Abstract
Recent research has suggested that frequent short bursts of activity characterize hyperactivity associated with attention deficit hyperactivity disorder (ADHD). This study determined whether such pattern is also visible in schedule-induced polydipsia (SIP) in the spontaneously hypertensive rat (SHR), an animal model of ADHD. Male SHR, Wistar Kyoto (WKY) and Wistar rats were exposed to 40 sessions of SIP using a multiple fixed-time (FT) schedule of food delivery with FT 30-s and FT 90-s components. Stable performance was analysed to determine the extent to which SIP-associated drinking is organized in bouts. The Bi-Exponential Refractory Model (BERM) of free-operant performance was applied to schedule-induced licks. A model comparison analysis supported BERM as a description of SIP episodes: licks were not produced at a constant rate but organized into bouts within drinking episodes. FT 30-s induced similar overall licking rates, latencies to first licks and episode durations across strains; FT 90-s induced longer episode durations in SHRs and reduced licking rate in WKY and Wistar rats to nearly baseline levels. Across schedules, SHRs made more and shorter bouts when compared to the other strains. These results suggest an incentive-induced hyperactivity in SHR that has been observed in operant behavior and in children with ADHD.
Keywords: Schedule-induced polydipsia, spontaneously hypertensive rats, hyperactivity, fixed time, bout, excessiveness, BERM
Introduction
Recent research suggests that children with attention deficit hyperactivity disorder (ADHD) engage in frequent but short bouts of operant behaviour (Taylor et al., 2010). The spontaneously hypertensive rat (SHR), an animal model of ADHD (Sagvolden et al., 2000; Sanabria and Killeen, 2008), shows a similar pattern of operant performance (Hill et al., 2012). It thus appears that this pattern of behaviour constitute a key component of the behavioural phenotype of ADHD.
The present study was aimed at determining whether frequent but short bouts of responding were also observed in SHRs in a non-operant behavioural preparation, schedule-induced polydipsia (SIP). SIP consists of excessive drinking that occurs on a schedule in which food is intermittently delivered, usually observed in partially food-deprived rats (Falk, 1961). SIP can be produced in a wide range of behavioural schedules, including conditions in which animals are exposed to intermittent delivery of food regardless of their behaviour, such as fixed time (FT) schedules (Falk, 1966, 1967).
The principal characteristic of SIP is its excessiveness, which distinguishes it from others behaviours performed throughout inter-food intervals. Thus, SIP serves as the prototype of so-called adjunctive behaviour (Falk, 1971; also referred to as interim behaviour: Staddon, 1977). In relation to ADHD, SIP successfully selects among experimental subjects those that display characteristics associated with excess behaviour (Riley & Wetherington, 1987; Gilpin et al., 2008) and other disorders associated with deficient impulse control (Cardona et al., 2006; López-Grancha et al., 2008), such as substance abuse (Myracle et al., 2005), obsessive compulsive behaviour (Plat et al., 2008; Van Kuyck et al., 2008; Moreno & Flores 2012; Moreno et al., 2012; Flores et al., 2014), and schizophrenia (Hawken et al 2011 a and b, 2013).
Comparisons of SIP between SHR, Wistar and Wistar Kyoto (WKY) rats have shown that (a) there are no significant difference in SIP among strains when the inter-food interval is short (30 s), but (b) whereas SIP declines with longer inter-food intervals (approximately 60 s) in WKY and Wistar rats, SIP in SHR remains relatively high (Íbias & Pellón, 2011, 2014). Reduced SIP in control strains may occur because of (a) a reduction in the proportion of inter-food intervals that contain drinking episodes, (b) an increase in the latency to initiate drinking episodes, (c) a reduction in the duration of drinking episodes, and/or (d) a reduction in the rate of licking during a drinking episode. To the extent that licking rate is, like operant behaviour, organized in bouts (Shull et al., 2004; Brackney et al., 2011), licking rate may decline because (a) licking bouts occur less often, (b) licking bouts are shorter, and/or (c) licking rate within bouts declines.
The present study aimed at examining these potential differences in SIP performance across three strains of rats, SHR, Wistar and WKY, and two food schedules, FT 30-s and FT 90-s. The organization of drinking episodes in bouts of licks was of particular interest, not only because of its ostensible link to ADHD, but also because parameters of bout organization have been associated with motor, motivational and learning variables (Brackney et al., 2011). Therefore, differences in parameter estimates across strains may suggest novel hypotheses about the behavioural mechanisms that underlie the differences in performance across strains, as well as on the behavioural mechanisms involved in SIP and related phenomena.
Method
Subjects
Twenty-four male rats of three strains - 8 SHR, 8 WKY and 8 Wistar - obtained from Charles River Laboratories (Lyon, France) were used. On arrival at the laboratory, animals were 10 weeks old; they were housed in groups, in an environmentally-controlled room with a 12-hour light-dark cycle (light from 08:00 to 20:00 hours), ambient temperature of 17 °C to 23 °C, and 60% relative humidity. Once habituated to the animal facility, rats were housed singly in 18 × 32.5 × 20.5 cm transparent Plexiglas cages, with a metal-grid detachable roof that allowed for food to be deposited and a water bottle to be fitted.
Rats were 12 weeks old at the start of the experiment. Their average weights were, for SHR, 292 g (range: 277–302 g); for WKY, 339 g (range: 306–361 g); for Wistar, 373 g (range: 359–384 g). Weights were reduced to 80%-85% of free-feeding weight by a controlled diet, and then maintained throughout the experiment in proportion to standard growth curves for each strain. Each rat was weighed daily before the experimental session. Twenty minutes after the experimental session, each animal received the appropriate food supplement to maintain its weight within the criterion-based range.
Apparatus
Eight Letica LI-836 conditioning chambers, measuring 29 × 24.5 × 35.5 cm, enclosed in soundproofed housing, equipped with its own ventilation and a small observation window at the front. The front panel of each conditioning chamber was made of aluminium, the left wall of transparent Plexiglas and the remaining walls of black Plexiglas. On the exterior of the chamber' right-hand wall, a water bottle was fitted, and the rat had access to the spout from the interior of the chamber, through a 3.2 × 3.9 cm aperture in the wall, situated 20 cm from the front panel and 7 cm from the floor. The spout was placed 2 cm towards the interior of the aperture to allow for licks rather than continuous drinking. Contact between the animal's tongue and the metal spout completed the electric circuit between the 12-bar metal grid that served as the floor and the water-bottle spout. Licks were recorded using a MED-PC-IV application under a Windows XP environment. Forty-five mg food pellets were dispensed (Bio-Serv, Frenchtown, NJ, USA) in an aperture in the chamber's front wall situated 3.7 cm from the floor, between the panel's two levers, which were retracted throughout the experiment. The chambers were lit by two 3W lamps situated on the front panel at either side of the food hopper and by an indirect 25W light fitted to the interior of the soundproof housing that insulated each chamber. Exterior noise was masked by a fan that produced an ambient noise of approximately 60 dB in each chamber. At the top of the front wall of each chamber was a speaker that produced sound signals when necessary.
Procedure
The SIP procedure was carried out using a multiple FT schedule, in which a food pellet was delivered at regular intervals regardless of the animal’s behaviour. Every experimental session contained two schedule components, FT 30-s and FT 90-s, and the first component to start each session was determined randomly. Rats were given 20 food pellets/session in each component. One of the components was always signalled with a continuous tone of 60 dB (while the other was signalled by its absence); assignment of tone-signalled component was randomized across animals. The experimental chamber lights were turned off for 60 s between FT components.
Forty experimental sessions were conducted. Once SIP performance was stable in both schedules, based on licking rate (licks/min) and volume of water consumed, 8 more sessions were conducted. Data from the last 8 sessions was used for the estimation of bout parameters. All data were collected with a resolution of 20 ms.
Estimation of bout parameters
Bout-parameter estimation was conducted only on rats that, during the last 8 sessions, drank more than the average daily level of prandial water consumption (2.75 ml). A total of 19 rats (all SHR rats, 6 WKY rats and 5 Wistar rats) were included in the analysis.
The following measures were obtained from each trial in which a rat produced at least two licks: latency to the first lick, duration of the drinking episode (i.e., time between first and last lick), and 3 parameters of the distribution of inter-lick intervals (ILIs): the rate at which licking bouts were initiated (b), the rate of licking within bouts (w), and the probability that a lick was not the last one in a bout [p; mean bout length = 1 / (1 – p)]. These parameters were estimated using the Biexponential Refractory Model (BERM) of free-operant performance (Brackney et al., 2011),
| (1) |
This equation indicates that there is a minimum time between consecutive licks, δ, and that ILIs longer than δ are sampled with probability p from an exponential distribution with mean (1 / w) + δ (the within-bout lick rate), and with probability 1 − p from another exponential distribution with mean (1 / b) + δ (the bout-initiation rate). Estimates of p, w and b were obtained using the method of least squares (Kessel & Luke, 2008), with δ fixed at 10 ms (half of the data resolution) for all rats. Goodness-of-fit of BERM parameters was computed (a) as the proportion of variance in the empirical survival function, expressed in log units (Shull et al., 2001), accounted for by the model (PVAF; Fleishman, 1980), and (b) as the improvement in likelihood (probability of the model given the data) relative to a single shifted-exponential distribution, correcting for the 2 extra free parameters in Equation 1 (the mixture weight p and the parameter of the second exponential). The latter computation was conducted using ΔAICc (Burnham & Anderson, 2002).
Statistical analysis
Licks per minute during the first 40 sessions was analysed using a 3 × 2 × 40 mixed-design ANOVA, with factors strain (SHR, Wistar and WKY), schedule (FT 30-and 90-s), and session (each of 40 sessions). The following dependent measures were analysed in the last 8 experimental sessions: the proportion of trials (i.e., inter-food intervals) with a drinking episode (2 or more licks), the median latency to start drinking episodes, the median duration of drinking episodes, and the mean number of bouts emitted per episodes (estimated from BERM parameters). Analysis was conducted using a 3 × 2 mixed-design ANOVA with factors strain and schedule. PVAF-weighted, log-transformed BERM parameter estimates were compared between groups using a similar ANOVA (the log transform follows suggestions that BERM parameters are log-normally distributed across rats; see Cheung et al., 2012).
Post-hoc comparisons were carried out using pairwise comparisons with a Bonferroni correction for p. The lowest p-value reported in every analysis was 0.01, with α = .05. All analyses were conducted using SPSS 19 © Software.
Results
SIP acquisition
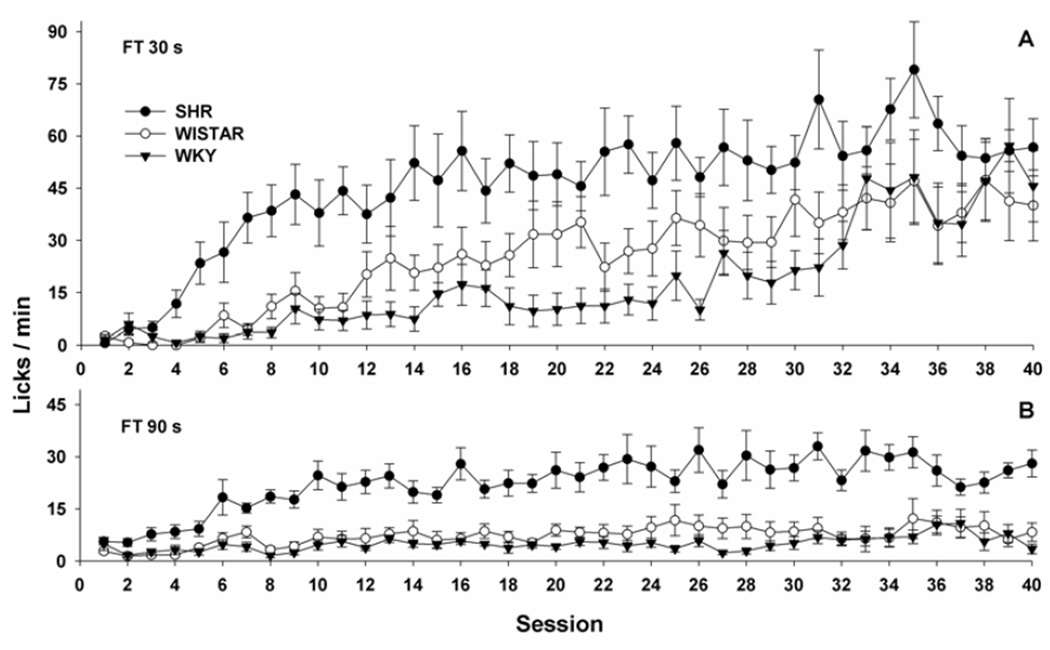
Figure 1A shows mean (±SEM) licks/min throughout 40 sessions of SIP acquisition for the three strains of rats in the FT 30-s schedule. A significant strain × session interaction effect was observed [F(78,819)= 2.146, p< 0.01]. Post hoc comparisons revealed that, in sessions 4 to 11, SHRs produced significantly more licks/min than Wistar and WKY rats (p< 0.05 for all comparisons). This difference persisted for SHR vs. WKY rats until session 31 (p< 0.05 for all comparisons). No significant difference in licks/min among strains was observed in the last 9 of the first 40 sessions of FT 30-s training.
Figure 1.
Mean (±SEM) licks/min over acquisition sessions of SIP in FT 30-s (Figure 1A) and FT 90-s (Figure 1B). In FT 30-s, SHR produced significantly more licks/min than Wistar rats in sessions 4 through 11, and more than WKY in sessions 4 through 31 (p< 0.05 for all comparisons). In FT 90-s, SHR produced significantly more licks/min than the other two strains from session 2 onwards (p< 0.05).
Figure 1B shows mean (±SEM) lick/min throughout 40 sessions of SIP acquisition for the three strains of rats in the FT 90-s schedule. A significant strain × session interaction effect was observed [F(78,819)= 3.265, p< 0.01]. Post hoc comparisons revealed that, from session 2 onwards, SHRs produced significantly more licks/min than Wistar and WKY rats (p< 0.05 for both comparisons).
The analyses reported in the remainder of this section are confined to the last 8 training sessions. Mean ±SD volumes of water consumed throughout these sessions were 13.0 ±0.6 ml for SHR, 10.5 ±0.9 ml for Wistar, and 7.6 ±0.8 ml for WKY. No significant differences in lick efficiency (volume of water per lick) were observed between strains. Mean ±SD estimations of lick efficiency were: 110.5 ±10.7 for SHR, 94.6 ±13.6 for Wistar, and 117.6 ±12.4 for WKY.
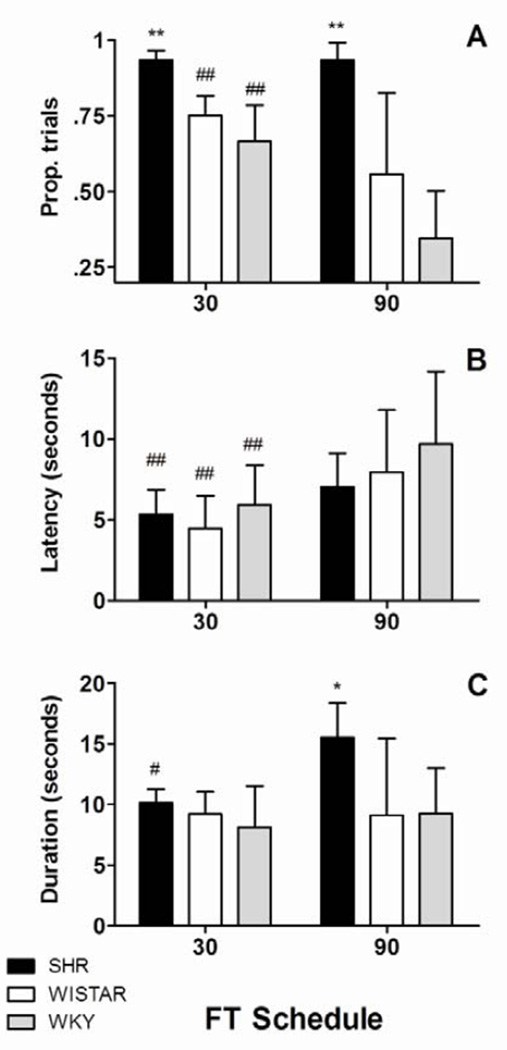
Figure 2A shows mean (±SEM) proportions of trials with at least 2 licks. An ANOVA of this dependent measure resulted in a significant strain × schedule interaction effect [F(2,16) =8.317, p <0.01]. Post hoc comparisons revealed that SHR rats had significantly more drinking episodes than the other strains in both FT schedules (p< 0.01 in all cases). These comparisons also showed a substantial decrease in the number of drinking episodes in Wistar and WKY rats in FT 90-s relative to FT 30-s schedules (p< 0.01 for both comparisons).
Figure 2.
A. Mean (±SEM) proportion of trials with at least 2 licks. Figure 2.B. Mean (±SEM) median latency to first lick. Figure 2.C. Mean (±SEM) median duration of drinking episodes (intervals between first and last lick). SHR produced more drinking episodes than Wistar and WKY in both schedules; this difference increased in FT 90-s. All strains started their drinking episodes later in FT 90-s than in FT 30-s. SHR produced longer episodes in FT 90-s relative to FT 30-s, and relative to other strains. Between strain differences: * = p< 0.05, ** = p< 0.01. Within strain differences: # = p< 0.05, ## = p< 0.01.
Figure 2B shows mean (±SEM) median latencies to first lick (±SEM) in all FT schedules for all groups. An ANOVA revealed a significant strain × schedule interaction effect [F(2,16) =4.064, p <0.04]. Post hoc comparisons showed that latencies increased for all strains between FT 30-s and FT 90-s (p< 0.01 for all comparisons).
Figure 2C shows mean (±SEM) median durations of drinking episodes. An ANOVA of episode durations revealed a significant strain × schedule interaction effect [F(2,16) =13.718, p <0.01]. Post hoc comparisons showed that SHR rats produced longer episode durations in FT 90-s than Wistar and WKY rats ( p< 0.02 and p< 0.04, respectively). Post hoc comparisons also revealed that SHRs produced longer episode durations in FT 90-s than in FT 30-s (p< 0.01).
In short, Figure 2 shows that, compared to control strains, SHRs produced more and longer drinking episodes that were more resilient to longer inter-food intervals. Despite this resiliency, SHR performance was sensitive to changes in inter-food interval: drinking episodes in SHR started later and lasted longer with longer inter-food intervals.
Effects on BERM parameters
Table 1 shows the goodness of fit of BERM. PVAFs were, in all cases, satisfactorily high, although the PVAF for WKY rats in FT 90-s was substantially lower than in any other strain × schedule cell. ΔAICc offered similar information. Nonetheless, even the worse fit of BERM provided a substantially superior fit than a single exponential distribution: BERM estimates were eΔAICc/2 = e2118 more likely than those from a single shifted exponential, given WKY performance in FT 90-s.
Table 1.
Mean (± SEM) goodness-of-fit of BERM parameters
| PVAF | ΔAICc* | |
|---|---|---|
| FT 30-s | ||
| SHR | 0,84 (0,03) | 8997 (1308) |
| WISTAR | 0,76 (0,08) | 5644 (1709) |
| WKY | 0,69 (0,09) | 5988 (1364) |
| FT 90-s | ||
| SHR | 0,93 (0,01) | 17247 (2725) |
| WISTAR | 0,84 (0,05) | 5095 (1983) |
| WKY | 0,63 (0,15) | 4237 (1584) |
Note. ΔAICc is the difference in likelihood of Equation 1 and a single-exponential model, after correcting for number of free parameters. Higher ΔAICc is indicative of a higher likelihood of Equation 1, given the data of each strain.
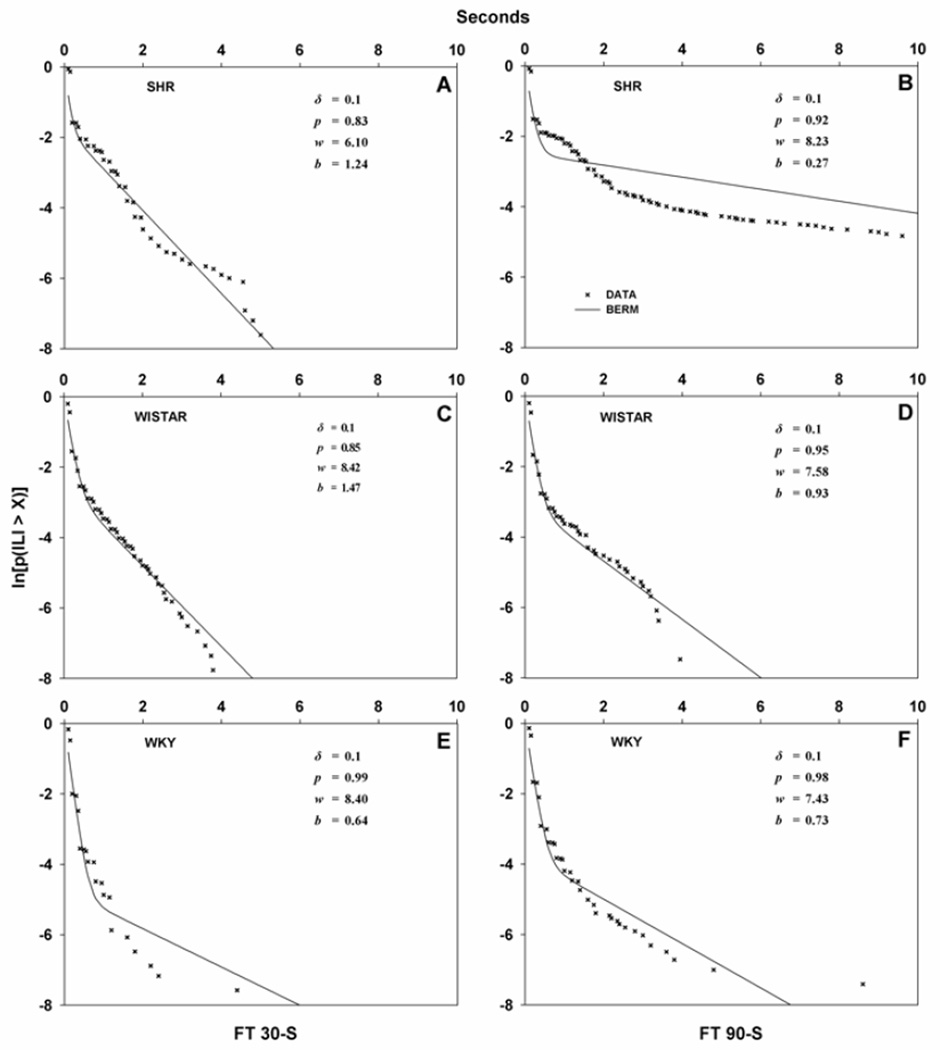
Figure 3 shows fits of BERM to individual rat data. BERM fit the data adequately, displaying the usual broken-stick survival function of ILIs also observed in free-operant inter-response times (IRTs: Shull et al., 2004; Brackney et al., 2011). This suggests that when rats were involved in SIP episodes, ILIs were sampled from two exponential distributions: more than 80% of ILIs were sampled from a distribution with a short mean (1/w estimates in median rats ranged between 12 and 16 ms), and the remainder were sampled from a distribution with a long mean (1/b estimates in median rats ranged between 81 and 370 ms).
Figure 3.
Sample fits of BERM (Equation 1; continuous curves) to individual log-survival plots (the natural log of the probability of an ILI greater than X) from selected SHR (top panels), Wistar (middle panels), and WKY rats (bottom panels). For each strain × schedule (FT 30-s on the left, FT 90-s on the right) cell, the rat that produced the median lick/min was selected. Each panel includes BERM parameter estimates. BERM provided an adequate account of individual SIP performance.
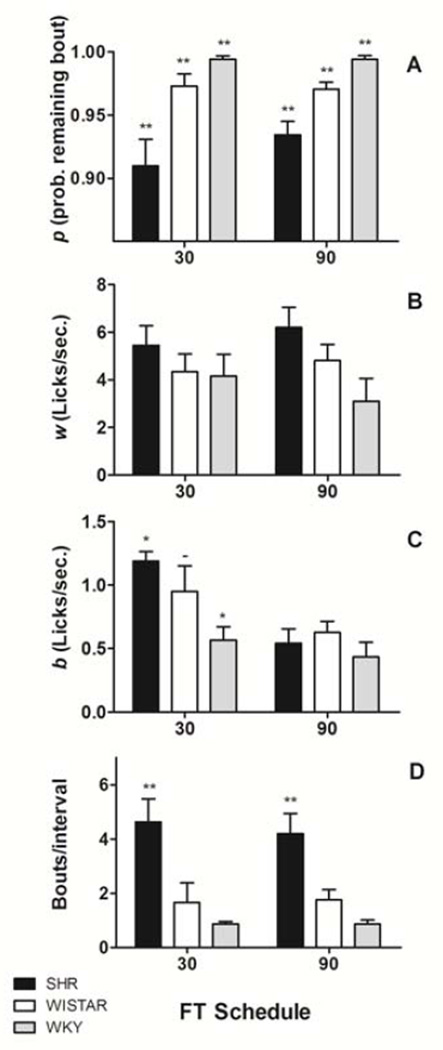
Figure 4 shows mean (±SEM) BERM parameters (p, w, and b) and the mean number of bouts per episode, estimated from drinking episodes. Although mean p estimates were greater than .90 (which translates into 10 licks/bout) for all strains and schedules, there were significant differences in estimates among strains revealed by a significant strain effect [F(2,32)= 20.796, p< 0.01]. SHRs generally produced lower p estimates than both WKY and Wistar (p< 0.01 and p< 0.02 respectively).
Figure 4.
Mean (±SEM) BERM parameters (Equation 1) estimated from drinking episodes. Figure 4A shows estimates of p, the probability of continuing a bout after every lick. Figure 4B shows estimates of w, the within-bout licking rate. Figure 4C shows estimates of b, the bout-initiation rate. Figure 4D shows estimations of the average number of bouts per episode, computed as w(bD + 1) / (w + Lb), where D is the mean episode duration, and L is the mean bout length = 1 / (1 – p). SHR produced shorter episodes (lower p) than WKY and Wistar rats. SHR produced bouts more frequently than WKY. SHR produced more bouts per episode than Wistar rats, and Wistar rats more than WKY in both schedules. Between strain differences: * = p< 0.05, ** = p< 0.01. Within strain differences: # = p< 0.05, ## = p< 0.01. − = p> 0.05.
No significant differences in estimates of w were observed among strains or between schedules. During a drinking bout, all rats licked, on average, 3 to 6 times per second.
An analysis of estimates of b revealed a significant strain [F(2,32)= 3.734, p< 0.04] and schedule effect [F(1,32)= 5.063, p< 0.03]. SHRs produced licking bouts at a significantly higher rate than WKY rats (p< 0.04), and rats generally produced licking bouts at a higher rate in FT 30-s than in FT 90-s. An analysis of estimates of the number of bouts per episode revealed a significant strain effect [F(2,32)= 36.814, p< 0.01]. Post hoc comparisons showed that SHRs produced more bouts per episode than Wistar rats (p< 0.01), and Wistars produced more than WKY rats (p< 0.01).
In short, Figure 4 shows that, compared to control strains, the drinking episodes of SHRs included more but shorter licking bouts. Within each bout, however, the rate of licking was very similar across strains.
Discussion
This study investigated the development and maintenance of SIP in SHR, Wistar and WKY rats in two FT schedules of food delivery. Results suggest a retardation in SIP acquisition in Wistar and WKY rats with respect to SHRs; the slowest acquisition was observed in WKY rats (Figure 1). After 40 experimental sessions, when SIP performance stabilized, no significant differences in overall licking rate were observed among strains in FT 30-s. In FT 90-s, control strains hardly developed SIP. In contrast, SHRs maintained their adjunctive drinking despite a reduction in feeding rate. These results are consistent with previous reports (Íbias & Pellón, 2011, 2014).
The finding of differences in acquisition of SIP by the three strains of rats is interesting from a theoretical standpoint on adjunctive behaviour. WKY has been proposed as a model of anxiety vulnerability, post traumatic-stress and depression (Solberg et al., 2001; Will et al., 2003), and there are theories of SIP that rely on its role as a coping strategy to mitigate the aversive conditions imposed by the intermittent presentation of food (e.g., Brett & Levine, 1981, observed reduced corticosterone levels as SIP training progressed). If SIP functions as an anti-anxiety behaviour, more licking should be expected in WKY than in the rest of rats. Results reported here were the opposite: WKY rats showed retardation in the acquisition of SIP in comparison to both SHR and Wistar rats, reaching with prolonged training (when developed) similar final levels of drinking. This learning deficit relates to other deficits observed in WKY rats under a variety of different behavioural tasks (e.g., McAuley et al., 2009). Conversely, SHRs developed SIP quicker than Wistar controls, and this could reflect less competition for expression from other behaviours within inter-food intervals (Íbias & Pellón, 2011) and/or an increased hyperactivity (Íbias & Pellón, 2014). Overall the present results seem to reflect that the functionality of SIP is more in line with adjustments of behaviour to reinforcement intermittency (see Falk, 1971; Killeen & Pellón, 2013) rather than with an anxiety-reduction function.
After 40 training sessions, not all rats acquired SIP above prandial levels, and were therefore excluded from further analysis. All excluded rats were Wistar and WKY; none were SHR. Even after excluding low responders, however, selected Wistar and WKY rats produced drinking episodes at a much lower rate than SHRs (Figure 2A). Furthermore, the episode durations of SHRs were longer than those of controls in FT 90-s (Figure 2C). Taken together, these results suggest that licking behaviour was stronger in SHR than in control strains, particularly in FT 90-s. Despite this difference, all selected rats produced enough ILIs in both schedules to demonstrate a superior fit of BERM relative to a more parsimonious alternative model (Table 1 and Figure 3).
The adequate fit of BERM suggests that ILIs are sampled from two exponentially distributed populations of ILIs, one with a shorter mean that corresponds to within-bout ILIs, and one with a longer mean that separates these bouts. This pattern of behaviour has 3 parameters: the proportion of ILIs sampled from the short distribution (p; which covaries positively with mean bout length), the within-bout licking rate (w), and the bout-initiation rate (b).
The pattern of differences in parameter estimates across strains is remarkably similar to the pattern of differences drawn from IRTs in variable-interval (VI) schedules of food reinforcement (Hill et al., 2012). This similarity is particularly striking when ILI and IRT distribution parameters are drawn from adult rats (Hill et al., 2012). Under these conditions, WKY produce very long bouts (i.e., high p) of both operant and adjunctive behaviour, compared to other strains (Figure 4A). Both operant and adjunctive bout lengths do not appear to be significantly affected by rate of reinforcement. No clear differences in within-bout response rate are observed across strains or schedules in either operant or adjunctive behaviour (Figure 4B). SHR produces substantially more operant and adjunctive bouts than Wistar, and Wistar produces more than WKY (Figure 4C). Taken together, these data suggest that differences across strains in the patterns of food-elicited behaviour are not dependent on response-food contingencies, even though bout-initiation rates are sensitive to the frequency of food delivery. The similarity of results across studies is consistent with the notion that the laws that govern adjunctive behaviours, such as SIP, are the same that govern operant behaviour (Killeen & Pellón, 2013).
Hill and colleagues (2012) infer from operant performance that operant hyperactivity (the inordinately high rate of responding at low rates of reinforcement) of SHR is due to a combination of steep reinforcement gradient (supporting shorter bouts) and, possibly, an enhanced responsiveness to reinforcement (supporting more frequent bouts). The present results extend these inferences to situations in which incentives are not contingent on behaviour. Thus, operant hyperactivity in SHR may be thought of as belonging to a wider class of hyperactive behaviour expressed by SHR, which may be called incentive-elicited hyperactivity.
Research Highlights.
Similar rate of licking induced by FT 30-s schedule in SHR, Wistar, and WKY rats
FT 90-s lengthened drinking episodes in SHR, reduced rate of licking in Wistar, WKY
Schedule-induced drinking occurred in bouts separated by pauses
SHR episodes comprised shorter, more frequent bouts of drinking
Acknowledgments
Research supported by Spanish Government grant PSI2011-29399 (Ricardo Pellón, Principal Investigator) (Ministerio de Economía y Competitividad, Secretaría de Estado de Investigación, Desarrollo e Innovación). Javier Íbias was under a UNED predoctoral research grant that supported 3 months stay at Arizona State University. Federico Sanabria was supported by grant MH094562 (National Institutes of Health, United States).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoric approach. 2nd ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- Brackney RJ, Cheung TH, Neisewander JL, Sanabria F. The isolation of motivational, motoric, and schedule effects on operant performance: a modelling approach. J Exp Anal Behav. 2011;96:17–38. doi: 10.1901/jeab.2011.96-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney RJ, Cheung TH, Herbst K, Hill JC, Sanabria F. Extinction learning deficit in a rodent model of attention-deficit hyperactivity disorder. Behav Brain Funct. 2012;13:8–59. doi: 10.1186/1744-9081-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett LP, Levine S. The pituitary-adrenal response to “minimized” schedule-induced drinking. Physiol Behav. 1981;26:153–158. doi: 10.1016/0031-9384(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Cardona D, López-Grancha M, López-Crespo G, Nieto-Escamez F, Sánchez-Santed F, Flores P. Vulnerability of long-term neurotoxicity of chlorpyrifos: Effect on schedule-induced polydipsia and a delay discounting task. Psychopharmacology. 2006;189:47–57. doi: 10.1007/s00213-006-0547-4. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Neisewander JL, Sanabria F. Extinction under a behavioral microscope: isolating the sources of decline in operant response rate. Behavioural processes. 2012;90(1):111–123. doi: 10.1016/j.beproc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Production of polydipsia in normal rats by an intermittent food schedule. Science. 1961;133:195–196. doi: 10.1126/science.133.3447.195. [DOI] [PubMed] [Google Scholar]
- Falk JL. Schedule-induced polydipsia as a function of fixed interval length. J Exp Anal Behav. 1966;9:37–39. doi: 10.1901/jeab.1966.9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Control of schedule-induced polydipsia: type, size, and spacing of meals1. J Exp Anal Behav. 1967;10(2):199–206. doi: 10.1901/jeab.1967.10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. The nature and determinants of adjunctive behavior. Physiol Behav. 1971;6:577–588. doi: 10.1016/0031-9384(71)90209-5. 1971. [DOI] [PubMed] [Google Scholar]
- Fleishman AI. Confidence intervals for correlation ratios. Educational and Psychological Measurement. 1980;40:659–670. [Google Scholar]
- Flores P, Sánchez-Kuhn A, Merchán A, Vilches O, García-Martín S, Moreno M. Schedule-Induced Polydipsia: Searching for the Endophenotype of Compulsive Behavior. World Journal of Neuroscience. 2014;4:253–260. [Google Scholar]
- Gilpin NW, Badia-Elder NE, Elder RL, Stewart RB. Schedule-induced Polydipsia in Lines of Rats Selectively Bred for High and Low Ethanol Preference. Behav Genet. 2008;38(5):515–524. doi: 10.1007/s10519-008-9224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken ER, Beninger RJ. The amphetamine sensitization model of schizophrenia symptoms and its effect on schedule-induced polydipsia in the rat. Psychopharmacology (Berl) 2013;231(9):2001–2008. doi: 10.1007/s00213-013-3345-9. [DOI] [PubMed] [Google Scholar]
- Hawken ER, Delva NJ, Beninger RJ. Increased drinking following social isolation rearing: implications for polydipsia associated with schizophrenia. PLoS One. 2013;8(2):e56105. doi: 10.1371/journal.pone.0056105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Herbst K, Sanabria F. Characterizing operant hyperactivity in the Spontaneously Hypertensive Rat. Behavioral Brain Functions. 2012;8(5):1–15. doi: 10.1186/1744-9081-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Íbias J, Pellón R. Schedule-induced polydipsia in the Spontaneously Hypertensive Rat and its relation to impulsive behaviour. Behav Brain Res. 2011;223:58–69. doi: 10.1016/j.bbr.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Íbias J, Pellón R. Different relations between schedule-induced polydipsia and impulsive behaviour in the Spontaneously Hypertensive Rat and in high impulisive Wistar rats: Questioning the role of impulsivity in adjunctive behaviour. Behav Brain Res. 2014;271:184–194. doi: 10.1016/j.bbr.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Kessel R, Lucke RL. An analtic form for the interrresponse time analysis of Shull, Gaynor, and Grimes with applications and extensions. Journal of the Experimental Analysis of Behavior. 2008;90:363–386. doi: 10.1901/jeab.2008.90-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P, Pellón R. Adjunctive behaviours are operants. Learn Behav. 2013;41:1–24. doi: 10.3758/s13420-012-0095-1. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, López-Crespo G, Sánchez-Amate MC, Flores P. Individual differences in schedule-induced polydipsia and the role of gabaergic and dopaminergic systems. Psychopharmacology. 2008;197:487–498. doi: 10.1007/s00213-007-1059-6. [DOI] [PubMed] [Google Scholar]
- McAuley JD, Stewart AL, Webber ES, Cromwell HC, Servatius RJ, Pang KCH. Wistar–Kyoto rats as an animal model of anxiety vulnerability: Support for a hypervigilance hypothesis. Behavioural Brain Research. 2009;204:162–168. doi: 10.1016/j.bbr.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Flores P. Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases. Psychopharmacology. 2012;219:647–659. doi: 10.1007/s00213-011-2570-3. [DOI] [PubMed] [Google Scholar]
- Moreno M, Gutierrez-Ferre VE, Ruedas L, Campa L, Sunol C, Flores P. Poor inhibitory control and neurochemical differences in high compulsive drinker rats selected by schedule-induced polydipsia. Psychopharmacology (Berl) 2012;219(2):661–672. doi: 10.1007/s00213-011-2575-y. [DOI] [PubMed] [Google Scholar]
- Myracle A, Lopez-Grancha M, Flores P, Glowa J, Riley AL. Differential effects of morphine and LiCl on schedule-induced polydipsia. Pharmacol Biochem Behav. 2005;80(1):195–202. doi: 10.1016/j.pbb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Plat B, Beber C, Schechter L, Rosenzweig-Lipson S. Schedule-induced polydipsia: a rat model of obsessive-compulsive disorder. Curr Protoc Neurosci. 2008;9:9–27. doi: 10.1002/0471142301.ns0927s43. [DOI] [PubMed] [Google Scholar]
- Riley AL, Wetherington CL. The differential effects of naloxone hydrochloride on the acquisition and maintenance of schedule-induced polydipsia. Pharmacol Biochem Behav. 1987;26(4):677–681. doi: 10.1016/0091-3057(87)90595-8. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24(1):31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sanabria F, Killeen PR. Evidence of impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;4:1–17. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: Effects of relative reinforcement and schedule type. Journal of the experimental analysis of behavior. 2001;75(3):247–274. doi: 10.1901/jeab.2001.75-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull RL, Grimes JA, Bennett JA. Bouts of responding: The relation between bout rate and the rate of variable-interval reinforcements. Journal of the Experimental Analysis of Behavior. 2004;81:65–83. doi: 10.1901/jeab.2004.81-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg L, Losee S, Turek F, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integ Comp Physiol. 2001;281:786–794. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- Staddon JER. Schedule-induced behavior. In: Honig WK, Staddon JER, editors. Handbook of Operant Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1977. pp. 125–152. [Google Scholar]
- Taylor D, Lincoln AJ, Foster SL. Impaired behavior regulation under conditions of concurrent variable schedules of reinforcement in children with ADHD. Journal of attention disorders. 2010;13(4):358–368. doi: 10.1177/1087054708329974. [DOI] [PubMed] [Google Scholar]
- Van Kuyck K, Brak K, Das J, Rizopoulos D, Nuttin B. Comparative study of the effects of electrical stimulation in the nucleus accumbens, the mediodorsal thalamic nucleus and the bed nucleus of the stria terminalis in rats with schedule-induced polydipsia. Brain Res. 2008;1201:93–99. doi: 10.1016/j.brainres.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiat. 2003;8:925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]