Abstract
While numerous studies indicate the involvement of the hippocampus in encoding and retrieval of spatial and temporal context, the neural basis of spatial and temporal processing within the hippocampal circuit remains unclear. We employed a novel paradigm in which participants encoded stores within a spatial layout by visiting them in a specific temporal order. Participants then underwent high-resolution functional magnetic resonance imaging (fMRI) targeting the hippocampus while retrieving details of the spatial or temporal context in alternating blocks. During retrieval, participants made judgments about either near or far intervals within the spatial layout or temporal sequence. Across both near and far intervals, we found that retrieving spatial layout and temporal order information resulted in comparable levels of activation in the hippocampus that was not preferentially localized to a specific subfield. Furthermore, using a multivariate approach called multivariate pattern similarity analysis (MPSA), we found that correct near judgments vs. correct far judgments differed in their patterns of activity for spatial vs. temporal order judgments. Despite these differences in MPSA patterns, we did not find any specific subfields differentially recruited for spatial vs. temporal order retrieval. We discuss our results in terms of their relation to computational models of hippocampal subfield function and suggest mechanisms by which the hippocampus could process space and temporal order without the need for specific contributions from hippocampal subfields.
Keywords: Hippocampus, fMRI, Spatial, Temporal, Navigation, Multivariate
1. Introduction
Details regarding where and when an event occurred form critical parts of our episodic memory [1–4]. For example, where we were or what time we met a friend that day often serve as potent cues for remembering what we had for dinner last night. As part of its role in storage and retrieval of episodic memories [3,5–8], recent evidence strongly implicates the hippocampus and surrounding cortices in processing spatial and temporal context. Lesion evidence suggests the human medial temporal lobes support aspects of processing both spatial and temporal details of recently learned information [9,10]. fMRI evidence demonstrates hippocampal and parahippocampal involvement, as indexed by increases in the BOLD signal, during encoding and retrieval of spatial [11–15] and temporal [16,17] context. Paradigms involving retrieval of both spatial and temporal context similarly demonstrate hippocampal involvement [18–22]. Finally, recent evidence suggests that the hippocampus contains not only ‘place cells,’ but also ‘time cells,’ [4,23–26]. Yet while there is fairly broad consensus that the hippocampus is important for storing and retrieving spatial and temporal context as part of episodic memory, the exact manner in which it processes spatial and temporal context remains unknown.
The hippocampus is comprised of cytoarchitechtonically distinct subfields, the dentate gyrus, CA3, CA1, and the subiculum. While several past studies have suggested the importance of hip-pocampal subfields to human episodic memory, particularly CA3 and CA1 [27–29], pinpointing their contributions to spatial and temporal episodic memory remains an important challenge. Gilbert et al. [30], for example, found a double dissociation in deficits for spatial distance discriminations (distinguishing two object-location pairs on a grid from the previous sample phase) and spatial temporal order discriminations (distinguishing two maze arms based on their presentation order from the previous sample phase) following DG and CA1 lesions. The authors concluded differential subregion involvement for spatial and (spatial) temporal processing [30]. An equivalent subfield distinction for spatial vs. temporal processing, however, has yet to be reported in the human fMRI literature [21,22]. Azab et al. found that judgments about spatial arrays and temporal order of presentation resulted in comparable levels of adaptation across hippocampal subfields. Similarly, Copara et al. found that activation patterns for spatial and temporal order judgments spanned multiple subfields but did not find differences between spatial and temporal retrieval, suggesting that spatial and temporal order judgments were not restricted to specific subfields.
One possibility, however, is that these two studies did not see differences in subfield involvement in spatial vs. temporal processing due to features of their task design. Specifically, one important factor not controlled for in the two studies is that of interval distance. For instance, the deficits present in Gilbert et al. vary according to distance in both the spatial and temporal tasks. Nearby trials in the [30] experiment were more difficult for rats to discriminate than more distant trials, which in turn showed greater deficits following CA3 and CA1 lesions, respectively. Additionally, several studies have shown that deficits and general performance depend on spatial and temporal distance [31–34], showing that discrimil nation is more difficult as distance decreases for both spatial and temporal judgments. Thus, it is unclear whether the distance discrimination in Copara et al. elicited similar task demands to that of Gilbert et al. For example, in the Copara et al. study, participants were asked which of two items was closer to a reference item. It is possible that the participants in the Copara et al. study consistently relied on the farther of the two distances in order to correctly respond. Similarly, Azab et al. did not explicitly manipulate spatial or temporal distance when participants viewed arrays that varied their spatial or temporal information during encoding. Thus, it could be that a task that better controls distance may show the distinction that Azab et al. and Copara et al. did not. By better controlling near vs. far intervals between spatial and temporal judgments, based on past animals studies [30], it would be reasonable to expect greater CA3/DG activation during spatial trials and greater CA1 activation for temporal trials. We might also expect differences as a function of near vs. far intervals.
To better understand how spatial and temporal context might be coded within the hippocampal circuitry, we employed high-resolution fMRI coupled with both a univariate and multivariate approach to our fMRI data. Univariate approaches are generally recognized to provide insight into the degree to which a brain area is recruited during a task (e.g., [35]). Multivariate pattern similarity [36,37], in contrast, is a technique involving comparison of patterns of activity in a collection of voxels between different trials. This necessitates correlating the pattern of voxel activity during different trials, which we did by comparing patterns of voxels during judgments involving multiple cued elements that were either nearby or faraway with regard to space or time. The pattern of voxels recruited may in turn provide insight into the nature of representations used to solve the task [37]. We employed high-resolution imaging to better localize signals within the medial temporal lobe to subareas such as PHC, CA1, and CA2/CA3/DG (CA2/CA3/DG cannot be segmented at the acquired resolution), using the same imaging sequence as our previous study [22]. By asking participants to judge whether the two probe stores were the same or different interval distance from the reference, we could inquire about the degree of activation as well as the patterns of activity evoked within the hippocampus for successful retrieval of spatial vs. temporal context during both near (contiguous) and far (non-contiguous) judgments. Judgments during which participants successfully determined that two probe stores were the same distance from the reference store better control for spatial or temporal distance than trials that involved different intervals, as used in our past study [22], and thus same interval trials were the primary focus of our analysis. This in turn allowed us to investigate (1) whether the degree of activation differed as a function of subfield during spatial vs. temporal order retrieval; (2) whether the degree of activation differed as a function of subfield during near vs. far judgments; (3) whether the (multivariate) patterns of activation differed during spatial vs. temporal order retrieval; (4) whether the (multivariate) patterns of activation differed during near vs. far judgments.
2. Materials and methods
2.1. Participants
We tested a total of 18 participants (10 female; ages 18–30) from the University of California-Davis and the surrounding communities; two participants were excluded due to below chance performance on all four conditions leaving a total of 16 participants. All were right-handed and screened for neurological disorders, and were paid for their participation. All procedures were in accordance with our Internal Review Board (IRB) Guidelines for experimental testing.
2.2. Behavioral design: encoding
Participants navigated a virtual city designed using the Landmarks 1.0 package for the Unity game development platform (Unity Technologies, San Francisco, CA). The city contained six stores unevenly spaced in a rectangular environment, such that each store was equidistant from at least one pair of stores, and of unequal distance from at least one pair of stores. This ensured that later retrieval of relative distances would be unbiased by the relative probability of an equidistant or unequally distant comparison. Field-of-view, depth-of-view, and the dimensions of the layout were designed such that active navigation of the whole environment was required to encode the relative locations of the stores and the geometry of the environment.
Participants were instructed to deliver to each store in a specified order, moving directly from one store to the next. This delivery order was designed to be uncorrelated with the spatial arrangement of the stores. We further designed the delivery order to avoid any spatial pattern to the temporal sequence (i.e., the order of deliveries defining a star shape), discouraging the use of spatial strategy in encoding the delivery order. Following one round of practice encoding and retrieval (consisting of colored, unlabeled block-shaped stores in a smaller city to avoid similarity to the testing environment), participants encoded the experimental city as part of the main experiment over six blocks (see Fig. 1A and B). These six blocks were broken into three consecutive encoding blocks during which participants were instructed to attend space and three consecutive blocks during which they were instructed to attend to temporal order. During the spatial encoding blocks, participants were instructed to pay attention to the locations of the stores, and the relative distances between them. After each ‘spatial’ block, participants sketched maps representing the layout of the stores to confirm the use of a spatial strategy for encoding distances. During the temporal encoding blocks, participants were instructed to pay attention to the order of the deliveries and the intervals between deliveries. After each ‘temporal’ block, participants wrote the six store names in the order of the delivery sequence to confirm the use of a temporal strategy for encoding intervals. The location of the stores and the delivery order were identical for each block. Participants were told that they would be later tested on this information. By having participants draw sketch maps and write out the serial order of deliveries of stores, we ensured participants encoded the two pieces of information as a spatial layout and temporal sequence, respectively. We counterbalanced the order of the encoding blocks (i.e., spatial first, or temporal first) equally across participants. We employed two cities, differing in store identity, layout, and delivery order. Stores in the unstudied city served as lures for the studied city, and vice versa.
Fig. 1.
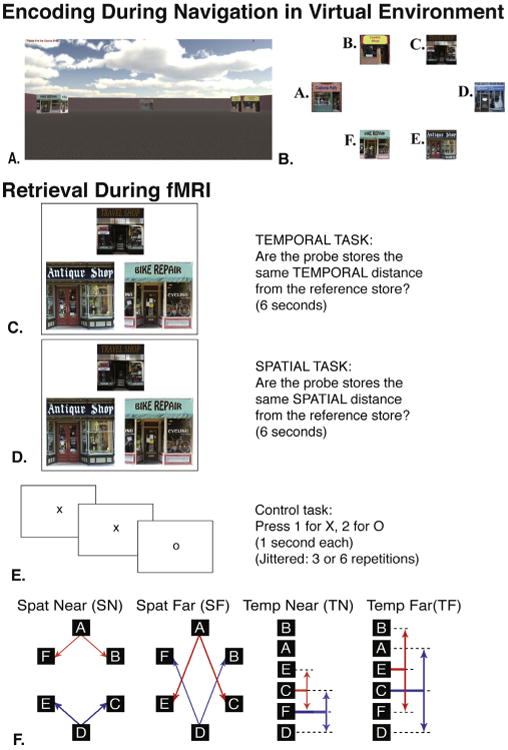
Encoding and retrieval. (A) An example view of what subjects might see during encoding within the virtual reality environment. (B) Overhead map of the virtual environment. (C and D) An example retrieval triad for temporal trials (C) and spatial trials (D). Subjects were shown the same triads of storefronts in both spatial and temporal blocks, but were given separate instructions before starting each run. The reference store is always the top store while source stores are the two bottom stores. Note, that the reference store is not the same spatial distance from each source store, but is the same temporal interval from each source store. (E) A control task was intermixed with encoding and was jittered to last 3 or 6 s. Subjects made responses to random sequences of X's and O's. (F) Trials of interest were binned into 2 distance/interval categories within both spatial and temporal blocks. Visual representations of the involved trials are shown with the red and blue arrows. Lettered store identifiers demonstrate the lack of correlation between spatial layout (left side) and delivery order (right side). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Behavioral design: retrieval during fMRI
We employed an event-related, blocked design paradigm. Retrieval was divided into five consecutive spatial blocks, and five consecutive temporal blocks. We structured the order of retrieval blocks (spatial or temporal) across participants for a fully counterbalanced design across encoding and retrieval. The spatial and temporal tasks were identical during retrieval. Each spatial and temporal block began with instructions before imaging began reminding participants whether they were beginning a spatial or temporal block.
For each retrieval trial, participants viewed a triad of stores with no accompanying text or other details (see Fig. 1C and D). Participants were instructed to compare the spatial distance or temporal interval between each (of two) ‘probe’ store (lower portion of stimuli) and a single ‘reference’ store (upper portion of stimuli). For spatial trials, participants indicated with a button press whether the distance from the left probe store to the reference store was the same as the distance from the right probe store to the reference store. Similarly, for temporal trials, participants indicated whether the interval between the delivery to the left probe store and to the reference store was the same as the interval between the delivery to the right probe store and to the reference store. Retrieval stimuli were presented for 6 s. Participants performed an active baseline task dispersed between trials (see Fig. 1E) to better model task-related hippocampal activations [38], in which they pressed “1” for the appearance ofan “X”, and “2” for the appearance of an “O”. Each letter appeared for 1 s and each baseline presentation varied between 3 or 6 letters/s as determined using opt-seq2 for optimizing event related design studies [39].
The same 180 trials were presented in corresponding spatial and temporal blocks. Seventy-two of these trials served as the focus of our investigation and had probe stores which shared an equal distance from the reference store. Of these, 36 trials were ‘near’ comparisons in which the source stores were one spatial or temporal “step” from the reference, and the other 36 trials involved ‘far’ comparisons in which the source stores were two spatial or temporal steps from the reference. Seventy-two trials presented ‘unequal’ comparisons in which the two source stores were an unequal spatial or temporal distance from the reference store. Additionally, 36 lure trials presented stores from the unstudied city. Participants were instructed to press button “1” for all ‘equal’ comparisons, button “2” for all ‘unequal’ comparisons, and button “3” for lure trials.
For spatial trials, equally ‘near’ probe stores were one arbitrary unit away from the reference store (a distance of roughly 6× the width of a store away from the reference store), and were adjacent to the references store. Spatial equally ‘far’ trials contained probe stores that were at least twice the distance of ‘near’ stores from the reference, and the reference had at least one other pair of probe stores positioned more closely to the reference. For temporal trials, equally ‘near’ meant each probe store was one step removed on the list from the reference store; one probe immediately before, and one probe immediately after the reference store (i.e., red temporal near trial E-C-F in Fig. 1B, C, D, F). Temporal equally ‘far’ trials had probe stores such that one probe occurred two deliveries before, and the other two deliveries after delivery to the reference store (i.e., blue temporal far trial A-C-D in Fig. 1F). For spatial and temporal blocks, any disparity in Euclidean distance or temporal interval, respectively, constituted an unequal trial. Trials were designed such that correct responses for spatial trials were uncorrelated with temporal trials. Stimuli were designed such that both pictorial arrangements for each triad were presented (i.e., probe-stores left-right position swapped as E-C-F and F-C-E). Each triad configuration (i.e., E-C-F) appeared nine times in each context (spatial and temporal). Additionally, trials were pseudo-randomized such that no identical trials appeared within 24 s of each other and the order of trials was identical for spatial and temporal blocks.
2.4. Imaging methods
All participants were tested immediately following encoding in the Siemens 64-Channel 3T “Skyra” scanner, located in the University of California-Davis Imaging Research Center in Davis (typically about 20 min after the completion of encoding to allow time for positioning in the scanner). High-resolution images were acquired employing T2-weighted turbo-spin echo (TSE) anatomical sequences (TR= 4200.0 ms, TE = 93.0 ms, FOV = 1.9 mm, flip angle = 139°, bandwidth = 199 Hz/pixel), with a resolution of .4 mm × .4 mm × 2 mm and an echo-planar imaging (EPI) sequence (TR = 3000 ms, TE = 29 ms, slices = 36, field of view (FOV) = 192 mm, flip angle = 90°, bandwidth = 1462 Hz/pixel), with a final resolution of 1.6 mm × 1.6 mm × 2 mm. Sequences were acquired perpendicular to the long axis of the hippocampus. An additional matched-bandwidth sequence was acquired to aid in registration of the EPI sequence to the high-resolution scan (TR = 3000 ms, TE = 38 ms, slices = 36, FOV = 245 mm, flip angle = 90°, bandwidth = 1446 Hz/pixel).
2.5. Analysis methods
Preprocessing and parameter estimation was performed in Statistical Parametric Mapping (SPM8, The Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). Each EPI sequence underwent slice-timing and motion correction before estimation using a canonical hemodynamic response function. All multivariate pattern similarity analyses were performed on unsmoothed data. Parameter estimates (betas) were analyzed in MATLAB using in-house scripts. Diffeomorphic warping was performed using Advanced Normalization Tools (ANTs) to warp each participants hippocampus to a representative subject [40].
2.6. Task vs. baseline univariate ROI analysis
To compare task related activity to our baseline task, a single parameter estimate for each block modeled all tasks against an implicitly modeled baseline, with an additional nuisance regressor modeling motion artifact. Parameter estimates were averaged across block and ROI. Testing blocks with below chance performance were not analyzed (an average of 0.6 blocks per subject ± 0.4 out of 10 total). Finally, activation levels were compared with baseline using a one-sample t test.
2.7. Trial type univariate analysis
Parameter estimates were generated using a design matrix including a separate regressor for each trial and an additional nuisance regressor modeling motion artifact. This generated a unique beta image for each of our 180 spatial and 180 temporal trials. Voxels 2 standard deviations from the mean were excluded from analysis. Correct trials were then binned and averaged according to block (spatial vs. temporal) and interval (near vs. far), leaving each participant with a mean beta image for each condition. Each participant's parameter estimates were then aligned to group space via a representative template participant using ANTs [40]. To ensure sub-region alignment, diffeomorphic warping parameters were generated using a registration protocol developed in our lab. This protocol makes use of ANTs ability to combine multiple similarity metrics into a single alignment metric. This alignment metric weighs the similarity of both structural images and centroid-weighted ROI information to achieve group level ROI alignment. All multivariate pattern similarity analyses were performed using the beta estimates described in this section.
2.8. Searchlight multivariate pattern similarity analysis
Multivariate pattern similarity analysis (MPSA) involves correlating parameter estimates between two different trials within a collection of voxels. We performed MPSA using a searchlight methodology [37], which involved correlated parameter estimates within condition (spatial near vs. spatial near) using a 2 voxel radius searchlight sphere. The searchlight sphere traversed through the entire MTL, and thus identified areas within the MTL without any pre-identification by subregion. As the searchlight volume traveled from one voxel to another, it calculated MPS for each condition at each new voxel to create a statistical map for each condition. These subject level maps were then warped to group space, contrasted, and clustered analogously to univariate group analysis techniques. Control of false positives is discussed later in this section.
2.9. Multivariate pattern similarity trial comparisons
In all our MPS analyses, correlations were made between two trials of the same condition. In our primary analysis, we performed correlations between all trials within the same trial category (e.g., spatial near correlated with other spatial near trials). Since correlations are inherently a pairwise comparison, many correlations were performed and then averaged together for a metric of within-condition similarity (Fig. 2). Specifically, we calculated all possible combinations of comparisons between independent trials that corresponded to a specific trial type, yielding a correlation matrix for each condition. The unique values (upper triangle) of this correlation matrix were then averaged together to give a metric of overall correlation within a trial type. Notably, within-block comparisons were excluded due to temporal autocorrelation of parameter estimates. Within and between triad analyses were performed following the above procedure with the exception that correlations were further segregated according to within-triad and between-triad. Correlating across all possible triads within spatial and temporal context provided insight into the extent to which elements within these two contexts share commonalities.
Fig. 2.
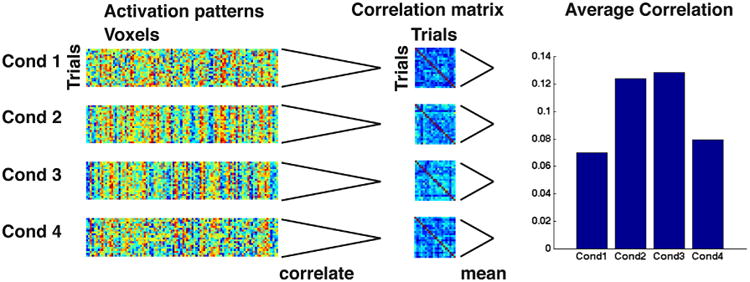
Visual representation of analysis method. This figure demonstrates the flow of each subject's data. (1) Parameter estimates of all trials are extracted within a collection of voxels and sorted according to condition. (2) Within each condition, all combinations of pairwise correlations are computed to give a trial × trial correlation matrix. (3) Within-block comparisons are removed from the matrix due to temporal autocorrelation. (4) The unique pairwise correlations (upper triangle, excluding within block correlations) are averaged together to generate an average correlation of each condition.
2.10. Control of false positives
As suggested in Kriegeskorte et al. [36], we used a bootstrap resampling procedure to estimate false positive levels. We did this by randomly shuffling trial labels between conditions before performing group-level ANOVAS at F ≥ 4.9. Performing 1000 label-shuffled-group-analyses gave distributions of the false positive cluster size for each contrast. We then plotted each contrast's distribution and determined the voxel volume cut-off corresponding to the top 1% of the distribution. This voxel volume served as the p < 01 threshold. Thus, clusters whose volume exceeded 99% of the bootstrapped false-positive distribution were considered significant and are indicated as p < .01 (corrected). For univariate analyses reported here, we employed alpha_sim, which uses the family wise error rate to simulate false positives. We employed a voxelwise p < .01, which corresponded to a cluster size of 52.
For both MPS and univariate analyses, we report only clusters that exceeded our false positive rate above.
3. Results
3.1. Faster and more accurate responses for near than far trials
All participants performed significantly above chance on item recognition and performed overall above chance for spatial and temporal trials (Table 1). Performance on “equal” trials, the trials of interest in which the intervals between the reference and probe stores were equal, differed according to spatial and temporal contextual retrieval and near and far discriminations. An ANOVA revealed a main effect of block (spatial vs. temporal retrieval, F(1,15) = 19.749, p < 0001), with better performance for spatial trials (see Table 1). There was also an effect of distance (near > far judgments, F(1,15) = 7.661, p = .014). Thus, near trials were more often judged correctly than far trials (and done so more quickly, see Table 1). Given some of these differences in performance, all subsequent fMRI analyses involved only correct trials. To further control for this effect, we included performance as a covariate whenever possible.
Table 1.
Performance and reaction time. All conditions were significantly greater than chance. Group level 2 way (spatial vs. temporal by near vs. far) ANOVA on same trials revealed a main effect of block (spatial vs. temporal, F(1,15)= 19.749, p < .0001), with better performance for spatial trials and a main effect of distance (near > far judgments, F(1,15) = 7.661, p = .014), with better performance for near trials.
| Same spat near | Same spat far | Same temp near | Same temp far | Diff spat | Diff temp | |
|---|---|---|---|---|---|---|
| Performance | 98.28 ± 0.56 | 92.89 ± 2.85 | 88.93 ± 4.37 | 82.92 ± 4.57 | 91.71 ± 2.21 | 92.48 ± 2.70 |
| Reaction Time | 2.178 ± 0.143 | 2.633 ± 0.256 | 2.974 ± 0.113 | 3.503 ± 0.110 | 2.899 ± 0.150 | 2.869 ± 0.192 |
3.2. Behavioral independence of spatial layout and temporalorder information during retrieval
Although we designed our encoding task to minimize participants confounding between the spatial layout and temporal order, the possibility remains that the spatial retrieval facilitated temporal retrieval, or vice versa. For example, retrieval of the layout may facilitate retrieval of the order of individual delivery routes, or retrieval of individual routes may facilitate retrieval of the layout. To determine whether spatial and temporal retrieval supported each other, we tested whether probabilities of correct retrieval for matched trials were statistically independent. Such independence requires that the conditional probabilities be equal to the marginal probabilities [41]. We found no significant difference between Pspatial|temporal and Pspatial (.91 vs. .92; t(30) = 2, p = .8), nor between Ptemporal|spatial and Ptemporal (.86 vs. .87; t(30) = .4, p = .7). These findings support the independence of spatial and temporal representations at retrieval. Additionally, we conducted Monte Carlo simulations based on each participant's actual hit rate and modeled a random distribution of spatial and temporal correct responses across trials. We found no significant difference between simulated and actual rates of conjunctive spatial and temporal order retrieval, suggesting again that these representations are treated as functionally separate information during our task.
3.3. Univariate effects: spatial and temporal trials activate the hippocampal subfields at statistically indistinguishable levels
We first addressed whether spatial and temporal retrieval resulted in activation above baseline within the hippocampus, which we fully anticipated based on our past studies [20,22]. To address this issue, we performed a univariate group-space cluster analysis to assess activation within the PHC and hippocampus generally (see methods, task vs. baseline). Fig. 3 shows the results of task vs. baseline (one-sample t-test) broken down by each condition. Results revealed two large clusters in left (1402 voxels) and right (1299 voxels) MTL showing activity greater than baseline (t = 2.6, p < .01). Analysis of this cluster revealed that it extended throughout the MTL and encapsulated large percentages of CA3DG, CA1, and PHC (CA3DG: 93.39 ±2.10%, CA1: 98.12 ±0.36%, PHC: 98.40 ±0.49%). No subfields differed in terms of their activation levels.
Fig. 3.
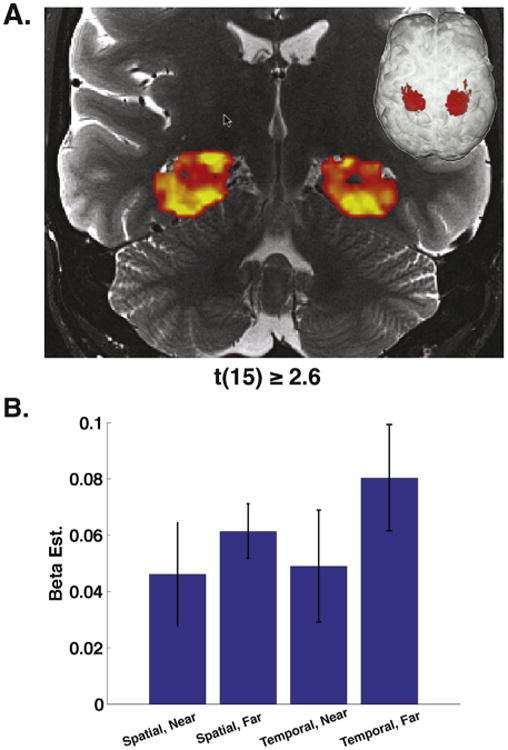
Univariate results. Group level contrast of task activation greater than baseline. (A) Coronal cross section of both left (1402 voxels) and right (1299 voxels) hemisphere clusters exhibiting task greater than baseline (t > 2.6, p < .01 corrected). (B) Average activations broken down by condition. Left and right hemisphere clusters were pooled, then voxel activations were averaged to find the average activation for each condition for each subject.
Next, we wanted to determine whether the conditions of our task recruited similar hippocampal networks, or rather if certain subfields were recruited differentially by either interval (trials with near or far interval) or block (spatial or temporal) using a univariate analysis. To investigate these issues, we conducted a 2 × 2 block by distance ANOVA (see methods, univariate analysis) using a group analysis. No clusters reached significance. These findings suggest that there were no univariate effects of block or interval in our data and that our task recruited a similar network of MTL sub-fields regardless of block or interval distance. Thus, although retrieving both spatial layout and temporal order information activated the hippocampus, we did not find any (statistically significant) differential pattern of activation as a function of spatial vs. temporal or near vs. far judgments.
3.4. Multivariate pattern similarity analysis for spatial vs. temporal order retrieval: no difference in patterns of activation across subfields
We next used within condition MPSA to determine whether the patterns of activation differed as a function of either behavioral task or subfield. To address this issue, we employed a 2 × 2 block (spatial vs. temporal) by distance (near vs. far judgment) ANOVA using a searchlight (see methods). We computed MPS within correct spatial or temporal trials involving near (probe stores contiguous with the reference store) or far distances (probe stores non-contiguous with the reference store). This method allowed us to simultaneously address whether there were differences as a function of block (e.g., spatial > temporal), distance (near > far), or differences between distances and spatial vs. temporal blocks (i.e., interaction effect). It also allowed us to identify any differences across subfields. This analysis revealed one significant cluster showing a significant (p < .01, corrected) interaction effect: this cluster spanned multiple subfields, including anterior CA, subiculum, CA23DG, and CA1 (23 voxels). For this cluster, near trials displayed higher mean MPS than far trials for temporal blocks while spatial blocks showed higher MPS for far compared to near trials (Fig. 4B; F(1,15) = 15.8, p < .001). No other clusters reached significance nor did any main effects (spatial vs. temporal, near vs. far) reach significance. Post hoc two-tailed t-tests revealed that temporal near trials showed greater MPS than far trials (t(15) = 2.1, p < .05). In contrast, spatial far trials showed greater MPS than near trials (t(15) = 4.6, p < .001).
Fig. 4.
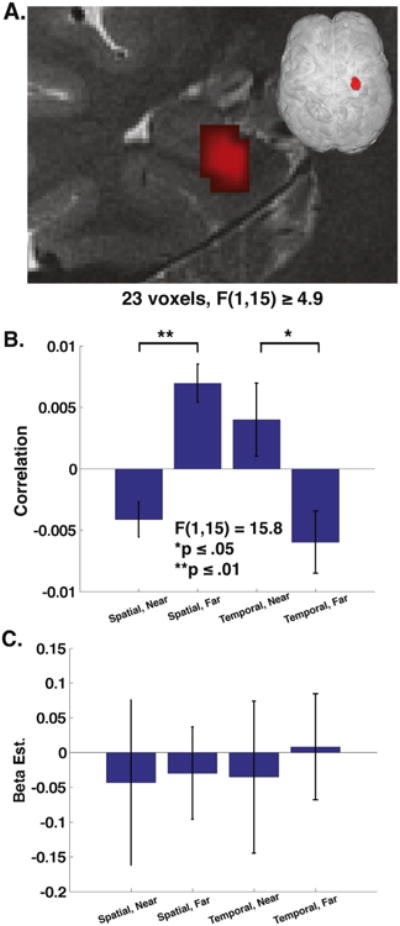
MPSA interaction effect. Group level 2 way (spatial vs. temporal by near vs. far) ANOVA revealed a significant cluster of 23 voxels displaying interaction effect with F ≥ 4.9. (A) Coronal cross section of right hemisphere shows cluster comprised of anterior hippocampal CA fields and subiculum. (B) Bar graph of within cluster MPSA for spatial near (SN), spatial far (SF), temporal near (TN), and temporal far (TF). Direction of interaction effect revealed by t test with spatial far > spatial near p ≤ .01 and temporal near > temporal far p ≤ .05. (C) Average parameter estimates reveal no significant differences by condition.
Further analysis of this cluster revealed there were no significant differences in the average parameter estimates (beta weights) across the cluster (2 × 2 ANOVA, all effects p >.76, Fig. 4C), suggesting that the differences in MPS within this cluster could not be accounted for by differences in activation levels between blocks or trials. Additionally, the interaction effect remained significant when participant performance was entered as a covariate (F(1,14) = 9.2, p < .01). Analysis of the location of the cluster determined that the cluster was present to a greater extent in hippocampus proper compared to PHC (%volume in hippocampus > %volume in PHC, t(15) = 31, p < <.001). These results suggested three important findings: (1) spatial and temporal retrieval resulted in differences in the relationship of MPS for near vs. far judgments; (2) these patterns could not be attributed to a single subfield; (3) this effect was more present in hippocampus than PHC.
To further investigate this effect we conducted two follow-up analyses that segregated each condition into their component parts. In our original MPS searchlight, each condition included correlations between repeated presentations of identical triads (within-triad, e.g., comparisons between different red triads and comparisons between different blue triads, Fig. 1F), and comparisons between presentations of non-identical triads (between-triad, e.g., comparisons between red and blue triads, Fig. 1F). To ensure that the effect was not driven by item differences, our follow up analysis separated these effects to give us a within-triad and between-triad measure for each condition (spatial near, spatial far, temporal near, and temporal far). We conducted separate 2 × 2, block by distance ANOVA searchlights for the within-triad and between-triad conditions. Both the within-triad (Fig. 5A and B) and between-triad searchlights revealed interaction effect clusters that overlapped with the cluster from our original analysis. The within-triad analysis revealed a 6 voxel cluster showing a significant interaction effect (Fig. 5B), F(1,15) = 10.1764, p < .01. The between-triad analysis revealed an 8 voxel cluster showing a significant interaction effect F(1,15) = 10.4906, p < .01.
Fig. 5.
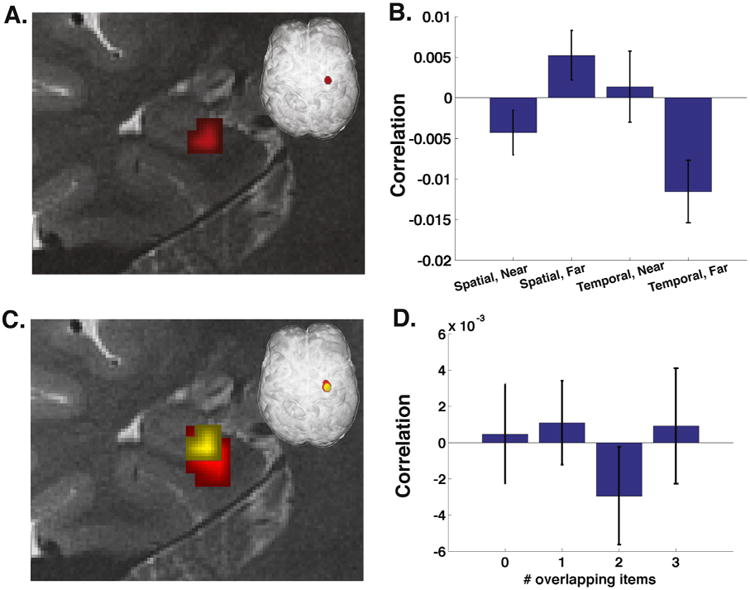
Control for item repetition. (A–C) Referring to Fig. 1f for each condition, pairwise correlations are computed between repetitions of red triads and between repetitions of blue triads, but not between red and blue triads. (A) This analysis reveals a similar but smaller cluster in the same location. (B) Bar graph of each condition's within triad pattern similarity. (C) Cluster from the within triad analysis (yellow) is laid on top of the primary analysis (red). (D) Overlap analysis within the 23 voxels from Fig. 3. If the area were sensitive to item identity, we would expect a linear increasing trend. Computing MPSA between all trials, and binning as a function of the number of items common in each comparison reveals no significant effect of item identity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Although the presence of the interaction effect in the within-triad condition argues against item-related effects driving the near/far vs. spatial/temporal interaction effect in our earlier analyses, we performed a final MPS analysis to attempt to rule out this potential confound. Due to the nature of this paradigm, item overlap was not held constant across all conditions. For example, in Fig. 1F, temporal near trials demonstrate an overlap in store identity of two items between red and blue triads. This means that the higher MPS of temporal near trials could be driven, in part, by specific items. To investigate the interaction effect cluster's sensitivity to item repetition, we conducted a control analysis which calculated MPS between store triads involving either no overlap in store identity, 1 overlapping store, 2 overlapping stores, or 3 overlapping stores (Fig. 5D). These analyses were conducted such that correlations were made between trials within spatial or temporal trials but did not include comparisons between spatial and a temporal trials, leaving room for the possibility that items could be encoded differently with regard to spatial or temporal context. If the cluster were sensitive to item identity we would expect an increase in MPS as we move from 0 item overlap to 3 item overlap. We did not find any effects of MPS as a function of item overlap, suggesting our effects were not driven by differences in item overlap.
4. Discussion
The current study examined the behavioral and neural characteristics of memory for spatial and temporal contextual judgments in which participants had to utilize their knowledge about the relative distance of items experienced within these two contexts. First, and most importantly, participants performed well above chance on all aspects of our paradigm, suggesting that they accurately retrieved spatial and temporal order information for both nearby and further away items within spatial and temporal context. Our behavioral data also showed that participants responded more accurately and faster for items nearby within both spatial and temporal contexts compared to those faraway. This suggests that participants were most likely using an associative strategy to retrieve spatial and temporal order involving remembering adjacent items to retrieve items that were further away, also known as chaining [42]. Finally, our behavioral data showed that spatial and temporal contextual retrieval did not significantly facilitate each other, suggesting that, at least as far as our behavioral data revealed, retrieving the spatial layout did not significantly influence the probability of retrieving the temporal sequence, and vice versa.
Analyzing correct trials in which participants retrieved items that were either nearby or further away within the spatial or temporal layout, we found significant levels of activation using a univariate approach when we compared correct spatial and temporal order retrieval with our baseline (control task). This cluster spanned multiple subfields and a contrast of spatial vs. temporal order retrieval did not show differential subfield involvement. These findings suggested that neither spatial nor temporal order retrieval could be unambiguously assigned to a single subfield, at least in our task (see also: [21,22]). These findings largely mirror (and thus generally replicate) our past work on this issue [20,22] and extend them to a task showing behavioral effects consistent with an associative retrieval strategy. No other univariate effects were significant, suggesting that near vs. far spatial and temporal judgments similarly did not activate specific subfields, at least within our task. Together, these findings suggest that while spatial and temporal order retrieval involve the hippocampus, no specific subfields differentially performed these decomposable functions in our task, such as spatial vs. temporal order retrieval or near vs. far judgments, at least as measured with fMRI.
Using a multivariate approach, we also correlated response patterns between respective trials (spatial near vs. spatial near, temporal near vs. temporal near, spatial far vs. spatial far, and temporal far vs. temporal far). Because this approach involved pooling correlated voxel response patterns between multiple stimulus triads together, it necessitated additional contrasts to try to rule out the alternative explanation that our effects were driven by store-specific responses. We found a large (23 voxels) and significant cluster of activity showing differences in multivariate pattern similarity for spatial vs. temporal retrieval as a function of interval (near vs. far). No other main effects were significant (spatial vs. temporal or near vs. far). Importantly, this cluster spanned multiple subfields (anterior CA, subiculum, CA23DG, and CA1). Our follow-up analysis showed that this cluster could not be explained by stimulus-specific representations amongst the different triads or differences in performance. It is important to note that using similar high-resolution sequences, other studies have reported sub-field specificity [22,43–46]. Thus, our data add an important piece to this literature by suggesting that retrieving information regarding the spatial or temporal proximity of elements within spatial or temporal context results in similar patterns of activity spanning multiple subfields. Thus, overall, our findings are more consistent with models that posit similar roles for hippocampal subfields generally in retrieving contextual information [47,48] compared to models positing differences in spatial vs. temporal order processing for specific subfields [49,50].
There are also several important caveats that we would like to mention regarding our results. Past models hypothesizing differences in subfield involvement in spatial vs. temporal order representation have focused specifically on the issue of pattern separation [49]. In our task, however, it is not clear the extent to which pattern separation might be involved in successful retrieval of either near or far pieces of information from recently learned spatial vs. temporal context. Pattern separation is often defined as a process that is constrained to encoding which involves separating two representations so they can be better discriminated during retrieval [51,52]. Because we scanned at retrieval, it is unlikely that we are viewing active pattern separation processes consistent with this definition. Another conceptualization of pattern separation, however, is the minimization of connections that mutually represent two events [53]. Based on this definition then, it may be possible to measure the degree of pattern separation at reinstatement using methods such as multivariate pattern analysis or adaptation. Thus, while pattern separation processes would appear more likely to occur during encoding, our multivariate pattern similarity results may speak to the degree that pattern-separated events were reinstated at retrieval [22].
The issue of pattern separation during spatial and temporal order retrieval is further complicated by our behavioral results. Typically, distance judgments with cued items demonstrate that reaction times are slower and performance is worse for nearby compared to far away items [30,31]. These findings are consistent with a strength-based, or recency-based retrieval strategy in which trace strength is used to judge interval distance [54]. Furthermore, it has been argued that successful use of this retrieval strategy relies on the ability to pattern separate [30]. Spatial and temporal contextual details in our task could conceivably be retrieved using this strategy. However, our behavioral results demonstrate the opposite pattern of what might be expected if participants employed a recency-based strategy. Instead, we find higher performance and lower reaction time for nearby than far trials. This indicates that participants may have represented non-cued, intermediary items via an associative rather than a recency-based strategy. This would appear to indicate that participants retrieved distance using associative information, presumably via chaining [42]. Unfortunately, it is not yet clear how these more complicated retrieval processes might involve pattern separation, making the neural results difficult to interpret. Because pattern separation remains a theoretical construct to explain one of the many complex steps involved in memory, we can only speculate on the degree to which our task involved this process. Given the finding of an interaction effect in the MPSA results, however, we feel it is more likely that this finding indicates differences in contextual representation than pattern separation per se. It may be difficult to say whether our MPSA findings speak directly to models such as the Rolls and Kesner model because they are based on results that assume lower performance for near vs. far pairs, the opposite of our behavioral results. In this case, our univariate results would simply suggest that we could find no subfield specificity for spatial layout vs. temporal order retrieval, as suggested in past human studies [21,22] and that our results cannot be extended to either refute or bolster computational models of subfield function involving pattern separation.
Nonetheless, it is instructive to consider how our MPSA results might imply how spatial and temporal context might be processed within the hippocampal circuit without the need for subfield specific representations. Although we must be cautious in linking our findings directly to representations of specific stores, in part because our task involved multiple stimuli and representations simultaneously during retrieval, we think it is reasonable to speculate how they might relate to the larger issue of spatial vs. temporal order contextual representation. As argued above, some form of spatial or temporal context representation was needed to correctly respond to questions. Consistent with this assumption, studies in the rodent literature have interpreted findings with regard to representation of multiplexed contextual information [55,56]. At a basic level, then, our results could indicate that spatial and temporal contextual representations differed with regard to a common variable: nearness. This could indicate potential differences in the associative structure of how a spatial layout is organized in memory compared to a temporal sequence. For example, theoretical proposals on temporal order representation suggest a chained association model of encoding and retrieval. According to these models, items during encoding are associated with each other and then retrieved based on a “chaining” mechanism in which the first item cues the second item, and so forth [42]. This may, in part, be due to a drifting temporal context, which continuously changes over the task and may be an additional factor influencing how items are encoded and retrieved during serial order learning [57,58]. In contrast, most theoretical proposals regarding spatial coding suggest that space involves a holistic, multidimensional representation in which details of the environment become integrated across multiple viewings and time points [59–63]. Behavioral studies support this assertion, suggesting that forming a cognitive map involves integrating multiple trajectories through space across time [59,60,64], which creates a more holistic, less elementized representation [59]. Thus, one possible interpretation of our findings is that near and far items differed in their representation for spatial vs. temporal context due to differences in how a temporal vs. spatial layout is stored associatively. Specifically, temporal order involves discrete, elementized representations over time, suggesting more distant representations would be more completely differentiated. In contrast, a spatial layout might involve a more holistic representation, predicting more similarity for near vs. far elements within the context.
Another important possibility, however, is that differences in retrieval strategies are responsible for our results. Strategy use in contextual memory retrieval is currently not well understood, however, it could be that participants used fundamentally different strategies to retrieve near and far elements of spatial layout vs. temporal order. For example, while temporal contiguity in free recall is thought to be supported by context cued episodic recall, temporally non-contiguous recall may be supported by a more semantic search strategy [65]. Partially analogous to these strategy differences in temporal context, spatial near and far judgments could differentially rely on egocentric and allocentric strategies. Specifically, far judgments could be facilitated by using a more holistic allocentric strategy while near spatial judgments might be easier to solve using an egocentric strategy based on recent spatial views [66]. Because our current paradigm did not investigate representations for individual stores, we can only speculate on whether our results stem from representational differences between the two contexts vs. differences in how participants go about retrieving these two bits of information in the first place. While representation and strategy are almost certainly interrelated during memory retrieval, our paradigm does not allow a clear separation of these two theoretical constructs due to our use of multiple store probes during retrieval.
In conclusion, we investigated how retrieving either spatial or temporal context would affect activations and patterns of activations within the hippocampus. Our findings suggest that while the specific subfields recruited during spatial vs. temporal order retrieval did not differ, the patterns of activity, as measured with MPS, did differ. These data thus provide greater insight into how the hippocampus processes spatial and temporal information as well as how the same brain region (the hippocampus) might be able to store and process different contextual representations involved in the same events. Our past work suggested that the hippocampus might represent a convergence zone for processing spatial and temporal processing, which might differentiate within the cortex according to the neural oscillatory frequency at which they occur [67]. Our current findings suggest that within the hippocampal convergence zone, although the specific subfields involved did not differ for spatial vs. temporal order retrieval, the patterns of activated voxels did, which we speculate could in turn relate to representational differences between the two contexts. Although we can only speculate on these issues, we hope to explore this issue in greater depth in future work.
Highlights.
We probe spatial and temporal order memory retrieval within the human hippocampus.
We employ high-resolution imaging to visualize subfields of the hippocampus.
Multiple hippocampal subfields involved in retrieving spatial and temporal context.
Hippocampal similarity shows unique patterns for spatial and temporal distance.
References
- 1.Godden DR, Baddeley AD. Context-dependent memory in 2 natural environments – land and underwater. Br J Psychol. 1975;66:325–31. [Google Scholar]
- 2.Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–49. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 7.Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–41. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 8.Rugg MD, Johnson JD, Park H, Uncapher MR. Encoding-retrieval overlap in human episodic memory: a functional neuroimaging perspective. Prog Brain Res. 2008;169:339–52. doi: 10.1016/S0079-6123(07)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124:2476–89. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- 10.Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6:823–9. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 12.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–88. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 13.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci. 2003;23:5945–52. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumann O, Chan E, Mattingley JB. Dissociable neural circuits for encoding and retrieval of object locations during active navigation in humans. Neuroimage. 2010;49:2816–25. doi: 10.1016/j.neuroimage.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Ekstrom AD. Human neural systems underlying rigid and flexible forms of allocentric spatial representation. Hum Brain Mapp. 2013;34:1070–87. doi: 10.1002/hbm.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki M, Fujii T, Tsukiura T, Okuda J, Umetsu A, Nagasaka T, et al. Neural basis of temporal context memory: a functional MRI study. Neuroimage. 2002;17:1790–6. doi: 10.1006/nimg.2002.1303. [DOI] [PubMed] [Google Scholar]
- 17.Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Haberg AK. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–84. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staresina BP, Davachi L. Mind the gap: binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–76. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A. 2010;107:14466–71. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;2011:18. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Azab M, Stark SM, Stark CE. Contributions of the human hippocampal subfields to spatial and temporal pattern separation. Hippocampus. 2014;24:293–302. doi: 10.1002/hipo.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, Ekstrom AD. Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. J Neurosci. 2014;34:6834–42. doi: 10.1523/JNEUROSCI.5341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 24.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–8. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 25.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–7. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus BJ, Robinson RJ, II, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78:1090–101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci. 2005;25:3280–6. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–66. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328:1412–5. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–36. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 31.Moyer RS, Landauer TK. Time required for judgements of numerical inequality. Nature. 1967;215:1519–20. doi: 10.1038/2151519a0. [DOI] [PubMed] [Google Scholar]
- 32.Chiba AA, Kesner RP, Reynolds AM. Memory for spatial location as a function of temporal lag in rats – role of hippocampus and medial prefrontal cortex. Behav Neural Biol. 1994;61:123–31. doi: 10.1016/s0163-1047(05)80065-2. [DOI] [PubMed] [Google Scholar]
- 33.Madsen J, Kesner RP. The temporal-distance effect in subjects with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 1995;9:94–100. doi: 10.1097/00002093-199509020-00006. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–10. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friston KJ, Price CJ. Modules and brain mapping. Cogn Neuropsychol. 2011;28:241–50. doi: 10.1080/02643294.2011.558835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci U S A. 2006;103:3863–8. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–62. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kreyszig E. Advanced engineering mathematics. USA: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 42.Murdock BB., Jr . Human memory: theory and data. Potomac, MD: Lawrence Erlbaum Associates; 1974. [Google Scholar]
- 43.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–2. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J Neurosci. 2009;29:10512–9. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2010;18:15–8. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22:389–98. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–4. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–25. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Katz Y, Kath WL, Spruston N, Hasselmo ME. Coincidence detection of place and temporal context in a network model of spiking hippocampal neurons. PLoS Comput Biol. 2007;3:e234. doi: 10.1371/journal.pcbi.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends Cogn Sci. 2002;6:505–10. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- 52.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–91. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 54.Hintzman DL. Memory strength and recency judgments. Psychon Bull Rev. 2005;12:858–64. doi: 10.3758/bf03196777. [DOI] [PubMed] [Google Scholar]
- 55.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 56.Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence forCA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–27. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–99. [Google Scholar]
- 58.Howard MW, Natu VS. Place from time: reconstructing position from a distributed representation of temporal context. Neural Netw. 2005;18:1150–62. doi: 10.1016/j.neunet.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 60.Siegel AW, White SH. The development of spatial representations of large-scale environments. In: Reese HW, editor. Advances in child development and behavior. New York: Academic; 1975. [DOI] [PubMed] [Google Scholar]
- 61.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 62.Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon Bull Rev. 2005;12:1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- 63.Buzsaki G. Rhythms of the brain. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 64.Arnold AE, Burles F, Krivoruchko T, Liu I, Rey CD, Levy RM, et al. Cognitive mapping in humans and its relationship to other orientation skills. Exp Brain Res. 2013;224:359–72. doi: 10.1007/s00221-012-3316-0. [DOI] [PubMed] [Google Scholar]
- 65.Kahana MJ. Associative retrieval processes in free recall. Mem Cognit. 1996;24:103–9. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Zherdeva K, Ekstrom AD. Different “routes” to a cognitive map: dissociable forms of spatial knowledge derived from route and cartographic map learning. Mem Cognit. 2014;42:1106–17. doi: 10.3758/s13421-014-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013;16:349–56. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]


