Abstract
Urothelial carcinoma of the bladder and upper tract pose significant diagnostic and therapeutic challenges. White light endoscopy plays a central role in the management of urothelial carcinoma but has several well-recognized shortcomings. New optical imaging technologies may improve diagnostic accuracy, enhance local cancer control, and better stratify treatment options. Confocal laser endomicroscopy enables dynamic imaging of the cellular structures below the mucosal surface and holds promise in providing real time optical diagnosis and grading of urothelial carcinoma. A variety of imaging probes are available that are compatible with the full spectrum of cystoscopes and ureteroscopes. We review the underlying principles and technique of confocal laser endomicroscopy in the urinary tract, with emphasis on specific application towards urothelial carcinoma. While the available data are largely related to urothelial carcinoma of the bladder, the lessons learned are directly applicable to the upper tract, where the clinical needs are significant. Ongoing efforts to optimize this technology offer an exciting glimpse into future advances in optical imaging and intraoperative image guidance.
Keywords: Urothelial carcinoma, Bladder cancer, Upper tract, Optical imaging, Microscopy
Introduction
Urothelial carcinomas are common cancers that pose significant management challenges and healthcare burdens. Urothelial carcinoma of the bladder (UCB) accounts for > 95 % of bladder cancer, which ranks as the sixth most common cancer in the US, with 74,690 new cases and 15,580 deaths expected in 2014 [1,2]. UCB is a heterogeneous disease, ranging from low-grade cancer that does not recur after initial resection, to high-grade variants that progress to metastatic, lethal disease [3]. Approximately 80 % of UCB is non-muscle-invasive at initial diagnosis, while the remaining presents with muscle-invasive or metastatic cancer [4]. UCB recurrence is among the highest of all cancers, reaching up to 61 % at one year and 78 % at five years [5], thereby requiring costly lifelong surveillance [4,6]. In comparison, upper tract urothelial carcinoma (UTUC) appears histologically indistinct from UCB and accounts for 5–10 % of all urothelial carcinomas [7]. Studies have shown that 8 to 13 % of UTUC is present with concomitant UCB [8,9], and 15–50 % of patients treated for UTUC go on to manifest recurrence in the bladder [10].
Direct visualization of the urothelium via endoscopy is integral in the management of urothelial carcinoma. In the bladder, white light cystoscopy (WLC) is used in the initial diagnosis and subsequent surveillance to determine tumor size, number, and location. Despite its central role, WLC has well-documented limitations [11,12]. Visualization of papillary tumors is straightforward, but differentiation of non-papillary or flat carcinomas, such as carcinoma in-situ (CIS), from benign inflammatory lesions are frequently challenging [11,13]. Several studies have shown that only 58–68 % of CIS are detected by WLC [14–16]. WLC also guides transurethral resection (TUR) and local staging of UCB. Indistinct borders and difficulty in visualizing submucosal tumor margins, however, may lead to incomplete tumor resection [17]. The presence of multifocal lesions further increases the likelihood that some will go undetected on initial TUR, and indeed, ‘residual’ cancer can be found in 40 % of patients at the time of second TUR [18,19]. Unrecognized and incompletely resected tumors, in addition to growth of microscopic lesions to detectable sizes, cancer cell implantation, and new growth from aggressive tumors, all contribute to the high recurrence rate of UCB [20,21]. Given the suboptimal detection of cancerous lesions using WLC, there is considerable interest in developing adjunctive imaging modalities to improve the effectiveness of WLC and TUR.
Ureteroscopy and ureteroscopic biopsy are the standards in establishing the initial diagnosis of UTUC, and radical nephroureterectomy with a bladder cuff excision remains the standard for definitive treatment [22]. Despite significant advances in endourology over the last 20 years, ureteroscopic biopsy remains technically challenging with suboptimal yield. Recognized shortcomings include insufficient tissue quantity for histology, sampling variability from heterogeneous lesions, tumor under-grading, and lack of staging information [23–25]. Up to 25 % of renal pelvic or ureteral biopsies may be nondiagnostic due to insufficient quantities of tissue [26]. In addition, 15 % of UTUC found to be low grade from ureteroscopic biopsy are up-graded after nephroureterectomy [24,25]. In patients with solitary kidney, bilateral UTUC, and severe underlying renal disease, nephroureterectomy may be contraindicated. These challenging clinical scenarios, coupled with advances in endourology, have encouraged the evolution of alternative strategies of UTUC treatment [27]. For selected patients with UTUC (i.e., low grade), renal-sparing endoscopic management based on ablation has become a viable option, with cancer-specific survival rates comparable to nephroureterectomy [28,29]. Taken together, these studies highlight the need for improved optical diagnosis for UTUC, particularly to better stratify patients for renal-sparing treatment options, as well as for long-term endoscopic surveillance.
To address the shortcomings of standard white light endoscopy, numerous optical imaging technologies are under active investigation for applications in the urinary tract [12,30]. The primary goal of these new strategies is to better identify and characterize urothelial pathology beyond white light. Imaging technologies are classified based on field of view. Macroscopic imaging modalities survey a large area of mucosa similar to white light endoscopy, and provide additional contrast enhancement to highlight suspicious lesions and distinguish them from surrounding non-cancerous mucosa. Examples include photodynamic diagnosis (PDD) (i.e., fluorescence cystoscopy, blue light cystoscopy) for the bladder and narrow band imaging (NBI) for the bladder and upper tract. Microscopic modalities are commonly referred to as optical biopsy technologies that are capable of high-resolution, subsurface characterization of suspected lesions. With optical biopsy, information is provided on tissue microarchitecture and cellular morphology that is not possible with macroscopic imaging. Examples include optical coherence tomography (OCT), spectroscopy, and confocal laser endomicroscopy (CLE), the focus of the current review.
Confocal Laser Endomicroscopy: Principles
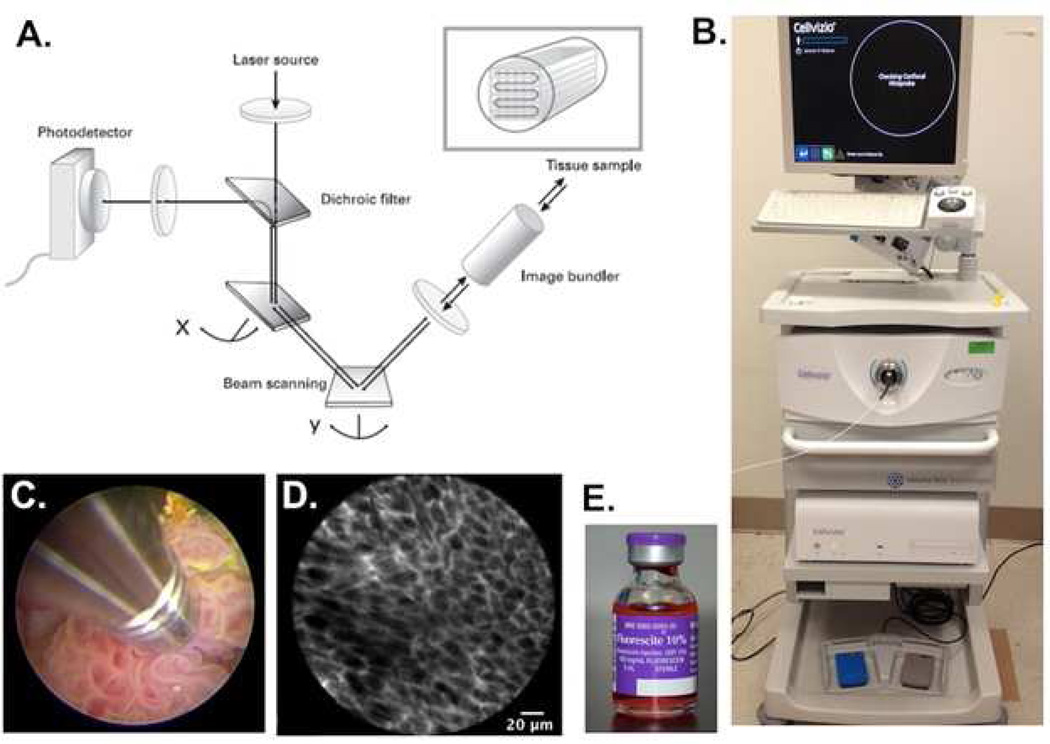
CLE is based on confocal microscopy, a mainstay tool of basic science research for high-resolution cellular and subcellular imaging. Recent advances in fiber-optics technology have enabled packaging of a confocal microscope into a small probe format compatible with standard endoscopes (Fig. 1) [31]. A 488 nm low-power laser scans a tissue section of interest below the surface. The tissue is nonspecifically stained with fluorescein, a Food and Drug Administration (FDA)-approved contrast agent that can be administered intravenously or topically [32]. Under excitation, the fluorescein emits light that is filtered through a pinhole so that only in-focus light is measured by a photodetector while the out-offocus light is rejected, resulting in optical sectioning of the regions of interest with micron-scale resolution comparable to histology [31]. In contrast to standard hematoxylin and eosin (H&E) histology, nuclear features are not routinely visualized, since fluorescein highlights the extracellular matrix and does not cross intact cell membranes. CLE images are acquired as video sequences at a rate of 12 frames per second via direct contact of the probe with tissues of interest.
Fig. 1.
Mechanism and method of confocal laser endomicroscopy. A) Schematic representation of the technology underlying CLE. A laser beam is directed to the tissue sample via an image bundler. The resulting emission of light is retrieved and filtered through a pinhole before reaching the photodetector; B) Clinical grade CLE system (Cellvizio) from Mauna Kea Technologies. The workstation includes a laser scanning unit for probe attachment and a computer with software for image acquisition and processing. C) Direct probe contact of a papillary tumor for image acquisition. D) High-resolution image of subsurface cellular features acquired from CLE. The fluorescence signal is coming from the extracellular matrix stained by fluorescein. E) Fluorescein, an FDA-approved contrast agent, can be administered intravenously or topically for CLE imaging
A probe-based CLE system (Cellvizio, Mauna Kea Technologies) is available clinically and is approved for gastrointestinal endoscopy and bronchoscopy [32,33]. Since the initial ex vivo and in vivo feasibility studies in 2009 [31,34], CLE has been under investigation in the urinary tract. CLE recently received approval for applications in the bladder and upper tract in Europe and the US. Imaging probes are available, ranging from 0.85 to 2.6 mm in diameter with varying optical specifications in spatial resolution, field of view, and compatibility with existing endoscopes (Fig. 2). The 2.6 mm probe is compatible with the working port of standard 22 French (Fr) or larger cystoscopes and resectoscopes, while the 1.4 mm probes are compatible with the larger scopes, as well as the working channel of flexible cystoscopes (~15 Fr). The newest 0.85 mm probe is compatible with all standard scopes for the lower and upper urinary tracts. CLE is best implemented for in-depth characterization of the tissue of interest after an initial, broad-based survey with WLC or other wide field technologies such as fluorescence cystoscopy or narrow band imaging. The small field of view is offset by the ability of CLE to visualize microscopic, cellular features and tissue organization that approach the characterization seen upon H&E histology, the current standard for cancer diagnosis. Given the in vivo characterization, CLE offers the added benefit of real-time imaging of physiological parameters, including vascular blood flow that is not possible with H&E [13].
Fig. 2.
Comparison of available probes for confocal laser endomicroscopy. Probes range in diameter from 2.6 mm to 0.85 mm. Decreasing spatial resolution by downsizing the probes is offset by greater compatibility with a larger array of endoscopes, including flexible scopes. The field of view is most narrow with the 2.6 mm probe and widest with the 1.4 mm probe, with the 0.85 mm probe falling in between
Technical Considerations
The technique of CLE in the lower urinary tract has been described in a step-by-step video [35]. In the bladder, fluorescein can be delivered intravesically with a Foley catheter using 300–400 ml 0.1 % fluorescein diluted in saline, or intravenously with a 0.5 ml 10 % fluorescein injection. Intravenous fluorescein is FDA-approved for use in diagnostic angiography of the retina. It has also recently been shown to be a safe, nontoxic contrast agent for CLE in the gastrointestinal tract. In over 2,000 patients undergoing CLE operations, IV fluorescein was administered and shown to be well tolerated, with no serious adverse events reported. Patients experienced temporary yellow skin discoloration lasting a couple of hours, as well as other minor side effects, including transient hypotension without shock, nausea, injection site reaction, diffuse rash, and mild epigastric pain [36]. Moreover, prior work done by our group on CLE in the urinary tract has shown that both IV and intravesical fluorescein demonstrate good safety profiles, with transient fluorescently tinged urine as the primary minor side effect [31].
Intravesical fluorescein is left indwelling for 5 min to allow uptake of the dye in the mucosa, whereas IV fluorescein distributes rapidly and can be administered immediately before CLE imaging [13]. Both intravesical and IV fluorescein enables visualization of papillary tumors, flat tumors, erythematous patches, and the border between normal mucosa and neoplastic lesions. Observation of the resection bed as well as the prostatic and penile urethra requires IV fluorescein administration. Following imaging, the CLE probes can be sterilized and reused.
The majority of the published work to date has been done using the 2.6 mm probe. As previously mentioned, WLC is first used for general surveillance of the bladder, followed by detailed imaging with CLE at specific regions of interest. Increasing and decreasing the pressure of the probe against the bladder surface enables sectional visualization of varying depths of mucosa. Biopsies of suspicious lesions are taken and sent for histopathologic analysis and postoperative comparison with acquired CLE images [35]. Due to the narrow field of view of CLE, post-processing of the images involves a video mosaicing technique that can stitch together overlapping, serial frames into a static, composite piece. This image reconstruction method can expand the field of view up to fivefold and remove motion distortions and artifacts without sacrificing resolution, providing a more complete view of the imaged tissue for analysis [13,37].
Imaging Diagnostic Criteria for Bladder Cancer
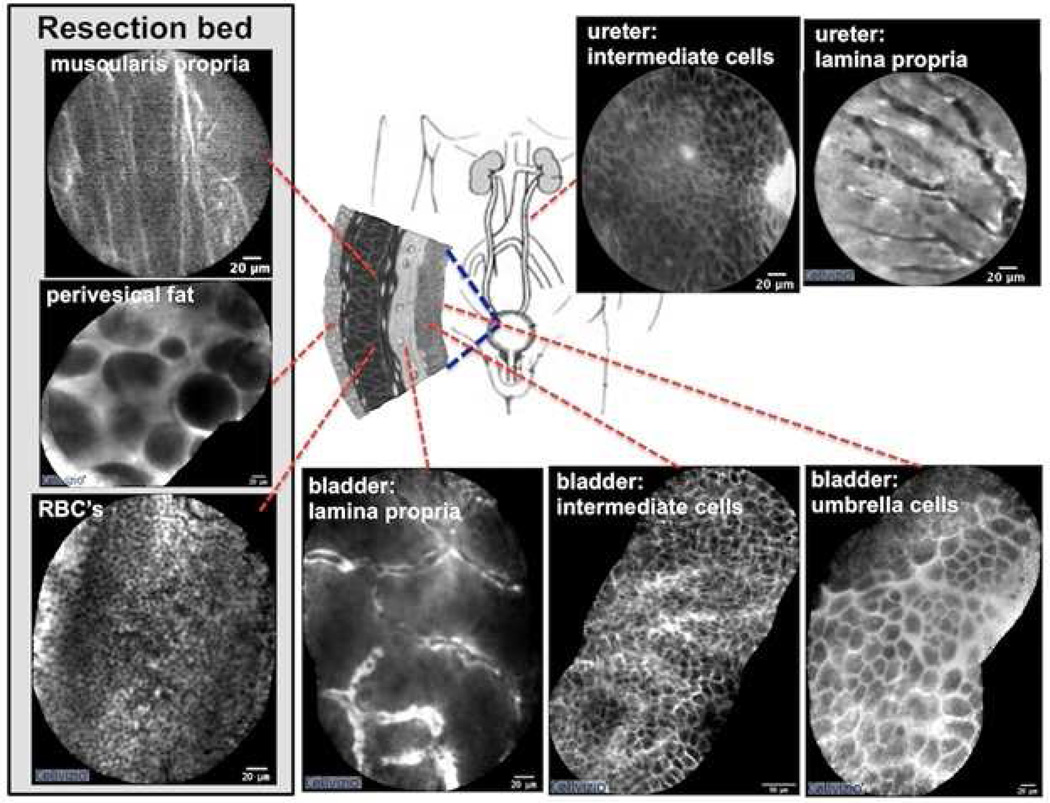
Prior works by our group have established a preliminary set of optical diagnostic criteria for normal urothelium, benign inflammatory lesions, low-grade tumors, and high-grade tumors [13,31, 38]. Characteristic microarchitectural (flat vs. papillary, tissue organization, and vasculature) and cellular (morphology, cohesiveness, borders) features for each tissue type have been described [38]. Representative images documenting the various diagnostic constituents have been compiled into an optical imaging atlas in an ongoing effort to refine the diagnostic criteria (Fig. 3). Notably, given the limited depth of penetration, CLE is unable to visualize the muscularis propria from the muscosal surface [31]. It is, however, possible to image the muscularis propria (and perivesical fat) after tumor resection through IV administration of fluorescein or secondary instillation of topical fluorescein.
Fig. 3.
Confocal images of normal anatomic landmarks within the urinary tract. Normal urothelium is characterized by superficial polygonal-shaped umbrella cells and underlying intermediate cells. The lamina propria is characterized by a vacular network with a relatively acellular matrix. Characteristic findings of the tumor resection bed include multi-directional fibers consistent with the muscularis propria, large adipocytes within perivesical fat, and copious red blood cells. The resection bed was imaged after the tumor resection. In the upper urinary tract, images of the urothelium are similar to the lower tract. Images of the lower urinary tract were acquired in vivo while those of the upper urinary tract were obtained ex vivo. Video mosaicing was utilized to expand the field of view for select images
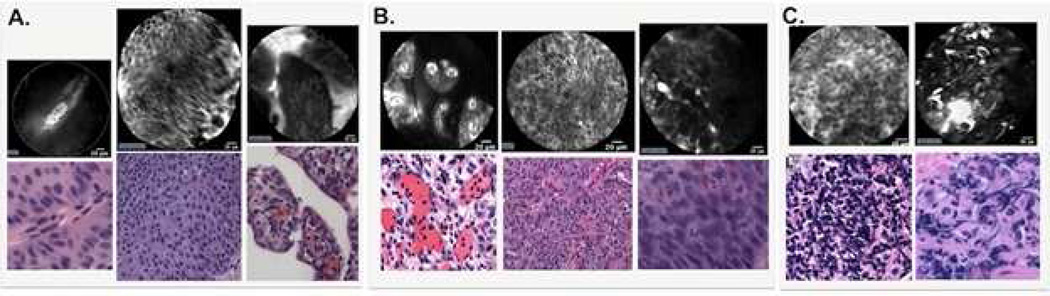
Under CLE, normal mucosa is characterized by layers of superficial, polygonal-shaped umbrella cells and homogeneous, smaller intermediate cells located more deeply. Within the lamina propria, dense capillary networks are commonly seen populated with flowing erythrocytes (Fig. 3). These elements of normal morphology are absent in cancerous lesions (Fig. 4). Low-grade papillary tumors demonstrated crowding of monomorphic cells, papillary structures, and the presence of fibrovascular stalks, vessels with a thickened endothelial layer (Fig. 4A). High-grade tumors are identified by a decidedly disorganized appearance, with pleomorphic cells arranged haphazardly around distorted vasculature. Delineating cell boundaries has proven difficult given the general loss of cellular cohesion and tissue organization in high-grade tumors (Fig. 4B). In benign, inflammatory urothelium, small monomorphic cells are observed loosely distributed in the lamina propria, but fibrovascular stalks are notably absent (Fig. 4C).
Fig. 4.
Diagnostic imaging criteria for urothelial carcinoma. A) Low-grade cancer are characterized by organized, densely-packed, monomorphic cells, the absence of umbrella cells, papillary structures, and fibrovascular stalks. B) High-grade cancer show disorganized, pleomorphic cells, indistinct borders with loss of cellular cohesion, the absence of umbrella cells, and fibrovascular stalks with distorted vasculature. C) Benign, inflammatory lesions feature small, infiltrative, monomorphic cells in the lamina propria loosely arranged and absent fibrovascular stalks. Normal urothelium is described in Fig. 3. Using confocal laser endomicroscopy adjunct to white light cystoscopy, these diagnostic imaging criteria demonstrated substantial agreement among urologists familiar with the technology (ĸ 0.80) and moderate agreement among novice users (ĸ 0.59). Sensitivity and specificity of the diagnostic criteria were reported as 89 % and 88 %, respectively
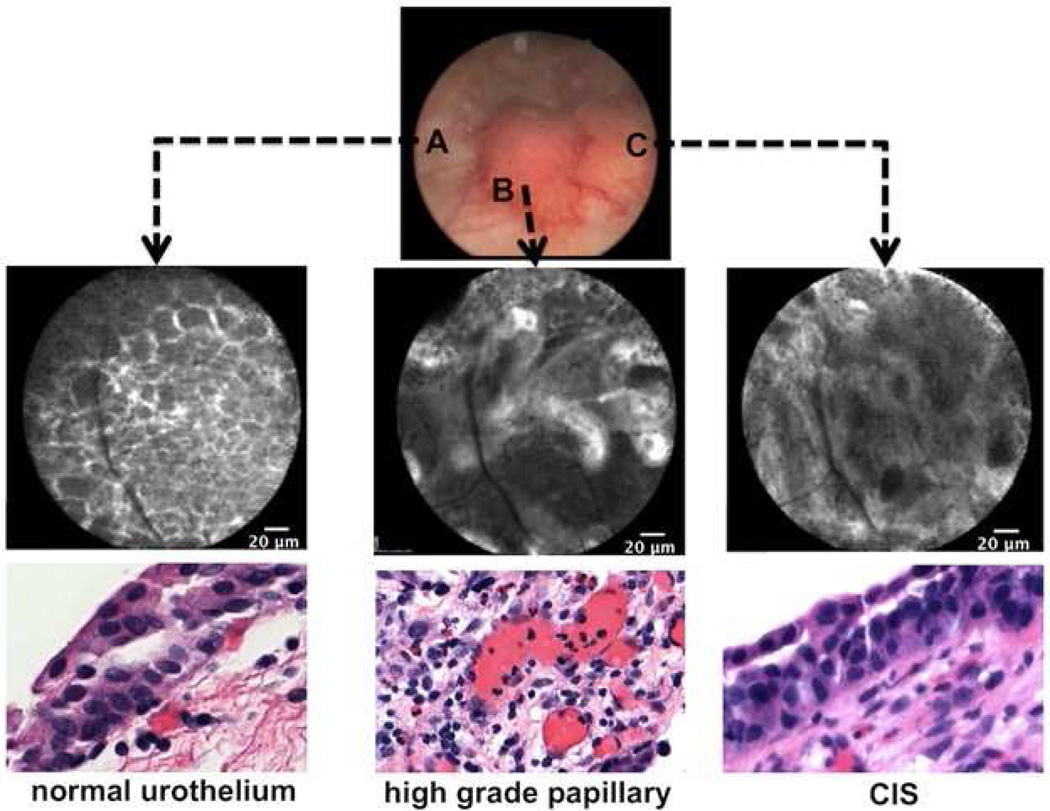
High-grade carcinomas can appear as papillary, sessile, and flat. Importantly, flat lesions typified by high-grade CIS are difficult to diagnosis under WLC, given its close mimicry to erythematous patches of benign, inflammatory origin. Under H&E histology, CIS is known to be a heterogeneous entity, with different morphological features and a degree of inflammatory infiltrate [39]. As seen in Fig. 5, CIS appears as pleomorphic cells with indistinct cellular borders and absence of organized microarchitecture. Given the heterogeneity of CIS, additional evaluation is catalog optical imaging characteristics for CIS with histopathological correlation. Computer-aided imaging diagnosis may prove to be helpful, as described in CLE applications in the gastrointestinal (GI) tract [40].
Fig. 5.
Intraoperative image guidance of a tumor seen under white light with corresponding confocal imaging and histology. A) Normal urothelium surrounding the papillary tumor highlighting organized, monomorphic cells. B) High-grade, papillary tumor with papillary structures and distorted microvasculature. C) High-grade, carcinoma-in-situ featuring pleomorphic cells and disorganized microarchitecture
Inter-Observer Variance and Diagnostic Accuracy
Novel imaging technologies necessitate inter-observer agreement studies to evaluate variability among observers in interpreting acquired images. High precision in a diagnostic test indicates reproducibility of results and reliability for consistent analysis [41]. This in turn predicts the value of the imaging modality for clinical applications. Endoscopic diagnosis and grading of cancers using CLE imaging demonstrate short learning curves as well as good to substantial inter-observer agreement in colorectal cancers [42,43], and substantial to excellent agreement in Barrett’s esophagus [44–46]. Most recently, inter-observer agreement in CLE image interpretation of bladder cancers was determined to range from moderate in novice CLE users to substantial in expert observers [38]. CLE is therefore a highly adoptable technology for the diagnosis of various cancers. Using bladder cancer as a model with CLE, current work is focused on expanding this technology into related urologic cancers. Measures of accuracy using CLE has a sensitivity of 88–98 %, specificity of 92–96 %, and accuracy of 92–97 % in Barrett’s esophagus and gastrointestinal neoplasia [40,45]. Diagnostic accuracy of bladder cancer using WLC together with CLE, as clinically relevant, has been reported as having 89 % sensitivity and 88 % specificity [38].
CLE in the Upper Tract
Previously, ex vivo CLE imaging of the renal pelvis and proximal ureter showed similarity between upper tract and bladder urothelial cells and lamina propria (Fig. 3) [13]. With the recent availability of a 0.85 mm imaging probe compatible with standard semirigid and flexible ureteroscopes (Fig. 6), our group has initiated a feasibility study of CLE in the upper urinary tract [47]. With IV administration of fluorescein, normal urothelium and papillary UTUC have been imaged with CLE. Imaging of UTUC showed characteristic features of tumors, including papillary structure, pleomorphic cells, and fibrovascular stalks. In comparison to the 2.6-mm probe, the 0.85-mm probe expectedly demonstrated lower resolution in identifying diagnostic features in the bladder. Additional works are pending to expand this initial feasibility study.
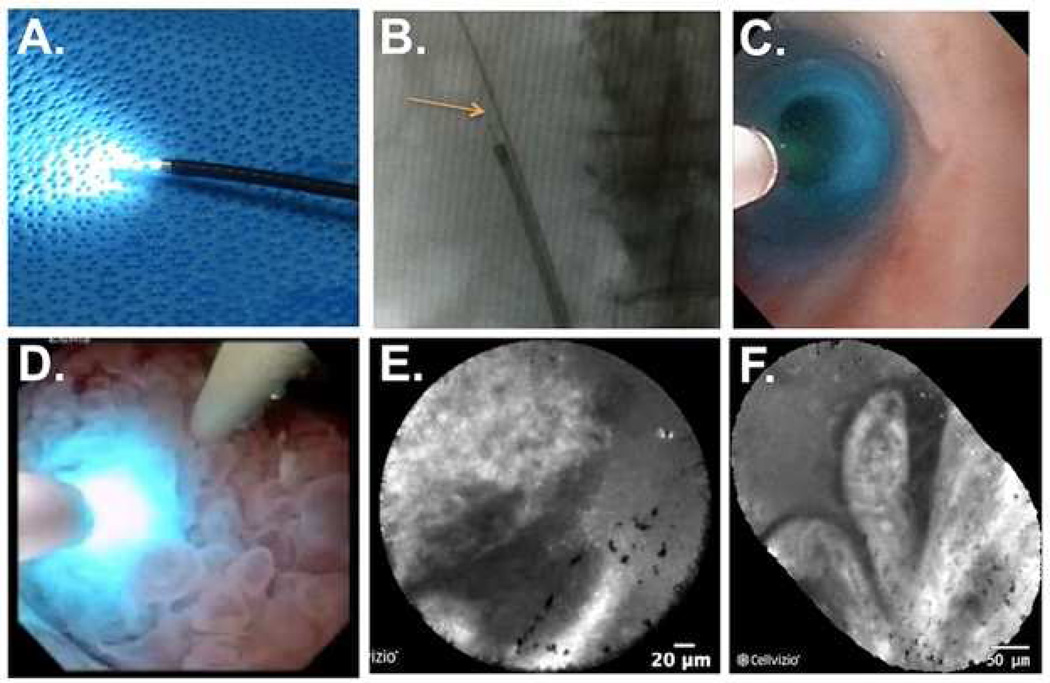
Fig. 6.
Application of confocal laser endomicroscopy to the upper urinary tract. A) A 0.85 mm probe inserted into a flexible ureteroscope. B) Fluoroscopy image of the flexible ureteroscope in the ureter. The arrow is pointing to the confocal probe fitted through the ureteroscope. C) Ureteroscopic view of the confocal probe inside a normal ureter. D) Confocal probe in direct contact with a large, papillary tumor in the renal pelvis. E) CLE image of tumor in D showing the papillary border. F) Mosaic image of the papillary tumor showing the papillary structure of the tumor shown in D. Fine streaks seen in the papillary structures represent the fibrovascular stalk present in cancerous lesions
Future Outlooks
Optical diagnosis of urothelial carcinoma using CLE, while promising, remains at the early stage of clinical integration. Recent approval for clinical use will facilitate prospective multicenter studies to validate the clinical efficacy and determine optimal clinical indications. In UCB, outstanding questions include diagnostic accuracy of CLE for papillary and non-papillary tumors, clinical utility of resection bed imaging after TUR, and to determine the role of CLE in bladder cancer surveillance. In UTUC, additional clinical experience is needed to investigate the feasibility of the 0.85 mm probe.
To decrease the learning curve and facilitate clinical studies, the development of a computer-based ‘smart atlas’ for urothelial carcinoma may be beneficial through collaborative efforts among urology, pathology, and imaging scientists. Wu et al. have established the basis for an optical imaging atlas of bladder cancer, with representative images describing the various grades acquired from CLE [13]. An exciting avenue for future work involves expanding the bladder cancer atlas into an automated smart atlas to broaden the range of users who would benefit from this evolving technology. André et al. have pioneered this in the GI tract, with the development of a content-based, image retrieval classification algorithm that matches real-time CLE images with those stored in the database. The goal is to provide objective assistance in the diagnosis of colonic polyps, and initial results are promising [48,49]. Such a system would naturally develop into a more accurate tool with the addition of more CLE images to the source database, detailing the various morphologies and appearances of colorectal lesions.
Moreover, an attractive feature of CLE is the ease with which it integrates into surgical practice, given its compatibility with existing endoscopes. This advantage can be further harnessed using molecular imaging to target tissues of interest. Though fluorescein is currently the preferred contrast agent for CLE, it is a nonspecific dye that broadly stains the extracellular matrix, with diffuse uptake in both normal and neoplastic tissues. Conjugating monoclonal antibodies to fluorescein for targeted binding of cancer-specific antigens has the potential to significantly improve tumor detection. However, immunogenic responses to antibodies preclude more widespread clinical use [50–52]. Instead, peptide markers, which are less immunogenic and smaller in size, are believed to be more favorable. In contrast to IV fluorescein, topical application of fluorescent peptides can reduce inter-observer subjectivity in image interpretation and guard against incomplete tumor resection by demarcating tumor boundaries [50]. Additionally, the high affinity and selectivity of fluorescent peptide probes to cancer cell receptors reduces the amount of the agent needed for imaging, augmenting image quality by increasing the signalto- noise ratio [51].
Refinement of fluorescence confocal imaging with conjugated peptide probes is just beginning. Preclinical and early clinical studies of colorectal cancer demonstrated significantly greater signal in cancer cells over normal mucosa when a fluorescein-conjugated peptide marker specific for dysplastic colonocytes was administered [50,53]. Similarly, a fluorescently labeled peptide used in imaging for esophageal adenocarcinomas revealed a 3.8-fold increase in signal intensity as compared to Barrett’s esophagus or normal epithelium [52]. Preclinical studies evaluating a fluorescently labeled deoxyglucose agent for CLE detection of Barrett’s esophagus capitalizes on the metabolic changes that occur with cancer and shows high sensitivity and specificity for differentiating between metaplastic and neoplastic sites [54]. These preliminary results suggest a logical path of exploration to enhance the detection and diagnosis of bladder cancer using CLE and contrast agents with molecular specificity.
Conclusion
The advent of novel imaging technologies has a transformative potential in cancer detection and management. For urothelial carcinomas, confocal laser endomicroscopy has become one of the leading candidates as an adjunctive imaging modality to WLC to improve endoscopic visualization of tissues. Studies have demonstrated the reliability of CLE in bladder cancer diagnosis and the general adoptability of the technology. Recently, application of CLE has extended to optical imaging of the upper urinary tract as well. With efforts under way to increase the usability of this tool through the development of a smart atlas and targeted molecular probes, CLE promises to achieve better surgical outcomes and more effective management for patients with urothelial carcinoma.
Acknowledgement
The authors thank current and past members of the Liao Laboratory, particularly Katherine Wu and Kathy Mach, for technical support and helpful discussions. Funding support was provided in part by Stanford University School of Medicine MedScholars Fellowship (to S.P.C.) and NIH R01 CA160986 (to J.C.L.).
Footnotes
Conflict of Interest
Dr. Stephanie P. Chen and Dr. Joseph C. Liao each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.U.S. Cancer Statistics Working Group. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. United States Cancer Statistics, 1999–2010 Incidence and Mortality Web-based Report. [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA. Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Kirkali Z, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TM, Clark PE. Bladder cancer. Curr. Opin. Oncol. 2010;22:242–249. doi: 10.1097/CCO.0b013e3283378c6b. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. discussion 475–477. [DOI] [PubMed] [Google Scholar]
- 6.Cauberg Evelyne CC, de la Rosette JJMCH, de Reijke TM. Emerging optical techniques in advanced cystoscopy for bladder cancer diagnosis: A review of the current literature. Indian J. Urol. IJU J. Urol. Soc. India. 2011;27:245–251. doi: 10.4103/0970-1591.82845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouprêt M, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur. Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Hall MC, et al. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 9.Linton KD, Catto JW. Upper tract urothelial carcinoma. J. Clin. Urol. 2013;6:272–279. [Google Scholar]
- 10.Azémar M-D, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol. Oncol. 2011;29:130–136. doi: 10.1016/j.urolonc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Lee CSD, Yoon CY, Witjes JA. The past, present and future of cystoscopy: the fusion of cystoscopy and novel imaging technology. BJU Int. 2008;102:1228–1233. doi: 10.1111/j.1464-410X.2008.07964.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu J-J, Droller MJ, Liao JC. New optical imaging technologies for bladder cancer: considerations and perspectives. J. Urol. 2012;188:361–368. doi: 10.1016/j.juro.2012.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu K, et al. Dynamic Real-time Microscopy of the Urinary Tract Using Confocal Laser Endomicroscopy. Urology. 2011;78:225–231. doi: 10.1016/j.urology.2011.02.057. This paper describes the suggested optical diagnostic criteria for normal urothelium, benign inflammatory urothelium, low grade urothelial carcinoma, and high grade urothelial carcinoma.
- 14.Schmidbauer J, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J. Urol. 2004;171:135–138. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 15.Fradet Y, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J. Urol. 2007;178:68–73. doi: 10.1016/j.juro.2007.03.028. discussion 73. [DOI] [PubMed] [Google Scholar]
- 16.Jocham D, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J. Urol. 2005;174:862–866. doi: 10.1097/01.ju.0000169257.19841.2a. discussion 866. [DOI] [PubMed] [Google Scholar]
- 17.Kolozsy Z. Histopathological ‘self control’ in transurethral resection of bladder tumours. Br. J. Urol. 1991;67:162–164. doi: 10.1111/j.1464-410x.1991.tb15100.x. [DOI] [PubMed] [Google Scholar]
- 18.Babjuk M, Soukup V, Petrík R, Jirsa M, Dvorácek J. 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int. 2005;96:798–802. doi: 10.1111/j.1464-410X.2004.05715.x. [DOI] [PubMed] [Google Scholar]
- 19.Daniltchenko DI, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J. Urol. 2005;174:2129–2133. doi: 10.1097/01.ju.0000181814.73466.14. discussion 2133. [DOI] [PubMed] [Google Scholar]
- 20.Klän R, Loy V, Huland H. Residual tumor discovered in routine second transurethral resection in patients with stage T1 transitional cell carcinoma of the bladder. J. Urol. 1991;146:316–318. doi: 10.1016/s0022-5347(17)37779-0. [DOI] [PubMed] [Google Scholar]
- 21.Brausi M, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur. Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 22.Margulis V, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 23.Straub J, Strittmatter F, Karl A, Stief CG, Tritschler S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. Urol. Oncol. 2013;31:1166–1170. doi: 10.1016/j.urolonc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Wang JK, Tollefson MK, Krambeck AE, Trost LW, Thompson RH. High rate of pathologic upgrading at nephroureterectomy for upper tract urothelial carcinoma. Urology. 2012;79:615–619. doi: 10.1016/j.urology.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Smith AK, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78:82–86. doi: 10.1016/j.urology.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Tavora F, et al. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am. J. Surg. Pathol. 2009;33:1540–1546. doi: 10.1097/PAS.0b013e3181aec42a. [DOI] [PubMed] [Google Scholar]
- 27.Cutress ML, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. 2012;110:614–628. doi: 10.1111/j.1464-410X.2012.11068.x. [DOI] [PubMed] [Google Scholar]
- 28.Elliott DS, Segura JW, Lightner D, Patterson DE, Blute ML. Is nephroureterectomy necessary in all cases of upper tract transitional cell carcinoma? Long-term results of conservative endourologic management of upper tract transitional cell carcinoma in individuals with a normal contralateral kidney. Urology. 2001;58:174–178. doi: 10.1016/s0090-4295(01)01109-8. [DOI] [PubMed] [Google Scholar]
- 29.Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS., Jr Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J. Urol. 2010;183:2148–2153. doi: 10.1016/j.juro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Lopez A, Liao JC. Emerging endoscopic imaging technologies for bladder cancer detection. Curr. Urol. Rep. 2014;15:406. doi: 10.1007/s11934-014-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonn GA, et al. Optical Biopsy of Human Bladder Neoplasia With In Vivo Confocal Laser Endomicroscopy. J. Urol. 2009;182:1299–1305. doi: 10.1016/j.juro.2009.06.039. This paper was the initial feasibility study of in vivo confocal laser endomicroscopy in the urinary tract.
- 32.Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388–392. 392.e1–392.e2. doi: 10.1053/j.gastro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Thiberville L, Salaün M. Bronchoscopic advances: on the way to the cells. Respir. Int. Rev. Thorac. Dis. 2010;79:441–449. doi: 10.1159/000313495. [DOI] [PubMed] [Google Scholar]
- 34.Sonn GA, et al. Fibered confocal microscopy of bladder tumors: an ex vivo study. J. Endourol. Endourol. Soc. 2009;23:197–201. doi: 10.1089/end.2008.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang TC, Liu J-J, Liao JC. Probe-based confocal laser endomicroscopy of the urinary tract: the technique. J. Vis. Exp. JoVE. 2013:e4409. doi: 10.3791/4409. This on-line video paper demonstrates a step-by-step approach of confocal laser endomicroscopy in the lower urinary tract.
- 36.Wallace MB, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 37.Becker V, et al. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video) Gastrointest. Endosc. 2007;66:1001–1007. doi: 10.1016/j.gie.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 38. Chang TC, et al. Interobserver agreement of confocal laser endomicroscopy for bladder cancer. J. Endourol. Endourol. Soc. 2013;27:598–603. doi: 10.1089/end.2012.0549. This paper describes the interobserver agreement of confocal laser endomicroscopy of bladder lesions and provides an updated optical diagnostic criteria.
- 39.Aron M, et al. Utility of a triple antibody cocktail intraurothelial neoplasm-3 (IUN-3-CK20/CD44s/p53) and α-methylacyl-CoA racemase (AMACR) in the distinction of urothelial carcinoma in situ (CIS) and reactive urothelial atypia. Am. J. Surg. Pathol. 2013;37:1815–1823. doi: 10.1097/PAS.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 40.Wallace MB, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest. Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam. Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 42.Kuiper T, Kiesslich R, Ponsioen C, Fockens P, Dekker E. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest. Endosc. 2012;75:1211–1217. doi: 10.1016/j.gie.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 43.Gómez V, et al. Interobserver agreement and accuracy among international experts with probe-based confocal laser endomicroscopy in predicting colorectal neoplasia. Endoscopy. 2010;42:286–291. doi: 10.1055/s-0029-1243951. [DOI] [PubMed] [Google Scholar]
- 44.Lee YC, et al. Interobserver reliability in the endoscopic diagnosis and grading of Barrett’s esophagus: an Asian multinational study. Endoscopy. 2010;42:699–704. doi: 10.1055/s-0030-1255629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiesslich R, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Gastroenterol. Assoc. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Gaddam S, et al. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett’s esophagus. Am. J. Gastroenterol. 2011;106:1961–1969. doi: 10.1038/ajg.2011.294. [DOI] [PubMed] [Google Scholar]
- 47.Bui D, Mach KE, Lopez A, Liu JJ, Chang T, Lavelle J, Leppert JT, Liao JC. Optical biopsy of upper tract urothelial carcinoma with confocal laser endomicroscopy. Eur. Urol. 2014;13:e630. [Google Scholar]
- 48.André B, Vercauteren T, Buchner AM, Wallace MB, Ayache N. A smart atlas for endomicroscopy using automated video retrieval. Med. Image Anal. 2011;15:460–476. doi: 10.1016/j.media.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 49.André B, et al. Software for automated classification of probe-based confocal laser endomicroscopy videos of colorectal polyps. World J. Gastroenterol. WJG. 2012;18:5560–5569. doi: 10.3748/wjg.v18.i39.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiung P-L, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker A, et al. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat. Biotechnol. 2001;19:327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 52.Sturm MB, et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Sci. Transl. Med. 2013;5:184ra61. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller SJ, et al. In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PloS One. 2011;6:e17384. doi: 10.1371/journal.pone.0017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thekkek N, et al. Pre-clinical evaluation of fluorescent deoxyglucose as a topical contrast agent for the detection of Barrett’s-associated neoplasia during confocal imaging. Technol. Cancer Res. Treat. 2011;10:431–441. doi: 10.7785/tcrt.2012.500220. [DOI] [PMC free article] [PubMed] [Google Scholar]