Abstract
Background
Exosomes play an important role in intercellular signaling and exert regulatory function by carrying bioactive molecules. This study investigated the cardioprotective capabilities of exosomes derived from mesenchymal stem cells (MSC) overexpressing GATA-4 (MSCGATA-4) and its underlying mechanisms through delivering miroRNAs (miRs) to regulate the target proteins in recipient cells.
Methods and Results
Exosomes were isolated and purified from MSCGATA-4 (ExoGATA-4) and control MSCs (ExoNull). Cell injury was investigated in primary cultured rat neonatal cardiomyocytes (CM) and in the rat heart. Exosomes contributed to increased CM survival, reduced CM apoptosis, and preserved mitochondrial membrane potential in CM cultured under a hypoxic environment. Direct intramyocardial transplantation of exosomes at the border of an ischemic region following ligation of the left anterior descending coronary artery significantly restored cardiac contractile function and reduced infarct size. Real-time PCR revealed that several anti-apoptotic miRs were highly expressed in ExoGATA-4. Rapid internalization of ExoGATA-4 by CM was documented using time-lapse imaging. Subsequent expression of these miRs, particularly miR-19a was higher in CM and in the myocardium treated with ExoGATA-4 compared to those treated with ExoNull. The enhanced protective effects observed in CM were diminished by inhibition of miR-19a. The expression level of PTEN, a predicted target of miR-19a, was reduced in CM treated with ExoGATA-4, which resulted in the activation of the Akt and ERK signaling pathways.
Conclusions
ExoGATA-4 transferred miRs into damaged CM, triggering activation of the cell survival signaling pathway.
Keywords: exosomes, miRs transfer, target proteins, bone marrow stem cells, cardioprotection, GATA-4
1. Introduction
Regional myocardial ischemia subsequent to a significant reduction or cessation of coronary arterial blood flow induces cardiomyocyte (CM) injury and can lead to heart failure. Exploration of stem cells as potential therapeutic agents has increased markedly in the past decade as a novel approach in both animal models and human subjects to reverse myocardial remodeling by reducing infarct size and thereby restoring cardiac pump function. [1–5] Bone marrow-derived mesenchymal stem cells (MSCs) are among the most promising cell types for the treatment of ischemic heart disease. [1, 2] We aimed to enhance this therapeutic potential by genetic modification of MSCs to overexpress the GATA-4 protein. Our previous studies have suggested that overexpression of GATA-4 not only increases MSC differentiation into cardiac cell phenotypes [6] but also promotes the survival of MSC in ischemic environments.[7, 8] Moreover, MSCs overexpressing GATA-4 (MSCGATA-4) increase CM survival, reduce CM apoptosis [9], and enhance angiogenesis in the ischemic myocardium and consequently improve cardiac function significantly.[7]
The therapeutic effects of MSCs may not only be mediated by their ability to differentiate into cardiac phenotypes to replace damaged cardiac tissues, [10] but may also be related to their salutary paracrine effects. [5, 11, 12] The soluble factors secreted from stem cells include a variety of growth factors, cytokines, and chemokines that orchestrate interactions within the microenvironment to inhibit apoptosis, stimulate proliferation, promote vascularization, and mobilize endogenous cardiac stem cells (CSCs).[13] In addition to these factors, the paracrine effect depends on the transfer of other proteins, bioactive lipids, and genetic material including mRNA, microRNAs (miRs), and other non-coding RNA. Subsequent work has affirmed that the paracrine effect of stem cell is also mediated by secreted extracellular vesicles (EVs). EVs secreted from MSC are small, spherical membrane fragments and can be divided into exosomes and shedding vesicles, which include microvesicles and apoptotic bodies. These vehicles display a wide range of biological activities.[14] In particular, exosomes have been identified as a cardioprotective component in MSC paracrine secretion and have been demonstrated to reduce myocardial ischemia/reperfusion (I/R) injury.[14–16]
Exosomes appear to function as one of several modes of intercellular communication by capturing and delivering complex cargos of signaling molecules to recipient cells. [8, 17, 18] Most exosomes have an evolutionarily conserved set of binding proteins that have an affinity to other ligands on cell membranes or within the extracellular matrix. These proteins include members of the tetraspanin family (e.g., CD9, CD63 and CD81) and other proteins unique to the tissue/cell type. [15] In 2007, Valadi and colleagues [19] reported for the first time that exosomes released from mast cells contain miRs and mRNAs. Since that finding, numerous groups have confirmed the existence of miRs, as well as proteins in exosomes. [17, 20] Accumulating evidence suggests that exosomes serve as vectors for miR communication between different cell types. [18, 21] miRs are evolutionarily conserved and consist of 18- to 25-nucleotides, non-protein coding transcripts that repress mRNA translation or modulate mRNA degradation by binding to the 3′-untranslated region of target mRNAs. Exosome-delivered miRs can regulate target protein expression in recipient cells, which is an important signaling transfer mechanism among neighboring cells. [22] In an earlier study, we confirmed that exosomes were rapidly internalized by CM, resulting in a transfer of miRs and regulation of protein synthesis in CM. [9]
Exosomes were directly and systematically investigated whether derived from MSCGATA-4 could deliver anti-apoptotic miRs into cultured CM in vitro and into the ischemic myocardium in vivo to produce a greater cardioprotective effect. miR-19a was selected as a representative miR to test the hypothesis that anti-apoptotic miRs play an important role in ExoGATA-4-mediated cardioprotection. We confirmed that there was a substantially greater regulation of target proteins and an activation of one or more cell survival signaling pathways.
2. Methods
All animals were treated according to the guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, Revised 1996). All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
2.1. MSC Culture and Transduction with GATA-4
MSCs were isolated from the femurs and tibias of Sprague Dawley (SD) rats (2–4 months-old) and sacrificed with an anesthetic overdose as previously described. [23] Bone marrow MSCs were cultured with Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 15% fetal bovine serum (FBS) at 37°C in humidified air with 5% CO2. The second passage of MSCs was transduced with recombinant GATA-4, which was constructed using a pMSCV retroviral expression system (Clontech) based on our previous report. [7]
2.2. Isolation and Characterization of Exosomes
Conditioned medium (CdM) was collected from various MSCs cultured in IMDM with 10% exosome-free FBS for 48 hours and centrifuged to remove whole cells and debris. After filtration with a 0.2-μm pore filter, the supernatant was transferred to a 100-kDa molecular weight cut-off ultra-filtration conical tube (Amicon Ultra-15) and centrifuged (3,000 × g) at 4°C for 45 minutes. Exosomes were isolated from concentrated CdM using ExoQuick-TC Exosome Precipitation Solution (SBI) according to the manufacturer’s instructions. Exosome pellets were fixed in 2% paraformaldehyde in PBS, pH 7.4 and the morphology of the exosomes was observed using transmission electron microscopy (JEOL). The expression of three exosome-specific biomarkers: CD9, CD63, and heat shock protein 70 (HSP70) was analyzed.
2.3. miRs Inhibition and Quantification
MSCsGATA-4 were transfected with 50 nM mirVana™ miR-19a inhibitor (si-miR-19a) (Ambion) using the Lipofectamine RNAiMAX system (Invitrogen). MirVana™ miRNA Inhibitor Negative Control #1 (si-miR-scramble) was used as a negative control. After 48 hours of transfection, the culture media were replaced with fresh media supplemented with 10% exosome-free FBS (SBI) for subsequent exosome collection.
Total RNA from MSCs, exosomes, cultured CM, and hearts was extracted using the mirVana™ isolation kit (Ambion). Next, cDNAs corresponding to different miRs were synthesized using the miScript Reverse Transcription Kit (Qiagen). Quantitative PCR was performed with specific primers on an iQ5 real-time PCR system (Bio-Rad) with miScript SYBR Green PCR Kit (QIAGEN). U6 snRNA was used as an internal control. The relative expression of miRs was calculated based on the threshold cycle with a specific efficiency for each different primer.
2.4. Electroimmunoblotting and Immunostaining
Proteins were extracted from different cells or exosomes using the Qproteome Mammalian Protein Prep Kit (QIAGEN). Denatured proteins (30 μg) were separated using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with the following primary antibodies: polyclonal rabbit anti-PTEN, anti-BIM, anti-HSP70, anti-β-actin (Cell Signaling), anti-CD63 (Santa Cruz), and anti-CD9 (Abcam) at 4°C overnight. This was followed by incubation with HRP conjugated goat anti-rabbit secondary antibody (Cell Signaling). The membrane was washed and developed using the ECL plus kit (GE Healthcare). Densitometric analysis of the blots was performed using FluoChem SP software (Alpha Innotech).
For immunostaining, cells cultured on cover slides and sections of the myocardium were first incubated with mouse monoclonal anti-sarcomeric α-actinin antibody (Sigma) or rabbit polyclonal anti-GATA-4 antibody (Santa Cruz). Secondary antibody of goat anti-mouse IgG (FITC-conjugated) or goat anti-rabbit IgG (Alexa-568 conjugated) was then applied. Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Fluorescent images were obtained using an Olympus BX 71 microscope equipped with a digital camera (Olympus).
2.5. Hypoxia Model to Simulate Ischemic Myocardium in vitro
CM were isolated from neonatal rat ventricles (1- to 3-days-old) using a commercially available neonatal CM isolation kit (Worthington Biochemical Co.). The dissociated cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and penicillin (100 U/ml)/streptomycin (100 μg/ml) following pre-plating for 2 hours to exclude cells other than CM.
An in vitro hypoxic model was used to mimic in vivo myocardial ischemic injury. CM cultured in low glucose medium was placed into a hypoxia chamber (CO2/O2 incubator, MCO-18M, Sanyo) with 1% O2, 5% CO2, and 94% N2 at 37°C. Exosomes obtained from various MSC populations were added into the culture medium before CM were placed in the hypoxic incubator. The release of lactate dehydrogenase (LDH) from CM into the surrounding medium was measured using a commercially available kit (Promega). The number of surviving CM was estimated using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (MTS) (Promega). To quantify the number of apoptotic cells, CM were stained with terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) using the In situ Cell Death Detection Kit, TMR red (Roche). The mitochondrial membrane potential (ΔΨm) of CM was monitored by incubation with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolocarbocyanin iodide (JC-1) (5 μmol). The JC-1 green (monomer) was excited at a wavelength of 488 nm and the fluorescence emission signal amplitude was measured from 505 to 530 nm. The JC-1 red (aggregate) was excited at 543 nm and the subsequent fluorescence was detected at wavelengths above the 560-nm cut-off filter. The intensities of both fluorescence dyes were read using a microplate M3 spectrophotometer (Molecular Devices). The ratio of aggregate to monomer was interpreted as the ΔΨm of the CM.
2.6. Regional Myocardial Ischemia/Infarction Rodent Model
An acute regional left ventricular myocardial infarction was generated in 2- to 3-month-old female SD rats by permanent ligation of the left anterior descending (LAD) coronary artery. Animals were anesthetized with ketamine/xylazine (100/5 mg/kg, IP) and mechanically ventilated. The LAD was ligated with a 6-0 Ethicon suture and the animals were divided into three groups: saline control, ExoGATA-4, and ExoNull. After LAD ligation, each rat received a 50-μl saline intramyocardial injection containing exosomes harvested from 4×106 MSCs or with saline alone as a control. The fourth group consisted of the sham-operated rats that had undergone all surgical procedures except LAD ligation.
Cardiac (left ventricular) function was determined by performing echocardiography 4-weeks post-ligation using HDI 5000 SonoCT (Phillips) with a 15-MHz probe. The left ventricular (LV) parameters were recorded from two-dimensional images using the M-mode interrogation in the long-axis view. The infarct size was determined in post-mortem samples by standard Masson’s trichrome staining using image J analysis software (version 1.6065; NIH).
2.7. Detection of Exosomes in Vitro and in Vivo
To document the internalization of exosomes, purified exosomes pre-labeled with PKH26 (ExoPKH26) using the red fluorescent labeling kit (Sigma–Aldrich) were added into CM cultured in specialized Hi-Q4 dishes. Time-lapse images were captured in both the phase-contrast and red fluorescence channels from the Biostation IM-Q System (Nikon). Subsequently, after 24 hours of incubation with exosomes, the cells were fixed and incubated with mouse monoclonal anti-α-actinin antibody (Sigma).
To localize the ExoPKH26 after injection into myocardium in vivo, animals were euthanized with an anesthetic overdose on the second day following exosome injection. The myocardium was embedded in OCT and processed for 5-μm sections. The CM in these sections were immunostained using mouse monoclonal anti-α-actinin antibody (Sigma).
2.8. Statistical Analysis
All statistical analyses have been reviewed and all our data sets were submitted to a normal distribution test before subjecting them to ANOVA and t-tests. Comparisons between two or more groups were performed using the mean of unpaired Student’s t-test and analysis of variance (ANOVA), respectively. If ANOVA demonstrated a significant interaction between variables, then post hoc analyses were performed using the multiple-comparison Bonferroni correction test. Statistical significance was defined at p<0.05.
3. Results
3.1. Characterization of MSCGATA-4
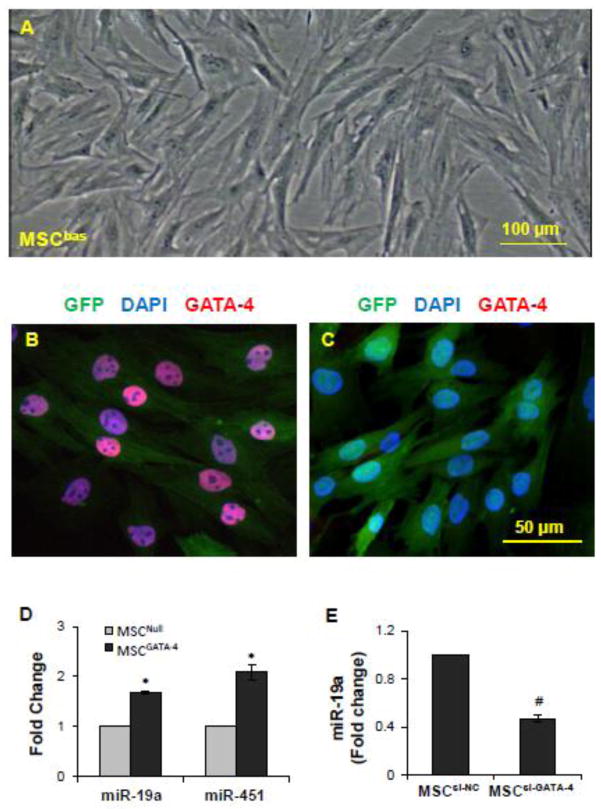
Basal MSCs (MSCbas) were isolated from the bone marrow of rats. GATA-4 was transduced into the 2nd passage of MSCbas (Figure 1A). The transfection efficiency approached 100% as evaluated using fluorescence microscopy for GFP and immunostaining of GATA-4 after selection with puromycin. No significant morphological differences were observed between the MSCGATA-4 and vector-empty control (MSCNull). However, MSCGATA-4 showed a strong positive immunostaining of GATA-4 (Figure 1B) compared to MSCNull (Figure 1C), and a high expression of miR-19a and other miRs (Figure 1D). Loss-of-function analysis was performed by introducing GATA-4-siRNA into MSCs (MSCsi-GATA-4) to study whether the difference in miR expression in MSC was due to an overexpression of GATA-4. MiR-19a was selected as an example to investigate the role of GATA-4 in miR expression. The expression of miR-19a was significantly reduced in MSCsi-GATA-4 compared to MSCs transfected with negative control-siRNA (MSCsi-NC) (Figure 1E), suggesting that up-regulation of miR-19a in MSCGATA-4 was partially related to the overexpression of GATA-4.
Figure 1.
Characterization of MSCs derived from bone marrow and MSCs transduced with GATA-4 (MSCGATA-4). A, Second passage of basal bone marrow stem cells. B and C, Immunostaining of GATA-4 in MSCGATA-4 (B) and MSCNull (C). GFP was expressed in both MSCNull and MSCGATA-4, but GATA-4 was only expressed in MSCGATA-4. D, Real-time PCR results show that the expression of miR-19a and miR-451 was up-regulated in MSCGATA-4 compared with MSCNull. E, Expression of miR-19 was down-regulated in MSCs that were transfected with GATA-4-siRNA (MSCsi-GATA-4) compared with MSCs transfected with the negative control siRNA (MSCsi-NC). *, p<0.05 vs MSCNull and #, p<0.05 vs MSCsi-NC.
3.2. ExoGATA-4 Increased the Resistance of CM against Hypoxia
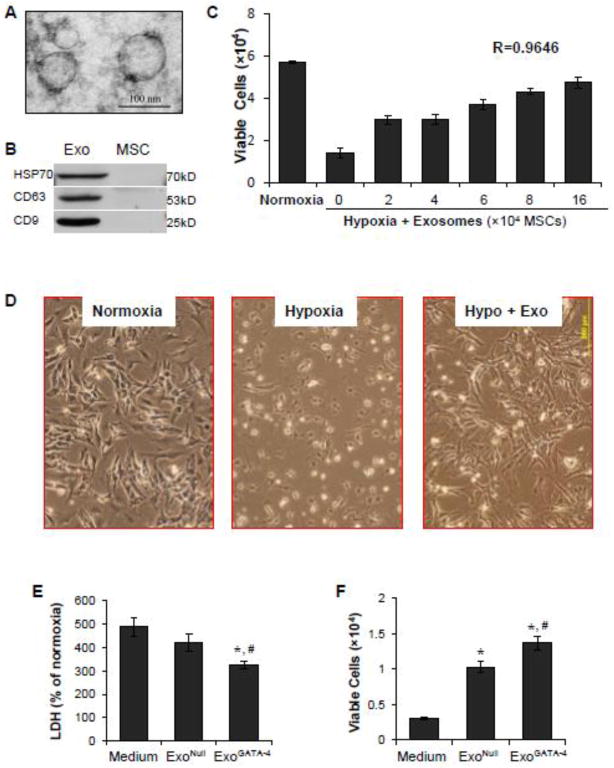
Exosomes isolated from cultured MSCs were observed as spheroids with an average size of ~100 nm using microscopy (Figure 2A). The expression of the exosome markers, CD63, CD9, and HSP70, was significantly higher in exosomes compared to parent MSCs (Figure 2B).
Figure 2.
Cardioprotective effect of exosomes. A, Ultrastructural features of exosomes derived from MSCs. B, Western blotting analyses revealed that exosomes highly expressed HSP70, CD63, and CD9 compared to MSCs; C-F, Exosomes protect CM against ischemic injury induced by exposure of CM to hypoxia for 48 hours. Panel C: Exosomes increased CM survival in a concentration-dependent manner. D, CM morphology in different treatments. Bright dots: dead or dying cells. E, LDH release from CM. F, The number of survived CM. *, p<0.05 vs medium control; #, p<0.05 vs ExoNull treatment.
CM cultured for three days and subsequently exposed to a hypoxic environment for 48 hours displayed a shrunken morphology. There was a marked decrease in the number of cells and many of the remaining cells were apoptotic. The number of surviving cells was significantly decreased compared to cells cultured under normoxic conditions (Figure 2C). The addition of exosomes, which were generated from up to 16 × 104 cultured MSCs, partially prevented hypoxia-induced death of CM in a concentration-dependent manner (r = 0.9646) (Figure 2D). Changes in cell morphology were also minimized with exosome treatment. ExoGATA-4 exhibited a greater effect than ExoNull in reducing LDH release from CM (Figure 2E) and this finding was associated with more viable CM in the ExoGATA-4-treated group (Figure 2F).
3.3. ExoGATA-4 Reduced CM Apoptosis and Maintained ΔΨm
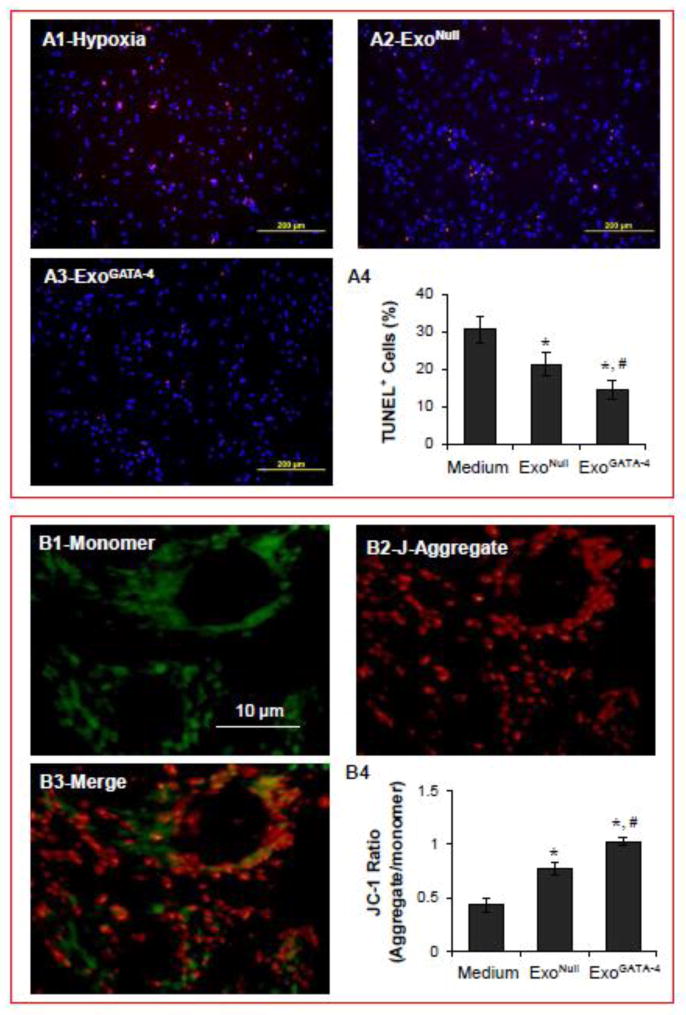
The fraction of TUNEL-positive (i.e., apoptotic) CM was significantly increased (>30%) when CM were exposed to hypoxia compared to CM maintained under normoxic conditions (<10%). Moreover, the number of apoptotic CM was significantly reduced when CM were cultured with ExoGATA-4 (Figure 3A). ΔΨm plays a pivotal role in maintaining mitochondrial integrity. The mitochondria were labeled with JC-1 in two colors to distinguish the state of membrane polarization. Depolarized mitochondria (monomer labeled green) were localized near the nucleus, whereas hyperpolarized mitochondria (aggregated labeled red) were confined to the cell periphery (Figure 3B). The ratio of aggregated to monomer mitochondria in normal control CM was approximately 1.29. However, this ratio was decreased to 0.43 in CM exposed to hypoxia for 24 hours, indicating that ΔΨm was reduced. ΔΨm was restored to near normal values in CM treated with ExoGATA-4.
Figure 3.
Exosomes significantly reduced CM apoptosis and maintained ΔΨm after CM exposure to hypoxia for 24 hours. A, Representative images of TUNEL staining in CM and positive cell quantification data. B, Representative JC-1 fluorescence imaging of mitochondria and quantitative data of the ratio of J-aggregate to monomer fluorescence. Green and red fluorescence indicate monomeric and J-aggregate mitochondria, respectively. *, p<0.05 vs medium control, and # p<0.05 vs ExoNull, respectively.
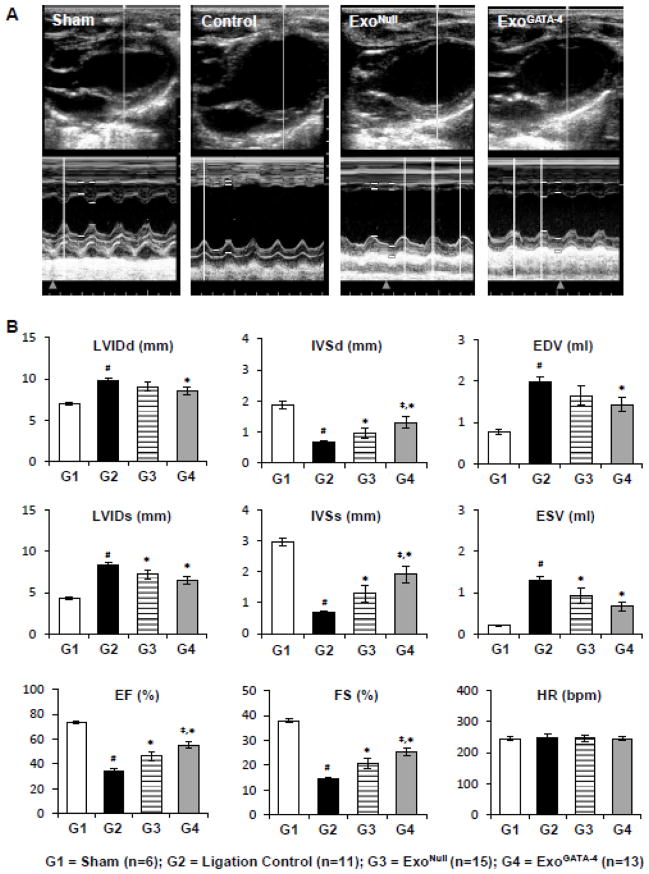
3.4. ExoGATA-4 Promoted Cardiac Function Recovery
Echocardiography measurements were performed to assess the left ventricular function four weeks post-surgery (Figure 4A). The global performance metrics of LV ejection fraction (EF) and fractional shortening (FS), and the specific morphological metrics of intraventricular septum systolic thickness (IVSs), and intraventricular septum diastolic thickness (IVSd) were significantly reduced 4 weeks post-LAD ligation. In contrast, other global dimensional metrics, including the LV minor axis internal chamber dimensions during systole (LVIDs) and diastole (LVIDd), as well as the computed estimates of LV end diastolic volume (EDV) and end systolic volume (ESV) were significantly increased in rats following LAD ligation. No significant changes were observed in heart rate (HR). Animals treated with exosomes, which were injected intramyocardially at the perimeter of the infarct region, particularly in the rats treated with ExoGATA-4, demonstrated significant preservation of cardiac function (Figure 4B). Infarct size in heart tissue was significantly decreased in the ExoGATA-4 group compared with rats treated with saline alone or with ExoNull (Figure 4C).
Figure 4.
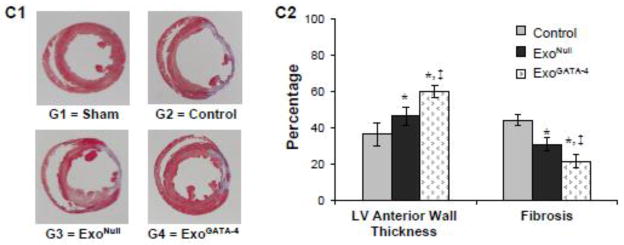
ExoGATA-4 improves cardiac function and reduces infarction size after transplantation for 4 weeks. A, Representative echocardiography images. B, Parameters of cardiac function calculated from M-mode echocardiograms. C, Heart sections stained with Masson-Trichome (C1) and quantitative data of LV thickness and fibrosis (C2). #, p < 0.05 vs sham animals; *, p < 0.05 vs ligation control; ‡, p < 0.05 vs ExoNull treatment, respectively.
3.5. MiRs Transfer Played a Role in ExoGATA-4-Mediated Cardioprotection
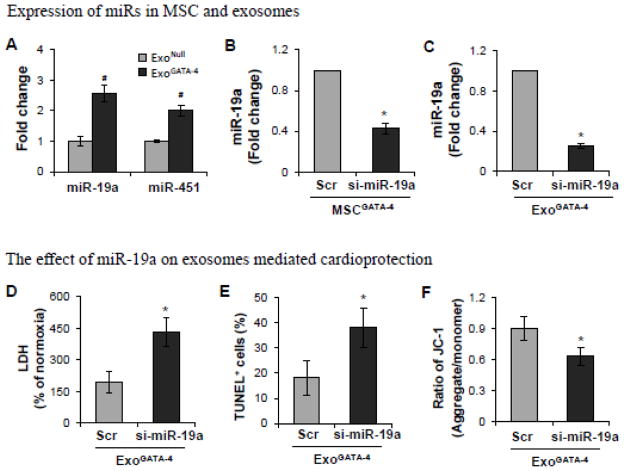
Accumulating evidence implicates miRs as one of the most important factors controlling cell survival-related gene expression. The expression of several miRs, e.g., miR-19a and miR-451 was significantly higher in ExoGATA-4 compared to ExoNull (Figure 5A). MiR-19a was selected to test the role of miR in ExoGATA-4-mediated cardioprotection. Inhibition of miR-19a was induced via transfection of si-miR-19a into MSCGATA-4 (MSCGATA-4-si-miR-19a). si-miR-scramble was transfected into MSCGATA-4 as a negative control (MSCGATA-4-si-miR-scr). The expression of miR-19a was down-regulated in MSCGATA-4-si-miR-19a compared with MSCGATA-4-si-miR-scr (Figure 5B). The expression of miR-19a in exosomes derived from MSCGATA-4-si-miR-19a was also lower compared to those derived from MSCGATA-4-si-miR-scr (Figure 5C). Moreover, inhibition of miR-19a partially abolished ExoGATA-4-mediated cardioprotection, including a reduction in LDH release from CM and the number of TUNEL-positive CM, and maintenance of ΔΨm (Figure 5D–5F).
Figure 5.
The expression of miRs and the role of miRs in ExoGATA-4 mediated cardioprotection. A, Real-time PCR expressed as the fold change of miR-19a and miR-451 in exosomes. #, p<0.05 vs ExoNull. B and C, The expression of miR-19a in MSC (B) and in exosomes (C) following transfection with si-miR-19a. *, p<0.05 vs scramble si-miR transfected. D~F, The effect of exosomes obtained from MSCGATA-4 transfected with si-miR-19a on LDH release (D), TUNEL-positive cells (E), and ΔΨm (F). *, p<0.05 vs scramble si-miR transfected, respectively.
3.6. ExoGATA-4 Delivered miRs into CM
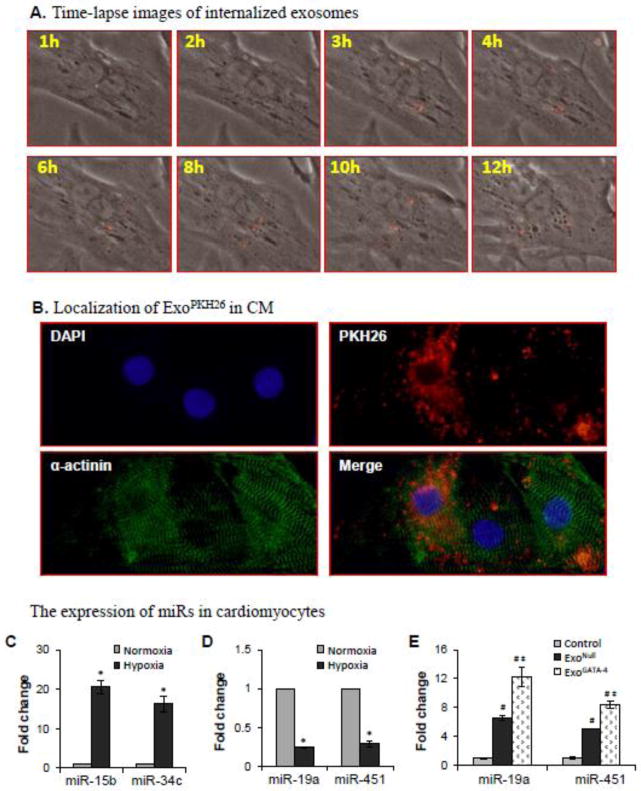
The internalization of exosome derived from MSC by CM was monitored using a state-of-art time-lapse imaging system, which showed direct evidence of the dynamic shedding course of exosomes into extracellular space. The internalization of MSC-MVs by CM was assayed by adding exosomes pre-labeled with PKH26 (ExoPHK-26) into cultured CM. One-hour interval time-lapse images of CM for 24 hours following the addition of ExoPHK-26 were taken. No clear red Exo could be seen in CM during the first 2 hours. Negligible red fluorescent spots appeared in CM by hour 3. ExoPHK-26 were clearly visible in many CM by hour 8 (Figure 6A). To confirm whether Exo were transferred into CM, cultured cells were fixed and labeled with anti-α-actinin. ExoPKH-26 were internalized in CM (α-actinin positive cells) exposed to ExoPKH-26 for 24 hours (Figure 6B).
Figure 6.
The internalization of exosomes and the effect of internalized exosomes on the expression of miRs in CM. A, Time-lapse images following the addition of ExoPKH-26 to CM culture. B, Immunostaining shows the red fluorescence (ExoPKH26) was inside α-actinin-positive cells. C-E, Expression of miRs in CM after exposure to hypoxia for 24 hours and treatment with exosomes. *, p<0.05 vs normal control; #, p<0.05 vs hypoxia, and ‡, p<0.05 vs ExoNull treated, respectively.
The expression of pro-apoptotic miRs (e.g., miR-15b and miR-34c) was up-regulated (Figure 6C) and the expression of anti-apoptotic miRs (e.g., miR-19a and miR-451) was down-regulated in CM (Figure 6D) after exposure to hypoxia for 24 hours. However, the expression of miR-19a and miR-451 were significantly increased in CM cultured with exosomes, particularly in the ExoGATA-4 treated group (Figure 6E). These results suggested that hypoxia up-regulated pro-apoptotic miRs and down-regulated anti-apoptotic miRs in CM, while exosomes delivered anti-apoptotic miRs into CM.
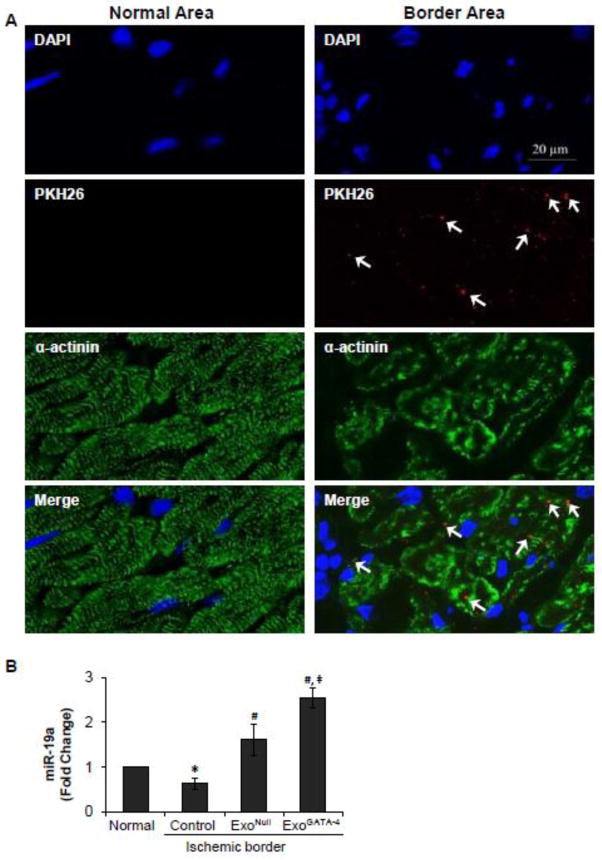
The internalization of exosomes and miR-19a up-regulation were also observed in the ischemic myocardium. Rats injected with ExoPKH-26, ExoNull and ExoGATA-4 were sacrificed on the second day post-LAD ligation. The myocardium was immunostained with anti-α-actinin, and images were obtained from the infarct border area. ExoPKH-26 were primarily located inside damaged myocytes (Figure 7A). The expression of miR-19a was significantly decreased in the ischemic myocardium. However, miR-19a was up-regulated in the border area of the ischemic rat myocardium in vivo following exosome treatment, which was more significantly enhanced in CM treated with ExoGATA-4 (Figure 7B).
Figure 7.
Exosome distribution in the myocardium and their effect on the expression of miRs 2 days post-transplantation. A, ExoPKH-26 was mainly located in the ischemic boarder area (white arrows). B, Real-time PCR of miR-19a in normal and ischemic border myocardium. *, p<0.05 vs normal control; #, p<0.05 vs medium treated; and ‡, p < 0.05 vs ExoNull treated, respectively.
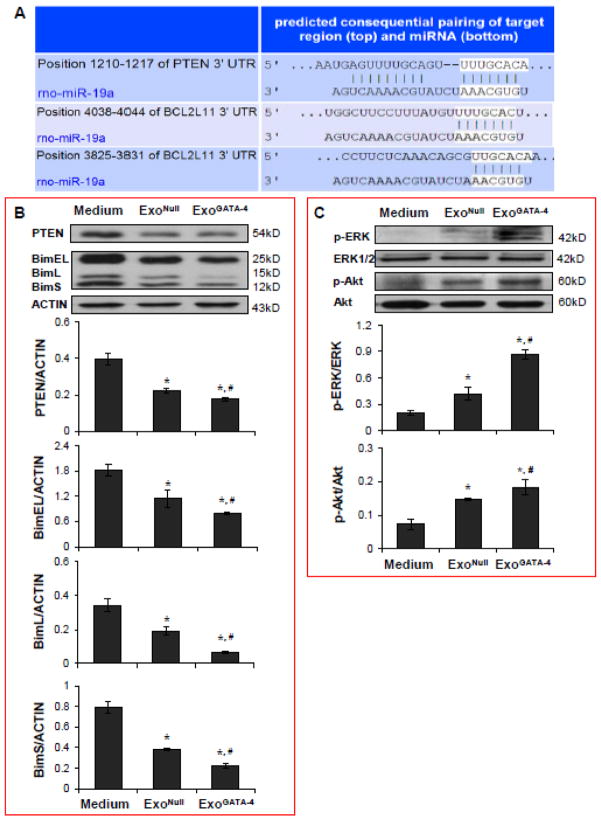
3.7. Transferred miR-19a Downregulates PTEN and BIM in CM
The target proteins of miR-19a were identified using TargetScan (Figure 8A). The 3’-UTR of PTEN and BIM (Bcl2l11) genes are perfectly complementary to the seed region of miR-19a at positions 1210–1217, and 3825–3831 and 4038–4044, respectively. The expression of PTEN and BIM (including three subtypes: BimEL, BimL, and BimS) were significantly reduced in CM treated with exosomes compared to CM treated with control medium following exposure to hypoxia for 24 hours. The reduction in PTEN and BIM expression was particularly significant in the CM treated with ExoGATA-4 (Figure 8B). PTEN and BIM are proposed mediators controlling cell survival through the Akt and ERK signaling pathways. ERK and Akt phosphorylation was significantly upregulated in CM treated with exosomes and, particularly, in CM treated with ExoGATA-4 (Figure 8C).
Figure 8.
Figure 8. Exosomes regulate PTEN, BIM, p-ERK and p-Akt signaling in CM. A, TargetScan shows the 3’ UTR of PTEN and BIM (BCL2L11) containing the conserved miR-19a binding site. B, Western blotting analyses of PTEN and BIM, and corresponding semi-quantitative data in CM. C, Western blotting analyses of p-ERK and p-Akt, and corresponding semi-quantitative data in CM. *, p<0.05 vs medium control, and #, p<0.05 vs ExoNull treated, respectively.
4. Discussion
The cardioprotective effect of exosomes derived from MSCGATA-4 was systematically investigated in this study. It was shown that: 1) Exosomes generated from MSC dramatically increased the resistance of CM to hypoxic injury. Intramyocardial injections of exosomes promoted cardiac functional recovery and reduced infarction size. 2) Exosomes transferred miRs into CM via rapid internalization by CM. ExoGATA-4 enhance cardioprotection, which may be related to ExoGATA-4 enriched anti-apoptotic miRs, e.g., miR-19a. Moreover, the cardiac protection of ExoGATA-4 was partially abolished by transfection of si-miR-19a. 3) miR-19a transferred by ExoGATA-4 downregulated the expression of target proteins, PTEN and BIM in CM, and resulted in activation of the Akt and ERK signaling pathway. ExoGATA-4 may provide an attractive safer therapeutic alternative to cell therapy in the treatment of myocardial infarction. We have previously reported in 2 published papers on some of the features whereby MSCs overexpressing GATA-4 can significantly improve ischemic myocardial function. We observed that the paracrine effect plays an important role in MSCGATA-4 mediated cardioprotection. [7] We also reported that cardioprotection by MSCGATA-4 may be regulated in part by a transfer of certain anti-apoptotic miRs contained within extracellular vesicles. [9] In this study, for the first time we provide evidence that elucidates one novel mechanism by which MSCGATA-4 produces salutary cellular and regenerative tissue effects in vivo; by secreting exosomes from MSCGATA-4 which contain higher anti-apoptotic miRs, particularly miR-19a (previously we reported on the role of miR-221), and can be transferred to cardiomyocytes. Transferred miRs, most specifically miR-19a, further regulate important cell regeneration signaling pathways. Moreover, in this paper, we have also demonstrated for the first time that exosomes can deliver a specific miR (miR-19a) to ischemic myocardium to induce myocardial protection and promote in vivo ischemic myocardium repair.
Recent studies have suggested that paracrine factors play a critical role in stem cell-mediated therapeutic potential. We previously demonstrated that CdM obtained from MSCs reduces hypoxic injury. [3, 7] It has been identified that the bioactive components in CdM include exosomes, microvesicles, and soluble proteins. Exosomes have been reported to rapidly activate multiple cardioprotective pathways, which can lead to infarct size reduction and the prevention of heart function deterioration after myocardial ischemic reperfusion injury. [14–16] It was demonstrated that purified exosomes, in a concentration-dependent manner, directly increased the resistance of CM against hypoxic injury. Intramyocardial injection of exosomes into the ischemic border of the left ventricle significantly improved cardiac function and reduced infarct size. Our results are consistent with previous reports in which a single intravenous administration of MSC-derived exosomes restored bioenergetics, reduced oxidative stress and activated pro-survival signaling. [14] Exosomes generated from cardiac progenitor cells can efficiently protect CM against apoptosis from oxidative stress as well as in mouse models of acute myocardial ischemia and reperfusion. [16]
Exosomes, a subtype of membrane vesicle, mediate endogenous cell-to-cell communication. The delivery of exosomes to target tissues is important for their therapeutic applications. In this study, we investigated the mode of exosome delivery to CM using a time-lapse imaging system and immunostaining. Exosomes were internalized rapidly by the CM. The tetrapannin membrane proteins, CD9 and CD81, functioned primarily to mediate cellular penetration, invasion and fusion events. These proteins could facilitate the cellular uptake of exosomes by specific cell types, including CM, which contain the co-immunoprecipitating complexes of CD81 and CD9. In addition, integrins on exosomes could facilitate homing of exosomes to CMs, which express the intercellular adhesion molecule-1 (ICAM1), a ligand of integrins, after ischemic injury. In our study, we confirmed that exosomes were localized more frequently inside damaged cells in the ischemic area than in normal myocardial cells. Exosome release and uptake is increased at low pHs compared with a neutral pH in buffered conditions. [24] This observation might explain the preferential conditions under which exosomes exert their cardioprotective effects because ischemic CM have a low intracellular pH and are also typically immersed in a low extracellular fluid pH. [25]
Exosomes because of their capability of traversing biological barriers and of transporting functional bioactive molecules between cells become a novel and exciting delivery therapeutic vehicle. In addition to delivering cytokines and growth factors, exosomes contain mRNA and miRs. [17, 26] Moreover, the exosome secretion profile can be improved by preconditioning or genetic manipulation of the parent cells.
It is well known that ischemic preconditioning is a potent approach to enhance survival and regeneration of MSCs in an ischemic environment. [27] We have shown that the number and expression level of miRs were different in exosomes secreted from MSCs subjected to ischemic preconditioning (ExoIPC). [28] In particular, miR-22 was highly upregulated in ExoIPC. The delivery of miR-22 reduced apoptosis in ischemic cardiomyocytes, ameliorated fibrosis, and improved cardiac function post-myocardial infarction. [28] The treatment of infarcted hearts with ExoIPC resulted in a better cardiac outcome as compared to treatment with the miR-22 mimic or Mecp2. [28]
Considering that genetically modified MSCs were widely tested in various studies, it has been hypothesized that exosomes derived from pretreated MSCs could be used as ideal vehicles for gene delivery to facilitate gene and cell therapy. The CD34+ hematopoietic stem cells have shown significant promise against myocardial ischemia by promoting angiogenesis that helps preserve the functionality of ischemic myocardium. Unfortunately, both viability and angiogenic attributes of autologous CD34+ cells decline with advanced age and diminished cardiovascular health. To offset age- and health-related angiogenic declines in CD34+ cells, Dr. Losordo’s group [29] explored whether the therapeutic efficacy of human CD34+ cells could be enhanced by augmenting their secretion of the known angiogenic factor, sonic hedgehog (Shh). Shh-modified CD34+ cells (CD34(Shh)) protected against ventricular dilation and cardiac functional declines associated with acute myocardial infarction. Treatment with these cells also reduced infarct size and increased border zone capillary density. CD34(Shh) primarily stored and secreted Shh protein in exosomes which transfered functional Shh to elicit induction of the canonical Shh signaling pathway in recipient cells. [29]
We have demonstrated previously that MSC overexpressing GATA-4 release more growth factors and promote endothelial cell mediated angiogenesis. [7] GATA-4 can regulate miR expression in MSC and increase MSC survival in ischemic environment. [8] In this study, ExoGATA-4 abundantly expressed various miRs, particularly anti-apoptotic miRs. Moreover, these miRs were significantly increased in CM cultured with ExoGATA-4 compared to that cultured with ExoNull. To address whether the miRs are associated with exosomes mediated cardiopotection, we firstly detected the expression of miRs listed on miScript miRNA PCR Array Rat Apoptosis Kit (QIAGEN) in hypoxic CM w/o exosomes treatment by using quantitative real-time PCR. A number of miRs were upregulated in CM treated with exosomes as compared with CM in the control normoxic medium. Five of these miRs were highly expressed in hypoxic CM treated with EXOGATA-4 compared to those treated with EXONull. miR-19a is one of these five miRs. To investigate the beneficial effect of these miRs in EXOGATA-4 mediated cardioprotection, we selected and focused on only miR-19a in this study. Nevertheless we are not excluding the effects of other miRs they may have on cardioprotection. Our in vivo studies also demonstrated that miR-19a expression was upregulated in the myocardium following intramyocardial ExoGATA-4 injection. ExoGATA-4 showed a higher potential in reducing CM apoptosis, maintaining ΔΨm, reducing infarct size and improving cardiac function. We further confirmed that knockdown of miR-19a by transfection of si-miR-19a partially abolished ExoGATA-4-mediated cardioprotection.
miR-19a is one member of the miR17-92 cluster, which consists of a cluster of six miRs on chromosome 13. Several studies support a key role for this miR-17-92 cluster in cardiogenesis. [30] Furthermore, miR-17-92 is also a critical regulator of CM proliferation, and these miRs could become therapeutic targets for cardiac repair and heart regeneration.[31] Knockdown of miR-19a also inhibited cell growth and induced cell apoptosis. [32] miR-19a is predicted to target the 3′ untranslated region of PTEN and BIM mRNA. Our study supports the notion that the levels of PTEN and BIM were downregulated in CM treated with ExoGATA-4. PTEN plays a crucial role in regulating the self-renewal and differentiation of human embryonic stem cells and hematopoietic stem cells. [33] It is well known that Akt plays a major role in cell survival signal in many cell types. PTEN also downregulates cell proliferation and promotes apoptosis by suppressing Akt activation.[34] Reduced PTEN and increased Akt signaling also enhance cardiomyogenesis, thereby attenuating adverse left ventricular remodeling. [34] In addition, the mitogen-activated protein kinases (MAPK), including ERK, are known to play fundamental roles in cell survival, proliferation and apoptosis. We also found that the levels of p-Akt and p-ERK were significantly increased in CM treated with ExoGATA-4. Akt negatively regulated the pro-apoptotic Bcl-2 homology domain (BH3)-only proteins, including BIM. ERK can also regulate the transcription of pro-apoptotic genes, including BIM. [35] Quantitative data showed that the three isoforms of BIM (BimEL, BimL, and BimS) were decreased significantly in CM treated with ExoGATA-4. These proteins mediated their effects by binding to and inactivating the pro-survival Bcl-2 family members, which resulted in permeabilization of the outer mitochondrial membrane and activation of the caspase cascade. [36] In the present study, increased resistance of CM to hypoxia in vitro or ischemia in vivo when treated with ExoGATA-4 might be associated with the reduction of PTEN and BIM caused by the action of miR-19a. Consequently, exosomes may be valuable for the therapeutic delivery of RNA interference and miR regulatory molecules.
We also demonstrated that ExoGATA-4 also carry other highly concentrated miRs (e.g., miR-451, miR-221) [9] and growth factors (e.g., IGF-1) (data not shown). MiR-451 has previously been reported to protect CM against ischemia/reperfusion-induced CM death.[37] Overexpression of miR-221/222 increased cell survival, which was associated with the regulation of PUMA expression. [38, 39] Undoubtedly, miR-19a, miR-451, and other anti-apoptotic miRs and paracrine factors have a synergistic effect in ExoGATA-4-mediated cardioprotection. Taken together, our results suggest that exosomes serve an important source and vehicle for the transfer of miRs and other bioactive molecules into CM or other cell targets.
In this study, we used the most current methods and tests to evaluate the differences in exosomes with respect to their miR content, other state-of-the-art methods to identify relevant biomarkers, as well as state-of-the-art echocardiography methods to assess the functional outcomes (global left ventricular performance) of exosome treatment in an in vivo model of ischemic cardiomyopathy. We used a gene engineering technique to increase the regeneration capacity of stem cells in repairing ischemic myocardium. To overcome the limitations of stem cell therapy, we isolated the exosomes from gene engineered stem cells in conditioned medium. We documented that these exosomes from such gene engineered stem cells convey a beneficial effect culminating in improved cardiac function compared to exosomes obtained from native stem cells. In this study, we also found that the exosomes derived from gene engineered stem cells highly expressed several anti-apoptotic miRs which, when delivered to target cells and tissues, regulate several signaling pathways leading to cardiac protection. This study further implicates a novel role for exosomes derived from gene engineered MSC as potential candidates for adjunctive therapy for patients recovering from acute myocardial infarction. In future study, we would like have a systematic in vivo study related to the use of exosomes as a therapeutic bioactive molecule delivery system. We will not only observe the time course of the retention of exosomes, but we also intend to administer exosomes through different routes (e.g., intravenous, intracoronary, etc.) that may be more relevant than intramyocardial injection to clinical administration. We will examine the effect of exosomes in repairing ischemic myocardium, as well as the temporal nature of this effect.
5. Conclusions
This study highlights the importance of MSC derived exosomes and their role in cardioprotection. These exosomes can act as vehicles for cytokines, growth factors and miRs which may participate in cardioprotection or regeneration in the ischemic myocardium. This study further suggests a novel role for MSC-derived exosomes as potential candidates for adjunctive therapy for patients suffering from acute myocardial infarction.
Highlights.
This study highlights the importance of MSC overexpressing GATA-4 derived exosomes (ExoGATA-4) in ischemic myocardium regeneration.
ExoGATA-4 have a higher potential in cardiac protection.
ExoGATA-4 enrich and transfer anti-apoptotic miRs, which results in a regulation of signaling pathways.
Exosomes are potential candidates for adjunctive therapy for acute myocardial infarction.
Acknowledgments
This work was supported by the National Institutes of Health grants HL105176 and HL114654 (M. Xu) and R37HL 074272(M. Ashraf). The authors thank Christian Paul for technical assistance.
Footnotes
7. Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathiasen AB, Haack-Sorensen M, Jorgensen E, Kastrup J. Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina--final 3-year follow-up. International journal of cardiology. 2013;170:246–51. doi: 10.1016/j.ijcard.2013.10.079. [DOI] [PubMed] [Google Scholar]
- 2.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF) American heart journal. 2011;161:1078–87. e3. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation research. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 4.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, et al. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circulation research. 2013;113:153–66. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento DS, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling following myocardial infarction by pro-angiogenic, anti-apoptotic and endogenous cell activation mechanisms. Stem cell research & therapy. 2014;5:5. doi: 10.1186/scrt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Zuo S, Pasha Z, Yu B, He Z, Wang Y, et al. GATA-4 promotes myocardial transdifferentiation of mesenchymal stromal cells via up-regulating IGFBP-4. Cytotherapy. 2011;13:1057–65. doi: 10.3109/14653249.2011.597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, et al. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. American journal of physiology Heart and circulatory physiology. 2010;299:H1772–81. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu B, Gong M, He Z, Wang YG, Millard RW, Ashraf M, et al. Enhanced mesenchymal stem cell survival induced by GATA-4 overexpression is partially mediated by regulation of the miR-15 family. The international journal of biochemistry & cell biology. 2013;45:2724–35. doi: 10.1016/j.biocel.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, et al. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PloS one. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Annals of the New York Academy of Sciences. 2003;996:152–7. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 11.DeSantiago J, Bare DJ, Semenov I, Minshall RD, Geenen DL, Wolska BM, et al. Excitation-contraction coupling in ventricular myocytes is enhanced by paracrine signaling from mesenchymal stem cells. Journal of molecular and cellular cardiology. 2012;52:1249–56. doi: 10.1016/j.yjmcc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:95–102. doi: 10.1016/j.healun.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 14.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem cell research. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochemical and biophysical research communications. 2013;431:566–71. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic acids research. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–8. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 19.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 20.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biology of reproduction. 2009;81:717–29. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 21.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells. 2012;30:1556–64. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–65. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 24.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrader J. Mechanisms of ischemic injury in the heart. Basic research in cardiology. 1985;80 (Suppl 2):135–9. [PubMed] [Google Scholar]
- 26.Montecalvo A, Larregina AT, Morelli AE. Methods of analysis of dendritic cell-derived exosome-shuttle microRNA and its horizontal propagation between dendritic cells. Methods in molecular biology. 2013;1024:19–40. doi: 10.1007/978-1-62703-453-1_3. [DOI] [PubMed] [Google Scholar]
- 27.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovascular research. 2008;77:134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PloS one. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circulation research. 2012;111:312–21. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danielson LS, Park DS, Rotllan N, Chamorro-Jorganes A, Guijarro MV, Fernandez-Hernando C, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27:1460–7. doi: 10.1096/fj.12-221994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circulation research. 2013;112:1557–66. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Yu SL, Wang Y, Guo GY, Ding Q, An RH. MicroRNA-dependent regulation of PTEN after arsenic trioxide treatment in bladder cancer cell line T24. Tumour Biol. 2011;32:179–88. doi: 10.1007/s13277-010-0111-z. [DOI] [PubMed] [Google Scholar]
- 33.Choorapoikayil S, Kers R, Herbomel P, Kissa K, den Hertog J. Pivotal role of Pten in the balance between proliferation and differentiation of hematopoietic stem cells in zebrafish. Blood. 2014;123:184–90. doi: 10.1182/blood-2013-05-501544. [DOI] [PubMed] [Google Scholar]
- 34.Yan B, Singla RD, Abdelli LS, Singal PK, Singla DK. Regulation of PTEN/Akt pathway enhances cardiomyogenesis and attenuates adverse left ventricular remodeling following thymosin beta4 Overexpressing embryonic stem cell transplantation in the infarcted heart. PloS one. 2013;8:e75580. doi: 10.1371/journal.pone.0075580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. The Journal of biological chemistry. 2005;280:20814–23. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–6. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, et al. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. Journal of molecular and cellular cardiology. 2010;49:841–50. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer. 2010;9:229. doi: 10.1186/1476-4598-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma AD, Narain N, Handel EM, Iken M, Singhal N, Cathomen T, et al. MicroRNA-221 regulates FAS-induced fulminant liver failure. Hepatology. 2011;53:1651–61. doi: 10.1002/hep.24243. [DOI] [PubMed] [Google Scholar]