Abstract
The Fragile X-related disorders (FXDs) are members of the group of diseases known as the Repeat Expansion Diseases. The FXDs result from expansion of an unstable CGG/CCG repeat tract in the 5’ UTR of the FMR1 gene. Contractions are also seen, albeit at lower frequency. We have previously shown that ERCC6/CSB plays an auxiliary role in promoting germ line and somatic expansions in a mouse model of the FXDs. However, work in model systems of other Repeat Expansion Diseases has suggested that CSB may protect against expansions by promoting contractions. Since FXD mice normally have such a high expansion frequency, it is possible that such a protective effect would have been masked. We thus examined the effect of the loss of CSB in an Msh2+/− background where the germ line expansion frequency is reduced and in an Msh2+/− background where expansions do not occur, but contractions do. Our data show that in addition to promoting repeat expansion, CSB does in fact protect the genome from germ line expansions in the FXD mouse model. However, it likely does so not by promoting contractions but by promoting an error-free process that preserves the parental allele.
Keywords: ERCC6, CSB, Fragile X syndrome, FXS, FMR1, FX-associated tremor and ataxia syndrome, FX-associated primary ovarian insufficiency
Introduction
The Repeat Expansion Disorders are a large group of human genetic diseases that result from the intergenerational expansion of an unstable short tandem repeat tract or microsatellite somewhere in the affected gene (Fry and Usdin, 2006; Mirkin, 2006). The Fragile X-related disorders (FXDs) are members of this group of diseases resulting as they do from expansion of a CGG/CCG-repeat tract in the 5’ untranslated region of the FMR1 gene (MIM# 309550; reviewed in (Chonchaiya, et al., 2009)). The FXDs comprise three distinct disorders, Fragile X-associated primary ovarian insufficiency (FXPOI; MIM# 311360) and Fragile X-associated tremor and ataxia syndrome (FXTAS; MIM# 300623) that occur in carriers of alleles with 54-200 repeats, so-called premutation (PM) alleles while Fragile X syndrome (FXS; MIM# 300624), the leading heritable cause of intellectual disability (ID) is seen in carriers of full mutation (FM) alleles, alleles that have >200 repeats.
While instability at these loci shows a strong expansion bias, some contractions are also seen. In the case of the FXDs this results in considerable tissue mosaicism that likely complicates the clinical presentation (Moutou, et al., 1997; Nolin, et al., 1994; Petek, et al., 1999; Pretto, et al., 2014; Prior, et al., 1995; Reyniers, et al., 1999; Schmucker and Seidel, 1999; Shapiro, et al., 1994; Tabolacci, et al., 2008; Wohrle, et al., 1998). Many unanswered questions remain as to how this instability arises, including whether expansions and contractions share a common mechanism, and whether the expansion mechanisms differ in the male and female germ line or between the germ line and somatic cells (Dragileva, et al., 2009; Hubert, et al., 2011; Tome, et al., 2011).
While the sequence of the disease-associated repeat varies with the disease, all the repeats share the ability to form unusual secondary structures of one sort or another (Fry and Usdin, 2006; Mirkin, 2006). Very GC-rich repeat units, present in the FXDs and diseases like amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD), have the potential to form hairpins containing a mixture of Watson-Crick and Hoogsteen base pairs, as well as a variety of quadruplex structures (Fojtik and Vorlickova, 2001; Fry and Loeb, 1994; Grigg, et al., 2014; Haeusler, et al., 2014 2014; Mitas, et al., 1995; Renciuk, et al., 2009; Usdin, 1998; Usdin and Woodford, 1995; Yu, et al., 1997). Many of these sequences also form persistent R-loops, consisting of an RNA:DNA hybrid and a displaced strand of DNA (Grabczyk, et al., 2007; Groh, et al., 2014; Loomis, et al., 2014). It is unclear at this point whether the hairpins/quadruplexes favor R-loop formation or vice versa. In any event, current thinking in the field is that one or more of these structures are the substrates upon which the expansion and contraction processes act.
We have previously shown that CSB (approved symbol, ERCC6; MIM# 609413), a protein essential for Transcription Coupled Repair (TCR), plays an auxiliary role in the process that generates maternally transmitted expansions and somatic expansions in males (Zhao and Usdin, 2014). The effect of CSB on female somatic expansion was not examined since somatic expansion occurs much less frequently in females (Lokanga, et al., 2014a). However, in contrast to a role of CSB in generating expansions seen in the FXD mouse, data from a mouse model of Huntington Disease (HD), a CAG/CTG-Repeat Expansion Disease, suggests that CSB protects against germ line repeat expansion by promoting repeat contractions (Kovtun, et al., 2011). This conclusion is consistent with the observation that in tissue culture models of CAG/CTG-repeat contractions knockdown of CSB and other TCR factors, led to fewer contractions (Lin and Wilson, 2007). Identification of a similar mechanism in the FXD mouse model would be significant since while contractions are frequently seen both in FX families and the FXD mouse model, the mechanism responsible for generating them is unknown. In fact, to date no specific pathway that protects against or competes with the expansion pathway has been identified in the FXD mouse model.
To test whether CSB also protects against repeat expansions in the FXD mouse model, we have taken advantage of our previous demonstration that the mismatch repair protein MSH2 (MIM# 609309) is required for all germ line and somatic expansions in a mouse model of the FXDs (Lokanga, et al., 2014b). We thus examined the effect of the loss of CSB on somatic and germ line contractions in an Msh2+/− background. In this background expansions can still occur but at a reduced frequency that should allow any positive effect of the loss of CSB on expansions to be seen. To test the role of CSB in promoting repeat contractions we examined the effect of the loss of CSB in an Msh2−/− background, where no expansions occur but where ~50% of transmitted alleles have undergone contractions. Taken together with our previous work (Zhao and Usdin, 2014), the results of these experiments suggest that CSB has paradoxical effects on CGG/CCG-repeat instability in the FXD mouse model, promoting expansions in some cases and protecting against them in others. However, we show here that CSB’s role in protecting against expansions is likely not mediated via the ability to generate contractions, as reported for other Repeat Expansion Disease models, but rather via the ability to carry out error-free repair to preserve the original allele.
Materials and Methods
Mouse maintenance
The generation of the FXD mice was described previously (Entezam, et al., 2007). Msh2−/− mice were a kind gift of Tak Mak (University Health Network, Toronto, Canada). Csb−/− mice were a kind gift of Vilhelm A. Bohr (National Institutes of Health, Baltimore, MD, USA). Mice were maintained in accordance with the guidelines of the NIDDK Animal Care and Use Committee and with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996). The Csb mutant mice were crossed to Msh2 mutant mice with PM allele (Lokanga, et al., 2014b) to generate FXD mice that were either Msh2−/−, Csb−/− or Msh2+/−, Csb−/−. To study the effect of loss of CSB on germ line expansion we examined the tail DNA of the progeny of crosses where only one parent carried the PM allele and either both parents were Msh2+/−, Csb−/− or both were Msh2−/−, Csb−/−. Msh2+/−, Csb+/+ and Msh2−/−, Csb+/+ breeding pairs were used as controls. Offspring of these crosses were used to study somatic expansion.
Genotyping and repeat analysis
Genomic DNA was isolated from the mice using the KAPA Mouse Genotyping Kit (KAPA Biosystems, Wilmington, MA). The Csb and Msh2 genotyping was carried out using the PCR reagents provided with the kit and primer pairs that we have described previously (Lokanga, et al., 2014b; Zhao and Usdin, 2014). The identification of mice carrying the PM allele and the determination of the repeat number present in the PM allele was determined using a fluorescent PCR assay as described previously (Lokanga et al., 2013). The PCR products were subjected to electrophoresis on a 3730XL Genetic Analyzer (Applied Biosystems, Foster City, CA) and the resultant fsa files analyzed using GeneMapper® 4.0 software as described previously (Lokanga et al., 2013). Statistical analysis was carried out using a web-based version of the GraphPad QuickCalcs Software (http://www.graphpad.com/quickcalcs) and VassarStats (http://vassarstats.net).
Somatic instability analysis
Genomic DNA from the organs of 6-12 month old animals was extracted using a Maxwell®16 Mouse tail DNA purification kit (Promega, Madison, WI) according to the manufacturer’s instructions. The repeat profile was assessed using the same fluorescent PCR assay/GeneMapper analysis referred to above. The somatic instability index (SII) was determined as previously described (Lee, et al., 2010) and used to evaluate the extent of somatic expansion in adult mice.
Results
The loss of CSB results in an increase in the intergenerational expansion frequency in an Msh2+/− background
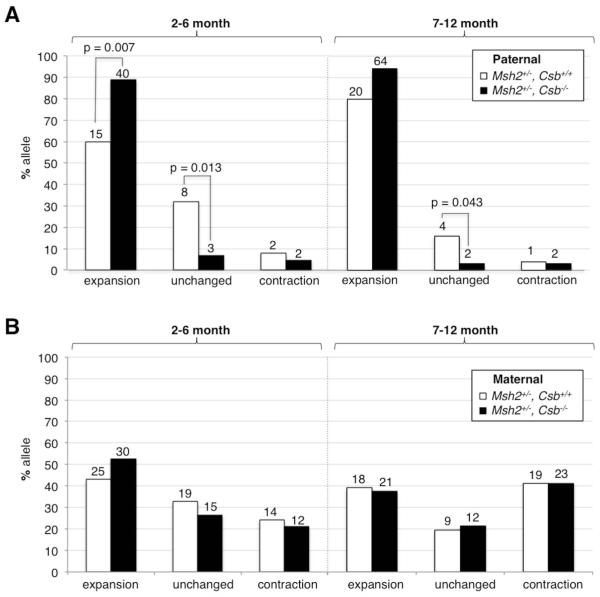
We had previously shown that there was no significant difference in the expansion frequency seen in the progeny of Csb−/− and Csb+/+ males in an Msh2+/+ background (Zhao and Usdin, 2014). We were thus able to conclude that CSB is not required for paternal expansions in a WT background. However, as the expansion frequency in the progeny of Msh2+/+ males was close to 100%, any protective effect against expansions by CSB might have been difficult to discern. To address a potential role of CSB in preventing expansions, we examined the effect of the loss of CSB in an Msh2+/− background where the expansion frequency is much lower. In this background the expansion frequency seen in the progeny of young Csb−/− males was ~89% compared to ~60% in Csb+/+ males of the same age (Fig. 1A, left panel; p=0.007). This increase in expansions was associated with a statistically significant reduction in the number of unchanged alleles (p=0.013). No significant change was seen in the number of contracted alleles.
Fig. 1. The effect of the loss of CSB on the intergenerational expansion frequency in Msh2+/− mice.
The frequency of A) paternally transmitted and B) maternally transmitted alleles that were expanded, unchanged or contracted was examined in the tail DNA of the progeny of Msh2+/−, Csb+/+ and Msh2+/−, Csb−/− taken at weaning. The data for transmitting parents that were 2-6 months old and 7-12 months old were plotted separately. The number above each bar corresponds to the number of animals observed in that category.
In the progeny of fathers 7-12 months old the distribution of allele sizes was similar to that seen in the progeny of younger fathers. However, the difference in the number of expanded alleles transmitted by Csb−/− males compared to Csb+/+ males just failed to reach statistical significance (Fig. 1A, right panel, p=0.055), while the decrease in the number of unchanged alleles barely did so (p=0.043). The smaller effect on expansion in these mice may reflect the confounding effect of the age-related accumulation of expansions in the male germ line that results in very high baseline of expansions in older males (Zhao and Usdin, 2014).
No significant increase was seen in the frequency of expansions in the progeny of Msh2+/−, Csb−/− females (Fig. 1B), perhaps due to the higher contraction frequency in females (Zhao and Usdin, 2014), that offsets any increase in expansions.
Taken together our data from Msh2+/−, Csb−/− animals suggests that CSB, in addition to promoting expansion as we previously demonstrated (Zhao and Usdin, 2014), can also play a role in reducing germ line expansions, an effect that is most prominent in young Msh2+/− males. Since the protective effect of CSB is not seen in Msh2+/+ animals, it suggests that in the WT genetic background the expansion pathway is dominant over the pathway that protects against expansions.
The loss of CSB reduces the extent of somatic instability in an Msh2+/− background
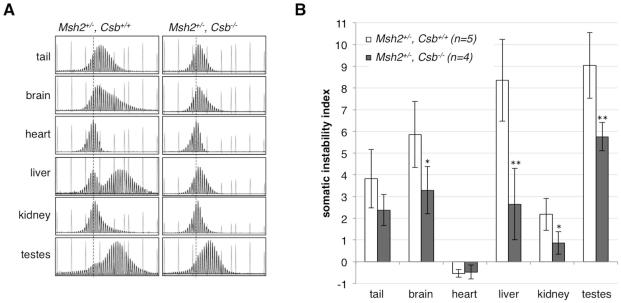
To test whether CSB has a similar protective effect in somatic cells we examined the extent of somatic expansion in various organs of Msh2+/−, Csb−/− males. However, rather than an increase in the extent of somatic expansion, we found that expansion was less extensive in most tissues of Msh2+/−, Csb−/− mice as reflected in the GeneMapper profiles and the somatic instability index (SII), a quantitative measure of the extent of somatic instability (Lee, et al., 2010) (Fig. 2B). This is consistent with the decrease in the SII that we had previously seen in Msh2+/+, Csb−/− animals that was attributed to the role that CSB plays in promoting somatic expansion (Zhao and Usdin, 2014). Thus, the CSB-dependent pathway that protects against expansions in the germ line of the Msh2+/− FXD mouse either does not operate in somatic cells or is masked by the higher efficiency of the expansion pathway in these tissues.
Fig. 2. The effect of the loss of CSB on the extent of somatic expansion in multiple organs of Msh2+/− mice.
A) GeneMapper profiles of 12-month-old Msh2+/−, Csb+/+ and Msh2+/−, Csb−/− mice each with an inherited allele having ~140 repeats. The dotted line indicates the size of the inherited allele based on the allele size in heart. The short grey peaks are derived from the 1200 LIZ® dye size standard. B) The average SII of different organs of mice with 130-140 repeats at 12 months of age. The data represents the average of the individual SIIs from 5 Msh2+/−, Csb+/+ and 4 Msh2+/−, Csb−/− mice. The asterisks indicate the organs in which the differences in SII were statistically significant (* p < 0.05; ** p < 0.01).
Loss of CSB does not significantly affect the intergenerational or somatic contraction frequency in an Msh2−/− background
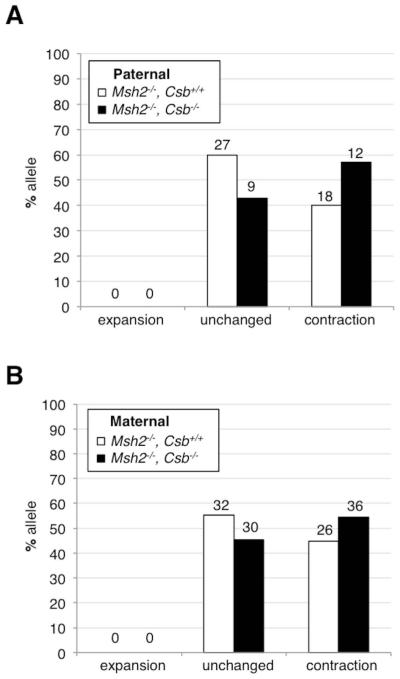
We have previously shown that MSH2 is required for all germ line and somatic expansions in the FXD mouse model and thus that the progeny of Msh2−/− mice only have alleles that either contracted or are the same size as the parental allele (Lokanga, et al., 2014b). Thus the generation of Msh2−/−, Csb−/− mice allowed us to specifically examine the effect of the loss of CSB on the frequency of germ line contractions independent of any confounding expansions. We could thus specifically address the question of whether CSB protects against expansions by promoting contractions. As can be seen in Fig. 3, instead of decreasing the number of contractions as would be expected if CSB was involved in generating contractions, the loss of CSB caused a modest, albeit not statistically significant, increase in the contraction frequency seen on both maternal and paternal transmission of the PM allele.
Fig. 3. The effect of the loss of CSB on the intergenerational contraction frequency in Msh2−/− mice.
The frequency of A) paternally transmitted and B) maternally transmitted alleles that are expanded, unchanged or contracted was examined in the tail DNA of the progeny of Msh2−/−, Csb+/+ and Msh2−/−, Csb−/− parents. Since Msh2−/− mice die young, only data from parents that were 2-6 months old were plotted. The number above each bar corresponds to the number of animals observed in that category.
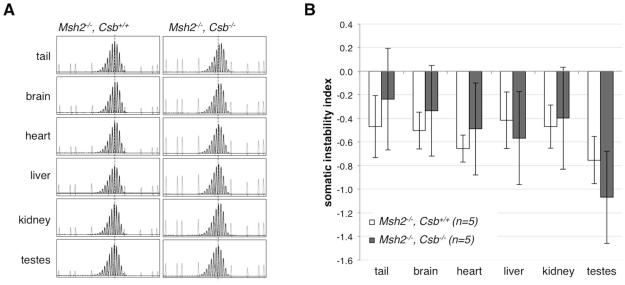
The loss of CSB also had no effect on the GeneMapper profiles or SII, of DNA isolated from different organs of 6-month-old Msh2−/− Csb−/− male mice (Fig. 4). Thus even in the absence of a functional somatic expansion pathway, loss of CSB does not result in the appearance of somatic contractions. This may reflect the fact that few, if any, somatic contraction events occur in adult animals and lends weight to our original conclusions that the lower SIIs seen in CSB-deficient animals reflects a role for CSB in promoting expansions rather than in preventing contractions (Zhao and Usdin, 2014).
Fig. 4. The effect of the loss of CSB on the extent of somatic expansion in multiple organs of Msh2−/− mice.
A) GeneMapper profiles of 6-month-old Msh2−/−, Csb+/+ and Msh2−/−, Csb−/− mice each with an inherited allele having ~140 repeats. The dotted line indicates the size of the inherited allele based on the allele size in heart, an organ that shows little or no somatic instability (Lokanga, et al., 2013). The short grey peaks are derived from the 1200 LIZ® dye size standard. B) The average SII of different organs of mice with 130-140 repeats at 6-9 months of age. The data represents the average of the individual SIIs from 5 Msh2−/−, Csb+/+ and 5 Msh2−/−, Csb−/− mice. The differences were not statistically significant. The SII for all organs is slightly negative. However, this does not reflect the presence of somatic contractions, since the SII is similar throughout the animal’s lifespan. Rather, these small negative values are thought to reflect presence of “stutter products” resulting from strand slippage during PCR.
Discussion
Our study of the effect of the loss of CSB in MSH2-deficient backgrounds has allowed us to uncover an apparently paradoxical role for CSB in repeat instability in a mouse model of the FXDs. Not only does CSB promote maternal germ line expansions as we previously reported (Zhao and Usdin, 2014), but under some circumstances CSB also protects the paternal germ line against repeat expansions. As shown in Fig. 1, loss of CSB in an Msh2+/− background, results in an increase in the number of intergenerationally transmitted expansions. These expansions occur at the expense of alleles that are the same size as the parental allele rather than at the expense of contracted ones. This result, taken together with our demonstration that CSB does not promote contractions (Fig. 3), suggests that CSB is not acting to reduce expansions in the FXD mouse model by promoting contractions as reported for the HD mouse (Kovtun, et al., 2011). Instead, we speculate that CSB acts by competing with the expansion pathway to preserve the original allele as part of an error-free repair process. The pathway that results in the contractions that are seen particularly in older females, or in younger males and females when the expansion pathway is compromised (Fig. 3), still remains to be identified, but is likely to be distinct from either the expansion or the error-free pathway.
The apparent paradox that CSB both promotes and prevents expansion can be resolved if it is assumed that CSB acts as a component of one pathway to promote expansions and via a different pathway to prevent them. However, the identity of these pathways is still an open question. We had previously suggested that the role played by CSB in the generation of expansions involved a process other than TCR, since CSB, which is essential for TCR, seemed to be playing an auxiliary rather than obligatory role in expansion (Zhao and Usdin, 2014). CSB has many activities that would be compatible with such a role (reviewed in (Lake and Fan, 2013). However, since TCR involves removal of the damaged region XPG, an endonuclease that is insensitive to secondary structures (Hohl, et al., 2007), it may be less prone to the incorporation of supernumerary bases into the “repaired” strand than repair processes (e.g., base excision repair) that use endonucleases like FEN1.
The ability of CSB to protect against germ line expansions in Msh2+/− mice is masked in WT animals by the relative efficacy of the expansion process. It remains to be seen whether the same is true in humans. It has been previously suggested that down-regulation of the expansion pathway could have therapeutic potential for reducing expansion risk (Dragileva, et al., 2009; Gonitel, et al., 2008; Halabi, et al., 2012; Lopez Castel, et al., 2010). Upregulation of the protective pathway we have identified here may likewise be useful in reducing the likelihood of expansion.
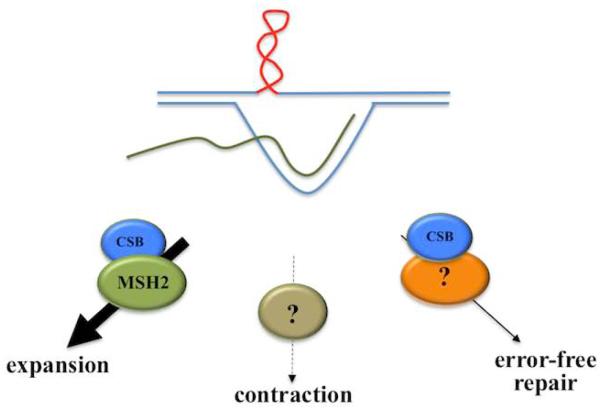
The data shown here, along with our previously published data on the role of MSH2 (Lokanga, et al., 2014b) and CSB (Zhao and Usdin, 2014), supports a view in which three different pathways compete for the instability substrate as illustrated in Fig. 5. There is an MSH2-dependent, CSB-facilitated pathway that generates expansions, an error-free pathway involving CSB that restores the original allele and a third pathway that generates contractions, whose components have yet to be identified. The extent of expansion in different cells or in males and females could depend, at least in part, on how effectively the three pathways compete for the chance to process the instability substrate.
Fig. 5. Model for the generation of expansions, contractions and unchanged PM alleles in the FXD mouse model.
The secondary structure that is thought to be the substrate for expansion, shown in red on the top strand, can arise any time the repeat is unpaired. This could be during transcription as drawn here with the FMR1 mRNA shown in green, or during other DNA processing events. This structure can then be processed one of three ways, via a MSH2-dependent process in which CSB plays an auxiliary role to generate an expansion, via a contraction pathway whose constituents are currently unknown, or via a error-free process that involves CSB that preserves the original allele.
Acknowledgments
The Usdin lab would like to acknowledge all the hard work by the people that take care of the mice used in this study. Without their help, this work would not have been possible. The work described in this manuscript was funded by a grant from the Intramural Program of the NIDDK to KU (DK057808-07)
Grant Sponsor: Intramural program of the NIDDK, NIH (DK057808)
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Chonchaiya W, Schneider A, Hagerman RJ. Fragile X: a family of disorders. Adv Peds. 2009;56:165–86. doi: 10.1016/j.yapd.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragileva E, Hendricks A, Teed A, Gillis T, Lopez ET, Friedberg EC, Kucherlapati R, Edelmann W, Lunetta KL, MacDonald ME. Intergenerational and striatal CAG repeat instability in Huntington's disease knock-in mice involve different DNA repair genes. Neurobiol Dis. 2009;33(1):37–47. doi: 10.1016/j.nbd.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395(1-2):125–34. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojtik P, Vorlickova M. The fragile X chromosome (GCC) repeat folds into a DNA tetraplex at neutral pH. Nucleic Acids Res. 2001;29(22):4684–90. doi: 10.1093/nar/29.22.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Loeb LA. The fragile X syndrome d(CGG)n nucleotide repeats form a stable tetrahelical structure. Proc Natl Acad Sci U S A. 1994;91(11):4950–4. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Usdin K. Human Nucleotide Expansion Disorders. Springer; Heidelberg: 2006. [Google Scholar]
- Gonitel R, Moffitt H, Sathasivam K, Woodman B, Detloff PJ, Faull RL, Bates GP. DNA instability in postmitotic neurons. Proc Natl Acad Sci U S A. 2008;105(9):3467–72. doi: 10.1073/pnas.0800048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabczyk E, Mancuso M, Sammarco MC. A persistent RNA.DNA hybrid formed by transcription of the Friedreich ataxia triplet repeat in live bacteria, and by T7 RNAP in vitro. Nucleic Acids Res. 2007;35(16):5351–9. doi: 10.1093/nar/gkm589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JC, Shumayrikh N, Sen D. G-quadruplex structures formed by expanded hexanucleotide repeat RNA and DNA from the neurodegenerative disease-linked c9orf72 gene efficiently sequester and activate heme. PLoS One. 2014;9(9):e106449. doi: 10.1371/journal.pone.0106449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh M, Lufino MM, Wade-Martins R, Gromak N. R-loops Associated with Triplet Repeat Expansions Promote Gene Silencing in Friedreich Ataxia and Fragile X Syndrome. PLoS Genet. 2014;10(5):e1004318. doi: 10.1371/journal.pgen.1004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler AR, Donnelly CJ, Periz G, Simko EA, Shaw PG, Kim MS, Maragakis NJ, Troncoso JC, Pandey A, Sattler R. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507(7491):195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabi A, Ditch S, Wang J, Grabczyk E. DNA mismatch repair complex MutSbeta promotes GAA.TTC repeat expansion in human cells. J Biol Chem. 2012;287(35):29958–67. doi: 10.1074/jbc.M112.356758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Dunand-Sauthier I, Staresincic L, Jaquier-Gubler P, Thorel F, Modesti M, Clarkson SG, Scharer OD. Domain swapping between FEN-1 and XPG defines regions in XPG that mediate nucleotide excision repair activity and substrate specificity. Nucleic Acids Res. 2007;35(9):3053–63. doi: 10.1093/nar/gkm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert L, Jr., Lin Y, Dion V, Wilson JH. Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1. Hum Mol Genet. 2011;20(24):4822–30. doi: 10.1093/hmg/ddr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun IV, Johnson KO, McMurray CT. Cockayne syndrome B protein antagonizes OGG1 in modulating CAG repeat length in vivo. Aging (Albany NY) 2011;3(5):509–14. doi: 10.18632/aging.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake RJ, Fan HY. Structure, function and regulation of CSB: a multi-talented gymnast. Mech Ageing Dev. 2013;134(5-6):202–11. doi: 10.1016/j.mad.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zhang J, Su AI, Walker JR, Wiltshire T, Kang K, Dragileva E, Gillis T, Lopez ET, Boily MJ. A novel approach to investigate tissue-specific trinucleotide repeat instability. BMC Syst Biol. 2010;4:29. doi: 10.1186/1752-0509-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wilson JH. Transcription-induced CAG repeat contraction in human cells is mediated in part by transcription-coupled nucleotide excision repair. Mol Cell Biol. 2007;27(17):6209–17. doi: 10.1128/MCB.00739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokanga AR, Zhao X-N, Entezam A, Usdin K. X inactivation plays a major role in the gender bias in somatic expansion in a mouse model of the Fragile X-related Disorders: implications for the mechanism of repeat expansion. Hum Mol Genet. 2014a;23(18):4985–4994. doi: 10.1093/hmg/ddu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokanga RA, Entezam A, Kumari D, Yudkin D, Qin M, Smith CB, Usdin K. Somatic expansion in mouse and human carriers of Fragile X premutation alleles. Hum Mutat. 2013;34(1):157–66. doi: 10.1002/humu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokanga RA, Zhao X-N, Usdin K. The mismatch repair protein, MSH2, is rate-limiting for repeat expansion in a Fragile X premutation mouse model. Hum Mutat. 2014b;35:129–136. doi: 10.1002/humu.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis EW, Sanz LA, Chedin F, Hagerman PJ. Transcription-Associated R-Loop Formation across the Human FMR1 CGG-Repeat Region. PLoS Genet. 2014;10(4):e1004294. doi: 10.1371/journal.pgen.1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Castel A, Cleary JD, Pearson CE. Repeat instability as the basis for human diseases and as a potential target for therapy. Nat Rev Mol Cell Biol. 2010;11(3):165–70. doi: 10.1038/nrm2854. [DOI] [PubMed] [Google Scholar]
- Mirkin SM. DNA structures, repeat expansions and human hereditary disorders. Curr Opin Struct Biol. 2006;16(3):351–8. doi: 10.1016/j.sbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Mitas M, Yu A, Dill J, Haworth IS. The trinucleotide repeat sequence d(CGG)15 forms a heat-stable hairpin containing Gsyn. Ganti base pairs. Biochemistry. 1995;34(39):12803–11. doi: 10.1021/bi00039a041. [DOI] [PubMed] [Google Scholar]
- Moutou C, Vincent MC, Biancalana V, Mandel JL. Transition from premutation to full mutation in fragile X syndrome is likely to be prezygotic. Hum Mol Genet. 1997;6(7):971–9. doi: 10.1093/hmg/6.7.971. [DOI] [PubMed] [Google Scholar]
- Nolin SL, Glicksman A, Houck GE, Jr., Brown WT, Dobkin CS. Mosaicism in fragile X affected males. Am J Med Genet. 1994;51(4):509–12. doi: 10.1002/ajmg.1320510444. [DOI] [PubMed] [Google Scholar]
- Petek E, Kroisel PM, Schuster M, Zierler H, Wagner K. Mosaicism in a fragile X male including a de novo deletion in the FMR1 gene. Am J Med Genet. 1999;84(3):229–32. [PubMed] [Google Scholar]
- Pretto DI, Mendoza-Morales G, Lo J, Cao R, Hadd A, Latham GJ, Durbin-Johnson B, Hagerman R, Tassone F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J Med Genet. 2014;51(5):309–18. doi: 10.1136/jmedgenet-2013-102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior TW, Papp AC, Snyder PJ, Sedra MS, Guida M, Enrile BG. Germline mosaicism at the fragile X locus. Am J Med Genet. 1995;55(3):384–6. doi: 10.1002/ajmg.1320550327. [DOI] [PubMed] [Google Scholar]
- Renciuk D, Zemanek M, Kejnovska I, Vorlickova M. Quadruplex-forming properties of FRAXA (CGG) repeats interrupted by (AGG) triplets. Biochimie. 2009;91(3):416–22. doi: 10.1016/j.biochi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Reyniers E, Martin JJ, Cras P, Van Marck E, Handig I, Jorens HZ, Oostra BA, Kooy RF, Willems PJ. Postmortem examination of two fragile X brothers with an FMR1 full mutation. Am J Med Genet. 1999;84(3):245–9. doi: 10.1002/(sici)1096-8628(19990528)84:3<245::aid-ajmg16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schmucker B, Seidel J. Mosaicism for a full mutation and a normal size allele in two fragile X males. Am J Med Genet. 1999;84(3):221–5. [PubMed] [Google Scholar]
- Shapiro LR, Simensen RJ, Wilmot PL, Fisch GS, Vibert BK, Fenwick RG, Tarleton J, Phelan MC. Asymmetry of methylation with FMR-1 full mutation in two 45,X/46,XX mosaic females associated with normal intellect. Am J Med Genet. 1994;51(4):507–8. doi: 10.1002/ajmg.1320510443. [DOI] [PubMed] [Google Scholar]
- Spiro C, Pelletier R, Rolfsmeier ML, Dixon MJ, Lahue RS, Gupta G, Park MS, Chen X, Mariappan SV, McMurray CT. Inhibition of FEN-1 processing by DNA secondary structure at trinucleotide repeats. Mol Cell. 1999;4(6):1079–85. doi: 10.1016/s1097-2765(00)80236-1. [DOI] [PubMed] [Google Scholar]
- Tabolacci E, Pomponi MG, Pietrobono R, Chiurazzi P, Neri G. A unique case of reversion to normal size of a maternal premutation FMR1 allele in a normal boy. Eur J Hum Genet. 2008;16(2):209–14. doi: 10.1038/sj.ejhg.5201949. [DOI] [PubMed] [Google Scholar]
- Tome S, Panigrahi GB, Castel AL, Foiry L, Melton DW, Gourdon G, Pearson CE. Maternal germline-specific effect of DNA ligase I on CTG/CAG instability. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr099. [DOI] [PubMed] [Google Scholar]
- Usdin K. NGG-triplet repeats form similar intrastrand structures: implications for the triplet expansion diseases. Nucleic Acids Res. 1998;26(17):4078–85. doi: 10.1093/nar/26.17.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K, Woodford KJ. CGG repeats associated with DNA instability and chromosome fragility form structures that block DNA synthesis in vitro. Nucleic Acids Res. 1995;23(20):4202–9. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohrle D, Salat U, Glaser D, Mucke J, Meisel-Stosiek M, Schindler D, Vogel W, Steinbach P. Unusual mutations in high functioning fragile X males: apparent instability of expanded unmethylated CGG repeats. J Med Genet. 1998;35(2):103–11. doi: 10.1136/jmg.35.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Barron MD, Romero RM, Christy M, Gold B, Dai J, Gray DM, Haworth IS, Mitas M. At physiological pH, d(CCG)15 forms a hairpin containing protonated cytosines and a distorted helix. Biochemistry. 1997;36(12):3687–99. doi: 10.1021/bi9625410. [DOI] [PubMed] [Google Scholar]
- Zhao X-N, Usdin K. Gender and cell-type specific effects of the transcription coupled repair protein, ERCC6/CSB, on repeat expansion in a mouse model of the Fragile X-related disorders. Hum Mutat. 2014;35(3):341–349. doi: 10.1002/humu.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]