Abstract
The question pursued in this study was when neural activity appears in the cortico-basal ganglia system that could predict alternate behavioral responses in a reaction time (RT) task. In this protocol, rats first performed a nose poke to initiate a trial, depressed a lever when presented, and then released the lever after a tone cue. Multiple-channel, single-unit recordings (up to 62 units) were obtained simultaneously from the prefrontal cortex, the dorsal medial striatum, the globus pallidus, and the substantia nigra pars reticulata in a single rat during a session. Results indicated that (1) global alterations of neural activity appeared in clusters, which was associated with different behavioral components and observed in each of the targeted areas; (2) small independent subsets of neurons responded differently between error (lever was released before tone presentation) and correct trials (lever was released within 0.5 s after tone onset) during these behavioral episodes; (3) significant correlations between RTs and single units activities were found in the early preparation phases of the task. The results reveal that complex early preparatory activity exists several seconds before the final movements in a RT task, which may determine executive functions leading to rapid decoding of alternate behavioral performances.
Keywords: Striatum, Frontal cortex, Substantia nigra pars reticulata, Globus pallidus, Movement initiation, Timing
1. Introduction
Accurate performance of a reaction time (RT) task requires anticipation and coordination of sensory and motor functions. With the ability to rapidly decode cues that affect initiation of movement, the frontal cortico-basal ganglia system is thought to be a flexible system involved in processing and integrating sensorimotor information into an executive role over many complex and diverse cognitive functions. These include not only movements required by simple RT tasks, but delayed responses requiring working memory task components (Chang et al., 2002; Deadwyler et al., 1996; Hampson et al., 1996, 1999, 2000; Hampson and Deadwyler, 1996), coding of place and directional information (Crutcher et al., 1990; Callaway and Henriksen, 1992; Lavoie and Mizumori, 1994; Pratt and Mizumori, 1998), the alteration of error versus correct task outcomes (Chang et al., 2002; Deadwyler et al., 1996; Hampson et al., 1996, 1999, 2000; Hampson and Deadwyler, 1996), seeking of specific reward (Chang et al., 1994, 1998, 2000; Mulder et al., 2000), and decoding of complex information required for an executive role in selection of a response (Takenouchi et al., 1999).
The basal ganglia are in a pivotal position to process descending information from cortical regions to regulate motor function (Alexander et al., 1990; Pennartz et al., 1994). Two basal ganglia-thalamocortical pathways are postulated to be involved in motor regulation. A ‘direct pathway’ consists of a GABAergic projection from the striatum to the entopeduncular nucleus (EP)/substantia nigra pars reticulata (SNr) while an ‘indirect pathway’ reaches the EP/SNr by relay through globus pallidus (GP) and subthalamic nucleus (Grofova et al., 1982; Kita and Kitai, 1991; Wilson et al., 1982). The output from EP/SNr projects back to the cortices via thalamic nuclei. Together, these circuits form a functional network for initiation and selection of movement. The question arises as to in which subsets of neurons, and in what temporal sequence the activity appears in this complex system that leads to the execution of specific behaviors.
This functional anatomic organization of the frontal cortico-basal ganglia system provides a possible basis for its involvement in regulation of a simple RT paradigm (lever release after tone cue) as examined in this study. Primate study indicates that when visuomotor associations have become well established through over-training, the task performance depends on connections between the basal ganglia and premotor cortex (Nixon et al., 2004). Lesions of frontal cortex generate longer RTs and additional errors, observed as both early (i.e., premature) and late (i.e., too late to get a reward) responses in similar tasks (Baunez et al., 1998; Muir et al., 1996). Striatum is also thought to be involved in the performance of a RT task since depletion of dopamine in the caudate nucleus impairs RT performance, and leads to more early and late responses (Amalric and Koob, 1987). Pharmacological agents that influence glutamatergic, GABAergic, and dopaminergic transmission within striatum, GP, or SNr can exert opposite effects on motor control since both sustained holding and prompt release may appear. A proper balance of action, between holding and timed release, appears to be necessary to optimize RT performance (Amalric et al., 1994, 1995b; Baunez and Amalric, 1996). In spite of extensive behavioral studies, few reports have been published describing neuronal activities during a RT task (Laubach et al., 1999, 2000) in which a specific requirement for rapid responding is imposed. A study indicates that in reaction time tasks, a perceptual choice is made when the firing rate of a selective cortical neural population reaches a threshold, which is sensitive to, and can be optimally tuned by the strength of cortico-striatal synapses (Lo et al., 2006).
In addition, could neural firing before movement completion predict behavior outcome? In a motor neuron study, a prominent anticipatory change was found during preparation period, suggesting that central influences act on motor neurons well before it is time to act (Duclos et al., 2008). Furthermore, it is reported that neural activity in mPFC and ACC was related to the level of preparatory attention and subsequent correct or incorrect choice (Totah et al., 2009). Another study also indicates that the electrical patterns in integrative motor areas before accurate performance differed from the patterns before inaccurate performance in adult brain (Gevins et al., 1987). In addition, the real-time transformations of neuronal population signals derived from multiple cortical areas in primates could be used to control prosthetic robot arm movements (Wessberg et al., 2000).
The aim of the current study is to reveal the neuronal ensemble activities within the cortical-basal ganglia circuit underlying the performance of the simple reaction time task. A question we tried to resolve in this study concerned precisely when activity arises in the cortical striatal system that is predictive of alternate modes of performance in a RT task. A statistical search technique was employed to reveal the differences in activity within the subsets of neurons related to RT performance. Our hypothesis was that the state of the neural network, such as attention for the go cue, and the readiness for execution of the response, might contribute to how well the animal can perform the reaction time task. Thus, we predicted that signals would arise both in the early phase and immediately before the movements that carry out early error responses or regulate fast versus slow responses.
2. Materials and methods
2.1. Animals
Six young adult male Sprague-Dawley rats weighing 300–350 g were used in the experiment. Animals were housed individually under reversed dark-light cycle (lights off from 7:00 to 19:00 h) for seven days. They were then water-deprived for 24 h before RT training. Animals’ body weights were maintained during the early stages of training at no less than 90% of the initial level by adjusting water intake. Training was processed in steps during a 1–2 week period, starting from a simple lever press for water reward to completion of the sequential actions of RT task. Animals were treated in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals.
2.2. Surgical procedures
Recording microwires were implanted after the completion of RT task training. Rats were given free access to water for three days before surgery. They were then anesthetized with ketamine (100 mg/kg, i.m.). After securing them in a stereotaxic apparatus, the skull was exposed and small holes were drilled in the skull for the electrodes, screws, and ground wires. Arrays of eight stainless steel Teflon-insulated microwires (in 2 × 4 or 3:3:2 arrangements, 50 μm in diameter, minimum 250 μm between each two wires, NB Labs, Denison, Texas) were lowered bilaterally into the medial prefrontal cortex (mPFC), dorsal medial striatum (DMS), GP, and SNr of each rat (eight wires on a strip connector per location). The coordinate positions were: 3.2 mm anterior (A) to bregma, 0.5 mm lateral (L) to the midline and 3.5 mm ventral (V) to the surface of cortex for the mPFC; 0.5 mm A, 2.5 mm L, and 3.7 mm V for the DMS; −1.5 mm A, 3.2 mm L, and 6.0 mm V for the GP; and −5.2 mm A, 2.0 mm L, and 7.8 mm V for the SNr, according to the atlas of Paxinos and Watson (1998). Stainless steel ground wires were positioned about 2-mm ventral to the cortical surface. The head stage was secured onto the cranium with dental cement using skull screws as anchors. The animals received enrofloxacin (2.5 mg/kg, i.m.) before surgery to prevent infection.
2.3. Apparatus and behavioral training
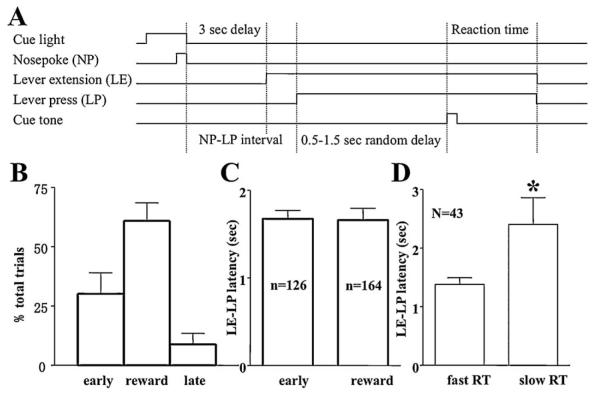
The reaction time task procedure is presented in Fig. 1A. Water-deprived rats were placed in an operant conditioning cage, which was enclosed in a sound-attenuating chamber. The base of the cage was 22 cm × 44 cm, and was 20 cm in height. A retractable lever was mounted on the wall 8 cm above the cage floor. The RT trial started with presentation of a cue light located above the nose poke device. The rat was required to make a nose poke at the device located 7 cm from the lever, which turned off the cue light. This gave the animals an easy control to initiate the trial by themselves, so that they would be more likely to focus on the task, and made necessary motor preparation for the later stage of the trial. The lever was extended after a 3-s delay. The reaction time sequence was initiated after the rat pressed the lever. A tone was presented randomly between 0.5 and 1.5 s after the lever press. The rat was required to release the lever in response to the tone within a short time window (<0.5 s) to obtain a drop of water (about 30 μl) at a spout. Error trials (releasing before the tone, or holding longer than 0.5 s after the tone) resulted in a 5-s time-out period with house light turned off. A new trial would start after a 5-s inter-trial interval signaled by illumination of a ceiling light.
Fig. 1.
Description of the reaction time task. (A) Diagram for the reaction time task. (B) Behavioral performances of the reaction time task showing percentage of trials that was correct, release too early, or too late in all six rats. (C) Mean lever extension-lever press intervals in early and correct response trials of a sample session of the rat, no significant difference could be detected between early versus correct responses. (D) Mean lever extension-lever press intervals in the same session, significant difference between the fast and slow quartile of reaction time in correct trials was detected (p < 0.001). Extremely long intervals (more than 2 folds of the standard deviation away from the mean) were considered outliers and excluded from analysis.
Daily experimental sessions began about two hours into the animal’s dark cycle and lasted about three hours. Extracellular recording of spike activities during the RT task was accomplished by connecting the FET (field effect transistors) headstage plugs and lightweight cabling onto the implanted microwire assembly. The cabling was in turn connected to a motor-assisted 80-lead commutator located in the center of chamber’s ceiling. The commutator was free to turn by a battery-operated motor that was activated by rotation of the cable. In this manner, the animal was able to move unrestrictedly in the operant chamber.
2.4. Electrophysiological recording
Electrophysiological recording during each RT task session started with a 200-s control period during which no cue light or lever was presented. Neuroelectric signals were passed from the headset assemblies to programmable amplifiers, filters (0.5 and 5 kHz, 6 db cutoffs), and a multi-channel spike-sorting device, and sampled at a sampling rate of 50 kHz. Spikes were monitored on a computer with a time resolution of 20 μs, and sorted by setting proper parameters for amplitude and duration with software MNAP 4.32. The identity of clearly sorted single units was verified by graphical capture of waveforms (see Fig. 3 for example). We also routinely computed inter spike interval (ISI) histograms of the spike train data. If the ISI plots reveal counts in bins close to zero, the unit will be rejected. The time stamps of these waveforms were then stored on a personal computer for offline analysis. Up to 62 neurons from the mPFC, DMS, GP, and the SNr were monitored concurrently from 64 microwires.
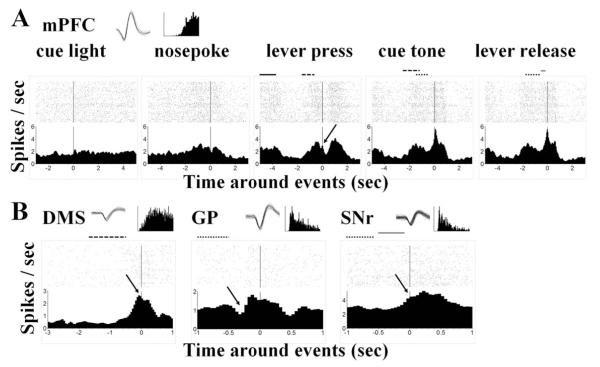
Fig. 3.
Rasters and perievent histograms showing neural activity around the behavioral events. (A) Change of activities of an mPFC neuron around cue light, nosepoke, lever press, cue tone, and lever release events. First panel: This neuron barely showed any response to the cue light. Second panel: It gradually fired more spikes for the preparation of the nosepoke with a dip around the event per se, followed by a gradual decrease of activity (see also the middle panel, with the solid bar indicating distribution of the nosepoke). Middle panel: It again picked up speed with the extension of the lever (dashed bar) towards the lever press, which happened on the down slope with a small but clear bounce back (indicated with the arrow). Forth panel: After the lever press (dotted bar), the firing rate increased rapidly and peaked slightly after the cue tone (50–100 ms after) with a peak of 5.63 Hz. Last panel: Although the peak is closer to the lever release event (−50 to 0 ms before), but the peak value is slight lower (5.56 Hz), so it can be viewed as a peak during the tone-release interval and aligned better to the cue tone event. (B) Examples of neuronal response from the other three recording areas. Left panel: A DMS neuron started to increase firing rate 0.5 s before lever press and peaked immediately before the event (indicated by the arrow). Middle panel: A GP neuron showed a dip (see arrow head) after the lever press (dashed bar) when aligned with the cue tone, indicating an inhibition during the lever-holding period. Right panel: A SNr neuron gradually increased its activity after the cue tone (gray bar) and reached a plateau with the lever release (see arrow head). Insets in each panel showed spike waveforms and inter-spike interval histograms of the example neuron.
Digital signals of spike activity, nose poke, tone, and lever release were monitored and controlled with data acquisition software Magnet (Biographics, Inc., Winston-Salem, NC) operating with a time resolution of 1 ms. Spike train activity was analyzed offline with commercially available PC-based programs STRANGER (Biographics Inc., Winston-Salem, NC) and Nex (Plexon, Inc., Dallas, TX).
2.5. Histology
At the conclusion of the final experimental session, the animal was subjected to the same anesthetization as described in the surgery session. A positive current of 10–20 μA was passed through selected microwires for 10–20 s to deposit iron ions. The animal was then sacrificed and perfused with 4% paraformaldehyde solution. Coronal sections were cut through the mPFC, DMS, GP, and SNr and mounted on slides. Incubation of the mounted sections in a solution of 5% potassium ferri-cyanide/10% HCl revealed iron deposits (recording sites) in the form of blue dots. Boundaries of the four brain areas were assessed with reference to the rat brain atlas of Paxinos and Watson (1998).
2.6. Data analysis
Rats were recorded for five to six sessions. Data were analyzed with 150–200 trials per session after each rat was well trained and the performance was stable. Spike counts of single units during a selected behavioral event (with a minimum of 0.05-s bin size) were calculated for each trial and exported as a matrix to MatLab. Neural activity was then calculated by a time window of typically 1-s duration and moving at 0.1-s steps across the sample for each trial and for the duration of the selected behavioral events. In some cases 0.25-s window width and smaller steps were used to examine extremely narrow peaks. The mean firing rates of each window were compared with those of the last two seconds of the 5-s inter-trial interval. Two arbitrary criteria were concurrently used to determine a ‘significant neural response’ as a change in firing rate: (i) there should be at least a 20% difference in the average firing rate, from the mean firing rate of the last 2 s of baseline, at least in one of the moving windows; and (ii) the difference in firing rate reached a statistically significant level (p < 0.001, student’s two-tailed t-test) in at least five successive time-steps of the moving window, thus to achieve a global significance of p < 0.05 (result of a Monte Carlo simulation with a program AlphaSim, see Ward (Ward, 2000), which might suffer certain systemic bias due to departures from normality if applied alone).
For a motor event, neural response may be slightly before (i.e., preparative), around, or even after the performance (i.e., post-executive learning). For a multi-event task as we currently employed, it is important to decide which event a given change of spike activity belongs to. We addressed this question by aligning spike activities of different trials to each of the adjacent events. The alignment resulted in the strongest firing rate change (i.e., larger peak or more significant difference) claimed that this very event drives the observed neural activity.
A sliding-window technique was also applied to compare the neural responses during events with different behavioral outcomes (e.g., correct versus error RT responses). These methods have been applied in the area of sensory coding (Wang et al., 2003, 2004) and motor activity study (Shi et al., 2004).
To show the patterns of neuronal excitation or inhibition, a cluster analysis technique (K-means, SPSS Inc.) was employed to sort neuronal responses based on the similarities in these patterns around lever press. Neuronal firing rates was first calculated with 0.1 s bin and smoothed over five bins with a boxcar kernel. They were then normalized as z-scores before entering the analysis. The z-scores were calculated by the following formula:
where x is the raw firing rate of a neuron computed within the moving window at a particular time point, and m and s are mean and standard deviation, respectively, of its firing rates throughout the whole time epoch (−3–1 s) around lever press event over the entire session. The clustering analyses were run with the initial assumption of 2, 3, 4, 5, 6, and 7 clusters, respectively. Two-way ANOVAs were performed to calculate the percentage of variance explained by clustering under each assumption. The results from an assumed number of clusters that explained the largest percentage of the variance or reached a plateau of the percentage were taken as the final result.
To evaluate whether a neuron is possibly involved in coding of reaction time, we calculated the nonparametric (Spearman’s) correlation coefficients between individual neuronal firing rates in a given 1-s time window and reaction times in the same trial with similar sliding-window methods. To verify whether the correlation coefficient (r) is statistically significant, a p-value was calculated for each of the correlation analysis to test the null hypothesis that r is actually zero. Thus, a small p value indicates that r is distinct from zero and hence the correlation is significant (Zar, 1984). RTs shorter than 100 ms were considered as performed using an anticipatory strategy. Hence those trials were excluded from analysis except for an extremely fast performer of R4, for which this limit was cut down to <50 ms.
3. Results
3.1. Behavioral performance of RT task
Six male Sprague-Dawley rats were used in this experiment. They were initially well trained to perform the RT task, in which rats were first required to make a nose poke to make a lever extended after a 3-s delay. After the lever was pressed, a tone was presented randomly between 0.5 and 1.5 s. Rats were required to release the lever in response to the tone within a short time-window (<0.5 s) to obtain a drop of water (Fig. 1A). A lever press usually happens 1–2 s after the lever was extended. During this period, rats usually wait in front of the lever for its accessibility. Performances of individual rats were shown in Table 1. All animals but one achieved more than 50% of correct performance. The exceptional R1 made 66% early response in the 3-h session, which far exceeded others. Most of the animals made only a few late responses, except for R2 which had 31% of trials in this category. The mean reaction times were generally 150–340 ms, except for the slow performer R2 which had a mean RT of 717±44 ms. The pooled performance of six rats in the RT task is shown in Fig. 1B. Rats released the lever within 0.5 s after the onset of cue tone to receive reward in 60% of the trials. They made anticipatory error responses (releasing before the tone) in about 30% of trials and late responses (holding longer than 0.5 s after the tone) in the remaining 10% of trials (Table 1, also see Fig. 1B).
Table 1.
Individual performance of reaction time task in a 3-h session.
| Rat ID | Reaction time (ms) |
Response category |
|||
|---|---|---|---|---|---|
| Early error | Correct response |
Late response |
Total | ||
| R1 | 249 ± 16 | 204 (66) | 97 (31) | 10 (3) | 311 |
| R2 | 717 ± 44 | 60 (17) | 187 (52) | 109 (31) | 356 |
| R3 | 284 ± 16 | 25 (10) | 205 (83) | 17 (7) | 247 |
| R4 | 151 ± 10 | 130 (44) | 165 (55) | 4 (1) | 299 |
| R5 | 340 ± 18 | 43 (13) | 262 (77) | 36 (10) | 341 |
| R6 | 205 ± 7 | 79 (33) | 161 (66) | 2 (1) | 242 |
| Sum | 541 (30) | 1077 (60) | 178 (10) | 1796 | |
Numbers show trials in each category of outcomes in a 3-h session. Numbers in parenthesis show percentage of that outcome in the session.
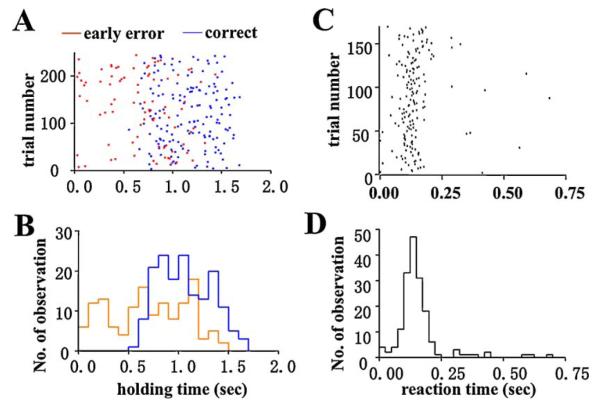
The lever extension–lever press intervals proceeding correct or early responses were similar (for an example, see Fig. 1C). However, the small difference in this interval, with a large number of trials, did reach a significant level of difference between the fast quartiles of trials (25 percentage of fast reaction times) and slow quartiles (1.4±0.1 and 2.4±0.5 s, respectively, t (43) = 2.159, p = 0.0364, see Fig. 1D) of the correctly performed trials, suggesting a slight difference in the preparation phase of the RT task for trials with fast vs. slow responses. In well-trained rats, there were few late responses and not enough for analysis. The distribution of total holding time (from lever press to release) showed that rats usually hold the lever for at least 0.5 s (see Fig. 2A and B for an example), and release the lever promptly after the onset of the tone. The majority of reaction times occurred in the range of 0.1–0.5 s, with an approximately Gaussian distribution; except for a rightward tail of late responses (see Fig. 2D for an example).
Fig. 2.
Distribution of reaction time and lever-holding time in a sample session. (A) Raster of the holding time (lever press at time = 0). Red and blue dots represented the events of release in error and correct trials, respectively. (B) Distribution of holding time in error (red line) and correct (blue line) trials. (C) Raster for lever release response (onset of tone at 0 s). (D) Distribution of lever release response. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.2. General neuronal responses during the RT task
Arrays in the bilateral DMS in one rat and in the unilateral SNr in three rats missed the target; hence those data were excluded from analysis. A total of 313 neurons (91 in the mPFC, 73 in the DMS, 92 in the GP, and 57 in the SNr) were recorded from the frontal cortico-basal ganglia regions of the six rats during a single RT task session included for each subject (for a breakdown of neural populations see Table 2). Their mean firing rates were 2.2±0.5 Hz for the DMS, 2.8±0.3 Hz for the mPFC, 4.6±0.7 Hz for the GP, and 5.6±1.4 Hz for the SNr. Table 3 summarizes the neuronal responses of four recorded areas during different stages of RT task. A substantial fraction of neurons (25–50%) showed minimal or no change in activity during the different behavioral events. However, a large proportion of neurons from the recorded areas showed “responses”, indicated by significant changes (greater than 20% and with p < 0.001 in five successive time points) compared with either the initial baseline during the inter-trial period (for lever press) or the preceding one-second period (as in the case of cue tone and lever release). The responses were found before lever press (74–77% neurons responded during 0–3 s before lever press), during lever holding (39–51% neurons responded 0–500 ms before tone), and before lever release (41–68% neurons responded 0–300 ms before lever release). Whenever the relationship of these responses with adjacent events were not clear, the amplitudes of changes with different alignments would be compared. The aligned event that showed larger firing alteration change would be considered responsible for the change. Examples of these responses were shown in Fig. 3 (see the figure legend for details).
Table 2.
Number of neurons recorded from each rat.
| Rat ID | Brain areas |
||||
|---|---|---|---|---|---|
| mPFC | DMS | GP | SNr | Total | |
| R1 | 15 | 13 | 14 | 8 | 50 |
| R2 | 15 | 16 | 15 | 16 | 62 |
| R3 | 12 | 15 | 15 | 10 | 52 |
| R4 | 15 | 15 | 14 | 7 | 51 |
| R5 | 18 | – | 18 | 5 | 41 |
| R6 | 16 | 14 | 16 | 11 | 57 |
| Sum | 91 | 73 | 92 | 57 | 313 |
Arrays of bilateral DMS in R5, and unilateral SNr in R1, R4, and R5 missed.
Table 3.
Number of neurons showed a response during RT task segments.
| Segments of RT task | Brain area recorded |
|||
|---|---|---|---|---|
| mPFC | DMS | GP | SNr | |
| Before lever press | 68 (74) | 54 (74) | 68 (74) | 44 (77) |
| During holding | 47 (52) | 28 (38) | 44 (48) | 26 (46) |
| Before lever release | 38 (42) | 30 (41) | 54 (59) | 39 (68) |
| Total neurons | 91 | 73 | 92 | 57 |
Numbers in parentheses showed the percentage of responding neurons in total recorded neurons of respective area.
The neural response patterns were displayed as plots of firing rates normalized for each neuron (z-scores) over the entire session (Fig. 4). These plots exhibited subgroups of neurons with similar peaks and valleys at different stages of the task. A clustering analysis (K-means, SPSS Inc.) demonstrated at least five clusters of neural responses patterns during the RT task. These clusters emerge from temporal patterns within normalized activity, independent of absolute firing rates (see Fig. 4A). Two-way analysis of variance revealed a significant cluster vs. time interaction, which explained 59% of total variance (F (160, 12628) = 122.7, p < 0.0001). However, no significant difference of the mean neural firing rates could be found among clusters (see Table 4, last column, one-way ANOVA, F (4,308) = 1.024, p > 0.05). Thus, distinct temporal patterns of activities exist among recorded neurons.
Fig. 4.
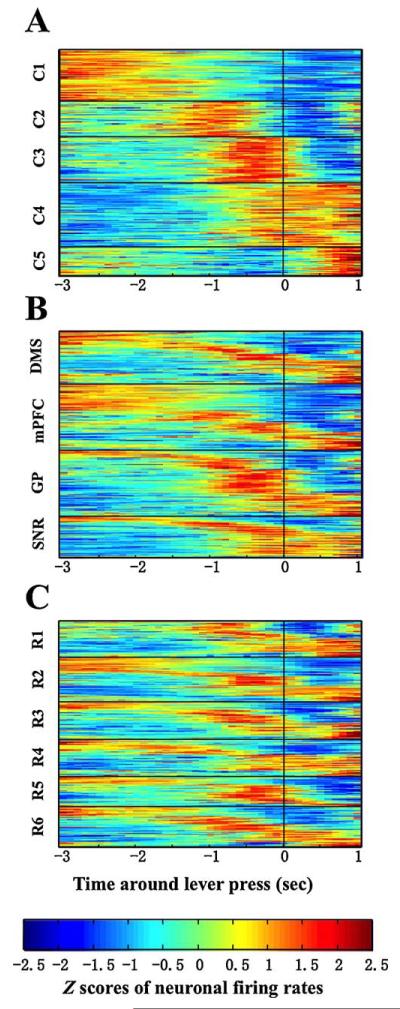
Activity of all valid neurons from all six rats aligned to the lever press event (time = 0). The firing rate was smoothed and normalized as Z-scores (red for highest and blue for lowest frequency). Each horizontal line represents one single neuron. (A) Data plotted according to a clustering analysis revealed five major response patterns across the entire time window of −3 to +1 s around the lever press. (B) Neurons were rearranged as clusters within each area, revealing multi-pattern response in each area. The early responses (3–2 s before lever press) largely occurred in the mPFC and the DMS while more lever release responses were observed in the SNr. (C) Neuronal activities were rearranged as clusters in each rat. Similar pattern of neural responses were found in six different rats. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Table 4.
Mean firing rates of neurons with different firing patterns.
| Clusters | Brain area recorded |
||||
|---|---|---|---|---|---|
| mPFC | DMS | GPa | SNrb | Cluster means | |
| C1 | 3.1 ± 0.4 (36) | 1.5 ± 0.2 (19) | 3.5 ± 0.9 (10) | 10.3 ± 5.2 (6) | 3.3 ± 0.6 (71) |
| C2 | 4.7 ± 1.3 (16) | 4.5 ± 2.7 (12) | 6.5 ± 1.7 (16) | 1.5 ± 0.3 (6) | 4.9 ± 0.9 (50) |
| C3 | 1.0 ± 0.3 (14) | 1.8 ± 1.2 (8) | 4.2 ± 1.4 (32) | 1.0 ± 0.4 (8) | 2.8 ± 0.8 (62) |
| C4 | 2.2 ± 0.5 (12) | 1.9 ± 0.5 (19) | 3.8 ± 0.8 (26) | 6.6 ± 2.1 (33) | 4.2 ± 0.8 (90) |
| C5 | 2.3 ± 0.5 (13) | 1.6 ± 0.5 (15) | 6.7 ± 4.0 (8) | 4.6 ± 1.4 (4) | 3.2 ± 5.5 (40) |
| Area means | 2.8 ± 0.3 (91) | 2.2 ± 0.5 (73) | 4.6 ± 0.7 (92) | 5.6 ± 1.4 (57) | |
Firing rates are shown as mean ± S.E.M. Numbers in parenthesis shows the number of neurons falls in that particular category. Chi-square test revealed significant difference of cluster distribution among brain areas (Chi-square (12) = 68.64, p < 0.0001).
p < 0.01, Chi-square test, compared with mPFC.
p < 0.001, Chi-square test, compared with mPFC.
The first group of neurons (Cluster C1) responded from −3 to −2 s before lever press, which is within the nosepoke-lever extension interval. Responses in cluster C2 started when the response in C1 faded away, and finished at 0.5 s before lever press, largely overlapped with the time of lever extension. Cluster C3 overlapped C2 at the beginning and continued till the lever press, which is most likely a preparation of the execution of lever-pressing. Cluster C4 followed C3 and continued during entire reaction time performance. Cluster C5 neurons responded largely with the lever release event (0.5–2 s after lever press, see Fig. 4A).
Although all clusters existed in each recorded area, they were not evenly distributed (Table 4). Chi-square test revealed significant difference between brain areas (Chi-square (12) = 68.64, p < 0.0001). The mPFC contained higher ratio of C1 neurons, while GP and SNr had more C3 and C4 neurons (also see Fig. 4B). Clusters distributed in all animals except R1 which lacked of C1 neuron. Interestingly, this is also the rat that made most early errors (66%) in the 3-h session (Fig. 4C). Spiking rates did not differ significantly among clusters, neither is there any cluster-brain area interaction, as revealed by a two-way ANOVA (interaction, F (12, 293) = 1.187, p > 0.05; cluster effect, F (4, 293) = 1.153, p > 0.05, see Table 4). The only meaningful difference of firing speeds were among brain zones (F (3, 293) = 3.026, p < 0.05). Thus, temporal pattern was where the major difference lay among these clusters.
3.3. Neural responses coding correct versus early error trials
To reveal neural activities that may underlie the performance of the task, neural responses were compared with a moving-window scanning between correct (i.e., lever was released within 0.5 s after the onset of cue tone) versus early-release error trials (i.e., lever was released before the onset of cue tone). Late error responses were rare in these well-trained rats and were thus not sufficient for statistical analysis. Mean spiking rates of each neuron during the 3-s period before lever press were analyzed for all six animals. Thirty-seven out of 91 (40%) of mPFC neurons met our criteria of significantly different activity before the lever press in correct and error trials (as indicated by a cluster of 5 points with p < 0.001) in the moving window analysis. This distinction of firing rates was found in 39% (28/73) of the DMS, 49% (44/92) of the GP, and 44% (25/57) of the SNr neurons recorded. No significant difference of this percentage could be detected among recorded areas (Chi-square test, p = 0.6293). Fig. 5A depicts the temporal function of neurons in each area demonstrating disparate spiking, plotted as the percentage over the total number of neurons that responded to the lever-pressing event in the area. The mPFC and the GP neurons showed dispersed differential responses before lever-pressing which increased rapidly after the event. The SNr and the DMS neurons, however, showed strong differential signal during the nosepoke-lever extension interval, with the former leading the latter for 0.5–1 s (−3 ~ −1.5 s for SNr, and −2.5 ~ −0.5 s for DMS, see Fig. 5A).
Fig. 5.
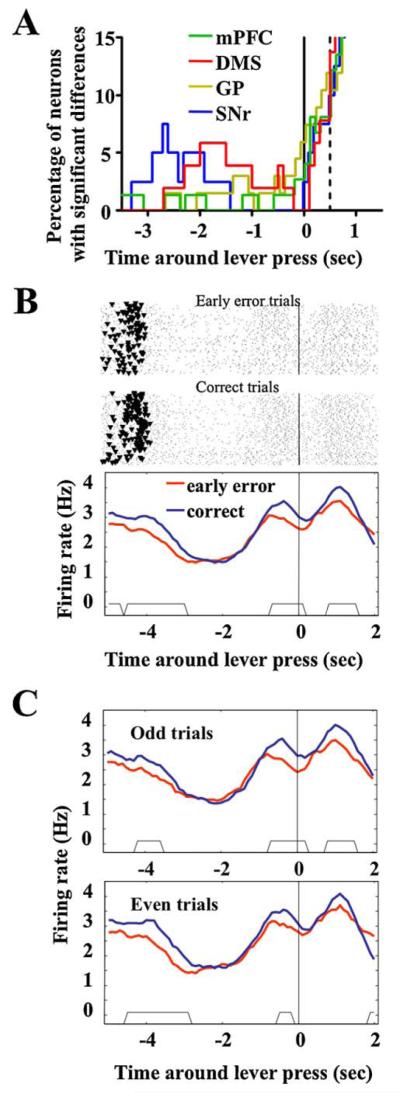
Differential neural responses around lever pressing behavior in early error and correctly performed trials. (A) Numbers of neurons with this differential response shown as percentage of all responding neurons in each recorded brain area and plotted as a function of time. Vertical solid line at time = 0 indicate the time when the lever was pressed. Dash line at time = 0.5 s indicates the earliest cue tone presentation, after which the difference of spiking between events could be the results of distinct behavioral context (i.e., in correct trials, rats was consuming water; while in the error trials, they were having a time-out) and thus should not be interpreted as preparative coding of the reaction time task. (B) As an example, the activity of an mPFC neuron was plotted as rasters and perievent histograms around lever press (time = 0) in the early error (top raster panel and red curve) and correctly performed trials (bottom raster panel and blue curve). To illustrate where the nose pokes occurs (as dark dots in the raster plot, 4–5 s before lever press), the x-axis was extended further to the earlier phase of the trial. The time period when two histograms differed significantly (revealed by the moving window comparison) were shown as the trapezoids at the bottom of the histogram panel. (C) This analysis seems robust, as indicated by the similar result obtained when only the odd (top panel) or even (bottom panel) trials were separately involved in the analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
An example of a neuron showing significant differences between error and correct trials were shown in Fig. 5B. This mPFC neuron fired 0.5 Hz faster in correct trials during both the early phase (3–5 s before lever press, including most of the nosepokes as indicated with the triangles in the raster panels, and 1 s into the nosepoke-lever extension interval) and the late phase (the lever extension-lever press interval, 0–1 s before lever press) of the RT task (Fig. 5B). The time periods where significant differences existed between correct and early error trials were revealed by the sliding-windows method and indicated with the trapezoid at the bottom of the histogram curves panel. These differences in firing rate could be reproduced in both odd and even trials, which suggested that this differential response detected by the sliding-window procedure is a robust pattern across all the trials (Fig. 5C).
3.4. Correlation of single neural firing rates with reaction time
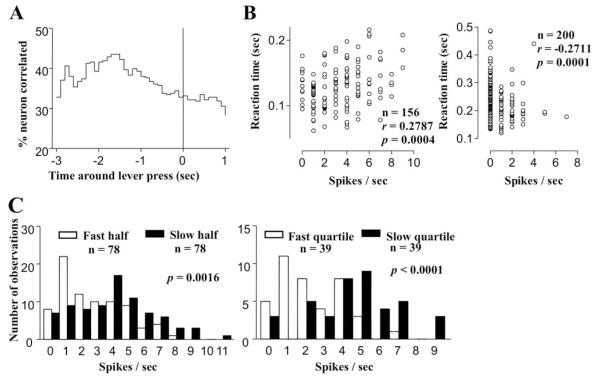
The major part of reaction time distribution was between 100 and 300 ms after the onset of tone (for an example of a fast-performer, see Fig. 2C and D). To determine whether neuronal activity prior to the lever release might underlie the speed of response, Spearman correlation coefficients were computed between the reaction times and neural firing rates of each neuron with a moving window scanning technique before lever press, during holding, and before lever release (Table 5). Significant correlation between neural firing rate and reaction time was detected in a subset of neurons in different behavioral episodes during the RT task. During the period 3 s prior to lever press, 29–42% of the neurons in different regions exhibited significant correlations. The firing rate of 18–33% of neurons during lever holding, and 29–30% of neurons before lever release also had significant correlation with the speed of reaction time. However, no special relation between this correlation and the aforementioned clusters of activation pattern was found (data not shown). The temporal function of the percentages of total neurons displaying this correlation was depicted in Fig. 6A. The peak mass correlation were found 1.5–2.2 s before lever press, in the middle of the nosepoke-lever press interval, with more than 40% of total neurons showing correlation with reaction time. This is significantly higher (p < 0.01) compared with after lever press. A scatter plot as an example of such a correlation was shown in Fig. 6B (left panel). This particular DMS neuron fired at a higher rate 1.5–2.5 s before lever presses that preceded a slower lever release responses. An example of negatively correlated neuron was also shown in Fig. 6B (right panel).
Table 5.
Number and percentage of neurons with correlation between firing rate and reaction time.
| Segments of RT task | Brain area recorded |
|||
|---|---|---|---|---|
| mPFC | DMS | GP | SNr | |
| Before lever press | 12/15 (29) | 9/12 (29) | 18/14 (36) | 11/13 (42) |
| During holding | 13/7 (22) | 6/7 (18) | 7/10 (19) | 12/7 (33) |
| Before lever release | 18/9 (29) | 7/4 (15) | 9/7 (18) | 13/4 (30) |
| Total neurons | 92 | 72 | 89 | 57 |
Numbers before and after the slash indicated the neurons for which the firing rates positively or negatively correlated with reaction times, respectively. Numbers in parentheses showed the percentage of all correlated neurons in total recorded neurons of the respective area.
Fig. 6.
Correlation between reaction times and individual neural firing rates in the same trial. (A) Temporal function of the percentage of neurons showing significant correlation (i.e., p < 0.001 in 5 successive time points) between firing rates and reaction times pooled from the results of all six rats. More than 40% of neurons showed this correlation 1–2 s before lever press, at the middle of the nosepoke-lever press interval. (B) Examples of positive (left panel) and negative (right panel) correlations shown as scatter plots of RTs vs. the mean firing rates of two DMS neurons, 1.5–2.5 s before lever press, and −0.8 to 0.2 s around lever press, respectively. For the first neuron, these two variables showed a Spearman correlation coefficient of 0.2787 across the 156 correct trials (p = 0.0004). Reaction time became longer when this particular DMS neuron fired at higher rates in this time window. For the second neuron, a Spearman r of −0.2711 was found across the 200 trials with a p value of 0.0001. (C) Frequency distribution histograms of the firing rates in this time-window for the faster and the slower half of 78 trials (blank and filled histograms, respectively, left panel), and for the fastest and the slowest quartile of 39 trials (blank and filled histograms, respectively, right panel) for the sample neuron with positive correlation. Mann-Whitney nonparametric test showed moderately different distribution in the former (p = 0.0014) but more remarkable difference in the latter (p < 0.0001).
To verify whether the difference of the spiking rate really exist between faster and slower trials, the distribution of firing rates for the data shown in Fig. 6B was plotted separately for the faster and slower halves of reaction times (see Fig. 6C, left panel). The two distributions showed small but highly significant differences (p = 0.0014, Mann-Whitney test). A similar plot for the fastest and slowest quartiles of trials showed additional significant differences (p < 0.0001, Mann-Whitney test, see Fig. 6C, right panel). Thus, small but significant differences in the distribution of neural activity between fast and slow reaction time did exist in the multiple regions recorded.
3.5. Histological localization of recording sites
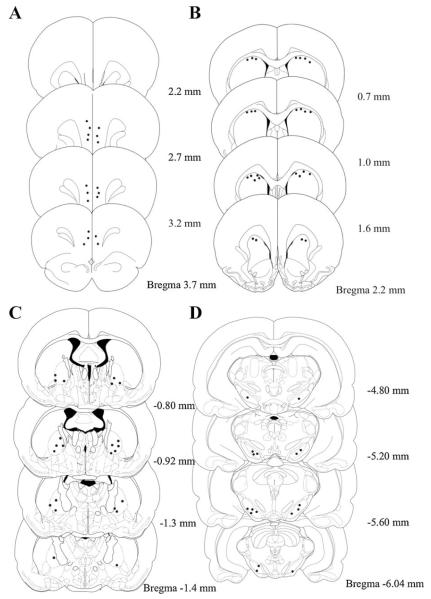
Brain slices were stained with potassium ferricyanide to reveal recording sites as blue dots in the DMS, mPFC, GP, and SNr. Fig. 7 showed the locations of recording sites. Tips that hit the striatum were located in dorsal medial part of striatum. In the mPFC, most of the tips of microwires were located in the prelimbic areas. A few recording sites were found in the infralimbic area of the mPFC. In the GP, most of the recording tips were located in the ventral lateral part of the area. In substantia nigra recording, tips were located in the reticulata part except for the missing wires. Data from missed wires were discarded and not included in analysis.
Fig. 7.
Location of recording sites in the mPFC (A), DMS (B), GP (C) and SNr (D) revealed by potassium ferrocyanide staining. Black dots in the diagram show the sites of staining within target area and hence been included in the final data analysis.
4. Discussion
The results partially answered the question initially posed regarding when and where the signals appear that predict the reaction time task performance. It was revealed that the widespread predictive signals appear within subsets of neurons at times up to several seconds prior to the final completion of the RT task instead of just at the time of the final movement. It was found that the earliest signals appeared in the SNr and then DMS in the middle of the nosepoke-lever press interval, several seconds prior to the lever press. Most differential signals in the mPFC and GP appeared immediately before as well as around the lever press, except for some sparsely distributed difference in the nosepoke-lever press interval. The results also indicated that subpopulations of neurons from all the four regions including the mPFC, the dorsal striatum, the GP, and the SNr, demonstrated variations in activity that were predictive of the RT task performance (see Table 5).
4.1. Temporal patterns within regions
Cluster analysis of normalized firing patterns over the task revealed groups of neurons in the frontal cortico-basal ganglia system that responded in a clear temporal sequence across the entire period of the RT task, especially around lever press (see Fig. 4A). In this period, rats already finished nose poking, thus all responses could be considered as preparation and execution of lever press in the RT task. Nearly half of the mPFC neurons, and slightly less of the DMS neurons, exhibited responses at the early preparation phase of the task (2–3 s before lever press, which is between nosepoke and lever extension), while the GP and the SNr were more active immediately before (<1 s) the lever press during the lever extension-lever press interval, considered the late preparation phase of the RT task. In a primate study, it was reported that 30% of neurons in putamen, caudate nucleus and GP exhibited changes in activity during the preparatory period in a precued reaching task and the preparatory activity in basal ganglia takes part in a process termed motor readiness (Jaeger et al., 1993). Another primate report indicates that 45% of GP exhibited firing change 50–150 ms before mechanically detected movement (Anderson et al., 1985). It was also revealed that 60% of neurons in putamen and pallid um in cats prior to movement onset could be related to the movement initiation, and only minor differences were found between the characteristics of the populations of neurons recorded in the putamen, the GP and the EP (Cheruel et al., 1994), which is consistent with our findings.
Although this temporal sequence of signals is consistent with the direction of information flowing within anatomic connections of the frontal cortico-basal ganglia looping system (Alexander et al., 1990; Pennartz et al., 1994), it is at a time scale far longer than the required propagation times of a few milliseconds to send impulses within the loop. Therefore, we view these patterns of activity as reflecting a slowly evolving sequence of preparatory states, which may involve multiple anatomically segregated loop circuits, appearing in large subsets of memory, that appear well before movements to press and release the lever (Jaeger et al., 1993, 1995). The question arises as to which extent this task-segment specific activity also includes information related to regulation of task performance.
4.2. Activity differentiates premature responses from correct responses
It is suggested that the movement variability arises before movements begin, or during motor preparation period even for highly practiced task. Small variations in preparatory neural activity were predictive of small variations in the upcoming reach (Churchland et al., 2006). So the question arises as to when and where activity patterns bifurcate in time to cause the lever to be released before versus after the arrival of the tone cue. The precise mechanism for the trigger of movement onset is unknown but may be viewed as a transition event arising from sustained activity patterns established over time within feedback loops (Beiser and Houk, 1998; Houk et al., 1993) that are influenced by the anticipatory states. The temporal response patterns do appear within the cortical striatal system at times when they may be able to regulate appropriate perseverant behavior (i.e., a premature response being made before the tone signal versus the lever being held until after the cue tone).
A comparison of the neuronal activities revealed that small subsets of neurons were predictive of the later behavior of a premature vs. correctly timed response. These appear from as early as 3 s before and until after lever press (Fig. 5A). The percentage of responding neurons were almost evenly distributed to the four-recorded areas (Chi-square test, p > 0.05, see Section 2.3), which showed that all the four recorded areas may contribute to the part of motor control during lever press holding and release. These early differential signal may reflect the state of preparation for the task, such as arousal, attention, etc.
This result is in line with those of other studies. For example, lesions of mPFC or frontal parietal cortex generated more premature or delayed responses, indicating a failure of proper control of perseverance (Baunez et al., 1998; Risterucci et al., 2003). Striatum was also reported (Amalric et al., 1995a) to play a similar role in regulating perseverant behavior, since bilateral depletion of dopamine fibers in this region generates concomitantly more anticipated and delayed responses. Disconnection of the corticostriatal circuit produced a significant reduction in the accuracy of a choice reaction time task performance (Christakou et al., 2001). And unilateral lesions of mPFC and CPu within the same hemisphere produced a severe and long-lasting contralesional neglect syndrome (Christakou et al., 2005). On the other hand, GP and SNr were reported to have opposite influences on RT performance. An intact GP was suggested to be important for fewer earlier responses, while normal function in SNr seemed to prevent later responses (Amalric et al., 1994; Baunez and Amalric, 1996). We interpret such findings as indicating that these regions help establish ongoing states of activity that influence the final neural events that trigger the movement in the task.
4.3. Activity patterns related to fast versus slow reaction times
We observed faster reaction time in the trial with shorter lever extension-lever press (LE-LP) interval (Fig. 1C), indicating attention or motivation maybe plays a role in the RT task performance. When the rats were more focused, they had more chance to perform faster. Thus, it is interesting to detect which part of the ensemble neural activity is predictive for the promptness of the task performance. A moving windows technique with Spearman correlation analysis revealed that the spiking rates of a small fraction of neurons were correlated with reaction time during certain phase of the RT task (see Table 5). The amount of neurons showing this correlation at any given time point was around 30–40% of total neurons (Fig. 6A). The maximum fraction of neurons (>40%) exhibiting significant correlations appeared around 1–2 s before lever press. Validation procedures as shown in Fig. 6C revealed that there exists significant difference between the distribution of spiking activities in fast and slow RT trials.
Conditions that regulate speed of reaction time may be widely distributed. Premotor intracortical microstimulation led to a highly specific increase in reach reaction time. The effects were largest when activity was disrupted around the time of the go cue (Churchland and Shenoy, 2007). Other workers (Amalric et al., 1983) have studied a similar task and reported that neurons from the posterior red nucleus of cats showed marked changes of firing time-locked with the go-signal and frequency-correlated with the duration of RT. It is also reported that amplitude of oscillatory pallidal activity occurring prior to the completion of a bimanual timing task was strongly correlated with eventual task duration, suggesting pallidum may be involved in the prediction of movement timings (Brown and Robbins, 1991). Primate study indicates that cortical neuronal ensemble activity could predict temporal intervals and was also informative for generating predictions that dissociated the delay periods of the task from the movement periods (Lebedev et al., 2008). These findings, together with what we observed in the current study, suggest that critical neural activity regulating rapid and correct responses in the reaction time task may take place, at least in part, in the early phase of the task. Given the limitations of the sampling procedure used, it seems that a subset of neurons consisting of a few in each observed area might operate as an ensemble, which manipulates the promptness of the performance at an early phase in a RT task trial.
In addition, other means may exist for detection of predictive information within single neurons. For example, it is demonstrated that response duration was more informative than the amplitude or spike count encoding reward prospects in the SNr and the GPe (Parush et al., 2008). This will be addressed in future studies.
4.4. Role of substantia nigra pars reticulata in the reaction time task
In the present study, we observed that SNr neurons, compared with other recording regions, exhibited more difference in firing between correct trials and error trials preceding the lever release, and more SNr neurons showed correlation between firing rate and reaction time before the completion of the RT task. We consider these observations are functional manifestations for SNr as an important output structure of basal ganglia. The basal ganglia are highly organized network, which interact with the cerebral cortex through a complex series of loop circuits, contributing to movement control, associative and limbic function. Integrative properties are a characteristic of the basal ganglia (Yelnik, 2008).
SNr is an important mesencephalic nucleus that functions as a relay area for BG output, receiving projection from striatum, global pallidus, subthalamic nucleus, and send projections to thalamus, brain stem motor nucleus. Behavioral studies indicate that infusion of NMDA receptor antagonist into SNr induced a dramatic increase in the number of premature lever releases and decreased mean reaction time (Baunez and Amalric, 1996); injections of GABAA antagonist into SNr produced a dose-related suppression of operant responding (Correa et al., 2003). Electrophysiological studies displayed that a large proportion of SNr neurons showed responses which were related to memory, attention, or movement preparation (Wichmann and Kliem, 2004): and a significant positive correlation was found between the discharge rate in primate SNr neurons and elbow movement velocity as well as amplitude, some SNr cells discharged in anticipation of the EMG (Magarinos-Ascone et al., 1992), which are consistent with our findings. Morphological studies demonstrate that SNr neurons usually extended the dendritic arborizations within the adjacent striatal projection fields (Mailly et al., 2001), which may be one of the structure substrates for SNr integration function.
Both SNr and GPi/EP are basal ganglia output structures. It is implied that the two nuclei should not be considered as the same functional entity in the organization of BG outflow, as infusion of NMDA receptor antagonist into SNr and EP induced different effects in reaction time task (Baunez and Amalric, 1996). In addition, the arrangement of striatopallidal and striatonigral projections are primarily parallel and convergent, respectively, suggesting SNr could integrate more information in processing corticostriatal signal (Kaneda et al., 2002). So SNr may be involved in higher motor functions, and GPi may be preferentially concerned with elemental movement control (Wichmann and Kliem, 2004).
4.5. Functional implications of neural activity during reaction time task
It appears that the states of brain activity that influence the behavior are set up many seconds prior to the movement, and within distributed regions, at the time of the nose poke, and do not emerge limited in time to the moment of movement generation. One implication is that lesions or drugs that influence glutamate, GABA, dopamine, or norepinephrine may have correspondingly widespread targets of action over a considerable time span. Many agents can then exert significant influences on response latencies or failure to respond to the cue (Amalric and Koob, 1987; Amalric et al., 1994, 1995a,b; Baunez et al., 1995; Baunez and Amalric, 1996; Brasted et al., 1998, 2000; Brown and Robbins, 1991; Granon et al., 2000). The fact that signs of neural regulation appear across most of the response cycle reflects the complex-interactions between different neurotransmitters detected in the basal ganglia regions (Huang et al., 1998; Walters et al., 1987; Waszczak and Walters, 1986).
Traditional studies of RT have involved relatively complex tasks with intervening mental events or conditions. For example, detection of a light cue at a specific location or within a rule-based context may be followed by a series of movements to a distant position to break a photocell (Brasted et al., 1997, 1998; Brown and Robbins, 1991; Inglis et al., 2001). Impaired movement onset in Parkinson’s disease may involve failure to evaluate criteria for responding in complex situations (Gauntlett-Gilbert and Brown, 1998). Movements in these tasks may appear after hundreds of millisecond latencies during which intervening sensory-motor and cognitive processes may assert influences (Posner, 1980). Event related potential studies (Mangun and Hillyard, 1991) in human have suggested that very early attention in a complex RT task is critical to prepare the brain for rapid completion of any required intervening mental activity. Our view is that the very early anticipatory/regulatory signals we have seen even in this simplest task, are nevertheless quite complex. This basic structure of anticipatory states (Beiser and Houk, 1998) may provide a basic substrate that can be expanded as the task complexity increases. Similar preparatory states of brain activity may mediate early planning with an equivalent role in other functions of the frontal system. These may include (Chang et al., 1998, 1999, 2000, 2002) delayed responses involving working memory, reward anticipation, coding of temporal determinations, with each requiring a bridge be created to the future.
4.6. Conclusion
In summary, our study has shown that preparatory neuronal activities that predict the precision and speed of the performance of a simple reaction time task can exist several seconds before actual performance of the task. This result provided further evidence that the proper execution of a simple motor task may require seconds of preparation of the cortical striatal system.
Acknowledgments
This work was supported by the Tourette Syndrome Association, AA-10337, DA-2338, and NS-40628 to D.J. Woodward, DA-10370, NS-43441, and TW-06144 to J.-Y. Chang, and by the National Natural Science Foundation of China (30770688 and 30970959), a Beijing NNSF grant (5082008), the 100 Talented Plan of the Chinese Academy of Sciences, the National Hi-Tech Research and Development Program of China (Grant No. 2008AA022604), and a grant from NIH Fogarty International Center (R03-TW 008038) to F. Luo.
Abbreviations
- DMS
dorsal medial striatum
- EP
entopeduncular nucleus
- GP
globus pallidus
- mPFC
medial prefrontal cortex
- RT
reaction time
- SNr
substantia nigra pars reticulata
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Amalric M, Conde H, Dormont JF, Farin D, Schmied A. Cat red nucleus changes of activity during the motor initiation in a reaction time task. Exp. Brain Res. 1983;52:210–218. doi: 10.1007/BF00236629. [DOI] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Depletion of dopamine in the caudate nucleus but not in nucleus accumbens impairs reaction-time performance in rats. J. Neurosci. 1987;7:2129–2134. doi: 10.1523/JNEUROSCI.07-07-02129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Farin D, Dormont JF, Schmied A. GABA-receptor activation in the globus pallidus and entopeduncular nucleus: opposite effects on reaction time performance in the cat. Exp. Brain Res. 1994;102:244–258. doi: 10.1007/BF00227512. [DOI] [PubMed] [Google Scholar]
- Amalric M, Baunez C, Nieoullon A. Does the blockade of excitatory amino acid transmission in the basal ganglia simply reverse reaction time deficits induced by dopamine inactivation? Behav. Pharmacol. 1995a;6:508–519. [PubMed] [Google Scholar]
- Amalric M, Moukhles H, Nieoullon A, Daszuta A. Complex deficits on reaction time performance following bilateral intrastriatal 6-OHDA infusion in the rat. Eur. J. Neurosci. 1995b;7:972–980. doi: 10.1111/j.1460-9568.1995.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Baunez C, Nieoullon A, Amalric M. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J. Neurosci. 1995;15:6531–6541. doi: 10.1523/JNEUROSCI.15-10-06531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Amalric M. Evidence for functional differences between entopeduncular nucleus and substantia nigra: effects of APV (DL-2-amino-5-phosphonovaleric acid) microinfusion on reaction time performance in the rat. Eur. J. Neurosci. 1996;8:1972–1982. doi: 10.1111/j.1460-9568.1996.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Baunez C, Salin P, Nieoullon A, Amalric M. Impaired performance in a conditioned reaction time task after thermocoagulatory lesions of the frontoparietal cortex in rats. Cereb. Cortex. 1998;8:301–309. doi: 10.1093/cercor/8.4.301. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J. Neurophysiol. 1998;79:3168–3188. doi: 10.1152/jn.1998.79.6.3168. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Humby T, Dunnett SB, Robbins TW. Unilateral lesions of the dorsal striatum in rats disrupt responding in egocentric space. J. Neurosci. 1997;17:8919–8926. doi: 10.1523/JNEUROSCI.17-22-08919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted PJ, Dobrossy MD, Robbins TW, Dunnett SB. Striatal lesions produce distinctive impairments in reaction time performance in two different operant chambers. Brain Res. Bull. 1998;46:487–493. doi: 10.1016/s0361-9230(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Dunnett SB, Robbins TW. Unilateral lesions of the medial agranular cortex impair responding on a lateralised reaction time task. Behav. Brain Res. 2000;111:139–151. doi: 10.1016/s0166-4328(00)00147-9. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Simple and choice reaction time performance following unilateral striatal dopamine depletion in the rat. Impaired motor readiness but preserved response preparation. Brain. 1991;114(Pt 1B):513–525. doi: 10.1093/brain/114.1.513. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Henriksen SJ. Neuronal firing in the nucleus accumbens is associated with the level of cortical arousal. Neuroscience. 1992;51:547–553. doi: 10.1016/0306-4522(92)90294-c. [DOI] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J. Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J. Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Chen L, Woodward DJ. Neural responses in the frontal cortical and hippocampal circuits during timing behaviors in freely moving rats. Soc. Neurosci. 1999;25:876. Abstr. [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp. Brain Res. 2002;142:67–80. doi: 10.1007/s00221-001-0918-3. [DOI] [PubMed] [Google Scholar]
- Cheruel F, Dormont JF, Amalric M, Schmied A, Farin D. The role of putamen and pallidum in motor initiation in the cat. I. Timing of movement-related single-unit activity. Exp. Brain Res. 1994;100:250–266. doi: 10.1007/BF00227195. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav. Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prolonged neglect following unilateral disruption of a prefrontal cortical-dorsal striatal system. Eur. J. Neurosci. 2005;21:782–792. doi: 10.1111/j.1460-9568.2005.03892.x. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron. 2006;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J. Neurophysiol. 2007;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- Correa M, Mingote S, Betz A, Wisniecki A, Salamone JD. Substantia nigra pars reticulata GABA is involved in the regulation of operant lever pressing: pharmacological and microdialysis studies. Neuroscience. 2003;119:759–766. doi: 10.1016/s0306-4522(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J. Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos Y, Schmied A, Burle B, Burnet H, Rossi-Durand C. Anticipatory changes in human motoneuron discharge patterns during motor preparation. J. Physiol. 2008;586:1017–1028. doi: 10.1113/jphysiol.2007.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Brown VJ. Reaction time deficits and Parkinson’s disease. Neurosci. Biobehav. Rev. 1998;22:865–881. doi: 10.1016/s0149-7634(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Morgan NH, Bressler SL, Cutillo BA, White RM, Illes J, Greer DS, Doyle JC, Zeitlin GM. Human neuroelectric patterns predict performance accuracy. Science. 1987;235:580–585. doi: 10.1126/science.3810158. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J. Comp. Neurol. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Byrd DR, Konstantopoulos JK, Bunn T, Deadwyler SA. Hippocampal place fields: relationship between degree of field overlap and cross-correlations within ensembles of hippocampal neurons. Hippocampus. 1996;6:281–293. doi: 10.1002/(SICI)1098-1063(1996)6:3<281::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Ensemble codes involving hippocampal neurons are at risk during delayed performance tests. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13487–13493. doi: 10.1073/pnas.93.24.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Deadwyler SA. Distribution of spatial and non-spatial information in dorsal hippocampus. Nature. 1999;402:610–614. doi: 10.1038/45154. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Hedberg T, Deadwyler SA. Differential information processing by hippocampal and subicular neurons. Ann. N. Y. Acad. Sci. 2000;911:151–165. doi: 10.1111/j.1749-6632.2000.tb06724.x. [DOI] [PubMed] [Google Scholar]
- Houk JC, Keifer J, Barto AG. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993;16:27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Huang KX, Bergstrom DA, Ruskin DN, Walters JR. N-methyl-D-aspartate receptor blockade attenuates D1 dopamine receptor modulation of neuronal activity in rat substantia nigra. Synapse. 1998;30:18–29. doi: 10.1002/(SICI)1098-2396(199809)30:1<18::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav. Brain Res. 2001;123:117–131. doi: 10.1016/s0166-4328(01)00181-4. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW. Primate basal ganglia activity in a precued reaching task: preparation for movement. Exp. Brain Res. 1993;95:51–64. doi: 10.1007/BF00229653. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW. Neuronal activity in the striatum and pallidum of primates related to the execution of externally cued reaching movements. Brain Res. 1995;694:111–127. doi: 10.1016/0006-8993(95)00780-t. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections. J. Neurophysiol. 2002;88:1420–1432. doi: 10.1152/jn.2002.88.3.1420. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res. 1991;564:296–305. doi: 10.1016/0006-8993(91)91466-e. [DOI] [PubMed] [Google Scholar]
- Laubach M, Shuler M, Nicolelis MA. Independent component analyses for quantifying neuronal ensemble interactions. J. Neurosci. Methods. 1999;94:141–154. doi: 10.1016/s0165-0270(99)00131-4. [DOI] [PubMed] [Google Scholar]
- Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature. 2000;405:567–571. doi: 10.1038/35014604. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Mizumori SJ. Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain Res. 1994;638:157–168. doi: 10.1016/0006-8993(94)90645-9. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, O’Doherty JE, Nicolelis MA. Decoding of temporal intervals from cortical ensemble activity. J. Neurophysiol. 2008;99:166–186. doi: 10.1152/jn.00734.2007. [DOI] [PubMed] [Google Scholar]
- Magarinos-Ascone C, Buno W, Garcia-Austt E. Activity in monkey substantia nigra neurons related to a simple learned movement. Exp. Brain Res. 1992;88:283–291. doi: 10.1007/BF02259103. [DOI] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Mahon S, Menetrey A, Thierry AM, Glowinski J, Deniau JM. Dendritic arborizations of the rat substantia nigra pars reticulata neurons: spatial organization and relation to the lamellar compartmentation of striato-nigral projections. J. Neurosci. 2001;21:6874–6888. doi: 10.1523/JNEUROSCI.21-17-06874.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J. Exp. Psychol. Hum. Percept. Perform. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Nordquist R, Orgut O, Pennartz CM. Plasticity of neuronal firing in deep layers of the medial prefrontal cortex in rats engaged in operant conditioning. Prog. Brain Res. 2000;126:287–301. doi: 10.1016/S0079-6123(00)26020-2. [DOI] [PubMed] [Google Scholar]
- Nixon PD, McDonald KR, Gough PM, Alexander IH, Passingham RE. Cortico-basal ganglia pathways are essential for the recall of well-established visuomotor associations. Eur. J. Neurosci. 2004;20:3165–3178. doi: 10.1111/j.1460-9568.2004.03788.x. [DOI] [PubMed] [Google Scholar]
- Parush N, Arkadir D, Nevet A, Morris G, Tishby N, Nelken I, Bergman H. Encoding by response duration in the basal ganglia. J. Neurophysiol. 2008;100:3244–3252. doi: 10.1152/jn.90400.2008. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Characteristics of basolateral amygdala neuronal firing on a spatial memory task involving differential reward. Behav. Neurosci. 1998;112:554–570. doi: 10.1037//0735-7044.112.3.554. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur. J. Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Neural responses in multiple basal ganglia regions during spontaneous and treadmill locomotion tasks in rats. Exp. Brain Res. 2004;157:303–314. doi: 10.1007/s00221-004-1844-y. [DOI] [PubMed] [Google Scholar]
- Takenouchi K, Nishijo H, Uwano T, Tamura R, Takigawa M, Ono T. Emotional and behavioral correlates of the anterior cingulate cortex during associative learning in rats. Neuroscience. 1999;93:1271–1287. doi: 10.1016/s0306-4522(99)00216-x. [DOI] [PubMed] [Google Scholar]
- Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J. Neurosci. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- Wang JY, Luo F, Chang JY, Woodward DJ, Han JS. Parallel pain processing in freely moving rats revealed by distributed neuron recording. Brain Res. 2003;992:263–271. doi: 10.1016/j.brainres.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Wang JY, Zhang HT, Han JS, Chang JY, Woodward DJ, Luo F. Differential modulation of nociceptive neural responses in medial and lateral pain pathways by peripheral electrical stimulation: a multichannel recording study. Brain Res. 2004;1014:197–208. doi: 10.1016/j.brainres.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Ward BD. [Retrieved December 1, 2000];Simultaneous inference for fMRI data. 2000 From http://afni.nimh.nih.gov/afni/docpdf/Alphasim.pdf. Now available: http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf.
- Waszczak BL, Walters JR. Endogenous dopamine can modulate inhibition of substantia nigra pars reticulata neurons elicited by GABA iontophoresis or striatal stimulation. J. Neurosci. 1986;6:120–126. doi: 10.1523/JNEUROSCI.06-01-00120.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, Nicolelis MA. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA. Neuronal activity in the primate substantia nigra pars reticulata during the performance of simple and memory-guided elbow movements. J. Neurophysiol. 2004;91:815–827. doi: 10.1152/jn.01180.2002. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp. Brain Res. 1982;45:157–167. doi: 10.1007/BF00235775. [DOI] [PubMed] [Google Scholar]
- Yelnik J. Modeling the organization of the basal ganglia. Rev. Neurol. (Paris) 2008;164:969–976. doi: 10.1016/j.neurol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice-Hall, Inc; Englewood Cliffs, New Jersey: 1984. [Google Scholar]