Abstract
A novel transgenic mouse line that expresses codon-improved Cre recombinase (iCre) under regulation of the Endothelin-2 gene (edn2) promoter was developed for the conditional deletion of genes in Endothelin-2 lineage cells and for the spatial and temporal localization of Endothelin-2 expression. Endothelin-2 (EDN2, ET-2, previously VIC) is a transcriptionally regulated 21 amino acid peptide implicated in vascular homeostasis, and more recently in female reproduction, gastrointestinal function, immunology, and cancer pathogenesis that acts through membrane receptors and G-protein signaling. A cassette (edn2-iCre) was constructed that contained iCre, a polyadenylation sequence, and a neomycin selection marker in front of the endogenous start codon of the edn2 gene in a mouse genome BAC clone. The cassette was introduced into the C57BL/6 genome by pronuclear injection, and two lines of edn2-iCre positive mice were produced. The edn2-iCre mice were bred with ROSA26-lacZ and Ai9 reporter mice to visualize areas of functional iCre expression. Strong expression was seen in the periovulatory ovary, stomach and small intestine, and colon. Uniquely, we report punctate expression in the corneal epithelium, the liver, the lung, the pituitary, the uterus, and the heart. In the embryo, expression is localized in developing hair follicles and the dermis. Therefore, edn2-iCre mice will serve as a novel line for conditional gene deletion in these tissues.
Keywords: Endothelin-2, Cre recombinase, Transgenic, Ovary, BAC Clone
INTRODUCTION
The Endothelin system is made of three 21 amino acid peptides (EDN1, EDN2, EDN3), two receptors (EDNRA and EDNRB), and several activating peptidases including ECE1 and ECE2. The system is highly conserved throughout mammals, and within vertebrates as a whole (Braasch and Schartl, 2014). Though EDN2 (also seen as ET-2, previously vasoactive intestinal contractor VIC) differs from EDN1 by two amino acids and has the same receptor affinities, and differs from EDN3 by six amino acids and has the same affinity to EDNRB, there now exists a substantial body of evidence suggesting that EDN2 acts through unique regulation and synthesis from its familial isoforms (Ling et al., 2013). Previously, approaches using Cre/loxP-mediated recombination have demonstrated the importance of EDN1, EDN3, ENDRA, and EDNRB in mice, though with phenotypes unique from newly generated EDN2 deficient mice. It is now apparent that EDN2 has a unique role within the Endothelin system in female reproduction (Ko et al., 2012), and there is also strong evidence that it is involved in gastrointestinal function (Chang et al., 2013b; Takizawa et al., 2005), immune cell function (Grimshaw et al., 2002b), heart failure (Brown et al., 2000), and various cancers (Ling et al., 2013). It has been previously demonstrated that edn2 is expressed at low levels in the cerebellum, cerebrum, and lung; that it is more highly expressed in the testes and stomach, and it is particularly highly expressed in the intestines, ovary, and uterus (Uchide et al., 2002; Uchide et al., 1999). Embryonic expression begins by embryonic day 15 (e15) and persists until at least e17 (Chang et al., 2013b; Uchide et al., 1999). Global loss of edn2 is lethal, wherein mice expire around three weeks of age from internal starvation, hypothermia, and emphysema (Chang et al., 2013b) and also exhibit ovulatory defects (Cacioppo et al., 2014), thus demonstrating a critical functional role in the brain, lungs, GI tract, and ovary. Although a floxed edn2 mouse became recently available to selectively ablate edn2 (Rattner et al., 2013), no tool has yet been available to remove genes in those cells with edn2 expression. Thus we aimed to generate a novel mouse model that expresses codon-improved Cre recombinase (iCre) driven by the natural promoter for the edn2 gene. Two lines of edn2-iCre mice were produced and the sites of functional iCre expression and, presumably, localization of endogenous edn2 expression was determined using two lines of reporter mice: ROSA26-lacZ (Soriano, 1999) and Ai9 (Madisen et al., 2010).
RESULTS AND DISCUSSION
Little is known of the regulation of edn2, both from the transcriptional and peptide conversion perspectives. Although edn1 has been shown to be largely transcriptionally regulated through cis-acting promoter elements, and edn2 was thought to have similar regulation, it has been shown previously to be regulated by transcription factors such as epidermal growth factor, TNF-α, forskolin, HIF1-α, and pituitary gonadotropins within the reproductive system (Ko et al., 2012; Palanisamy et al., 2006). Hence a strategy that encompassed all potentially involved regulatory DNA regions was mandated to ensure correct mimicry of edn2 expression by Cre recombinase.
To this end, we chose to use a bacterial artificial chromosome (BAC) clone that contained the entire edn2 gene and neighboring sequences as the vector for iCre insertion (Figure 1). A cassette containing iCre, a polyadenylation sequence, and a neomycin selection marker was inserted in front of the ATG start codon of the edn2 gene (Figure 1). Following removal of the neomycin selection marker by FLP-mediated recombination, the entire 200Kbp vector was inserted into the C57BL/6 genome by pronuclear injection. Sixty-nine pups were produced, of which 12 contained the edn2-iCre vector in their genome (Figure 1). Copy number was not evaluated. Two of these 12 edn2-iCre positive mice, edn2-iCre#9 and edn2-iCre#12, showed no health issues or major fertility defects and were chosen for follow-up characterization of iCre expression. To validate that iCre expression mimics edn2 expression in edn2-iCre mice, we examined the ovaries of 28 day old mice following gonadotropin stimulation to induce ovulation. Expression of edn2 specifically occurs between 11 and 12 hours after hCG injection (Ko et al., 2006; Palanisamy et al., 2006), and we expected iCre expression to be limited to this time frame. Ovaries were examined at 0, 6, 12, 16, and 24 hours after hCG injection for iCre RNA in wild type (WT) mice and the two founder lines of transgenic mice. As expected, both founder lines demonstrated iCre expression only 12 hours after hCG injection concurrent with their own and WT edn2 expression (Figure 2). The gene Rpl19, encoding murine ribosomal 60S protein L19, was chosen as an internal control based on previous publications (Al-Bader and Al-Sarraf, 2005; Szabo et al., 2004). From these data, we parsimoniously conclude that iCre expression mimics edn2 temporally in the ovary, and that in addition to removing floxed genes at the times and locations of edn2 expression, edn2-iCre tissues can also be used to localize edn2 expression sites.
Figure 1. Transgenic vector construct and screening of edn2-iCre transgenic mouse.

(a) Schematic diagram of a part of chromosome 4 and the edn2-iCre BAC transgenic vector. Edn2 and its neighboring genes are shown. Exons are shown in blue boxes. Positions of PCR primers for screening transgenic mice are indicated by horizontal black arrows. The cassette containing the coding region of iCre, SV40 late polyadenylation signal, and frt-neo-frt cassette was inserted in front of ATG of edn2 by homologous recombination. The frt-neo-frt cassette was then deleted by Flp recombinase. (b) Screening of transgenic mice carrying the transgene iCre (a representative gel image). The transgene was inserted into the mouse genome by pronuclear injection to C57B/6 blastocysts. Lanes 1–8: founder mouse template genomic DNA (gDNA) from one litter; Lane 9: iCre sequence positive control; Lane 10: WT gDNA negative control; Lane 11: No template DNA negative control; Lane 12: 100bp ladder (Invitrogen).
Figure 2. Temporal mRNA expression of Edn2 and iCre in the edn2-iCre mouse ovary.

Twenty-five day old edn2-iCre9 and Edn2iCre12 mice were injected with PMSG/hCG for superovulation induction. Ovaries were collected at the indicated hours (h) after hCG injection, and mRNA expression levels were measured by semi-quantitative RT-PCR. Shown is a representative image of n=5 mice of each genotype. Note the temporal expression pattern of Edn2 and iCre mRNA. L19 was used as internal control. Bar graph indicates quantification at 12 hours after hCG administration and was normalized to L19. No significant differences were present between groups for edn2 expression.
To typify expression, transgenic mice were crossed with ROSA26-lacZ reporter mice, which express β-galactosidase under a universal promoter with a floxed stop codon (Soriano, 1999). Both founder lines demonstrated similar staining patterns with X-gal. Of these, edn2-iCre#9 was further characterized for localization of edn2 expression. This line was chosen because it provided an average number of healthy pups per litter for C57BL/6 mice, approximately eight, while line edn2-iCre#12 averaged only four pups per litter. All results described below, using either reporter mouse, are from this single founder line; other lines were euthanized and frozen sperm was stored for potential restoration of the line if necessary. Of note, positive staining marks cells that are expressing or have expressed edn2, or are descended from cells that have expressed edn2. No phenotypes are associated with homozygosity for the transgene insertion in these animals.
Pregnant mice were sacrificed and embryos of varying days of development were analyzed by X-gal staining. Positive staining was not present prior to day e12.5 (Figure 3), though placental tissue stained positively past day e8.5 including the umbilicus (data not shown). By day e13.5, faint positive staining was visible in the skin of the embryos; staining was present in a punctate pattern throughout the skin by day e15.5, and was readily apparent in the sinusoid hair follicles of the rostrum (whiskers). This skin staining consistently was present in the outer layer of cells of the developing dermis. Staining was also present on the surface of the digits of fore and hind limbs, the dorsum, and the tail. By day e17.5 near parturition, staining had expanded throughout the skin of the ventrum and was consistently present in the skin surrounding areas of the eye, mouth, and ear openings, and between digits (Figure 3). Staining eventually expands throughout the entirety of the skin by puberty. It is interesting to note early staining presence in those areas that must undergo early keratinization for function in neonates (Findlater et al., 1993), though these areas coincide with those of hair follicle development. This timing of expression is consistent with earlier findings that concluded EDN2 is important for normal embryonic development (Saida et al., 2000; Uchide et al., 2000a).
Figure 3. X-Gal characterization of edn2-iCre embryos.
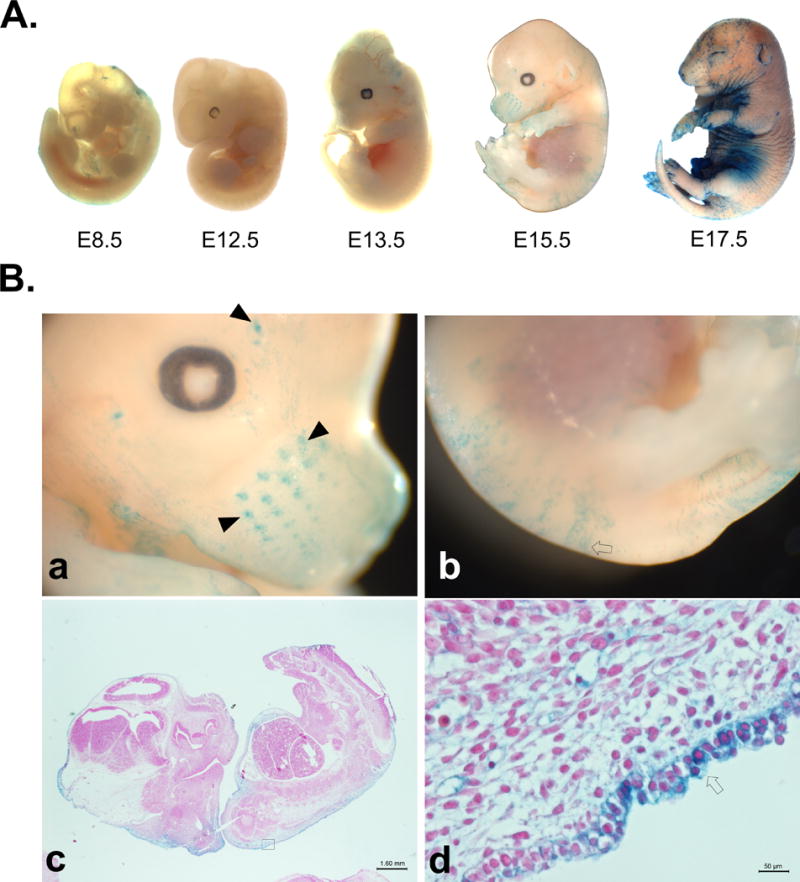
(A) X-Gal staining detects iCre expression and successful recombination in whole mount embryos of edn2-iCre mice crossed with ROSA26-lacZ reporter mice. Faint positive staining is consistently observed as early as e12.5 in the skin of the ventrum. It becomes noticeable throughout much of skin in a fragmented pattern by day e15.5. (B) Higher magnification of dissecting scope images of edn2-iCre fetuses at approximately embryonic day e15.5 after X-Gal staining. Images show a) rostrum, b) dorsal skin and tail, c) whole-mount histological staining 1.25×, and d) magnified histological staining of skin of embryo 40×. Staining of sinus follicles of rostrum is visible by day e15.5, while wide-spread staining of ventrum begins one to two days later. By day 17.5, staining is also visible at opening of mouth, eyes, and ears, and between digits. Closed arrowheads: sinus hair follicles; open arrows: staining in developing dermis/epidermis.
Twenty-eight day old healthy mice were sacrificed and individual organs previously implicated to be involved in EDN2 signaling were stained with X-gal to localize edn2 expression. In the majority of the organs examined, staining revealed a punctate pattern (Figure 4). Brains demonstrated intense staining around the olfactory bulb, although individual neurons stained positively throughout the cerebrum and cerebellum (cerebellum histology pictured). Heart staining revealed that the majority of the epicardium expressed edn2, though only a few scattered cardiomyocytes and endothelial cells stained positively in a seemingly random arrangement, complimenting previous findings (Kakinuma et al., 1999; Plumpton et al., 1993). A similar stippled pattern was visualized in the lung throughout the pneumocytes of the parynchema and the respiratory epithelium. In the kidney, staining was present in only some renal corpuscles and tubules, although staining was limited to the cortex and not present in the medulla. However, cells expressing iCre in the kidney may become more prevalent during renal stress or disease (Bot et al., 2012; Miyauchi et al., 2012). Interestingly, the entirety of the epidermis stained positively, as well as the cells surrounding the hair follicles and the inner root sheath of the follicles themselves. Beyond hair follicles (Chang et al., 2013a), this is a novel area of EDN2 expression, and presents a potential unique application for future gene removal. In the gastrointestinal tract, cross-sectional staining was limited to individual villi or to individual tubular glands, and was also present in patches on the exterior surface, which may be a reflection of the enteric nervous system as Endothelins are known to be involved in neural crest cell migration (Kunieda et al., 1996). Ovarian staining was highly prevalent, present throughout the organ stroma, capsule, and small follicles, in addition to large follicles and corpora lutea as previously reported (Ko et al., 2006; Palanisamy et al., 2006). Punctate staining was present in the epithelium of the oviduct, while individual myofibers of uterine myometrium stained to create a strippled appearance.
Figure 4. Localization of functional iCre expression in adult edn2-iCre mouse organs.

X-Gal staining detects iCre expression and successful recombination in whole organs of edn2-iCre mice crossed with ROSA26-lacZ reporter mice that have previously been shown to produce EDN2 peptide. Gross images are shown at left and histology images with nuclear fast red background staining are at right. Kidney tissue was bisected, ovary was punctured, and heart and colon were flushed prior to staining. The oviduct is visible in the bottom portion of the gross ovarian image. Symbol labeling is as follows: Brain: open arrowhead: olfactory bulb; open arrow: molecular layer; closed arrow: granule cell layer; solid arrowhead: purkinje cell layer. Heart: open arrowhead: epicardium; closed arrowhead: cardiomyocyte. Lung: solid arrow: pneumocytes of alveoli; open arrow: respiratory epithelium of bronchiole. Kidney: open chevron: cortex; closed chevron: medulla; closed arrowhead: renal corpuscle; open arrowhead: tubules of cortex. Skin: solid arrow: epidermis; open arrow: bulge stem cells; closed chevron: dermis. Colon: open arrowhead: muscularis interna; closed arrowhead: goblet cells of longitudinal mucosal fold. Ovary: closed arrowhead: oviduct; open arrowhead: oocyte of secondary follicle; open arrow: secondary follicle granulosa cells; closed arrow: theca cell layer and stromal cells. Uterus: solid arrowhead: endometrium; open arrowhead: uterine glands; open arrow: smooth muscle cells of myometrium.
X-gal staining may occur as a false positive in adult tissues through endogenous beta-galactosidase activity (Burn, 2012; Odgren et al., 2006), and may additionally create ‘edge-effect’ artifact staining on any tissue. Although the majority of tissues of interest showed little or no negative staining in mice lacking the edn2-iCre mutation, false positive staining was present in the gross view of the kidney, GI tract, portions of the uterus, and exterior of the skin (Figure S1) though little staining was present histologically. Additional false positive staining was present in the bone, adrenal, tarsal gland, and liver tissue (not pictured), though decreasing incubation temperature significantly reduced gross background x-gal staining. Similarly, control embryos showed no x-gal staining at any age except for slight edge-effect staining of the skin which does also not appear histologically (Figure S1).
To verify the sites observed as positive for X-gal staining and to identify other novel sites of edn2 expression, edn2-iCre mice were crossed with Ai9 reporter mice that produce red fluorescent protein in cells with functional iCre expression. Tissues from 28 day old males and females were visualized grossly under fluorescent light during dissection, and histological images were compared with H&E staining (Figure 5). Of interest, fluorescence was seen in the anterior and intermediate pituitary, the cornea and photocells of the eye, the detrusor muscle of the bladder, the male coagulation gland (anterior prostate), and the interstitium of the testes. The majority of expression appears in a punctate manner, excluding the epidermis, ovary, testicular interstitium, and coagulation gland. These areas are potential key areas for the utilization of the edn2-iCre transgenic mouse outside of localization of edn2 expression and removal of potential downstream Endothelin target genes. Eye expression is particularly interesting, given recent work by Rattner et al. showing that EDN2 can override VEGF signaling to inhibit retinal angiogenesis (Rattner et al., 2013). Expression of edn2 may act in the cornea and retina continually to inhibit angiogenesis, whereas loss of edn2 could eventually lead to vascularization and visual impairment. A summary of fluorescence observed grossly and histologically is listed in Table 1. No fluorescence was observed in control tissues.
Figure 5. Localization of iCre expression by red fluorescence in adult edn2-iCre mouse organs.

Edn2-iCre mice were crossed with Ai9 reporter mice for rapid visualization. Fluorescence intensity at low magnification (row 2) correlates with the number of edn2-iCre-expressing cells. Red fluorescence indicates areas of cell lineage expression of iCre (row 4). Top rows: gross images and fluorescence comparison. Bottom rows: histological images and fluorescence comparison. A–D: pituitary (female, PND28); E–F: Eye; G–H: Cornea of eye; I–L: Bladder (detrusor muscle); M–N: Gastrointestinal tract; O–P: Jejunum; Q–R: Male reproductive organs; S–T: Seminiferous tubules; U–V: Female reproductive organs; W–X: Ovary. Symbols are used to mark specific organs or cell types. Image A, B, C, D: solid arrow: anterior pituitary (adenohypophesis); open arrow: posterior pituitary (neurohypophysis). Image G, H: Solid arrowhead: corneal epithelium (anterior epithelium, outside of eye). Image K,L: solid arrow: smooth muscle cells of detrusor muscle (3 layers); open arrow: transitional epithelium of bladder facing lumen. Image M,N: Solid arrow: small intestines; open arrow: liver; solid arrowhead: large intestines. Image O,P: Solid arrow head: luminal epithelium of jejunum. Image Q, R: solid arrow: seminal vesicle; open arrow: ductus (vas) deferens; solid arrowhead: coagulation gland (anterior prostate); open arrowhead: testis; solid chevron: epididymis; open chevron: bladder. Image S, T: solid arrow: interstitial cells of testes. Image U, V: Solid arrowhead: ovary; open arrowhead: oviduct; open chevron: uterus. Image W, X: Solid arrowhead: secondary follicle; solid arrow: antral follicle.
Table 1.
Summary of Cre expression in multiple adult organs as determined by localization of red fluorescence after crossing with an Ai9 reporter mouse line.
| Tissue | Relative Fluorescence | Notes | Relevant Previous Literature |
|---|---|---|---|
| Adrenal | +++ | Punctate fluorescence throughout adrenal cortex; little to none in medulla | (Davenport et al., 1996) |
| Aorta | + | Very limited punctate fluorescence in tunica media in smooth muscle cells | (Bacon and Davenport, 1996; Howard et al., 1992; O’Reilly et al., 1993a; Yanagisawa et al., 1988) |
| Bladder | + | Punctate fluorescence in smooth muscle cells of detrusor muscle, not in epithelium | – |
| Blood (circulating) | − | No fluorescence seen (in all RBC and WBCs observed) | (Grimshaw et al., 2002a; Grimshaw et al., 2002b) |
| Bone (femur) | − | No fluorescence seen in osteocytes or bone marrow | (Briggs et al., 1998) |
| Brain | + | Mild fluorescence throughout cortex and cerebellum, extremely limited, 1–2/100 neurons. More intense in hypothalamus and optic chiasm. Greatest fluorescence in olfactory bulb. | (Masuo et al., 2003; Saida et al., 2002) |
| Coagulation gland (anterior prostate) | +++ | Fluorescence seen in all secretory cells | – |
| Colon | +++ | Punctate fluorescence throughout mucosa; cells of mucosa will either all fluoresce or not within one tubular gland (all or none expression by gland); not present in submucosa or muscularis; punctate pattern seen in mesothelial cells of serosa. | (Chang et al., 2013b; McCartney et al., 2002; Wang et al., 2013) |
| Duodenum | +++ | Fluorescence in groups of epithelial cells of individual villi with punctate expression of some submucosal glands; punctate serosal fluorescence present. | (Bianchi et al., 2012; Chang et al., 2013b; Takizawa et al., 2005) |
| Epididymis | ± | 0–1 cells/100 of epithelial cells in lumen of epididymis | – |
| Esophagus | − | No fluorescence visualized | – |
| Eye | + | Punctate/striped fluorescence in most outer cellular layer of corneal epithelium; fluorescence in photocell layer with fluorescence extending through all retinal layers in striped pattern in limited areas of retina approx. 0–1 per section, fluorescence in tarsal gland, no fluorescence in lens or iris | (Bramall et al., 2013; Braunger et al., 2013; Rattner et al., 2013) |
| Heart | + | Punctate fluorescence in cardiomyocytes (2–5/100) and the majority of epicardial cells | (Kakinuma et al., 1999; O’Reilly et al., 1993b; Plumpton et al., 1996; Plumpton et al., 1993; Uchide et al., 2000a) |
| Ileum | +++ | Fluorescence seen in villi similar to duodenum – punctate in groups of cells and often near crypts, fluorescence in individual cells within peyer’s patches (1–2/100), fluorescence in serosa cell layer in patches | (Bloch et al., 1991; Chang et al., 2013b; Fu et al., 1989) |
| Jejunum | +++ | Punctate fluorescence seen in villi and serosa similar to duodenum, ileum; may not extend entire villi if fluorescence seen in crypt | (Chang et al., 2013b) |
| Kidney | + | Limited punctate fluorescence in a few individual cells of corpuscle or proximal tubules, entirely limited to cortex | (Hocher et al., 1996; Karet and Davenport, 1996; Ohkubo et al., 1990) |
| Liver | + | Punctate fluorescence seen in parenchyma, limited to cells appearing stellate | (Battistini et al., 1994) |
| Lung | + | Moderate punctate fluorescence in respiratory epithelium; limited in alveoli/pneumocytes (0–1/100) | (Chang et al., 2013b; Marciniak et al., 1992; Uchide et al., 2000a) |
| Mouth/oral cavity | + | No fluorescence in teeth, present on entirety of gingival surface | – |
| Nerve (thoracic vagus) | − | No fluorescence visualized | (Yuen et al., 2013) |
| Ovary | +++ | Fluorescence seen encompassing all of ovarian capsule, majority of cells of stroma, corpora lutea, and granulosa cells; not seen in oocytes | (Cacioppo et al., 2014; Klipper et al., 2010; Ko et al., 2006; Palanisamy et al., 2006; Uchide et al., 2000a) |
| Oviduct | +++ | Punctate fluorescence pattern in epithelium; more prevalent fluorescence in epithelium of isthmus | (Al-Alem et al., 2007; Jankovic et al., 2010) |
| Pancreas | + | Seemingly random punctate fluorescence throughout parenchyma, 2–5 cells/100 | – |
| Penis | + | Seen in all cells of transitional epithelium of urethra and in the skin surrounding the prepuce | – |
| Pituitary | + | Punctate pattern throughout anterior and intermediate pituitary; not present in posterior pituitary | (Masuo et al., 2003) |
| Preputial gland | +++ | Fluorescence seen in the majority of acini in both basal and secretory cells | – |
| Rostrum | + | Visible in epidermis, in inner layer of dermal root sheath of large sinusoid hair follicles | – |
| Salivary gland | + | Punctate pattern present through some cells of acini; more prevalent in mucous than serous glands | (Lam et al., 2004; Lam et al., 1991) |
| Seminal vesicle | − | No fluorescence visualized | – |
| Skeletal muscle | − | No fluorescence visualized | – |
| Skin | +++ | Visible throughout epidermis, extending into hair follicles | (Chang et al., 2013a; Tanese et al., 2010) |
| Testes | + | Interstitial cells, potentially including Leydig cells; not seen within seminiferous tubules | (Ergun et al., 1999) |
| Uterus | + | Seen in some smooth muscle cells of the myometrium throughout the uterus in a banded pattern (Figure 4) | (Cameron et al., 1993; Uchide et al., 2000b) |
METHODS
Ethics Statement
This study was carried out in tight accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal protocol was approved by the University of Illinois Animal Care and Use Committee (Protocol: 11184), and all efforts were made to minimize animal suffering. Animal models generated in this study will be made readily available to the research community.
Construction of the iCre-polyA-FRT-neo-FRT cassette
E. coli SW105, pL253, pL451, and pICGN21 were obtained from Frederick National Laboratory for Cancer Research (Frederick, MD http://ncifrederick.cancer.gov/). An iCre-polyA-FRT-neo-FRT cassette was constructed by cloning the following DNA fragments between HindIII restriction site and AatII restriction site of pGEM7Zf(+) (Promega, Madison, WI): HindIII-EcoR1 fragment (1100 bp) containing the coding region of the codon-improved Cre recombinase (iCre) from pBluescript KS(+)-icre, EcoRI-KnpI fragment (283 bp) containing SV40 late polyadenylation signal sequence from pCS2+MT, KpnI-SfiI fragment(1500 bp) containing FRT-neo-FRT from pICGN21 and Sfi1/AatII adaptor sequence (5′-CGGCCCTGATCAGTGCTAGCGACGT-3′ annealed with 5′-CGCTAGCACTGATCAGGGCCGCCT-3′).
Insertion of iCre-polyA-FRT-neo-FRT cassette into the edn2 BAC clone
The iCre-pA-FRT-neo-FRT cassette was inserted into exon 1 of the edn2 gene of the BAC clone (ID RP23-98J9) that was purchased from Invitrogen (Carlsbad, CA). Recombineering was performed according to protocols provided by Frederick National Laboratory for Cancer Research (Jahrling et al., 2014; Parkitna et al., 2009; Thomason et al., 2014). Briefly, the iCre-polyA-FRT-neo-FRT cassette was inserted in front of the ATG initiation codon in the BAC plasmid (Gebhard et al., 2007). A homology arm (upstream from ATG) was amplified using primer pairs (5′-ACGCGTTCCTGAAGGTGTTGCAGAGAA-3′, 5′-AAGCTTAGCAGCAGCGGCAGAGTG-3′) and cloned between the Mlu1 site and the HindIII site upstream of iCre-polyA-FRT-neo-FRT cassette. Another homology arm downstream from ATG was amplified using primer pairs (5′-GGCCGAGGCGGCCATGGTCTCCGCCTGGTGTT-3′, 5′-GACGTCCCTTGGTGTTCAGGAACCAC-3′) and cloned the between SfiI site and the AatII site after the cassette. The cassette with the two homologies was cut with MluI and AatII and gel purified. Approximately 15ng of the gel-purified cassette was electroporated into red-induced E.coli SW105 carrying the BAC clone. The recombinants were selected on LB agar plates supplemented with kanamycin (25 μg/ml). The selected bacterial cells were then treated with arabinose (0.1%) for one hour to delete FRT-neo-FRT from the BAC. The recombinant BAC plasmid purification, plasmid integrity test, and pronulear microinjection were performed at the Transgenic Animal Model Core of University of Michigan. Fertilized eggs from C57BL/6 mice were microinjected with the BAC plasmid and implanted into pseudopregnant foster mothers (C57BL/6). Genomic DNA was extracted from the tails of 69 offspring from the pseudopregnant mothers. The presence of iCre was determined by PCR using the following primer pairs: Cre-F (5′-TCTGATGAAGTCAGGAAGAACC-3′) and Cre-R (5′-GAGATGTCCTTCACTCTGAATC-3′) (Bridges et al., 2008).
RT-PCR
Of those offspring that had correct construct insertions, founder lines #9 and #12 were used for further evaluation for ovarian edn2 expression. It is known edn2 is induced during ovulation; thus female 28-day old pre-pubertal edn2-iCre mice of these lines and control WT mice were injected in the intra-peritoneal cavity with 5 IU of pregnant mare serum gonadotropin (PMSG) at 25 days old to induce follicle development, and then 48 h later with 5 IU of human chorionic gonadotropin (hCG) to induce ovulation. Mice ovulate 12–14 hrs after hCG injection, and ovaries were collected at 0, 6, 12, 16, and 24 hrs after hCG injection (n=5). Animals were euthanized by CO2 asphyxiation and cervical luxation, ovaries were removed, and homogenized in Trizol solution (Life Technologies, Grand Island, NY) for RNA extraction. RNA was purified with an RNeasy kit (Qiagen, Germantown, MD), and cDNA was generated using a reverse transcription superscript VILO synthesis kit (Invitrogen). For RT-PCR, iCre was amplified using the above primer set; edn2 was amplified with (5′-CTCCTGGCTTGACAAGGAATG-3′) and (5′-GCTGTCTGTCCCGCAGTGTT-3′), and L19 was amplified as a control housekeeping gene with (5′-CCTGAAGGTCAAAGGGAATGTG-3′) and (5′-GTCTGCCTTCAGCTTGTGGAT-3′). PCR products were visualized on an agarose gel; band intensity was analyzed using ImageJ software (NIH, Bethesda, MD) for relative intensity.
Visualization of Transgene Expression
Animals were bred with B6;129S4-Gt(Makinoda et al.)26Sortm1Sor/J (ROSA26-lacZ) reporter mice to visualize edn2-expressing tissues by X-gal staining (Soriano, 1999). Mice were euthanized by CO2 asphyxiation; perfused intracardially with cold PBS solution and then a 4% paraformaldehyde (PFA) solution to assist in fixation. Tissues were then collected and washed 2 times with cold PBS; large and lipid-dense tissues (brain, liver, lungs, heart, stomach, kidney, tubular organs) were cut into sections with a scalpel to allow better fixative penetration. Tissues were placed in 4% PFA on ice for 1 hour. They were then washed twice with PBS and placed into individual vials with X-gal stain solution (Millipore, Billerica, MA), diluted 1:40 according to the manufacturer’s instructions. Tissues were incubated in the dark in at 4°C on a shaker for 24–48 hours, washed 3 times with PBS, incubated in the dark at room temperature for 1 hour with X-gal holding solution, and then fixed overnight in 4% PFA at 4C. Embryos were similarly fixed and stained. Gross images of tissues were taken of tissues following this final fixation. Tissues were then dehydrated, embedded in paraffin blocks, and sectioned by microtome at 5um thickness. Background staining was performed with nuclear fast red (Fisher Scientific, Pittsburg, PA) and images were taken with an Olympus BX51 microscope. The edn2-iCre animals were also bred with B6;129S6-Gt(Makinoda et al.)26Sortm9(CAG-tdTomato)Hze/J (Ai9) reporter mice (Madisen et al., 2010). Gross images were taken immediately after euthanasia under a Zeiss SV11 fluorescent microscope. Tissues were fixed overnight, embedded, and serially sectioned at 5uM; one section was used for fluorescence visualization and an adjacent slide was stained with hematoxylin and eosin (Thermo Scientific, Kalamazoo, MI).
Supplementary Material
X-Gal staining detects false-positive beta-galactosidase activity or edge-effect staining in (A,B) whole embryos or (C) whole organs of control mice crossed with ROSA26-lacZ reporter mice. For embryos in (A), higher magnification of dissecting scope images of edn2-iCre fetuses at approximately embryonic day 15.5 are shown in (B). Images show a) rostrum, b) dorsal skin, c) whole-mount histological staining 1.25x, and d) magnified histological staining of skin of embryo 40x. For adult tissues (C), gross images are shown at left and histology images with nuclear fast red background staining are at right. Brain, heart, and kidney tissue were bisected, ovary was punctured, and colon was flushed prior to staining. The oviduct and uterus are visible in the gross ovarian image. Note the light staining on the edges of the embryos, the brain, lung, and uterus. Additionally, there is staining throughout the cortex of the kidney and visible histological staining on the hair from the skin section and in the lumen of the colon.
Acknowledgments
The authors thank Dr. Lori Raetzman for technical advice on the use of reporter mice, Dr. Jing Yang for the pCS2+M2 plasmid, and Karen Doty and David Ko for histological assistance.
This work was supported by NIH (HD052694 and HD071875 to CK).
References
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Al-Bader MD, Al-Sarraf HA. Housekeeping gene expression during fetal brain development in the rat-validation by semi-quantitative RT-PCR. Brain Res Dev Brain Res. 2005;156:38–45. doi: 10.1016/j.devbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Bacon CR, Davenport AP. Endothelin receptors in human coronary artery and aorta. Br J Pharmacol. 1996;117:986–992. doi: 10.1111/j.1476-5381.1996.tb15292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistini B, O’Donnell LJ, Warner TD, Fournier A, Farthing MJ, Vane JR. Characterization of endothelin (ET) receptors in the isolated gall bladder of the guinea-pig: evidence for an additional ET receptor subtype. Br J Pharmacol. 1994;112:1244–1250. doi: 10.1111/j.1476-5381.1994.tb13217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Adur J, Takizawa S, Saida K, Casco VH. Endothelin system in intestinal villi: A possible role of endothelin-2/vasoactive intestinal contractor in the maintenance of intestinal architecture. Biochem Biophys Res Commun. 2012;417:1113–1118. doi: 10.1016/j.bbrc.2011.12.053. [DOI] [PubMed] [Google Scholar]
- Bloch KD, Hong CC, Eddy RL, Shows TB, Quertermous T. cDNA cloning and chromosomal assignment of the endothelin 2 gene: vasoactive intestinal contractor peptide is rat endothelin 2. Genomics. 1991;10:236–242. doi: 10.1016/0888-7543(91)90505-9. [DOI] [PubMed] [Google Scholar]
- Bot BM, Eckel-Passow JE, LeGrand SN, Hilton T, Cheville JC, Igel T, Parker AS. Expression of endothelin 2 and localized clear cell renal cell carcinoma. Hum Pathol. 2012;43:843–849. doi: 10.1016/j.humpath.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Schartl M. Evolution of endothelin receptors in vertebrates. Gen Comp Endocrinol. 2014 doi: 10.1016/j.ygcen.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Bramall AN, Szego MJ, Pacione LR, Chang I, Diez E, D’Orleans-Juste P, Stewart DJ, Hauswirth WW, Yanagisawa M, McInnes RR. Endothelin-2-mediated protection of mutant photoreceptors in inherited photoreceptor degeneration. PLoS One. 2013;8:e58023. doi: 10.1371/journal.pone.0058023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis. 2013;50:1–12. doi: 10.1016/j.nbd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Koo Y, Kang DW, Hudgins-Spivey S, Lan ZJ, Xu X, DeMayo F, Cooney A, Ko C. Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor alpha (Esr1) from the ovary and testis. Genesis. 2008;46:499–505. doi: 10.1002/dvg.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs PJ, Moran CG, Wood MB. Actions of endothelin-1, 2, and 3 in the microvasculature of bone. J Orthop Res. 1998;16:340–347. doi: 10.1002/jor.1100160310. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Sharma P, Stevens PA. Association between diastolic blood pressure and variants of the endothelin-1 and endothelin-2 genes. J Cardiovasc Pharmacol. 2000;35:S41–43. doi: 10.1097/00005344-200000002-00010. [DOI] [PubMed] [Google Scholar]
- Burn SF. Detection of beta-galactosidase activity: X-gal staining. Methods Mol Biol. 2012;886:241–250. doi: 10.1007/978-1-61779-851-1_21. [DOI] [PubMed] [Google Scholar]
- Cacioppo JA, Oh SW, Kim HY, Cho J, Lin PC, Yanagisawa M, Ko C. Loss of function of endothelin-2 leads to reduced ovulation and CL formation. PLoS One. 2014;9:e96115. doi: 10.1371/journal.pone.0096115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IT, Plumpton C, Champeney R, van Papendorp C, Ashby MJ, Davenport AP. Identification of endothelin-1, endothelin-2 and endothelin-3 in human endometrium. J Reprod Fertil. 1993;98:251–255. doi: 10.1530/jrf.0.0980251. [DOI] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013a;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I, Bramall AN, Baynash AG, Rattner A, Rakheja D, Post M, Joza S, McKerlie C, Stewart DJ, McInnes RR, Yanagisawa M. Endothelin-2 deficiency causes growth retardation, hypothermia, and emphysema in mice. J Clin Invest. 2013b;123:2643–2653. doi: 10.1172/JCI66735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Hoskins SL, Kuc RE, Plumpton C. Differential distribution of endothelin peptides and receptors in human adrenal gland. Histochem J. 1996;28:779–789. doi: 10.1007/BF02272151. [DOI] [PubMed] [Google Scholar]
- Ergun S, Harneit S, Paust HJ, Mukhopadhyay AK, Holstein AF. Endothelin and endothelin receptors A and B in the human testis. Anat Embryol (Berl) 1999;199:207–214. doi: 10.1007/s004290050221. [DOI] [PubMed] [Google Scholar]
- Findlater GS, McDougall RD, Kaufman MH. Eyelid development, fusion and subsequent reopening in the mouse. J Anat. 1993;183(Pt 1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Fu T, Chang W, Ishida N, Saida K, Mitsui Y, Okano Y, Nozawa Y. Effects of vasoactive intestinal contractor (VIC) and endothelin on intracellular calcium level in neuroblastoma NG108–15 cells. FEBS Lett. 1989;257:351–353. doi: 10.1016/0014-5793(89)81569-8. [DOI] [PubMed] [Google Scholar]
- Gebhard S, Hattori T, Bauer E, Bosl MR, Schlund B, Poschl E, Adam N, de Crombrugghe B, von der Mark K. BAC constructs in transgenic reporter mouse lines control efficient and specific LacZ expression in hypertrophic chondrocytes under the complete Col10a1 promoter. Histochem Cell Biol. 2007;127:183–194. doi: 10.1007/s00418-006-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw MJ, Naylor S, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002a;1:1273–1281. [PubMed] [Google Scholar]
- Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol. 2002b;32:2393–2400. doi: 10.1002/1521-4141(200209)32:9<2393::AID-IMMU2393>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hocher B, Liefeldt L, Thone-Reineke C, Orzechowski HD, Distler A, Bauer C, Paul M. Characterization of the renal phenotype of transgenic rats expressing the human endothelin-2 gene. Hypertension. 1996;28:196–201. doi: 10.1161/01.hyp.28.2.196. [DOI] [PubMed] [Google Scholar]
- Howard PG, Plumpton C, Davenport AP. Anatomical localization and pharmacological activity of mature endothelins and their precursors in human vascular tissue. J Hypertens. 1992;10:1379–1386. doi: 10.1097/00004872-199211000-00010. [DOI] [PubMed] [Google Scholar]
- Jahrling PB, Keith L, St Claire M, Johnson RF, Bollinger L, Lackemeyer MG, Hensley LE, Kindrachuk J, Kuhn JH. The NIAID Integrated Research Facility at Frederick, Maryland: a unique international resource to facilitate medical countermeasure development for BSL-4 pathogens. Pathogens and Disease. 2014;71:211–216. doi: 10.1111/2049-632X.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic SM, Jankovic SV, Lukic G, Canovic D, Folic M. The contractile effects of endothelins on isolated isthmic segment of human oviduct at the luteal phase of the menstrual cycle. Methods Find Exp Clin Pharmacol. 2010;32:91–95. doi: 10.1358/mf.2010.32.2.1428740. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y, Miyauchi T, Kobayashi T, Yuki K, Maeda S, Sakai S, Goto K, Yamaguchi I. Myocardial expression of endothelin-2 is altered reciprocally to that of endothelin-1 during ischemia of cardiomyocytes in vitro and during heart failure in vivo. Life Sci. 1999;65:1671–1683. doi: 10.1016/s0024-3205(99)00416-6. [DOI] [PubMed] [Google Scholar]
- Karet FE, Davenport AP. Localization of endothelin peptides in human kidney. Kidney Int. 1996;49:382–387. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- Klipper E, Levit A, Mastich Y, Berisha B, Schams D, Meidan R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: possible role in bovine corpus luteum formation. Endocrinology. 2010;151:1914–1922. doi: 10.1210/en.2009-0767. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Ko C, Meidan R, Bridges PJ. Why two endothelins and two receptors for ovulation and luteal regulation? Life Sci. 2012;91:501–506. doi: 10.1016/j.lfs.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Kunieda T, Kumagai T, Tsuji T, Ozaki T, Karaki H, Ikadai H. A mutation in endothelin-B receptor gene causes myenteric aganglionosis and coat color spotting in rats. DNA Res. 1996;3:101–105. doi: 10.1093/dnares/3.2.101. [DOI] [PubMed] [Google Scholar]
- Lam HC, Lo GH, Lee JK, Lu CC, Chu CH, Sun CC, Chuang MJ, Wang MC. Salivary immunoreactive endothelin in patients with upper gastrointestinal diseases. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S413–417. doi: 10.1097/01.fjc.0000166288.87571.ae. [DOI] [PubMed] [Google Scholar]
- Lam HC, Takahashi K, Ghatei MA, Warrens AN, Rees AJ, Bloom SR. Immunoreactive endothelin in human plasma, urine, milk, and saliva. J Cardiovasc Pharmacol. 1991;17(Suppl 7):S390–393. doi: 10.1097/00005344-199100177-00109. [DOI] [PubMed] [Google Scholar]
- Ling L, Maguire JJ, Davenport AP. Endothelin-2, the forgotten isoform: emerging role in the cardiovascular system, ovarian development, immunology and cancer. Br J Pharmacol. 2013;168:283–295. doi: 10.1111/j.1476-5381.2011.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinoda S, Hirosaki N, Waseda T, Tomizawa H, Fujii R. Granulocyte colony-stimulating factor (G-CSF) in the mechanism of human ovulation and its clinical usefulness. Curr Med Chem. 2008;15:604–613. doi: 10.2174/092986708783769740. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Plumpton C, Barker PJ, Huskisson NS, Davenport AP. Localization of immunoreactive endothelin and proendothelin in the human lung. Pulm Pharmacol. 1992;5:175–182. doi: 10.1016/0952-0600(92)90038-i. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ishikawa Y, Kozakai T, Uchide T, Komatsu Y, Saida K. Vasoactive intestinal contractor/endothelin-2 gene expression in the murine central nervous system. Biochem Biophys Res Commun. 2003;300:661–668. doi: 10.1016/s0006-291x(02)02872-3. [DOI] [PubMed] [Google Scholar]
- McCartney SA, Greaves RR, Warner TD, O’Donnell LJ, Domizio P, Farthing MJ. Endothelin content, expression, and receptor type in normal and diseased human gallbladder. Dig Dis Sci. 2002;47:1786–1792. doi: 10.1023/a:1016532228836. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Sakai S, Maeda S, Shimojo N, Watanabe S, Honma S, Kuga K, Aonuma K, Miyauchi T. Increased plasma levels of big-endothelin-2 and big-endothelin-3 in patients with end-stage renal disease. Life Sci. 2012;91:729–732. doi: 10.1016/j.lfs.2012.08.008. [DOI] [PubMed] [Google Scholar]
- O’Reilly G, Charnock-Jones DS, Cameron IT, Smith SK, Davenport AP. Endothelin-2 mRNA splice variants detected by RT-PCR in cultured human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 1993a;22(Suppl 8):S18–21. doi: 10.1097/00005344-199322008-00007. [DOI] [PubMed] [Google Scholar]
- O’Reilly G, Charnock-Jones DS, Morrison JJ, Cameron IT, Davenport AP, Smith SK. Alternatively spliced mRNAs for human endothelin-2 and their tissue distribution. Biochem Biophys Res Commun. 1993b;193:834–840. doi: 10.1006/bbrc.1993.1701. [DOI] [PubMed] [Google Scholar]
- Odgren PR, MacKay CA, Mason-Savas A, Yang M, Mailhot G, Birnbaum MJ. False-positive beta-galactosidase staining in osteoclasts by endogenous enzyme: studies in neonatal and month-old wild-type mice. Connect Tissue Res. 2006;47:229–234. doi: 10.1080/03008200600860086. [DOI] [PubMed] [Google Scholar]
- Ohkubo S, Ogi K, Hosoya M, Matsumoto H, Suzuki N, Kimura C, Ondo H, Fujino M. Specific expression of human endothelin-2 (ET-2) gene in a renal adenocarcinoma cell line. Molecular cloning of cDNA encoding the precursor of ET-2 and its characterization. FEBS Lett. 1990;274:136–140. doi: 10.1016/0014-5793(90)81348-r. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Parkitna JR, Engblom D, Schutz G. Generation of Cre recombinase-expressing transgenic mice using bacterial artificial chromosomes. Methods Mol Biol. 2009;530:325–342. doi: 10.1007/978-1-59745-471-1_17. [DOI] [PubMed] [Google Scholar]
- Plumpton C, Ashby MJ, Kuc RE, O’Reilly G, Davenport AP. Expression of endothelin peptides and mRNA in the human heart. Clin Sci (Lond) 1996;90:37–46. doi: 10.1042/cs0900037. [DOI] [PubMed] [Google Scholar]
- Plumpton C, Champeney R, Ashby MJ, Kuc RE, Davenport AP. Characterization of endothelin isoforms in human heart: endothelin-2 demonstrated. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S26–28. doi: 10.1097/00005344-199322008-00009. [DOI] [PubMed] [Google Scholar]
- Rattner A, Yu H, Williams J, Smallwood PM, Nathans J. Endothelin-2 signaling in the neural retina promotes the endothelial tip cell state and inhibits angiogenesis. Proc Natl Acad Sci U S A. 2013;110:E3830–3839. doi: 10.1073/pnas.1315509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K, Hashimoto M, Mitsui Y, Ishida N, Uchide T. The prepro vasoactive intestinal contractor (VIC)/endothelin-2 gene (EDN2): structure, evolution, production, and embryonic expression. Genomics. 2000;64:51–61. doi: 10.1006/geno.1999.6083. [DOI] [PubMed] [Google Scholar]
- Saida K, Kometani N, Uchide T, Mitsui Y. Sequence analysis and expression of the mouse full-length vasoactive intestinal contractor/endothelin-2 gene (EDN2): comparison with the endothelin-1 gene (EDN1) Clin Sci (Lond) 2002;103(Suppl 48):84S–89S. doi: 10.1042/CS103S084S. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Szabo A, Perou CM, Karaca M, Perreard L, Palais R, Quackenbush JF, Bernard PS. Statistical modeling for selecting housekeeper genes. Genome Biol. 2004;5:R59. doi: 10.1186/gb-2004-5-8-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S, Uchide T, Adur J, Kozakai T, Kotake-Nara E, Quan J, Saida K. Differential expression of endothelin-2 along the mouse intestinal tract. J Mol Endocrinol. 2005;35:201–209. doi: 10.1677/jme.1.01787. [DOI] [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Ishiko A, Sakamoto M. Endothelin-2 is upregulated in basal cell carcinoma under control of Hedgehog signaling pathway. Biochem Biophys Res Commun. 2010;391:486–491. doi: 10.1016/j.bbrc.2009.11.085. [DOI] [PubMed] [Google Scholar]
- Thomason LC, Sawitzke JA, Li X, Costantino N, Court DL. Recombineering: genetic engineering in bacteria using homologous recombination. Curr Protoc Mol Biol. 2014;106:1 16 11–11 16 39. doi: 10.1002/0471142727.mb0116s106. [DOI] [PubMed] [Google Scholar]
- Uchide T, Adur J, Fukamachi T, Saida K. Quantitative analysis of endothelin-1 and vasoactive intestinal contractor/endothelin-2 gene expression in rats by real-time reverse transcriptase polymerase chain reaction. J Cardiovasc Pharmacol. 2000a;36:S5–8. doi: 10.1097/00005344-200036051-00004. [DOI] [PubMed] [Google Scholar]
- Uchide T, Fujimori Y, Sasaki T, Temma K, Adur J, Masuo Y, Kozakai T, Lee YS, Saida K. Expression of endothelin-1 and vasoactive intestinal contractor genes in mouse organs during the perinatal period. Clin Sci (Lond) 2002;103(Suppl 48):167S–170S. doi: 10.1042/CS103S167S. [DOI] [PubMed] [Google Scholar]
- Uchide T, Masuda H, Lee YS, Makiyama Y, Mitsui Y, Saida K. Fluctuating gene expression and localized cellular distribution of vasoactive intestinal contractor (VIC) in mouse uterus. J Histochem Cytochem. 2000b;48:699–707. doi: 10.1177/002215540004800514. [DOI] [PubMed] [Google Scholar]
- Uchide T, Masuda H, Mitsui Y, Saida K. Gene expression of vasoactive intestinal contractor/endothelin-2 in ovary, uterus and embryo: comprehensive gene expression profiles of the endothelin ligand-receptor system revealed by semi-quantitative reverse transcription-polymerase chain reaction analysis in adult mouse tissues and during late embryonic development. J Mol Endocrinol. 1999;22:161–171. doi: 10.1677/jme.0.0220161. [DOI] [PubMed] [Google Scholar]
- Wang R, Lohr CV, Fischer K, Dashwood WM, Greenwood JA, Ho E, Williams DE, Ashktorab H, Dashwood MR, Dashwood RH. Epigenetic inactivation of endothelin-2 and endothelin-3 in colon cancer. Int J Cancer. 2013;132:1004–1012. doi: 10.1002/ijc.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988;85:6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Johnson KR, Miron VE, Zhao C, Quandt J, Harrisingh MC, Swire M, Williams A, McFarland HF, Franklin RJ, Ffrench-Constant C. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136:1035–1047. doi: 10.1093/brain/awt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
X-Gal staining detects false-positive beta-galactosidase activity or edge-effect staining in (A,B) whole embryos or (C) whole organs of control mice crossed with ROSA26-lacZ reporter mice. For embryos in (A), higher magnification of dissecting scope images of edn2-iCre fetuses at approximately embryonic day 15.5 are shown in (B). Images show a) rostrum, b) dorsal skin, c) whole-mount histological staining 1.25x, and d) magnified histological staining of skin of embryo 40x. For adult tissues (C), gross images are shown at left and histology images with nuclear fast red background staining are at right. Brain, heart, and kidney tissue were bisected, ovary was punctured, and colon was flushed prior to staining. The oviduct and uterus are visible in the gross ovarian image. Note the light staining on the edges of the embryos, the brain, lung, and uterus. Additionally, there is staining throughout the cortex of the kidney and visible histological staining on the hair from the skin section and in the lumen of the colon.


