Summary
A rapid and continuous rise in incidence of esophageal adenocarcinoma over the past four decades has been well documented in many developed countries across three continents. Among white males, who are in the highest risk demographic, incidence has risen 10-fold in the U.S. since the early 1970s. Incidence among males in the U.K. are among the highest in the world, and 50% higher than in the U.S. Unfortunately, treatments have not kept pace; unless their cancer is identified at a very early stage, most individuals will not survive a year after diagnosis. The beginnings of this widespread problem were first recognized over 25 years ago, yet rates have continued to rise against a backdrop of much improved understanding and management including the introduction of medical and surgical treatments aimed at reducing acid reflux, one of the most important risk factors; the availability of screening and surveillance programs for the precursor lesion Barrett’s metaplasia; and the development of endoscopic therapies for prevention or treatment of early invasive cancer. We estimate that only about 7% of the 10,000 cases of esophageal adenocarcinoma diagnosed annually in the U.S. are identified through current approaches to cancer control, and trace pathways by which the remaining 93% are “lost.” Based on emerging data on etiology and predictive factors coupled with new diagnostic tools, we suggest a five-tier strategy for prevention and control that begins with a wide population base and triages individuals into progressively higher risk strata, each with risk-appropriate prevention, screening and treatment options.
Background
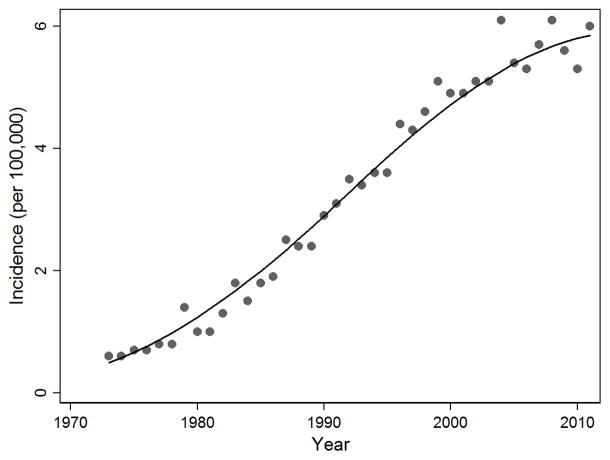
For nearly four decades, primary care physicians, gastroenterologists, surgeons and oncologists have been fighting a losing battle against a relentless opponent – esophageal adenocarcinoma. During this period, the cancer has undergone an impressive and continuous rise in incidence, from a true rarity in the early 1970s to the most common histologic type of esophageal cancer in many developed countries.1–3 The most recent data from the U.S. Surveillance Epidemiology and End Results (SEER) registries show more than an eight-fold rise (from 0.3 to 2.7 per 100,000) in overall annual incidence between 1973 and 2011, and a 10-fold increase (from 0.6 to 6.0 per 100,000) among white men (Figure 1).4 Some of the highest incidence rates of esophageal adenocarcinoma in the world have been observed in the U.K. (9.4 per 100,000 males in the 2008–2010 time period.)5 Treatment effectiveness has not substantially improved; currently about half of individuals newly diagnosed with the cancer succumb within a year, and fewer than 20% survive five years.6
Figure 1.
Esophageal adenocarcinoma incidence in white males, United States, 1973–2011. [Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, (with SEER Delay Factors) Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.]
These trends have occurred despite a background of improving medications and therapies that might have been expected to reduce risk of esophageal adenocarcinoma. For example H2-antagonists were introduced in the late 1970s, and the even more potent proton pump inhibitors a decade later. At appropriate doses these can essentially eliminate gastric acid production and minimize the symptoms of gastroesophageal reflux disease (GERD), which is a major risk factor for the cancer. Fundoplication was first described in the 1950s and gained popularity in the 1970s as a surgical means of strengthening the effectiveness of the lower esophageal sphincter and reducing or eliminating GERD. Unfortunately there is little evidence that these approaches substantially reduce incidence from esophageal adenocarcinoma.7–10
Increasingly, gastroenterologists have been focusing on the identification and long-term surveillance of persons with Barrett’s metaplasia to facilitate identifying and treating pre-invasive or early-stage cancer. Photodynamic therapy began to be used in the 1990s as an endoscopic method of eliminating metaplastic or dysplastic cells of the esophagus, but has largely been dropped because of frequent side effects and lack of clear benefit.11 More recently, endoscopic therapies such an endoscopic mucosal resection combined with ablation techniques such as radiofrequency ablation have advanced considerably, and become a common treatment for persons with high-grade dysplasia, a biomarker of high risk of neoplastic progression to esophageal adenocarcinoma, and have been proposed even for persons at lower risk.12,13 These procedures have largely replaced esophagectomy, with its high morbidity and mortality, for persons deemed at high risk of developing invasive cancer, or a poor risk for surgery. However, the jury is still out regarding the effectiveness of surveillance coupled with endoscopic therapy, as very little evidence is available regarding the ultimate goal of reducing population mortality from esophageal cancer.
While there is some room for optimism due to the improved treatments available, if Figure 1 is taken to heart, one can only conclude that primary and secondary prevention efforts in response to this mounting problem, which was first identified in the late 1980s,14 have had minimal impact, at least on a population basis. Where did we go wrong?
The road taken
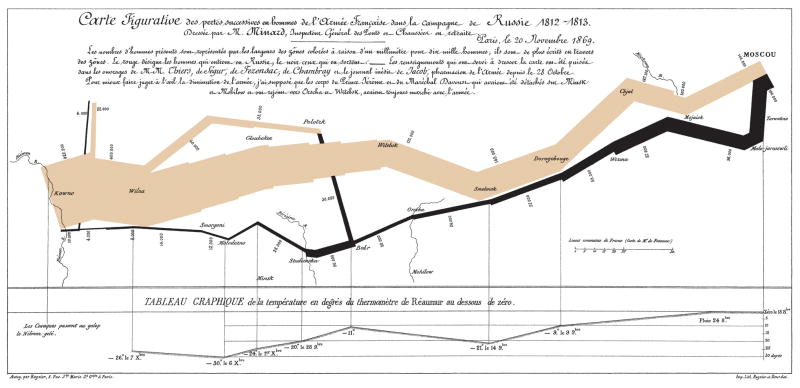
A key reason for the failure of the current paradigm is that we are not identifying the “at risk” population very effectively. The current strategy can be construed as representing not a war on esophageal adenocarcinoma, but rather a war on Barrett’s esophagus. However, for the majority of individuals, Barrett’s esophagus is a benign condition which usually remains undiagnosed. In fact there is evidence that the metaplastic epithelium protects against the inflammatory and erosive effects of bile and acid reflux.15,16 In addition, some cases of esophageal adenocarcinoma may occur without associated Barrett’s metaplasia, or in an ultra-short columnar tongue which may not be endoscopically visible. Far from a “lightning strike,” this war seems more akin to Napoleon’s long, cold and ultimately fruitless march on Moscow in 1812–1813.17 It is well known how that ended for soldiers and citizens on both sides, with vastly overstretched resources contributing to the rapidly diminishing size of Napoleon’s army over time, space and temperature, as famously depicted by Charles Minard (reproduced in Figure 2.)
Figure 2.
The size of Napoleon’s army, represented by the width of the line, dwindled from almost 500,000 to about 10,000 during his invasion of Russia (gray), and return to France (black) during the extremely cold winter of 1812–1813. (Charles Minard, 1869; public domain)
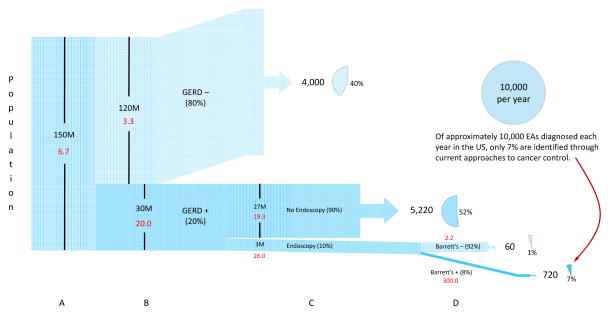
A similar graphical depiction (“Napoleogram”; Figure 3) can describe the current situation in terms of the overall burden of esophageal adenocarcinoma, the subgroups considered for diagnostic and surveillance endoscopy tests, and the rapidly diminishing number of esophageal adenocarcinoma cases which can be found in these subgroups, despite large expenditures for such endoscopic procedures.18 Figure 3 is divided into four panels estimating incidence rates and numbers of cases arising in: (A) the overall adult population, (B) those with and without recurrent symptoms of GERD, (C) those who do and do not receive screening endoscopy, and (D) those with and without diagnosed Barrett’s esophagus.
Figure 3.
The current approach to control of esophageal adenocarcinoma identifies only about 7% of the cases via screening and surveillance, with most of the remainder diagnosed because of alarm symptoms, usually indicative of later stage disease. Approximately half (52%) of the cases arise in persons with reflux symptoms who are not investigated through endoscopy or other means. Numbers in red indicate the estimated annual incidence rate in each population subgroup. See text for details.
Panel A
In the U.S. approximately 17,000 cases of esophageal cancer are diagnosed per year19 of which almost 60% (10,000) are adenocarcinomas.1 Over 99% occur in persons 40 years of age or older, a population of approximately 150M, yielding a crude incidence rate of 6.7 per 100,000.19,20
Panel B
Population-based studies in the U.S. and Sweden indicate that about 40% of esophageal adenocarcinoma cases (4,000 per year) occur among persons without chronic symptoms of GERD (i.e., heartburn and/or regurgitation once per week or more.)21,22 They comprise approximately 80% of the adult population (120M persons), and experience a crude annual incidence rate of 3.3 per 100,000 (upper section of panel B, labeled “GERD−”).23–25 The remaining cases (6,000) occur among the 20% of the adult population (30M) who report GERD symptoms at least once per week,23–26 yielding an annual incidence rate of 20 per 100,000 (lower section of panel B, labeled “GERD+”.) Comparing crude incidence rates in persons with and without chronic GERD symptoms yields a relative risk for esophageal adenocarcinoma of about six, which is consistent with published studies.25,26
Panel C
Among those with recurrent symptoms of GERD, only a small proportion, estimated at 10% (3M persons),18 undergo endoscopy in a given year to i) identify those with Barrett’s metaplasia for enrollment in surveillance, ii) follow up those known to have Barrett’s metaplasia, and/or iii) screen for early esophageal adenocarcinoma (or other treatable conditions such as ulcer) which has not yet given rise to alarm symptoms.27 Assuming that those who undergo endoscopy do not represent a random selection of those with GERD symptoms, but rather, those with slightly higher risk (estimated at 30% higher in Panel C) due to reasons such as symptom severity, clinical acumen, etc., their annual incidence would be 26 per 100,000. The remaining 27M persons with recurrent GERD symptoms who do not undergo endoscopic screening would therefore experience a slightly lower annual incidence of 19.3 per 100,000, yielding 5,220 cases in this group.
Panel D
Endoscopic investigation of persons with recurrent GERD symptoms generally reveals Barrett’s metaplasia to be present in at least 8% of initial endoscopies, depending on the population being considered.28–31 Assuming 8% prevalence of Barrett’s metaplasia (240,000 persons) and an annual rate of progression to EA of approximately 0.3% (300/100,000),32 then 720 cases (about 7%) will have been diagnosed as a consequence of current prevention activities. A few esophageal adenocarcinoma cases would still be observed among persons for whom Barrett’s is not found at endoscopy, perhaps due to Barrett’s being missed, a rapidly developing cancer or an adenocarcinoma not arising in Barrett’s epithelium. Given the numbers above (8% prevalence of Barrett’s and a progression rate of 0.3% in persons positive for Barrett’s), it can be calculated that 60 esophageal adenocarcinomas would arise in this group, reflecting an incidence rate of 2.2 per 100,000 per year. We are not aware of published incidence rates for comparison.
Challenges
Under- and over-diagnosis are increasingly being recognized as significant challenges for many cancers in devising screening and surveillance programs that are both economically sensible and effective in reducing mortality.33 These challenges are particularly apparent in the current approach to control of esophageal adenocarcinoma, as described in Figure 3, which paradoxically yields both over- and under-diagnosis.
Under-diagnosis occurs because more than 90% of cases arise outside of endoscopic diagnosis and surveillance programs.34 Current U.S. “best practice” advice states that, in the absence of alarm symptoms, endoscopic investigation is indicated only in those with recurrent GERD symptoms who do not respond to proton pump inhibitors.27 Thus, a large fraction of cases (about 40%) are missed simply because they do not report sufficient symptoms of GERD.25,26 Most of these 4,000 individuals will eventually be diagnosed only after they develop alarm symptoms, at which time the cancer is typically too far advanced for effective treatment. Another estimated 52% of cancer cases will have had recurrent GERD symptoms, but were never investigated. Reasons for this are not clear, but likely involve a combination of patient characteristics, physician characteristics, and issues of access to health care. They too are usually diagnosed at a late stage, and consequently have a very poor prognosis.
Conversely, over-diagnosis occurs because most (95%) individuals who do undergo periodic surveillance do not develop the cancer in their lifetime, and thus contribute to overstretching healthcare resources.16,33 Within the current paradigm, simply performing upper endoscopies on a larger proportion of persons with reflux symptoms would only worsen the over-diagnosis problem without materially improving population mortality from esophageal adenocarcinoma. Importantly, it is still not clear whether individuals diagnosed through surveillance programs in the community setting actually experience improved survival.34–37
A different direction
If Napoleon had paused as he neared Moscow to fully contemplate the likely outcome of his campaign, taking into account how poorly it had progressed up to that point, he might have changed course and chosen a different path, saving hundreds of thousands of lives in the process. An opportunity of similar magnitude presents itself today, as modeling indicates that the incidence of esophageal adenocarcinoma will continue to increase through 2030, and that approximately 160,000 deaths attributable to this cancer will occur in the U.S. alone over the next two decades.38 A lesson we can learn from Napoleon is the importance of recognizing when an approach is not working, and resources are stretched too thin, so that a different direction can be plotted and tried.
Analysis of the neglected and uninvestigated subpopulations depicted in Figure 3 might give us a new perspective regarding the individuals on whom we should focus our efforts in a renewed, more scientifically focused attempt to win this war. One promising approach to cancer prevention is to consider each risk factor, or predictor, in its most important and cost-effective context, i.e., “precision cancer prevention.” Current approaches presume that reflux is the single overriding risk factor.25,26 However, there are a number of other strong risk factors for esophageal adenocarcinoma that have been identified;39–46 hence it is now possible to categorize individuals into more precise risk groups, each of which can be targeted for further investigation and/or prevention interventions in a manner that is appropriate for their absolute risk (annual incidence) of esophageal adenocarcinoma.
Table 1 describes five separate risk strata that could be considered, ranging in annual cancer incidence from about 7/100,000 in the general population and the general practice settings, to 1,000-fold higher (7,000/100,000) among those in a tertiary care setting for management of those at highest risk. Incidence in the lowest two tiers is based on SEER data;4 whereas incidence in the highest tier (7% per year) represents an approximate minimum risk at which surgical or endoscopic interventions might be reasonably applied.47 Incidence in the third and fourth strata are set to be 10- and 100-fold higher than the general population to illustrate the separation in risk that might be achieved with current information together with resources that may be available in the near future. The sub-populations triaged to higher risk strata would be correspondingly reduced by 10-fold (or more) per level, depending on the sensitivity of such classification schemes.
Table 1.
Prevention approaches appropriate for specific strata of absolute risk (annual incidence) of esophageal adenocarcinoma.
| Stratum | Annual incidence | Setting (population) | Additional resources available | Additional risk factors assessed | Potential prevention activities |
|---|---|---|---|---|---|
| 1 | 7/100,000 | General population 40+ (150M) |
|
|
|
| 2 | 7/100,000 | Primary Care (150M) |
|
|
|
| 3 | 70/100,000 (.07%) | Primary care (15M) |
|
|
|
| 4 | 700/100,000 (.7%) | Secondary care (1.5M) |
|
|
|
| 5 | 7,000/100,000 (7%) | Tertiary care (150K) |
|
In the general population (stratum 1), the main modifiable risk factors include obesity, cigarette smoking and diet low in fruit and vegetables.48,49 It should be noted that although chronic GERD is a key risk factor, it is not easily addressed at the general population level.26 Given the well-recognized importance of these three factors for multiple other cancers, as well as for cardiovascular disease and other health outcomes, the marginal importance of the relatively rare occurrence of esophageal adenocarcinoma is quite small in driving public policy towards improvements in these adverse exposures. Nevertheless, it is still important to recognize that improvements in population healthy behaviors might actually have as large or larger impact on incidence and mortality from esophageal adenocarcinoma than the other more targeted interventions discussed below, as well as treatment of those with the cancer.50,51
In the office of the primary care provider (stratum 2), the absolute risk of a particular patient over 40 years of age might be considered about the same as in the general population, but here the possibilities are much greater for educating both provider and patient as to modifiable risk factors, and for those sufficiently motivated, actually encouraging modest behavioral changes.29,52 Given the additional information resources available in this setting, e.g., medical and family history,53 physical examination, blood/urine measures (as effective biomarkers are identified),54–57 and knowledge of their independent associations with cancer risk, a risk assessment tool, or risk calculator, can be used to educate and motivate, as well as to identify those at a higher level of risk who should be considered for additional investigation.51,58 While no highly penetrant germline mutations for esophageal adenocarcinoma have been identified, there is evidence for a significant polygenic hereditary component,59 and the list of common mutations conferring small differences in risk is growing.60–63 Eventually germline mutations might contribute to the risk prediction algorithms. This stratum is one context in which a substantial improvement in referral decisions can be made rather easily. Rather than relying primarily on reflux symptom history, ten or more risk factors, all easily assessed, can be integrated into a single risk calculation, and only those above a threshold (e.g., ten-fold higher is used as the example in Table 1) can be triaged to the next assessment stage.29
Traditionally, the next level of assessment has involved referral to a gastroenterologist for endoscopy in order to visualize abnormalities, including columnar epithelium, and to collect biopsies for histologic assessment. Ultra-thin transnasal endoscopes have been developed but they still require significant investment for the hardware and skilled operators more suited to the secondary care provider.64,65 Recently, several new less invasive non-endoscopic approaches have shown promise for use in primary care (stratum 3). A tethered capsule has been created using volume laser endomicroscopy which uses frequency domain optical coherence tomography that can rapidly scan the esophageal lumen at a thirty micron lateral resolution to allow this device to be used for screening for BE.66 In addition molecular screening of the esophagus using non-invasive methods soon may be shown to be practical.67 For example, Cambridge University have developed the Cytosponge which can be used in the office setting without sedation and then the retrieved esophageal epithelial cells are assessed in a diagnostic assay for BE which detects Trefoil Factor 3 (TFF3).68 This technique has a sensitivity of 80–90% depending on the length of the Barrett’s segment with a specificity of around 92%.69 It has also recently been shown to be able to identify lesions affecting the p53 gene, as an indicator of high grade dysplasia, with sensitivity of 86% and specificity of 100% and further clinical trials are ongoing. Such molecular tests might also detect early cancers that have not necessarily arisen in BE. 70,71 These emerging tests are still experimental but to the extent that tissue-based markers (e.g., cell surface or somatic genetic markers) from the capsule sponge or other non-invasive techniques, in concert with other risk factors,72–74 can be demonstrated to differentiate the minority with substantially increased risk (again, ten-fold higher is used as the example in Table 1) who can be referred to secondary care, from the majority whose absolute risk remains low, then a substantial improvement in clinical practice would be achieved.75 For those who remain in stratum 3, chemopreventatives (e.g., aspirin, statins) might be considered as well as stronger encouragement to modify lifestyle (e.g., smoking cessation, improved diet, weight loss, increased physical activity.)44,46,76–81
Those calculated to be at higher risk based all of the previously collected risk factor information would then be considered to have entered stratum 4 under care of a secondary care provider. This group would be much smaller in size than the number of persons with recurrent GERD symptoms in the general population (1.5M vs. 30M), but have a much higher average risk. Here, an absolute risk of 700/100,000 (0.7% per year) (for example) would likely prompt an endoscopy with multiple patterned biopsies as well as biopsies directed at visible abnormalities. Information based on endoscopy and biopsy, such as somatic genetic abnormalities70,74,82 and histologic findings83 would then define whether the patient could be referred back to stratum 3, entered into surveillance (remaining in stratum 4) and perhaps or be triaged to the highest risk stratum.71
Persons entering stratum 5 (tertiary care provider) would have an average absolute risk of developing esophageal adenocarcinoma of 7/100 (7%) per year, based on the example in Table 1. Surgical or endoscopic interventions proven to lower risk of mortality from esophageal adenocarcinoma could be offered to this group, who could then be returned to stratum 4 for continuing surveillance.
Conclusions
Efforts over the past several decades directed towards primary prevention and early diagnosis of esophageal adenocarcinoma have had very little impact at the population level in curtailing its rapidly increasing morbidity and mortality. Fortunately, much has been learned from epidemiologic and clinical studies during this period regarding etiologic and other predictive risk factors (e.g., lifestyle, demographics, anthropometry, genetic, and tissue-based biomarkers), while new techniques and devices are being developed which offer promising opportunities for integrating what is now known about the development of the cancer into a scientifically sound clinical practice protocol.
Such a protocol would be designed to start with a wide population base, including many who are ignored by current practices, and triage individuals into progressively higher risk strata, each with risk-appropriate prevention, screening and treatment options. Risk assessment methods would also be stratum-specific. At the lowest strata there would be minimal intervention and simple, cost-effective tools, whereas in the higher risk groups clinicians would make use of increasingly precise, but also increasingly invasive and expensive tools. Hence, the careful selection of tests and prevention activities at different stages should be favorable economically, in contrast to the current scenario of enrolling more and more patients into endoscopic surveillance with little regard to their absolute risk, and therefore little population benefit. Some of the technologies mentioned are still in research and development, and are not yet available for widespread adoption. In addition, development and validation of risk prediction algorithms for the middle three strata are important aspects of this approach. Such efforts have already begun, although they have been based on limited data and settings;29,84 thus a good deal of development and validation work in this area remains, but the potential reward is high.
Acknowledgments
TV was supported in part by National Cancer Institute Established Investigator Award in Cancer Prevention and Control (K05 CA124911).
References
- 1.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–62. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 3.Steevens J, Botterweck AAM, Dirx MJM, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. J Gastroenterol. 2010;22:669–678. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; SEER*Stat Database: Incidence - SEER 9 Regs Research Data, (with SEER Delay Factors) Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties. ( www.seer.cancer.gov) released April 2014, based on the November 2013 submission. at < http://seer.cancer.gov/data/seerstat/nov2013/>. [Google Scholar]
- 5.Oesophageal cancer incidence statistics. Cancer Research; UK: at < http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus/incidence/>. [Google Scholar]
- 6.Dubecz A, et al. Temporal Trends in Long-Term Survival and Cure Rates in Esophageal Cancer: A SEER Database Analysis. J Thorac Oncol Febr 2012. 2012;7:443–447. doi: 10.1097/JTO.0b013e3182397751. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 8.Wassenaar EB, Oelschlager BK. Effect of medical and surgical treatment of Barrett’s metaplasia. 2010;16:3773–3779. doi: 10.3748/wjg.v16.i30.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonka Z, et al. The effects of laparoscopic Nissen fundoplication on Barrett’s esophagus: long-term results. Scand J Gastroenterol. 2012;47:13–21. doi: 10.3109/00365521.2011.639081. [DOI] [PubMed] [Google Scholar]
- 10.Zaninotto G, et al. Long-term follow-up of Barrett’s epithelium: medical versus antireflux surgical therapy. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2012;16:7–14. doi: 10.1007/s11605-011-1739-8. discussion 14–15. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald RC, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 12.Phoa KN, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA J Am Med Assoc. 2014;311:1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JH, Kwon RS. Radiofrequency Ablation for Barrett’s Esophagus With Low-Grade Dysplasia: A Hammer Looking for a Nail. Gastroenterology. 2014;147:706–707. doi: 10.1053/j.gastro.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Yang PC, Davis S. Incidence of cancer of the esophagus in the US by histologic type. Cancer. 1988;61:612–617. doi: 10.1002/1097-0142(19880201)61:3<612::aid-cncr2820610332>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Lao-Sirieix P, et al. Physiological and molecular analysis of acid loading mechanisms in squamous and columnar-lined esophagus. Dis Esophagus. 2008;21:529–538. doi: 10.1111/j.1442-2050.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 16.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamoyski A. Moscow 1812: Napoleon’s Fatal March. Harper Collins; 2004. [Google Scholar]
- 18.Peery AF, et al. Burden of Gastrointestinal Disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer of the Esophagus - SEER Stat Fact Sheets. 2014 at < http://seer.cancer.gov/statfacts/html/esoph.html>.
- 20.Division, U. C. B. A. & Services, C. US Census Bureau Publications - Census of Population and Housing. at < http://www.census.gov/prod/www/decennial.html>.
- 21.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 22.Farrow DC, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–8. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 23.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruigómez a, et al. Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharmacol Ther. 2004;20:751–60. doi: 10.1111/j.1365-2036.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222–7. doi: 10.1111/j.1365-2036.2010.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook MB, et al. Gastroesophageal Reflux in Relation to Adenocarcinomas of the Esophagus: A Pooled Analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON) PLoS ONE. 2014;9:e103508. doi: 10.1371/journal.pone.0103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaheen NJN, et al. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2012;157:808–816. doi: 10.7326/0003-4819-157-11-201212040-00008. [DOI] [PubMed] [Google Scholar]
- 28.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–11. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein JH, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108:353–62. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex D, Cummings O, Shaw M. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramanian G, et al. Prevalence and predictors of columnar lined esophagus in gastroesophageal reflux disease (GERD) patients undergoing upper endoscopy. Am J Gastroenterol. 2012;107:1655–61. doi: 10.1038/ajg.2012.299. [DOI] [PubMed] [Google Scholar]
- 32.Desai TK, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 33.Esserman LJ, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15:e234–e242. doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat SK, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2014 doi: 10.1136/gutjnl-2013-305506. gutjnl–2013–305506. [DOI] [PubMed] [Google Scholar]
- 35.Corley DA, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–9.e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbeek RE, et al. Surveillance of Barrett’s Esophagus and Mortality from Esophageal Adenocarcinoma: A Population-Based Cohort Study. Am J Gastroenterol. 2014;109:1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 37.Whiteman DC. Does a prior diagnosis of Barrett’s oesophagus influence risk of dying from oesophageal adenocarcinoma? Gut. 2014:1–3. doi: 10.1136/gutjnl-2014-307171. [DOI] [PubMed] [Google Scholar]
- 38.Kong CY, et al. Exploring the Recent Trend in Esophageal Adenocarcinoma Incidence and Mortality Using Comparative Simulation Modeling. Cancer Epidemiol Biomarkers Prev. 2014;23:997–1006. doi: 10.1158/1055-9965.EPI-13-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook MB, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–53. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook MB, et al. Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744–753. doi: 10.1053/j.gastro.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyo C, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706–18. doi: 10.1093/ije/dys176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardikar S, et al. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PloS One. 2013;8:e52192. doi: 10.1371/journal.pone.0052192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubo A, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–91. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao LM, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–452.e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands cohort study. Int J Cancer J Int Cancer. 2011 doi: 10.1002/ijc.25928. [DOI] [PubMed] [Google Scholar]
- 46.Li W-Q, et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11:1130–1136.e2. doi: 10.1016/j.cgh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kastelein F, et al. Surveillance in patients with long-segment Barrett’s oesophagus: a cost-effectiveness analysis. Gut. 2014 doi: 10.1136/gutjnl-2014-307197. gutjnl–2014–307197. [DOI] [PubMed] [Google Scholar]
- 48.Olsen CM, Pandeya N, Green AC, Webb PM, Whiteman DC. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am J Epidemiol. 2011;174:582–590. doi: 10.1093/aje/kwr117. [DOI] [PubMed] [Google Scholar]
- 49.Engel LS, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 50.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thrift AP, Whiteman DC. Can we really predict risk of cancer? Cancer Epidemiol. 2013;37:349–52. doi: 10.1016/j.canep.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Rubenstein JH, et al. Influence of Malpractice History on the Practice of Screening and Surveillance for Barrett’s Esophagus. Am J Gastroenterol. 2008;103:842–849. doi: 10.1111/j.1572-0241.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- 53.Sun X, Chandar AK, Elston R, Chak A. What We Know and What We Need to Know About Familial Gastroesophageal Reflux Disease and Barrett’s Esophagus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2014:5–7. doi: 10.1016/j.cgh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Duggan C, et al. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11:934–43. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardikar S, et al. Inflammation and oxidative stress markers and esophageal adenocarcinoma incidence in a Barrett’s esophagus cohort. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-14-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook MB, et al. Association Between Circulating Levels of Sex Steroid Hormones and Barrett’s Esophagus in Men: a Case–Control Analysis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, et al. Esophageal Cancer Metabolite Biomarkers Detected by LC-MS and NMR Methods. PLoS ONE. 2012;7:e30181. doi: 10.1371/journal.pone.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, et al. Gastro-Esophageal Reflux Disease Symptoms and Demographic Factors as a Pre-Screening Tool for Barrett’s Esophagus. PloS One. 2014;9:e94163. doi: 10.1371/journal.pone.0094163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ek WE, et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett’s esophagus, and gastroesophageal reflux. J Natl Cancer Inst. 2013;105:1711–8. doi: 10.1093/jnci/djt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su Z, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett’s esophagus. Nat Genet. 2012;44:1131–6. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine DM, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nat Genet. 2013;45:1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buas MF, et al. Integrative post genome-wide association analysis of CDKN2A and TP53 SNPs and risk of esophageal adenocarcinoma. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palles C, et al. Polymorphisms Near TBX5 and GDF7 are Associated with Increased Risk for Barrett’s Esophagus. Gastroenterology. doi: 10.1053/j.gastro.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shariff MK, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc. 2012;75:954–961. doi: 10.1016/j.gie.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 65.Peery AF, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video) Gastrointest Endosc. 2012;75:945–953.e2. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gora MJ, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19:238–240. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varghese S, Lao-Sirieix P, Fitzgerald RC. Identification and clinical implementation of biomarkers for Barrett’s esophagus. Gastroenterology. 2012;142:435–441.e2. doi: 10.1053/j.gastro.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Kadri SR, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross-Innes C, et al. Evaluation of a minimally-invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s Esophagus: a multi-center case-control study. PLoS Med. 2015 doi: 10.1371/journal.pmed.1001780. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver JMJ, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet. 2014;46:837–843. doi: 10.1038/ng.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaughan TL. From genomics to diagnostics of esophageal adenocarcinoma. Nat Genet. 2014;46:806–807. doi: 10.1038/ng.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coleman HG, et al. Symptoms and endoscopic features at barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol. 2014;109:527–34. doi: 10.1038/ajg.2014.10. [DOI] [PubMed] [Google Scholar]
- 73.Gregson EM, Fitzgerald RC. Biomarkers for Dysplastic Barrett’s: Ready for Prime Time? World J Surg. 2014:1–10. doi: 10.1007/s00268-014-2640-x. [DOI] [PubMed] [Google Scholar]
- 74.Li X, et al. Temporal and Spatial Evolution of Somatic Chromosomal Alterations: A Case-Cohort Study of Barrett’s Esophagus. Cancer Prev Res (Phila Pa) 2014;7:114–127. doi: 10.1158/1940-6207.CAPR-13-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weaver JMJ, Ross-Innes CS, Fitzgerald RC. The ‘-omics’ revolution and oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:19–27. doi: 10.1038/nrgastro.2013.150. [DOI] [PubMed] [Google Scholar]
- 76.Vaughan T, Dong L, Blount P, Ayub K. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol. 2005;6:945–52. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 77.Kastelein F, et al. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology. 2011;141:2000–8. doi: 10.1053/j.gastro.2011.08.036. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 78.Singh S, Devanna S, Edakkanambeth Varayil J, Murad M, Iyer PG. Physical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2014;14:101. doi: 10.1186/1471-230X-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kantor ED, Onstad L, Blount PL, Reid BJ, Vaughan TL. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett’s esophagus. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21:456–61. doi: 10.1158/1055-9965.EPI-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharp L, Carsin AE, Cantwell MM, Anderson LA, Murray LJ. Intakes of Dietary Folate and Other B Vitamins Are Associated with Risks of Esophageal Adenocarcinoma, Barrett’s Esophagus, and Reflux Esophagitis. J Nutr. 2013;143:1966–1973. doi: 10.3945/jn.113.174664. [DOI] [PubMed] [Google Scholar]
- 81.Akiyama J, et al. Strategy for prevention of cancers of the esophagus. Ann N Y Acad Sci. 2014;1325:108–126. doi: 10.1111/nyas.12529. [DOI] [PubMed] [Google Scholar]
- 82.Dulak AM, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anaparthy R, Sharma P. Progression of Barrett oesophagus: role of endoscopic and histological predictors. Nat Rev Gastroenterol Hepatol. 2014;11:525–534. doi: 10.1038/nrgastro.2014.69. [DOI] [PubMed] [Google Scholar]
- 84.Thrift AP, Kendall BJ, Pandeya N, Whiteman DC. A Model to Determine Absolute Risk for Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:138–144.e2. doi: 10.1016/j.cgh.2012.10.026. [DOI] [PubMed] [Google Scholar]