Abstract
The prevalence of major depressive disorder and the limited efficacy of conventional drug treatments provide significant impetus to develop novel and more rapidly acting antidepressants for individuals with treatment resistant forms of depression. The primary goal of these studies was to ascertain whether buprenorphine (BPN), a medically available drug with mixed effects at opioid receptors, was effective in behavioral tests using the Wistar Kyoto (WKY) rat strain, a rodent model of exaggerated depressive and anxiety behaviors that demonstrates resistance to certain antidepressants. As WKY rats are maintained by different sources, we assessed the behavioral effects of BPN using the modified rat forced swim test (FST) and the emergence test in WKY rat colonies obtained from different vendors. BPN dose-dependently reduced immobility and increased swimming behavior in the FST and reduced emergence latencies in two WKY lines (Charles River (WKY/NCrl) and Harlan laboratories (WKY/NHsd)) that also showed high baseline immobility in the FST. WKY rats from Taconic (WKY/NTac) did not show high baseline immobility in the FST or anxiety as had been previously reported, suggesting drift in the phenotype of rats from this supplier. Furthermore, BPN did not reduce immobility in the FST or reduce latencies in the emergence test in WKY rats from Taconic. BPN also failed to produce antidepressant-like effects in Wistar and Sprague-Dawley rats. These results indicate a striking strain-selectivity for the effects of BPN, producing antidepressant and anxiolytic-like responses in WKY/NCrl and WKY/NHsd lines but not in the normosensitive control Wistar and Sprague-Dawley strains.
Keywords: Wistar Kyoto rats, buprenorphine, treatment-resistant depression, FST, emergence test
1. Introduction
Major depressive disorder (MDD) is a debilitating psychiatric disorder with a lifetime prevalence of ~ 17% in the United States [1]. Despite the wide range of therapies available to treat MDD, there are significant limitations associated with conventional antidepressants, including a delay in therapeutic efficacy of 3–4 weeks and successful remission is achieved in only 40–60 % of patients [2]. Those that fail to respond to two or more antidepressant treatments are considered to have a form of treatment resistant depression (TRD) [3]. Individuals with TRD complain of suicidal ideation and comorbid anxiety more frequently than other MDD patients [4] and generate a significantly greater economic burden due to higher medical costs due to there resistance to therapy [5]. Therefore, there is a pressing social, economic and medical need to develop novel antidepressants for the treatment of MDD.
Appropriate rodent models of depression are necessary to adequately evaluate the antidepressant potential and mechanism of action of novel therapeutics for MDD. One such model is the Wistar-Kyoto (WKY) rat strain. Originally developed as the normotensive control for the spontaneously hypertensive rat (SHR), WKY rats have consistently exhibited increased depressive-like behavior in the forced swim test (FST) and rapid development of learned helplessness [6–9]. WKY rats also displayed increased anxiety-like behavior in many behavioral tests, including the conditioned defensive burying test, open field, elevated plus maze and the novelty-induced hypophagia (NIH) test [9–14]. Furthermore, increased physiological responses to stress, as shown by prolonged activation of the hypothalamic–pituitary–adrenal (HPA) axis [15, 16] and increased development of stress-induced ulcers [17], has been reported in WKY rats. Additionally, WKY rats recapitulate resistance to the suppression of corticosterone by dexamethasone [15] and abnormalities in sleep architecture [18], characteristics commonly observed in patients with severe depression. WKY rats fail to exhibit behavioral responses following acute and chronic treatment with the most commonly prescribed class of antidepressants, selective serotonin reuptake inhibitors (SSRIs) [8, 19, 20], a trait shared by certain cohorts of treatment resistant MDD patients. Similarly, WKY rats did not exhibit behavioral responses to 5-HT1A receptor agonists and environmental enrichment in tests for behavioral domains relevant to depression and anxiety [8, 21, 22], These traits mark WKY rats as a genetic and pathological model of depression and anxiety [23].
Emerging evidence suggests that opioid receptors, particularly kappa (κ-ORs) and their endogenous κ-OR ligand dynorphin (DYN), may play a key role in the etiology of anxiety and depression [24, 25]. The κ-OR/DYN system is critical in the production of stress-induced aversion; this system is significantly upregulated by the release of corticotrophin-releasing factor following stress exposure [26]. Increased κ-OR/DYN signaling has been shown to induce depressive-like behavior, dysphoria and increased drug seeking in rodents [27–30]. Furthermore, WKY rats exhibit increased κ-OR expression in the locus coeruleus, piriform cortex and nucleus accumbens compared to Sprague-Dawley rats [14, 31]. Although not consistently apparent in non-stressed rodents, our laboratory has shown that the κ-OR antagonists, nor-BNI and DIPPA, effectively reduced immobility and increase swimming behavior in the FST in WKY rats [14, 32]. Critically these antidepressant-like effects of κ-OR antagonists persisted for 24 h after a single injection, a time frame longer than conventional antidepressants. Furthermore, these κ-OR antagonists effectively reduced anxiety-like behavior, as measured by a lower latency to approach and eat food in the NIH test and reduced defensive burying behavior in rats [14]. The κ-OR antagonists produced their effects more rapidly than conventional antidepressants, which require chronic administration for weeks to reduce approach latencies in the NIH test [33, 34].
Buprenorphine (BPN) is a relatively short-acting κ-OR antagonist [35] that is medically available and currently used to treat opiate addiction and chronic pain. The first suggestion that BPN may be of benefit in treating depression was in the early 1980’s [36]. Subsequently, two small studies conducted in TRD patients, indicated that BPN rapidly reduced depressive symptoms [37, 38]. Recent clinical evidence has outlined a significant clinical benefit of BPN in TRD patients following chronic administration at low doses [39]. Recently, our lab has shown that BPN reduced immobility time in the FST and reduced anxiety-like behavior in the novelty-induced hyponeophagia test in naïve mice [40]. The purpose of these studies was to examine the potential behavioral effects of BPN in a rodent model of pathological anxiety and depression, namely the WKY rat. As WKY rats can be sourced from a number of suppliers, we compared baseline depressive-like and anxiety-like behavior and the response to BPN in WKY rats obtained from Taconic (WKY/NTac), Charles River (WKY/NCrl) and Harlan laboratories (WKY/NHsd). Following these studies, we examined BPN-induced antidepressant and anxiolytic-like effects in Wistar and Sprague-Dawley rats as non-stressed control strains. The results highlight the potential of BPN as a novel antidepressant in a model of depression in which conventional therapies have previously failed, but also indicated a striking disparity between rat strains that were sensitive to the behavioral effects of BPN.
2. Materials and methods
2.1. Animals
Male WKY rats were obtained at age 6–7 weeks from three different sources, Taconic, (WKY/NTac, Cambridge City Facility, IN), Harlan laboratories (WKY/NHsd, Indianapolis, IN), and Charles River Laboratories (WKY/NCrl, Kingston, NY). Male Wistar (Crl:WI, Charles River Laboratories, Raleigh, NC) and Sprague-Dawley rats (Crl:SD, Charles River Laboratories, Kingston NY) were obtained at age 7 weeks. Rats were housed 3 per cage upon arrival and allowed at least 1 week to acclimate to the facility. All animals were maintained under a 12-h light cycle (lights on at 07:00 h) with room temperature of 22 ± 1 °C and food and water were provided ad libitum. All procedures were carried out in accordance the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Drug Preparation
Buprenorphine (BPN) hydrochloride was obtained from the National Institute on Drug Abuse and was freshly prepared on the morning of each experimental day. The compound was dissolved in sterile molecular grade deionized water and administered at a volume of 2 ml/kg subcutaneously. Doses were calculated according to the base weight. Vehicle groups received distilled water in an equivalent volume. All experiments were conducted during the light phase.
2.3. Study Design
2.3.1. Experiment 1
This experiment compared behavioral responses of WKY rats obtained from different suppliers. At the time of testing, all rats were 8 weeks old. WKY/NTac weighed approximately 280 g, WKY/NHsd weighed approximately 215 g, and WKY/NCrl rats weighed approximately 220 g. Two weight-matched groups of WKY rats per substrain were assigned to the vehicle (N=12) or 2.25 mg/kg BPN (N=12) condition. This dose was selected based on pilot data. All rats were transported to the experimental room 1 hour prior to testing. The effects of BPN were measured 24 h following injection because of previous reports that the behavioral effects of BPN and KOR antagonists are long-lasting [32, 40]. The emergence test was studied first. After a rest period of one week, the effects of BPN were determined in the FST.
2.3.2. Experiment 2
This experiment examined the effects of different doses of BPN in the FST, emergence test in WKY/NCrl and in two control strains, Wistar rats and Sprague-Dawley rats. At the time of testing all rats were 8 weeks old, WKY/NCrl weighed approximately 220 g; Crl:WI weighted approximately 275 g, and Crl:SD rats weighed approximately 265 g. Four sets of male WKY/NCrl rats (N=32), male Crl:WI (N=32) and Crl:SD rats (N=24) were used in order to study all of the strains simultaneously. The groups sizes were: N=8 for all WKY/NCrl groups, except for the FST vehicle group (n=7); N=8 for all Crl:WI groups; and N=6 for Crl:SD rats. All rats were transported to the experimental room 1 hour prior to testing sessions. First, rats were assessed in the emergence test 24 h following treatment. After a rest period of one week, rats were exposed to the FST and their behavior was evaluated. Locomotor activity was conducted to determine whether BPN induced hyperactivity 24 h post injection that could produce a false positive in the FST.
2.4. Emergence Test
The emergence test used here was based on earlier studies of emergence latency [41] and used a protocol similar to the home cage emergence test [42]. The premise for this task relies on the tendency of rats to remain within an enclosure when placed in a brightly lit open arena. The latency to emerge into the brightly lit arena from a red-tinted polycarbonate tunnel placed in the center of an open arena was assessed over three consecutive days following a single injection of BPN or vehicle 24 h prior to the first exposure. Rats were provided with a similar tunnel in their home cages on the first day of arrival in the facility, to ensure that each rat was familiar with the tunnels used on the test day. The emergence latency was scored only when the rat fully exited from the tunnel with four paws placed clearly in the arena. If the rat did not leave the tunnel within 300 s, the trial was ended and the rat was assigned a latency score of 300. Data is represented as an average latency over the three-day trial.
2.5. Forced Swim Test
The modified rat FST is based on a protocol previously published [43]. Rats were exposed to two swim sessions. The first session was a 15 min pretest session where rats were placed into a glass cylinder (20 cm diameter x 30 cm depth) filled with water (25°C ± 1°C) for 15 min. The second session was a 5 min test session conducted 24.5 h after the first session and 24 h after the injection. Rats were dried and returned to their home cage after each session. Both the 15-min pretest and 5-min test was recorded and scored for the frequency of immobility, swimming and climbing behaviors using a time-sampling method. A high inter-rater correlation (r = 0.905) was observed between the two raters for this test. Immobility was defined as the minimum movement required for rats to maintain their head above water in the absence of the other two active behaviors. Swimming was defined as horizontal movement throughout the cylinder. Climbing was defined as upward-directed movement of the forepaws aimed toward the sides of the cylinder.
2.6. Locomotor Activity
Locomotor activity was measured in a Plexiglas arena (L 46 cm x W 38 cm x H 38 cm) under a red light. Total distance traveled (cm) during the 15 min testing period was measured using the SMART video tracking system (San Diego Instruments, San Diego, CA).
2.7. Statistical Analysis
All statistical analyses and graphical representations were conducted using Graph Pad Prism 6. Overall two-way analysis of variance (ANOVA) were used to determine treatment*supplier interactions in experiment 1 and treatment*strain interactions in experiment 2 on behavioral performance. Pair-wise comparisons were conducted using Bonferroni tests. One-way ANOVA with Bonferroni posttest or Kruskal-Wallis with pairwise comparison of ranks were used to determine significant differences in the behaviors exhibited by the WKY substrains in the 15 min forced swim pretest. One-way ANOVA with Holm-Sidak multiple comparisons tests were used to compare FST behaviors between WKY/NTac rats studied currently in 2013 with cohorts from 2007 and 2010. Because of the divergent variances across strains in the FST of experiment 2, Dunnett’s or Holm-Sidak multiple comparisons test following overall ANOVA were used to compare the effect of individual doses with the vehicle control for each individual strain. A p value < 0.05 was deemed to be significant.
3. RESULTS
3.1. Experiment 1
3.1.1. Effect of WKY substrains in the emergence test
The WKY substrains differed in their baseline emergence latencies and in their response to BPN (see Fig. 1) as shown by a significant treatment*substrain interaction (F2,63=34.42, p=0.016) and significant main effect of treatment (F1,63=24.26, p<0.001) and substrain (F2,63=37.67, p<0.001). Post-hoc tests revealed that BPN significantly reduced the latency to emerge of WKY/NCrl (p<0.01) and WKY/NHsd (p<0.001) rats. However, BPN was ineffective in WKY/NTac rats, which exhibited significantly lower emergence latencies compared to the other substrains.
Fig 1.
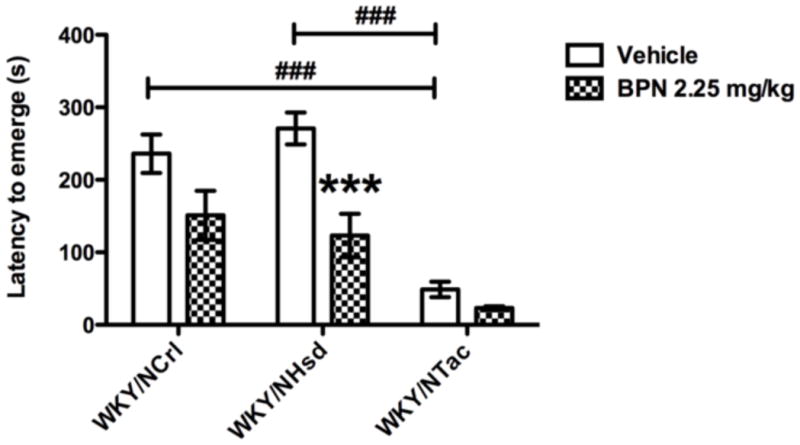
Performance in the emergence test compared between WKY substrains, WKY/NCrl, WKY/NHsd and WKY/NTac. WKY/NTac rats exhibited a low level of anxiety in this task; their latency to emerge was significantly lower than WKY/NCrl and WKY/NHsd rats. The symbol ### indicates p<0.001, where a significant difference exists between the WKY/NCrl or WKY/NHsd and the WKY/NTac vehicle groups. BPN significantly decreased the time to emerge 24 h post treatment in WKY/NCrl and WKY/NHsd rats. The symbol ** indicates p<0.01, where a significant effect of treatment was observed in WKY/NCrl rats. The symbol *** indicates p<0.001, where a significant effect of treatment was observed in WKY/NHsd rats.
3.1.2. Effect of WKY substrains in the forced swim pretest
Immobility, swimming and climbing were assessed per 5-minute segment of the 15-minute pretest session (Table 1). The WKY substrains exhibited significant differences in their behavior during the 15-minute pretest. A significant effect of strain was observed for immobility in the 1st (F2,27 =8.320, p=0.002), 2nd (F2,27 =14.43, p=0.001) and 3rd 5-minute segment of the test (F2,27 =11.85, p<0.001). Both WKY/NHsd (p<0.05) and WKY/NTac rats (p<0.05) exhibited lower levels of immobility compared to the WKY/NCrl strain in the 1st 5-minute segment. In the 2nd segment, WKY/NHsd (p<0.001) and WKY/NTac rats (p<0.001) continued to show less immobility than the WKY/NCrl substrain. During the final segment of the pretest, WKY/NHsd (p<0.01) and WKY/NTac rats (p<0.001) also exhibited less immobility than the WKY/NCrl substrain.
Table 1.
Pretest scores (mean ± SEM) for the WKY substrains, WKY/NCrl, WKY/NHsd and WKY/NTac.
| Segment | Behavior | WKY/NTac | WKY/NHsd | WKY/NCrl |
|---|---|---|---|---|
| 5 min | Immobility | 13.40 ± 3.63 | 13.60 ± 2.29 | 29.90* # ± 3.73 |
| Swimming | 28.50# ± 3.77 | 40.70 ± 2.89 | 27.20# ± 3.35 | |
| Climbing | 17.10 ± 3.82 | 5.50* ± 2.61 | 2.90** # ± 1.09 | |
| 10 min | Immobility | 15.10 ± 3.92 | 17.60 ± 2.77 | 39.30*** ### ± 3.72 |
| Swimming | 35.80 ± 3.87 | 40.50 ± 2.73 | 20.60* ## ± 3.70 | |
| Climbing | 9.10 ± 4.48 | 1.90* ± 1.48 | 0.00* ± 0.00 | |
| 15 min | Immobility | 17.40 ± 4.29 | 22.40 ± 3.53 | 41.50*** ## ± 3.17 |
| Swimming | 35.30 ± 4.34 | 36.10 ± 3.49 | 18.50* ± 3.17 | |
| Climbing | 7.30 ± 3.65 | 1.50* ± 0.92 | 0.00* ± 0.00 |
The symbol * indicates p<0.05, ** indicates p<0.01 and *** indicates p<0.001, where a significant strain difference exists between the WKY/NCrl or WKY/NHsd and the WKY/NTac substrain.
The symbol # indicates p<0.05, ## indicates p<0.01 and ### indicates p<0.001, where a significant difference in behavior was observed between WKY/NCrl or WKY/NTac and WKY/NHsd rats.
A significant effect of strain on swimming behavior was observed during the 1st (F2,27=4.926, p=0.015) 2nd (F2,27 =8.998, p=0.001) and the 3rd 5-minute segment of the test (F2,27 =7.217, p=0.003) of the test. WKY/NHsd rats exhibited more bouts of swimming during the 1st 5-minute segment of the test compared to both WKY/NTac (p<0.05) and WKY/NCrl rats (p<0.05). During the 2nd segment of the pretest, WKY/NTac (p<0.05) and WKY/NHsd rats (p<0.01) spent significantly more time swimming than WKY/NCrl rats. This pattern continued in the final segment of the test, where WKY/NTac (p<0.05) and WKY/NHsd rats (p<0.01) spent significantly more time swimming than WKY/NCrl rats Significant strain differences were observed for climbing behavior during 1st (H=10.06, p=0.006) 2nd (H=7.095, p=0.023) and the 3rd 5-minute segment of the pretest (H=6.570, p=0.004). WKY/NCrl rats did not exhibit climbing behavior during any of the segment. Furthermore, WKY/NTac rats exhibited more bouts of climbing behavior compared to the WKY/NCrl in all three segments (p<0.05). Similarly, the WKY/NTac substrain exhibited more climbing that the WKY/NHsd strain in the 1st segment of the pre-test during (p<0.01).
3.1.3. Effect of WKY substrains in the FST
The WKY substrains differed significantly in baseline behavior in the FST for all categories and responded distinctively to BPN. A significant treatment*substrain interaction (F2,65=3.93, p=0.024) and significant main effect of treatment (F1,65=20.88, p<0.001) and strain (F2,65=26.99, p<0.001) were measured for immobility in the FST (see Fig. 2a). WKY/NTac rats were less immobile compared to WKY/NCrl (p<0.001) and WKY/NHsd rats (p<0.001) following vehicle treatment. BPN was effective in reducing immobility levels of WKY/NCrl (p<0.05) and WKY/NHsd (p<0.001) rats but had no effect in the WKY/NTac substrain.
Fig 2.
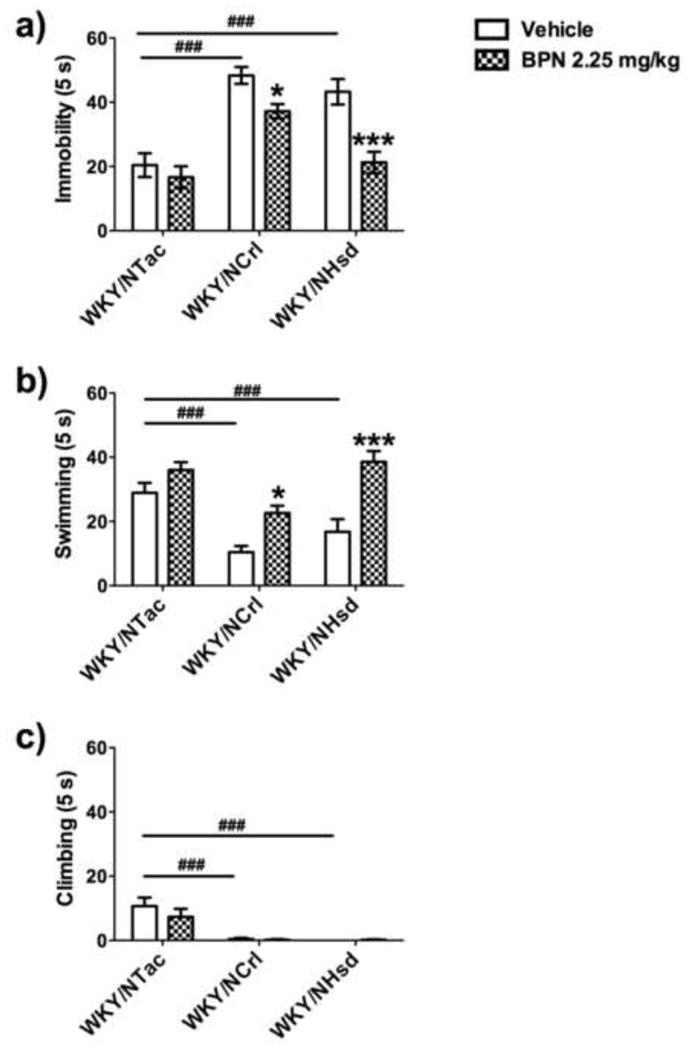
Comparison of behaviors in the FST between WKY substrains, WKY/NCrl, WKY/NHsd and WKY/NTac. A significant difference in baseline behavior was observed between the WKY/NTac and the other WKY strains. The symbol ### indicates p<0.001, where a significant difference exists between the WKY/NCrl or WKY/NHsd and the WKY/NTac vehicle groups. BPN significantly decreased immobility and increased swimming behavior 24 h post treatment. The symbol * indicates p<0.05, where a significant effect of treatment was observed in WKY/NCrl rats. The symbol *** indicates p<0.001, where a significant effect of treatment was observed in WKY/NHsd rats.
For swimming behavior (see Fig. 2b), ANOVA demonstrated a significant treatment*substrain interaction (F2,65=3.25, p=0.045) and significant main effect for treatment (F1,65=32.23, p<0.001) and substrain (F2,65=15.09, p<0.001). Post-hoc tests indicated that vehicle treated WKY/NTac rats exhibited higher levels of swimming compared to vehicle treated WKY/NCrl (p<0.001) and WKY/NHsd (p<0.001) rats. BPN increased swimming behavior in WKY/NCrl (p<0.05) and WKY/NHsd (p<0.001) rats but had no effect in WKY/NTac rats.
No treatment*substrain interaction or treatment effect was observed for climbing behavior. However, the WKY substrains differed significantly in overall frequency of climbing behavior (F2,65=22.28, p<0.001), as shown in Fig. 2c. Post-hoc tests determined that WKY/NTac rats exhibited more climbing behavior compared to WKY/NCrl (p<0.001) and WKY/NHsd rats (p<0.001).
3.2. Body weight gain in WKY substrains
Significant substrain differences were observed for body weight gain at age 7 weeks, (F4,615=472.3, p<0.001). WKY/NCrl (p<0.001) and WKY/NHsd (p<0.001) and Crl:WI rats (p<0.001) exhibited substantially lower weights than their age-matched WKY/NTac counterparts. A similar body weight gain was observed for Crl:SD and WKY/NTac at this age (Table 2).
Table 2.
Daily body weights (mean ± SEM) of WKY/NTac, WKY/NCrl, WKY/NHsd, Crl:SD and Crl:WI rats at age 7 weeks.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
|
WKY/NTac Average weight |
255.96 ± 5.43 | 258.17 ± 5.22 | 270.38 ± 5.32 | 279.42 ± 5.27 | 283.33 ± 5.18 |
|
WKY/NCrl Average weight |
205.16*** ± 1.95 | 209.84*** ± 1.96 | 210.04*** ± 1.82 | 215.72*** ± 2.09 | 221.76*** ± 2.12 |
|
WKY/NHsd Average weight |
199.52*** ± 1.54 | 203.64*** ± 1.52 | 210.32*** ± 1.61 | 213.40*** ± 1.68 | 216.56*** ± 1.75 |
|
Crl:WI Average weight |
238.88*** ± 1.73 | 246.79*** ± 2.13 | 256.29*** ± 2.50 | 264.08*** ± 2.68 | 274.13*** ± 2.93 |
|
Crl:SD Average weight |
234.94 ± 2.00 | 242.38 ± 1.83 | 250.50 ± 2.00 | 258.09 ± 2.15 | 265.72 ± 2.35 |
The symbol ### indicates p<0.001, where a significant difference in body weight existed compared to age-matched WKY/NTac rats.
3.3. Phenotypic shift in FST behaviors of WKY/NTac rats
FST performance of WKT/NTac rats in this study differed from their behavior analyzed by this laboratory and described in an earlier publication [32]. Therefore, original videotapes of WKY/NTac cohorts from 2007, 2010 and the current group tested in 2013 were rescored for direct comparison by the same rater (Figure 3). Statistically significant differences between these cohorts were obtained for all three behaviors: immobility (F2,29 =16.04, p<0.001; Fig. 3a), swimming (F2,29 =7.56, p=0.002; Fig. 3b) and climbing (F2,29 =5.11, p=0.013; Fig. 2c). Overall, WKY/NTac rats exhibited reduced bouts of immobility in the 2013 cohort compared to the 2007 (p<0.01) and the 2010 cohort (p<0.001), with no differences observed between the 2007 and 2010 WKY/NTac cohorts. Swimming behavior was significantly higher in the 2013 cohort compared to the 2010 cohort, but did not reach statistical significance compared to the 2007 cohort. Dramatic increases in climbing behavior were observed in the 2013 cohort compared to both the 2007 (p<0.05) and 2010 cohorts (p<0.05), which exhibited virtually no climbing behavior.
Fig 3.
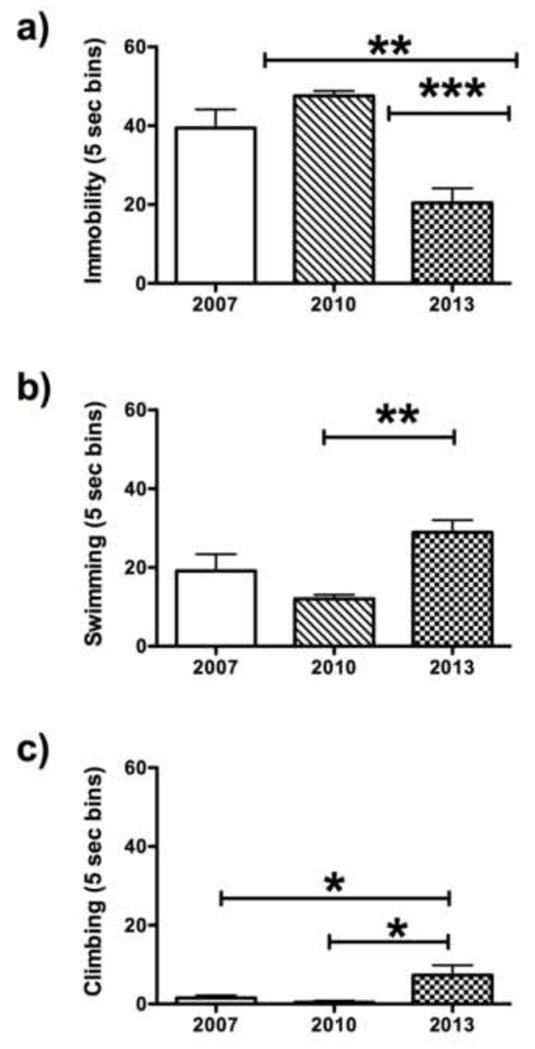
Comparison of FST performance from videotapes for a cohort of WKY/NTac rats tested in 2007, 2010 and 2013 under identical conditions. The WKY/NTac rats tested in 2013 exhibited a significant decrease in immobility and increased climbing behavior compared to WKY/NTac rats tested in 2007 and 2010 [32]. The symbol *** indicates p<0.001, and the symbol ** indicates p<0.01, and the symbol * indicates p<0.05, where a significant difference is observed between either the 2007 and 2010 cohort and WKY/NTac rats tested in 2013normo.
3.4. Experiment 2
3.4.1. Strain differences in the effects of BPN on the emergence test
The WKY/NCrl, Crl:SD and Crl:WI rats differed significantly in their baseline emergence latencies but only WKY/NCrl rats responded to BPN (Fig. 4). A significant treatment*strain interaction (F2,74=2.60, p<0.024) and a significant main effect of treatment (F2,74=3.66, p<0.0181) and strain (F2,74=254.90, p<0.001) were observed for emergence latency. Post-hoc tests indicated that BPN significantly reduced the emergence latency of WKY/NCrl rats at 0.75 mg/kg (p<0.001) and 2.25 mg/kg (p<0.001), but was ineffective in Crl:WI and Crl:SD rats.
Fig 4.
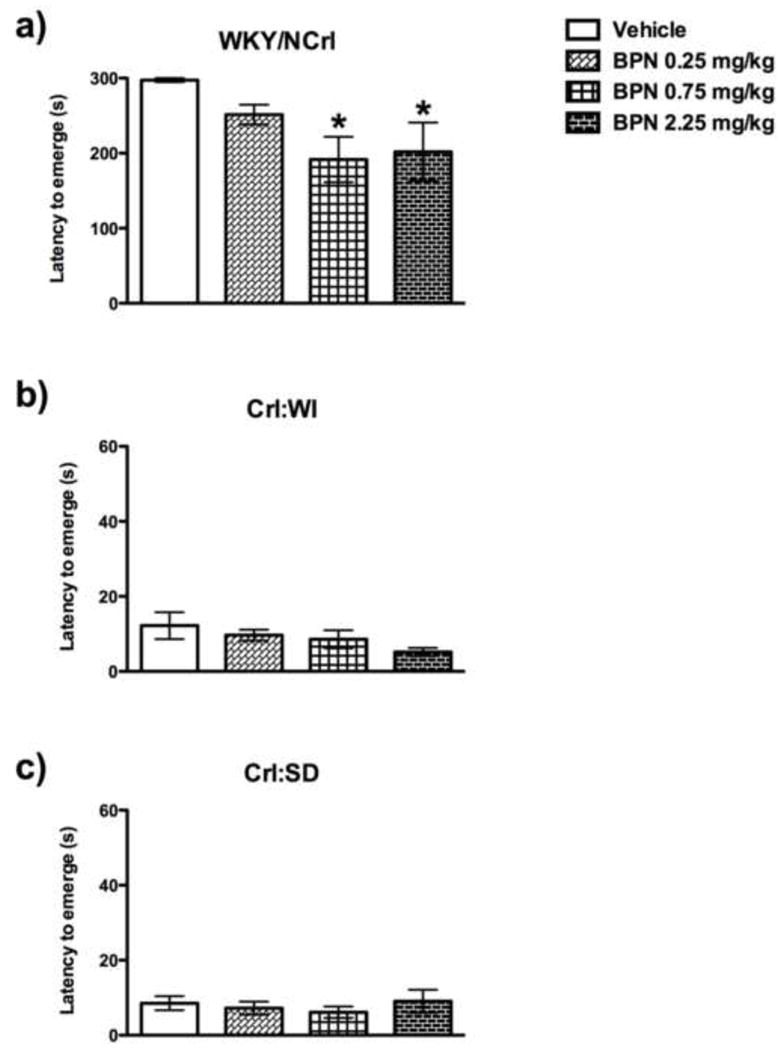
Effects of BPN in the emergence test in a) WKY/NCrl, b) Crl: WI and c) Crl: SD rats. BPN significantly decreased the latency to emerge in WKY rats, but not in Crl:SD or Crl:WI rats 24 h post treatment. The symbol ** indicates p<0.01, where 2.25 mg/kg BPN-treated WKY rats exhibited a decreased latency to emerge compared to the vehicle animals.
3.4.2. Strain difference in the effects of BPN in the FST
The rat strains differed significantly in baseline behavior in the FST for all categories and only WKY/NCrl rats responded to BPN. For immobility (Fig 5a), there was a significant effect of treatment in WKY/NCrl rats (F3,27=3.71, p=0.024). Post-hoc tests revealed that immobility levels were reduced BPN at 0.75 mg/kg (p<0.05) and 2.25 mg/kg (p<0.05) in WKY/NCrl rats only. BPN was ineffective in Crl:SD and Crl:WI rats.
Fig 5.
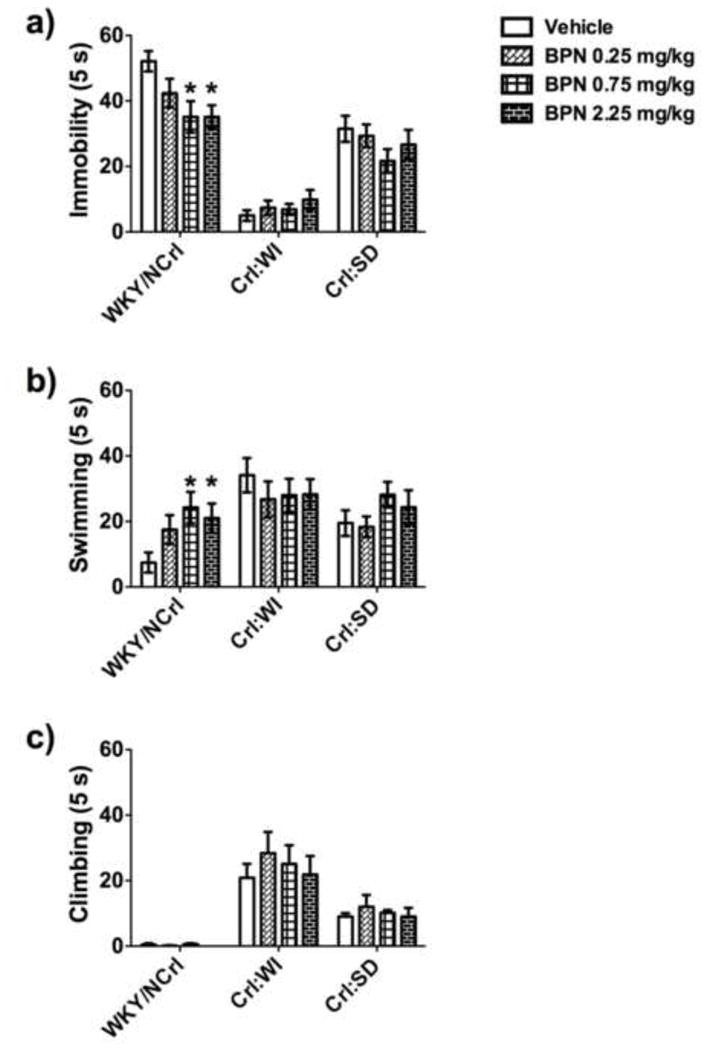
Performance in the FST and the effects of BPN treatment compared between WKY/NCrl, Crl:WI and Crl:SD rats. The symbol * indicates p<0.05, where a significant effect of treatment exists between BPN-treated and vehicle-treated WKY rats. The symbol *** indicates p<0.05, where a significant effect of treatment exists between BPN-treated and vehicle-treated WKY rats.
For swimming behavior (Fig. 5b), there was a significant effect of BPN treatment in WKY/NCrl rats (F3,27=3.03, p=0.046). Post-hoc tests determined that 0.75 mg/kg BPN (p<0.05) and 2.25 mg/kg BPN (p<0.05) significantly increased swimming behavior in WKY/NCrl rats 24 h after a single injection. No effect of BPN treatment was observed in Crl:SD and Crl:WI rats for swimming behavior.
There was no effect of BPN treatment for climbing behavior in any of the strains tested (Fig. 5c).
3.4.3. Strain differences in locomotor activity
No significant effect of BPN treatment was measured on total activity in any of the strains (Fig. 6).
Fig 6.
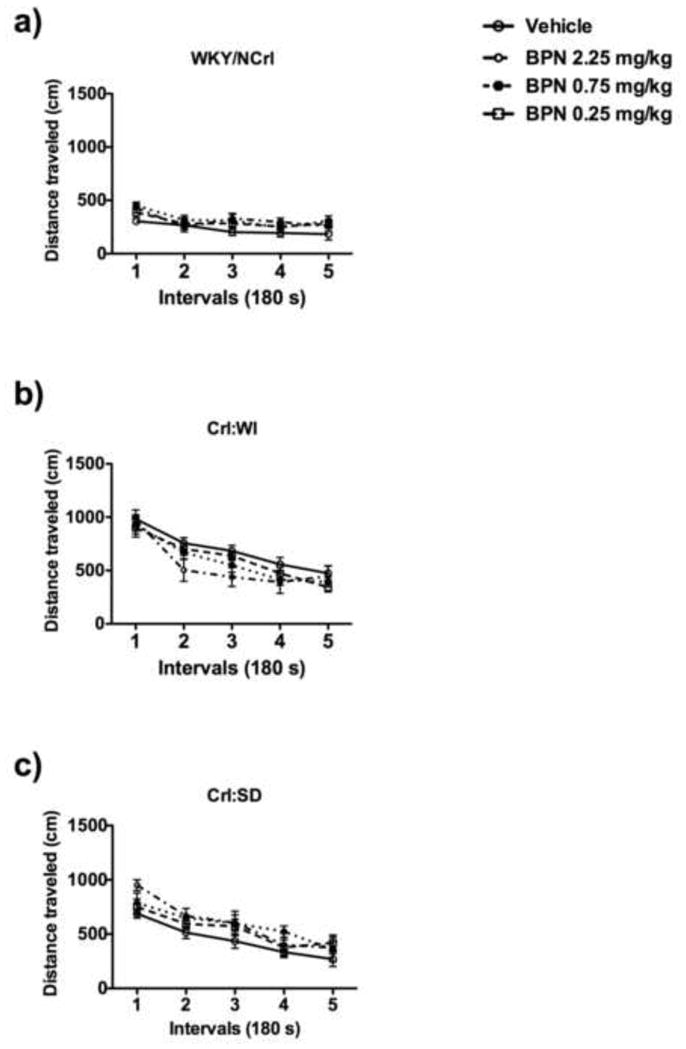
Locomotor activity of WKY/NCrl, Crl:WI and Crl:SD rats. Crl:WI rats exhibited higher locomotor activity over the 15-minute test compared to WKY rats. No significant difference was observed between Wistar and SD rats. BPN had no effect on locomotor activity 24 h post treatment in any of the three strains investigated.
4. Discussion
The purpose of these studies was to determine the efficacy of BPN in two behavioral tests, the FST used to assess the effects of antidepressant drugs and the emergence test used as a potential measure of the anxiolytic effects of BPN. Two important findings emerged from these studies: 1) the performance of WKY/NTac rats in the FST and emergence test differed significantly from other stress-sensitive WKY lines obtained from Charles River and Harlan laboratories. The present cohort of WKY/NTac rats exhibited a behavioral phenotype that resembled SD rats in the FST and differed from earlier cohorts of WKY/NTac rats assessed in 2007 and 2010. 2) BPN induced robust antidepressant-like and anxiolytic-like effects in the two stress-sensitive WKY substrains, but not in WKY/NTac rats and in the WKY genetic and behavioral controls, the Wistar and Sprague-Dawley (SD) strain respectively.
This is the first report of dramatic changes in the FST behavioral phenotype in WKY rats sourced from Taconic. As recently as 2010, WKY/NTac rats were shown to consistently exhibit the prototypical behavior of other WKY substrains in the FST, with higher immobility scores compared to SD rats [32]. However, data from cohorts we obtained in 2013 and tested in our laboratory under identical conditions, revealed a significant shift in the behavioral profile of WKY/NTac rats in the FST. The 2013 cohort showed a modest level of immobility and a striking increase in climbing behavior that was similar to SD rats. The WKY lines also differed in their behavior in the FST pretest. Typically, rats are very active through most of the pretest but adopt an immobile posture within minutes on the test day [44]. This pattern was observed in WKY/NHsd rats, which exhibited higher levels of immobility on the test day compared to the pretest. In contrast, WKY/NTac rats showed similar behavior in both the pretest and test, whereas WKY/NCrl rats developed high levels of immobility during the pretest that carried over into the test day, as described in earlier reports [15]. WKY/NTac rats also exhibited less anxiety in the emergence test compared to the other WKY lines. Furthermore, WKY/NTac rats were not sensitive to the behavioral effects of BPN in either the FST or the emergence test.
This dramatic difference in behavioral phenotype of the substrains is in line with previous reports that described significant physiological differences between WKY/NTac rats and the other WKY lines [45–47]. The substrain behavioral differences may be attributable to the maintenance policies of the WKY lines by the individual vendors. Originally, WKY rats bred to the F10 generation were provided by the Kyoto School of Medicine to the NIH in 1971 and transferred to all three vendors for their breeding colonies in 1974. The WKY/NHsd (harlan.com) and WKY/NCrl (criver.com) were maintained as inbred strains. However, Taconic did not derive their current WKY strain until 1982 and describe their stock as a partially inbred model (F10), which retains some residual heterozygosity (taconic.com). Such shifts in behavioral phenotype in rodents are not ideal but neither is it uncommon. Indeed a recent study conducted in the near isogeneic substrains of BALB/cJ and BALB/cByJ mice reported greater levels of aggression, depressive-like behavior in the tail suspension test and reduced anxiety-like behavior in the open field of BALB/cByJ mice [48]. Other potential causal factors that have been attributed to such shifts in behavioral phenotype include, 1) incomplete fixation of a genetic factor at specific loci, 2) impact of environmental stressors/enrichment and epigenetic variance of genetic factors, 3) inter-laboratory and inter-experimental differences. Overall the current dataset discourage the use of WKY/NTac rats as a reliable rodent model of increased anxiety and depressive behaviors. Considering these reports, choosing the appropriate WKY strain in which to model anxiety and depression may depend on multiple phenotypes. Indeed many investigators have chosen to breed their own colonies derived primarily from the Harlan stock [20]. In addition, it was reported that WKY/NCrl display higher levels of inattention compared to WKY/NHsd rats [49]. Furthermore, significant genomic differences (33.5%) are known to exist between the WKY/NCrl and WKY/NHsd lines, some of which may underlie the behavioral differences between these two substrains observed during the pretest [49, 50]. However, the data presented here established that both WKY/NCrl and WKY/NHsd rats show a high level of depressive-like and anxiety behavior.
BPN induced consistent and reproducible antidepressant and anxiolytic-like effects in WKY/NCrl and WKY/NHsd rats, but not in WKY/NTac, or in the outbred Wistar and SD strains. The pattern of results supports the use of WKY rats as a genetic model of depression and agrees with recent literature that WKY rats capture a range of functional domains relevant to clinical depression, including psychomotor retardation, behavioral inhibition, learned helplessness, social withdrawal and physiological dysfunction [9]. Moreover, the drug effects shown in WKY rats may be relevant to certain cohorts of TRD patients that exhibit resistance to SSRIs, as this rat strain also failed to display behavioral responses to SSRI treatment[15, 20, 51]. Although no effect of BPN was found in Wistar rats, often considered the genetic control for the WKY strain, the Wistar strain may provide ambiguous responses to antidepressants and anxiolytic drugs due to their hyperactivity. SD rats are a useful comparator strain to WKY rats, because many studies have demonstrated behavioral effects of conventional antidepressants and anxiolytic drugs using this strain [44]. Here we did not observe an effect of BPN treatment in SD rats. In previous studies, the response of unstressed SD rats to κ-OR antagonists in the FST has been variable, with some studies reporting no response after systemic administration of nor-BNI and DIPPA [32, 52]. However, systemic administration of the κ-OR antagonist ANTI [27] and JDTic [53] reduced immobility in the FST in a line of SD rats selected for high baseline immobility. It is problematic to interpret negative results when comparing drug effects between strains with different performance baselines because it is always possible that an effect of BPN may be produced in Wistar or SD rats when they are tested under different experimental conditions.
Recently it has been shown that κ-OR expression in an amygdala-anterior cingulate cortex-ventral striatal neural circuit mediates dysphoria in humans [54]. Additionally, postmortem data indicated significant impairment in prodynorphin expression in the amygdala of major depressed patients [55], particularly in the periamygdaloid cortex [56]. Furthermore, the κ-OR SNP 36G>T is associated with increased risk for opiate addiction and stress-responsivity [57]. These data suggest that abnormal DYN/κ-OR and increased responsiveness to stress may be involved in the pathophysiology of depression. It is plausible that regional differences in dynorphin/κ-OR signaling may underlie the differential and selective strain response of WKY rats to BPN. Indeed, WKY/NTac rats exhibited elevated dynorphin [32] and higher expression of the Oprk1 mRNA compared to SD rats [31]. Although the mechanisms involved in DYN/κ-OR signaling have been well delineated [58–60], the exact mechanism through which BPN induces its antidepressant-like and anxiolytic-like effects is uncertain. Further studies are required to characterize the regional alterations in dynorphin/κ-OR signaling in WKY rats at baseline and following BPN treatment. In addition, given the high prevalence of major depression in females and recent evidence for increased sensitivity of female WKY/NHsd rats to the antidepressant-like effects of low-dose ketamine [61], future studies should investigate whether or not female WKY rats exhibited increased sensitivity to low doses of BPN on tests for antidepressant activity.
Overall, these data clearly support the use of WKY/NCrl and WKY/NHsd as models of depression and anxiety. Furthermore, WKY rats offer a certain level of predictive validity for investigating potential antidepressants for MDD cohorts that do not respond to SSRIs. The dramatic decrease in depressive and anxiety phenotypes observed in WKY rats in this study indicates that BPN may have significant potential to treat patients with comorbid depression and anxiety. Further studies are required to characterize the exact mechanism and regions underlying the antidepressant-like and anxiolytic-like effects of BPN.
Highlights.
Buprenorphine produced antidepressant-like effects in the forced swim test in WKY rats.
Buprenorphine produced anxiolytic effects in the emergence test in WKY rats.
Wistar and Sprague-Dawley rats did not respond to buprenorphine.
WKY rats from different suppliers varied in behavior and response to buprenorphine.
Acknowledgments
This research was supported by USPHS grants R01 MH092412. We would like to acknowledge the assistance of Dr. Gregory Carr and Shivon A. Robinson in conducting some of the behavioral experiments.
Abbreviations
- WKY/NTac
Wistar Kyoto rats, Taconic
- WKY/NCrl
Wistar Kyoto rats Charles River
- WKY/NHsd
Wistar Kyoto rats Harlan
- Crl:SD
Sprague-Dawley rats Charles River
- Crl
WI, Wistar rats Charles River
- FST
forced swimming test
- κ-OR
kappa opioid receptors
- TRD
treatment resistant depression
- BPN
buprenorphine
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 3.Fava M, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–51. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, et al. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69(2):246–58. doi: 10.4088/jcp.v69n0211. [DOI] [PubMed] [Google Scholar]
- 5.Olchanski N, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512–22. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20(8):879–90. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 7.Pare WP. “Behavioral despair” test predicts stress ulcer in WKY rats. Physiol Behav. 1989;46(3):483–7. doi: 10.1016/0031-9384(89)90025-5. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22(2):191–9. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 9.Nam H, et al. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pare WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51(5):1051–6. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 11.Pare WP. Learning behavior, escape behavior, and depression in an ulcer susceptible rat strain. Integr Physiol Behav Sci. 1992;27(2):130–41. doi: 10.1007/BF02698502. [DOI] [PubMed] [Google Scholar]
- 12.Pare WP. Hyponeophagia in Wistar Kyoto (WKY) rats. Physiol Behav. 1994;55(5):975–8. doi: 10.1016/0031-9384(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55(3):433–9. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 14.Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology (Berl) 2010;210(2):295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rittenhouse PA, et al. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27(3):303–18. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 16.Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971(1):55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- 17.Pare WP, et al. Gender differences in acute and chronic stress in Wistar Kyoto (WKY) rats. Integr Physiol Behav Sci. 1999;34(4):227–41. doi: 10.1007/BF02688691. [DOI] [PubMed] [Google Scholar]
- 18.Dugovic C, et al. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11(3):627–31. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- 19.Tejani-Butt S, Kluczynski J, Pare WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 20.Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8(11):925–32. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- 21.Lahmame A, Armario A. Differential responsiveness of inbred strains of rats to antidepressants in the forced swimming test: are Wistar Kyoto rats an animal model of subsensitivity to antidepressants? Psychopharmacology (Berl) 1996;123(2):191–8. doi: 10.1007/BF02246177. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld A, Weller A. Behavioral effects of environmental enrichment during gestation in WKY and Wistar rats. Behav Brain Res. 2012;233(2):245–55. doi: 10.1016/j.bbr.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Solberg LC, et al. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R786–94. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- 24.Bruchas MR, et al. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4(12):e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Land BB, et al. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28(2):407–14. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mague SD, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305(1):323–30. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23(13):5674–83. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin JP, et al. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31(4):787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson KA, et al. Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology. 2006;31(11):2449–61. doi: 10.1038/sj.npp.1301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr GV, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35(3):752–63. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bechtholt AJ, Hill TE, Lucki I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl) 2007;190(4):531–40. doi: 10.1007/s00213-006-0615-9. [DOI] [PubMed] [Google Scholar]
- 34.Bodnoff SR, et al. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95(3):298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 35.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2(4):395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108–12. doi: 10.1111/j.1749-6632.1982.tb39483.x. [DOI] [PubMed] [Google Scholar]
- 37.Bodkin JA, et al. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28(5):593–5. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- 39.Karp JFBMA, Begley A, Miller MD, Lenze EJ, Blumberger D, Mulsant B, Reynolds CF., III Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in mid-life and older adults. J Clin Psychiatry. 2014 doi: 10.4088/JCP.13m08725. p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falcon EMK, Lucki I. Buprenorphine Produces Antidepressant-like and Anxiolytic Behavioral Responses in Mice. Psychopharmacology (Berl) 2014 p. Submitted. [Google Scholar]
- 41.Pare WP, Tejani-Butt S, Kluczynski J. The emergence test: effects of psychotropic drugs on neophobic disposition in Wistar Kyoto (WKY) and Sprague Dawley rats. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(8):1615–28. doi: 10.1016/s0278-5846(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 42.Lieben CK, et al. Acute tryptophan depletion induced by a gelatin-based mixture impairs object memory but not affective behavior and spatial learning in the rat. Behav Brain Res. 2004;151(1–2):53–64. doi: 10.1016/j.bbr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 44.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4–5):547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Henry R, Casto R, Printz MP. Diurnal cardiovascular patterns in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1990;16(4):422–8. doi: 10.1161/01.hyp.16.4.422. [DOI] [PubMed] [Google Scholar]
- 46.Pare WP, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physiol Behav. 1997;62(3):643–8. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- 47.Kurtz TW, et al. Molecular evidence of genetic heterogeneity in Wistar-Kyoto rats: implications for research with the spontaneously hypertensive rat. Hypertension. 1989;13(2):188–92. doi: 10.1161/01.hyp.13.2.188. [DOI] [PubMed] [Google Scholar]
- 48.Sittig LJ, et al. Phenotypic instability between the near isogenic substrains BALB/cJ and BALB/cByJ. Mamm Genome. 2014 doi: 10.1007/s00335-014-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagvolden T, et al. Behavioral and genetic evidence for a novel animal model of Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype. Behav Brain Funct. 2008;4:56. doi: 10.1186/1744-9081-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang-James Y, Middleton FA, Faraone SV. Genetic architecture of Wistar-Kyoto rat and spontaneously hypertensive rat substrains from different sources. Physiol Genomics. 2013;45(13):528–38. doi: 10.1152/physiolgenomics.00002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durand M, et al. Strain-dependent neurochemical and neuroendocrine effects of desipramine, but not fluoxetine or imipramine, in spontaneously hypertensive and Wistar-Kyoto rats. Neuropharmacology. 2000;39(12):2464–77. doi: 10.1016/s0028-3908(00)00088-5. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, et al. Central kappa-opioid receptor-mediated antidepressant-like effects of nor-Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol. 2007;570(1–3):89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beardsley PM, et al. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183(1):118–26. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 54.Pietrzak RH, et al. Association of In Vivo kappa-Opioid Receptor Availability and the Transdiagnostic Dimensional Expression of Trauma-Related Psychopathology. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.1221. [DOI] [PubMed] [Google Scholar]
- 55.Hurd YL. Subjects with major depression or bipolar disorder show reduction of prodynorphin mRNA expression in discrete nuclei of the amygdaloid complex. Mol Psychiatry. 2002;7(1):75–81. doi: 10.1038/sj.mp.4000930. [DOI] [PubMed] [Google Scholar]
- 56.Anderson SA, et al. Impaired periamygdaloid-cortex prodynorphin is characteristic of opiate addiction and depression. J Clin Invest. 2013;123(12):5334–41. doi: 10.1172/JCI70395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuferov V, et al. Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics. 2004;14(12):793–804. doi: 10.1097/00008571-200412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210(2):137–47. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruchas MR, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lemos JC, et al. Repeated stress dysregulates kappa-opioid receptor signaling in the dorsal raphe through a p38alpha MAPK-dependent mechanism. J Neurosci. 2012;32(36):12325–36. doi: 10.1523/JNEUROSCI.2053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tizabi Y, et al. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


