunction, n = 2) (18), count-poor images (n = 3), and hot spots (n = 6). However, the normalcy rate, a surrogate for specificity, was high (97%) and comparable to that described previously (12).
Left ventricular ejection fraction reserve during regadenoson stress is inversely related to the magnitude of reversibility and the magnitude of jeopardized myocardium on invasive coronary angiography. Notably, in this study, mean LVEF increased from rest to stress in the normal-MPI group but not the abnormal-MPI group. Further, patients with severe reversible perfusion defects or extensive myocardium at risk (high Duke Jeopardy Score) demonstrated significantly attenuated LVEF responses, compared with low-likelihood patients and patients with no obstructive CAD. Changes in LVEF with regadenoson stress are of interest, since a low LVEF reserve during vasodilator stress (predominantly dipyridamole) 82Rb PET is a useful risk marker for significant ischemically jeopardized myocardium (19,20) and worse risk-adjusted long-term prognosis (21). However, the duration of maximal hyperemia is significantly shorter with regadenoson than with dipyridamole, raising uncertainty about the value of regadenoson in assessing peak stress LVEF. After an intravenous injection of regadenoson, peak hyperemia is maintained for about 2.3 min, and a coronary flow velocity of 2 times or greater than that of baseline is maintained for about 8 min (4). However, regadenoson also increases left ventricular dp/dt (a measure of myocardial contractility) by 29% (22). Furthermore, myocardial oxygen consumption is increased because of the increase in heart rate with regadenoson and a doubling of the triple product (heart rate times systolic blood pressure times left ventricular dp/dt) (22). These effects may together increase coronary blood flow in patients with normal MPI results and increase the LVEF reserve via the Gregg effect (states that increased coronary blood flow is a potent stimulus for increased myocardial contractility) (23). Further research may be helpful to better understand the prognostic value of regadenoson 82Rb PET MPI and LVEF reserve.
The short duration of maximal hyperemia with regadenoson (2.3 min) (4), combined with the short half-life of 82Rb (76 s), are potential challenges to optimal regadenoson 82Rb imaging (24). However, a recent study showed that stress myocardial blood flow (2.2 ± 0.6 vs. 2.1 ± 0.6 mL/min/g, P = 0.39) and coronary flow reserve (2.9 ± 0.8 vs. 2.8 ± 0.7, P = 0.31) were similar in 52 matched patients who underwent regadenoson or dipyridamole stress (17). Likewise, in 32 patients who underwent a clinical dipyridamole 82Rb study and a repeated rest–regadenoson stress 82Rb MPI study within 45 d, there was a high degree of correlation between the summed stress scores, with minimal bias (r = 0.88) and no difference in the summed stress (12.9 ± 7.0 vs. 14.1 ± 6.4, P = 0.23) or summed difference scores (7.0 ± 6.8 vs. 7.6 ± 6.2, P = 0.4) between the regadenoson and dipyridamole studies (24). Together, these studies confirm that the hyperemic response of regadenoson is comparable to that of dipyridamole during 82Rb MPI (17). The results of the current study extend these findings by confirming the clinical efficacy of regadenoson as a vasodilator stress agent, when used with relative 82Rb MPI in a much larger cohort of patients, applying obstructive CAD on angiography as the gold standard.
Although our study was a single-center study with a relatively small cohort, 96 patients had invasive angiographic results available for correlation. Future studies on larger patient cohorts are needed to confirm these findings. As was true for most similar prior studies, patients were referred for stress PET because of clinical findings, and coronary angiography was performed on the basis of clinical and imaging findings. Thus, both pre- and posttest referral biases may have artificially inflated the test sensitivity and deflated the test specificity. Hence, we reported the normalcy rates of regadenoson 82Rb MPI and reported specificity including the low-likelihood patients. Also, instead of visual analyses, quantitative coronary angiography and absolute myocardial perfusion could have been used. Nonetheless, in routine clinical practice, downstream patient management is driven by visual analysis of percentage of coronary stenosis, and relative perfusion imaging, rather than by computer analyses or absolute myocardial blood flow. Thus, the findings of this study reflect clinical practice, making them more widely applicable.
Regadenoson offers several advantages with 82Rb PET MPI. It is ideal for stress testing while patients are in the scanner gantry, because the non–weight-based bolus dosing avoids lengthy tubing. Moreover, the fast-paced test may be better tolerated by patients referred for PET, as they typically have a high burden of comorbidities (rest–regadenoson stress 82Rb PET in 16–18 min). Furthermore, regadenoson may be used safely in certain patients with asthma, chronic obstructive pulmonary disease, and end-stage kidney and liver disease (25). Regadenoson is well studied with maximal exercise treadmill testing (25,26), and use of hybrid protocols (maximal exercise followed by regadenoson and injection of 82Rb) may allow measurement of immediate postexercise myocardial blood flow with PET. Finally, the estimated radiation dose to patients is much lower with 82Rb (~3.7 mSv) (15) than with 99mTc and 201Tl (~10–22 mSv) (27). Also, when ammonia is used for MPI, the rapid stress test with regadenoson facilitates the coordination between cyclotron production, delivery, and imaging without a loss of activity due to radioactive decay during administration of infusion stress agents, especially dipyridamole. However, as with most vasodilator stress agents, caffeine (12 h) and theophylline (48 h) must be withheld before regadenoson stress. Also, despite safety in certain patients with asthma or chronic obstructive pulmonary disease (stable medical regimen for 1 mo prior to the test, including steroid use), regadenoson is contraindicated in patients with poorly controlled asthma or active wheezing.
CONCLUSION
Regadenoson used as a vasodilator stress agent in conjunction with 82Rb PETMPI offers rapid testing and high diagnostic accuracy for the detection of obstructive CAD at a low radiation dose. In patients with single-vessel CAD, the test sensitivity of regadenoson 82Rb PET MPI is as good as in patients with multivessel CAD and higher than that reported with SPECT MPI. Left ventricular ejection fraction reserve is high in patients without significant ischemia or significant angiographic jeopardized myocardium.
Footnotes
DISCLOSURE
This study was supported in part by a National Heart, Lung and Blood Institute grant (K23HL092299) and by research grants from Astellas and Siemens. Marcelo F. Di Carli is a consultant and on the Advisory Board for Bracco Diagnostics. No other potential conflict of interest relevant to this article was reported.
References
- 1.Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imaging. 2008;1:307–316. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 3.Mahmarian JJ, Cerqueira MD, Iskandrian AE, et al. Regadenoson induces comparable left ventricular perfusion defects as adenosine: a quantitative analysis from the ADVANCE MPI 2 trial. JACC Cardiovasc Imaging. 2009;2:959–968. doi: 10.1016/j.jcmg.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Lieu HD, Shryock JC, von Mering GO, et al. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J Nucl Cardiol. 2007;14:514–520. doi: 10.1016/j.nuclcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–1480. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 6.Gould KL, Goldstein RA, Mullani NA, et al. Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VIII. Clinical feasibility of positron cardiac imaging without a cyclotron using generator-produced rubidium-82. J Am Coll Cardiol. 1986;7:775–789. doi: 10.1016/s0735-1097(86)80336-9. [DOI] [PubMed] [Google Scholar]
- 7.Grover-McKay M, Ratib O, Schwaiger M, et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am Heart J. 1992;123:646–652. doi: 10.1016/0002-8703(92)90502-m. [DOI] [PubMed] [Google Scholar]
- 8.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Demer LL, Gould KL, Goldstein RA, et al. Assessment of coronary artery disease severity by positron emission tomography: comparison with quantitative arteriography in 193 patients. Circulation. 1989;79:825–835. doi: 10.1161/01.cir.79.4.825. [DOI] [PubMed] [Google Scholar]
- 10.Go RT, Marwick TH, MacIntyre WJ, et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899–1905. [PubMed] [Google Scholar]
- 11.Stewart RE, Schwaiger M, Molina E, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303–1310. doi: 10.1016/0002-9149(91)90456-u. [DOI] [PubMed] [Google Scholar]
- 12.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–1058. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Mekkaoui C, Jadbabaie F, Dione DP, et al. Effects of adenosine and a selective A2A adenosine receptor agonist on hemodynamic and thallium-201 and technetium-99m-sestaMIBI biodistribution and kinetics. JACC Cardiovasc Imaging. 2009;2:1198–1208. doi: 10.1016/j.jcmg.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina: summary article—a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 15.Senthamizhchelvan S, Bravo PE, Esaias C, et al. Human biodistribution and radiation dosimetry of 82Rb. J Nucl Med. 2010;51:1592–1599. doi: 10.2967/jnumed.110.077669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Califf RM, Phillips HR, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–1063. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 17.Goudarzi B, Fukushima K, Bravo P, Merrill J, Bengel FM. Comparison of the myocardial blood flow response to regadenoson and dipyridamole: a quantitative analysis in patients referred for clinical 82Rb myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2011;38:1908–1916. doi: 10.1007/s00259-011-1853-6. [DOI] [PubMed] [Google Scholar]
- 18.Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 19.Brown TL, Merrill J, Volokh L, Bengel FM. Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated 82rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2008;35:336–342. doi: 10.1007/s00259-007-0603-2. [DOI] [PubMed] [Google Scholar]
- 20.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48:349–358. [PubMed] [Google Scholar]
- 21.Dorbala S, Hachamovitch R, Curillova Z, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2:846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trochu JN, Zhao G, Post H, et al. Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J Cardiovasc Pharmacol. 2003;41:132–139. doi: 10.1097/00005344-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Gregg DE. Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circ Res. 1963;13:497–500. doi: 10.1161/01.res.13.6.497. [DOI] [PubMed] [Google Scholar]
- 24.Cullom SJ, Case JA, Courter SA, McGhie AI, Bateman TM. Regadenoson pharmacologic rubidium-82 PET: a comparison of quantitative perfusion and function to dipyridamole. J Nucl Cardiol. 2012;20:76–83. doi: 10.1007/s12350-012-9636-4. [DOI] [PubMed] [Google Scholar]
- 25.Ghimire G, Hage FG, Heo J, Iskandrian AE. Regadenoson: a focused update. J Nucl Cardiol. 2013;20:282–288. doi: 10.1007/s12350-012-9661-3. [DOI] [PubMed] [Google Scholar]
- 26.Partington SL, Lanka V, Hainer J, et al. Safety and feasibility of regadenoson use for suboptimal heart rate response during symptom-limited standard Bruce exercise stress test. J Nucl Cardiol. 2012;19:970–978. doi: 10.1007/s12350-012-9562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]