Abstract
Conditioned place avoidance (CPA) paradigm has been used to investigate the affective component of pain. Although the anterior cingulate cortex (ACC) has been demonstrated to play an important role in the affective aspect of pain, whether the other prefrontal subdivisions are involved in pain-related aversion is unknown. The present study investigated the role of the prelimbic cortex (PL) and infralimbic cortex (IL) in the acquisition and expression of formalin-induced CPA (F-CPA) in rats. GABAA receptor agonist muscimol was bilaterally microinjected into PL/IL before or after the formalin-paired training, to explore the effect of temporary inactivation of PL/IL on the acquisition and expression of F-CPA, respectively. The results showed that inactivation of PL rather than IL impaired the acquisition and expression of F-CPA. Moreover, the PL inactivation did not block the acquisition of LiCl-induced CPA, suggesting that PL may be specifically implicated in the pain-emotion related encoding. These results indicate that PL but not IL is involved in the aversive dimension of pain.
Keywords: Prefrontal cortex, Conditioned place avoidance, Acquisition, Expression, Pain emotion, Rats
1. Introduction
Pain has sensory-discriminative and emotional–motivational components. Although the pathways of sensory transmission of pain have been extensively investigated, the mechanisms of the affective component of pain are far from being clarified. In recent years, conditioned place avoidance (CPA) paradigm has been used to investigate the affective dimension of pain [10,11]. Several brain areas have been reported to be involved in the acquisition of pain stimulus-induced CPA, including the anterior cingulate cortex (ACC) [10], the central and basolateral nuclei of the amygdala [24].
Recent imaging studies have found that the prefrontal areas were activated in the emotional and cognitive modulation of pain in healthy participants [14]. Functional and structural reorganizations in prefrontal cortex have also been found in chronic pain patients and spinal nerve ligated rats [2,15]. These studies indicate the critical role of prefrontal cortex in pain modulation. In rodents, medial prefrontal cortex (mPFC) is divided into cingulate cortex (Cg), prelimbic cortex (PL), and infralimbic cortex (IL) subdivisions [9]. Johansen and colleagues have found that lesions of ACC in rats disrupt the acquisition of formalin-induced CPA (F-CPA) [10] but not prevent the expression of F-CPA [11]. For classical fear conditioning, although amygdala is thought to mediate the acquisition of fear-motivated response [12], there is a general consensus that PL and IL integrate inputs from the amygdala and project excitatory and inhibitory outputs to the amygdala respectively to gate the expression of fear [23].
Clinical observations have demonstrated that pain emotion-induced avoidance behavior is an important factor in the occurrence and development of chronic pain [27]. Therefore, in the present study we aimed to investigate the role of prefrontal areas in the acquisition and expression of pain-related CPA in rats. We microinjected GABAA receptor agonist muscimol into the prefrontal regions (PL and IL) to temporarily inactivate PL/IL and attempted to answer the following questions: (1) whether PL and IL are necessary for the acquisition and expression of F-CPA and (2) whether they play different roles in mediating the aversive dimension of pain.
2. Materials and methods
2.1. Animals
One hundred and fifty-two male Sprague-Dawley rats (weight on arrival: 180–200 g, Laboratory Animal Center of the Academy of Military Medical Sciences, Beijing) were used in this study. Experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. The research protocol was approved by the Institutional Animal Care and Use Committee of Chinese Academy of Sciences.
2.2. Conditioned place avoidance (CPA) procedure
Experiment was conducted in a three-compartment plastic chamber (75 cm × 30 cm × 30 cm, length × width × height). Two compartments were “conditioning” compartments (i.e., formalin was paired with one or the other, 30 cm × 30 cm × 30 cm), and the third was a “neutral” compartment (15 cm × 30 cm × 30 cm). The three compartments were characterized by distinct tactile and visual cues. One conditioning compartment had hole floor with horizontal stripes on the walls, whereas the other had grid floor and vertical stripes. Walls of uniform color and no distinctive floor characterized the neutral compartment.
CPA procedure was performed 1 week after surgery, including three sessions: the baseline, conditioning, and test session. On the first two days (baseline session), rats were allowed to freely explore the three compartments for a total of 900 s. The time spent in each compartment and locomotor distance in the whole chamber were measured automatically (MacroAmbition S&T Development, Beijing, China) and averaged across the two days. No significant difference was detected between time spent in the two conditioning compartments. Days 3 and 4 were conditioning sessions. On day 3, for the F-CPA, rats were given an intraplantar injection of 5% formalin (50 μl) into the right hindpaw and were randomly confined to one of the conditioning compartments for 1 h. For the LiCl (lithium chloride)-CPA, similar procedure was conducted except that rats were injected intraperitoneally with LiCl (dissolved in saline, 125 mg/kg, 5 ml/kg). Muscimol or saline was microinjected into PL/IL/ACC 30 min before the CPA training to test the effect of PL/IL/ACC inactivation on the acquisition. The formalin-induced nociceptive behaviors were videotape recorded and calculated in every 5-min. On day 4, each rat was confined in the opposite compartment for 1 h. For the F-CPA, rats were given no treatment. For the LiCl-CPA, normal saline was given intraperitoneally in the same volume as LiCl. On the 5th day (test session), each rat was allowed to explore the three compartments freely. The time spent in each compartment and locomotor activity over 900 s were recorded. A similar procedure was applied to examine the effect of PL/IL/ACC inactivation on the expression of CPA, except that the microinjections of muscimol or NS were performed 30 min before the test session. The CPA scores were calculated by subtracting the time spent in the formalin-/LiCl-paired compartment during the test session from that during the baseline session.
To test whether intra-PL/IL infusion with muscimol per se had rewarding or aversive effect, additional rats received muscimol microinjection without aversive stimuli and confined in one conditioning compartment for 1 h. The next day, rats were confined in the opposite compartment for 1 h with intra-PL/IL saline infusion. The baseline and test sessions were the same as described above.
2.3. Surgery
Cannula implantation was performed 1 week before the CPA procedure. Animals (250–280 g) were deeply anaesthetized with sodium pentobarbital (50 mg/kg, i.p.). Bilateral stainless steel infusion guide cannula (30 gauge) were stereotaxically implanted into the ACC (AP, +2.9; ML, ±0.6; DV, −1.5), PL (AP, +2.9; ML, ±0.6; DV, −3.0) or IL (AP, +2.8; ML, ±0.6; DV, −4.2). The guide cannulae were fixed to the skull with three stainless steel screws and dental cement. Each cannula was kept patent with a sterile obturator until the time of drug administration.
2.4. Drug infusion
The GABAA receptor agonist muscimol was purchased from Sigma–Aldrich (St. Louis, MO) and dissolved in saline (0.9% NaCl, 0.55 nmol/μL). The injection needle (a thin dental needle with a 0.3-mm outer diameter) was introduced through the guide cannula such that its terminal protruded 1 mm below the guide cannula. The displacement of an air bubble inside a polyethylene catheter (PE-10) connecting the syringe needle to the intracerebral needle was followed to monitor the microinjection. Muscimol or saline was delivered at a rate of 0.1 μL/min (0.2 μL/side) for PL/IL/ACC bilaterally. After infusion, injectors were left in place for 1 min to allow the drug to diffuse. The dose of muscimol was selected on the basis of studies showing that this dose could avoid spreading to adjacent brain areas and did not impair freezing behavior [22].
2.5. Histology
Rats were perfused with 0.9% saline followed by 4% formalin in phosphate buffer (PB; pH 7.4). Brains were extracted and sunk in 20%, 30% sucrose dissolved in 0.1 M PB. Frozen coronal sections were cut 40 μm thick and the sections were stained with cresyl violet. The approximate location of injection cannulas tips was determined under a microscope according to Paxinos and Watson atlas [18].
2.6. Statistical analysis
GraphPad prism 5.0 was used to analyze data and draw graphs. Student’s t-test was used for comparing 2 groups. Data involving 2 factors was analyzed with two-way analysis of variance (ANOVA). Bonferroni posttest was employed for post hoc test. The data was presented as means ± SEM. The statistical significance was set at P < 0.05.
3. Results
3.1. Effect of PL/IL inactivation on the acquisition of F-CPA
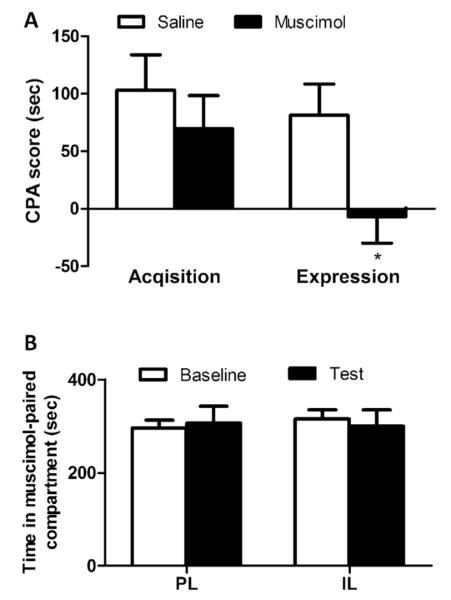
Microinfusion of muscimol into PL during acquisition stage significantly reduced the CPA score in the test (t-test, 1.8 ± 33.5 s vs. 121.9 ± 30.0 s, P < 0.05) (Fig. 1A), suggesting that PL inactivation impaired the acquisition of F-CPA. By contrast, inactivating IL with muscimol did not exert an effect on the acquisition of F-CPA (t-test, 106.5 ± 38.3 s vs. 117.7 ± 39.1 s, P > 0.05) (Fig. 1A). Additionally, infusion of muscimol into PL or IL had no effect on the formalin-induced licking behavior (two-way ANOVA, group × time effect: F(36, 444) < 1, P > 0.05; group effect: F(3, 37) < 1, P > 0.05) (Fig. 1B).
Fig. 1.
Effect of muscimol microinjection on the acquisition of F-CPA and formalin-induced nociceptive behavior. (A) CPA behavior. Significantly reduced avoidance behavior was found for muscimol microinjection into PL, suggesting that PL inactivation impaired the acquisition of F-CPA. By contrast, muscimol microinjection into IL had no effect on the acquisition of F-CPA and (B) formalin-induced nociceptive behavior. Inactivation of PL or IL did not exert an influence on the formalin-induced licking behavior. n = 9–12. *P < 0.05 vs. corresponding saline group.
3.2. Effect of PL/IL inactivation on the expression of F-CPA
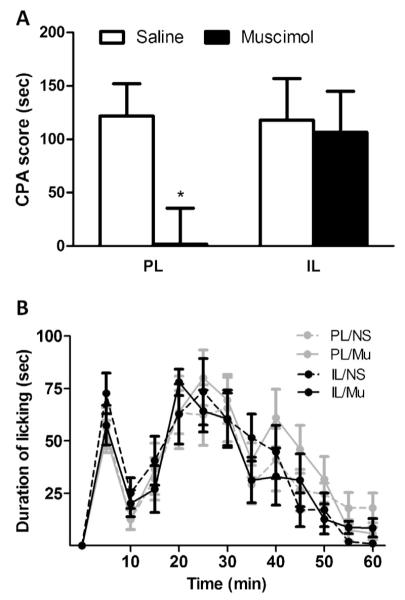
The effect of PL/IL inactivation on the expression of F-CPA was similar to that on the acquisition. As illustrated in Fig. 2A, intra-PL injection of muscimol before test blocked the avoidance behavior from the formalin-paired compartment (t-test, −32.6 ± 36.9 s vs. 138.1 ± 40.2 s, P < 0.01) (Fig. 2A), suggesting that PL is involved in the expression of F-CPA. However, inactivating IL with muscimol had no effect on the expression of F-CPA (t-test, 123.4 ± 41.6 s vs. 108.7 ± 34.3 s, P > 0.05) (Fig. 2A). In addition, infusion of muscimol into PL or IL did not exert an influence on the locomotor distance (two-way ANOVA, 20,657 ± 3122 s vs. 21,990 ± 3174 s, P > 0.05. for PL; 25,573 ± 3616 s vs. 21,659 ± 3224 s, P > 0.05 for IL) (Fig. 2B).
Fig. 2.
Effect of muscimol microinjection on the expression of F-CPA and locomotor activity. (A) CPA behavior. Similar to the acquisition phase, muscimol microinjection into PL attenuated the learned avoidance behavior; conversely, intra-IL muscimol microinjection did not exert an effect on the expression of F-CPA and (B) locomotor distance. No changes in the locomotor distance were found after inactivation of PL or IL. n = 8–10. **P < 0.01 vs. corresponding saline group.
3.3. Effect of PL inactivation on the acquisition and expression of LiCl-CPA
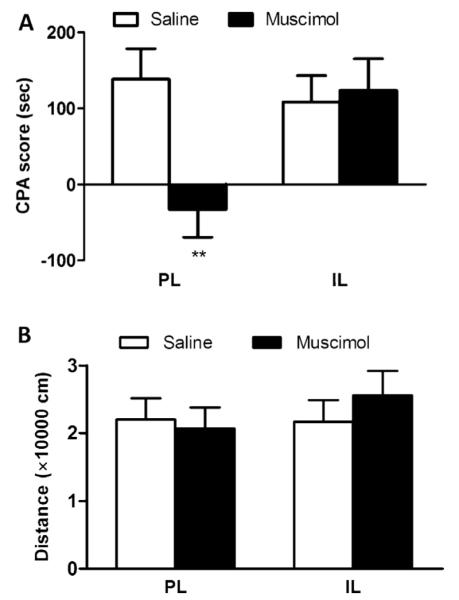
The PL inactivation during aversive learning did not reduce LiCl-induced avoidance behavior (t-test, 69.8 ± 28.6 s vs. 103.1 ± 30.9 s, P > 0.05), suggesting that PL is not involved in the acquisition of LiCl-CPA. In contrast, intra-PL infusion of muscimol before test significantly reduced the expression of LiCl-CPA (t-test, −6.9 ± 23.0 s vs. 81.2 ± 27.1 s, P < 0.05) (Fig. 3A). Additionally, inactivation of PL/IL in the absence of aversive stimulus did not produce preference or avoidance behavior (Fig. 3B), indicating that the muscimol injection per se had no rewarding/aversive effect.
Fig. 3.
Effect of PL inactivation on the acquisition and expression of LiCl-CPA. (A) Intra-PL injection of muscimol during the acquisition phase had no effect on the avoidance behavior. However, inactivation of PL disrupted the expression of LiCl-CPA and (B) no preference or avoidance behavior was observed after intra-PL/IL infusion of muscimol without aversive stimuli. n = 6–9. *P < 0.05 vs. corresponding saline group.
3.4. Effect of ACC inactivation on the acquisition and expression of F-CPA
As illustrated in Fig. 4, an attenuated avoidance behavior was observed for intra-ACC injection of muscimol during the acquisition of formalin-induced conditioning (t-test, −7.2 ± 36.3 s vs. 115.4 ± 33.5 s, P < 0.05). However, inactivation of ACC before test did not disrupt the expression of F-CPA (t-test, 107.2 ± 39.2 s vs. 84.5 ± 35.4 s, P > 0.05) (Fig. 4). These results indicate that ACC is implicated in the acquisition rather than the expression of F-CPA.
Fig. 4.
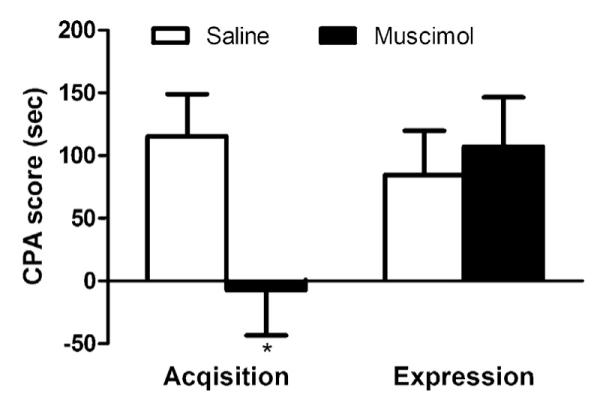
Effect of ACC inactivation on the acquisition and expression of F-CPA. Intra-ACC injection of muscimol before acquisition reduced the avoidance behavior toward pain-paired compartment. In contrast, ACC inactivation had no effect on the expression of F-CPA. n = 6–8. *P < 0.05 vs. corresponding saline group.
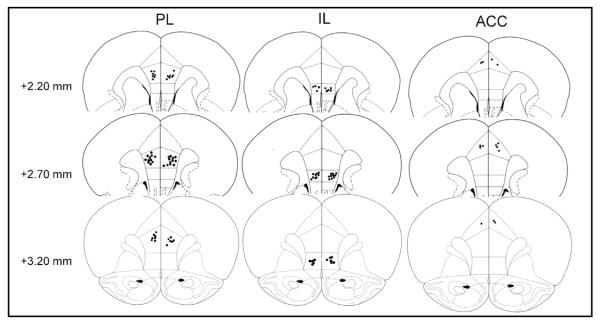
3.5. Histologically verified placement of cannulae
A schematic of coronal rat brain sections showing the sites of cannula placement is presented in Fig. 5. Six rats were excluded for improper cannula placement. All rats included in our data analysis had histologically verified placement of cannulae in the PL/IL/ACC.
Fig. 5.
Schematic drawings of the injection sites. Cannula placements within the PL/IL/ACC are shown in coronal sections modified from the atlas of Paxinos and Watson. The injection sites are marked by solid black circles.
4. Discussion
In the present study, we investigated the effects of temporary inactivation of the PL/IL cortex with muscimol on the acquisition and expression of F-CPA. The results showed that inactivation of PL rather than IL prevented the acquisition and expression of F-CPA. Besides, PL is not involved in the acquisition of LiCl-CPA, suggesting that PL is specifically implicated in the coding of pain emotion. Inactivation of PL also disrupted the expression of LiCl-CPA, indicating that PL may be involved in a general memory retrieval function.
Consistent with previous studies [8], we found that PL inactivation does not prevent the acquisition of LiCl-CPA, ruling out the possibility that the disrupted acquisition of F-CPA was attributed to the impaired conditioning learning ability. In addition, our results showed that infusions of muscimol into PL or IL did not affect the formalin-induced licking behavior in our study, in accordance with previous research showing that temporal activation or inactivation of vMPFC had no effect on the formalin-induced pain and tail flick test [1,21]. Thus, the most likely explanation for the important role of PL in the acquisition of F-CPA may be that PL itself is involved in the encoding of the aversive dimension of pain. Although PL is not essential in the acquisition of classical fear conditioning [23], it is required in the acquisition of some modified conditioning paradigms, such as olfactory conditioning [13]. The nature of the unconditioned stimulus (US) might be an important factor underlying this discrepancy. Compared with electric shock stimulus or LiCl, formalin-induced pain is much tenser. Formalin-induced pain, especially the second phase, depends on central sensitization and increases a wide range of Fos expression or regional cerebral blood flow in emotional pain-related regions such as the mediodorsal thalamus, insular cortex, amygdala, and ACC [5,17]. PL has been shown to have reciprocal projections with these areas [25]. Thus, the extensive interconnections also support a role for PL in the emotional processing of pain.
Another possible explanation may be that PL is the site where the association of nociceptive information and environmental cues is stored. In accordance with previous studies [10,11], we found the role of ACC in the acquisition rather than expression of F-CPA. ACC is necessary to provide the nociceptive aversive teaching signal, but it is not where the association stored [11]. ACC and PL are in close proximity and tightly interconnected [25]. It is possible that the two structures are involved in different components of pain-related memory traces [16]. During the acquisition of F-CPA, PL might receive pain-related aversive information from ACC where the emotional pain is encoded and integrate these nociceptive signals with environment cues. Therefore, inactivation of PL might block the synaptic plasticity of the association of pain-related aversion and environmental context.
In the present study, PL inactivation impaired the expression of LiCl-CPA as well as F-CPA. This is consistent with previous study indicating that PL is primarily involved in the selection of responses based on previously encoded associations [7]. In humans, ACC integrates the sensory and emotional components of pain and projects these information to PFC, suggesting the role of PFC in the initiation and planning of pain-related avoidance behavior [20]. Thus, during the expression of F-CPA, PL may be required to decide whether or not to mobilize the avoidance behavior according to the motivational effect of environmental cues. Furthermore, the essential role of PL in acquisition as well as expression of F-CPA is in accord with previous study, suggesting that the perception of pain-related negative affect and pain-related aversive learning are not possibly to be clearly separable at the neural level [6].
Previous studies have shown that the activity of IL is not essential for the acquisition or expression of fear conditioning [12,23]. Similarly, we demonstrated that IL is implicated in neither acquisition nor expression of F-CPA. IL and PL represent differential functions and reciprocal connections with other areas. IL is primarily involved in visceromotor functions, homologous to the mPFC of primates, and the PL (and ACC) is involved in limbic/cognitive activity, homologous to DLPFC of primates [26]. In chronic back pain reciprocal inhibitory responses in DLPFC and mPFC activity were found in fMRI studies [4]. Similarly, although IL has reciprocal interconnection with PL and ACC, its role in nociception seems to be different from PL and ACC. Millecamps et al. infused d-cycloserine into mPFC and found that the distances between cannulas and PL, ACC were negatively correlated to antinociception effect; however, for IL the correlation was positive [16]. Finally, some researchers have proposed that chronic pain is the result of persistence or inability to extinguish pain-related emotion evoked by an initial injury [3]. Thus the mechanisms underlying extinction of F-CPA is worth further exploration. Since previous studies have found that IL is involved in the extinction of fear conditioning and drug relapse [19], the role of IL in the extinction of F-CPA needs to be clarified in future studies.
5. Conclusion
In summary, our study demonstrated that temporary inactivation of PL rather than IL blocked the acquisition and expression of F-CPA. The results revealed the critical role of PL in pain-related aversion, and provided new evidence for the differential roles of PL and IL in pain emotion. Future studies should be designed to investigate the role of IL in the extinction of F-CPA, so as to better understand the involvement of the prefrontal cortex in emotional pain.
HIGHLIGHTS.
We explored the role of prelimbic cortex and infralimbic cortex in formalin pain-induced conditioned place avoidance.
Prelimbic cortex plays an essential role in the acquisition and expression.
Infralimbic cortex is not involved in either of them.
Acknowledgments
This work was funded by an NNSF Grant (31271092) and a Chinese Academy of Sciences Knowledge Innovation Project Grant (KSCX2-EW-Q-18) awarded to J.-Y.W., as well as NNSF Grants (30970959, 61033011, and 31171067), Chinese Academy of Sciences Knowledge Innovation Project Grants (YZ200944, KSCX2-YW-R-254, and KSCX2-EW-J-8), and a grant from the NIH Fogarty International Center (R03 TW008038) awarded to F.L. This research was also supported by the Key Laboratory of Mental Health at the Institute of Psychology, Chinese Academy of Sciences, China.
References
- [1].Altier N, Stewart J. Intra-VTA infusions of the substance P analogue, DiMe-C7, and intra-accumbens infusions of amphetamine induce analgesia in the formalin test for tonic pain. Brain Res. 1993;628:279–285. doi: 10.1016/0006-8993(93)90965-p. [DOI] [PubMed] [Google Scholar]
- [2].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apkarian AV. Pain perception in relation to emotional learning. Curr. Opin. Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baulmann J, Spitznagel H, Herdegen T, Unger T, Culman J. Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience. 2000;95:813–820. doi: 10.1016/s0306-4522(99)00478-9. [DOI] [PubMed] [Google Scholar]
- [6].Cao H, Gao YJ, Ren WH, Li TT, Duan KZ, Cui YH, Cao XH, Zhao ZQ, Ji RR, Zhang YQ. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 2009;29:3307–3321. doi: 10.1523/JNEUROSCI.4300-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- [8].Davis JF, Loos M, Di Sebastiano AR, Brown JL, Lehman MN, Coolen LM. Lesions of the medial prefrontal cortex cause maladaptive sexual behavior in male rats. Biol. Psychiatry. 2010;67:1199–1204. doi: 10.1016/j.biopsych.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- [12].Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci. Biobehav. Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J. Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- [15].Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. d-Cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2007;132:108–123. doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in fore-brain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain. 1998;75:355–365. doi: 10.1016/s0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates. Academic; Sydney: 2007. [DOI] [PubMed] [Google Scholar]
- [19].Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- [21].Resstel LB, Souza RF, Guimaraes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol. Behav. 2008;93:200–205. doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [22].Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur. J. Neurosci. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- [25].Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- [26].Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuro-science. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- [27].Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]